Abstract
Cancer immune surveillance is considered to be an important host protection process to inhibit carcinogenesis and to maintain cellular homeostasis. In the interaction of host and tumour cells, three essential phases have been proposed: elimination, equilibrium and escape, which are designated the ‘three E’s'. Several immune effector cells and secreted cytokines play a critical role in pursuing each process. Nascent transformed cells can initially be eliminated by an innate immune response such as by natural killer cells. During tumour progression, even though an adaptive immune response can be provoked by antigen-specific T cells, immune selection produces tumour cell variants that lose major histocompatibility complex class I and II antigens and decreases amounts of tumour antigens in the equilibrium phase. Furthermore, tumour-derived soluble factors facilitate the escape from immune attack, allowing progression and metastasis. In this review, the central roles of effector cells and cytokines in tumour immunity, and the escape mechanisms of tumour cells during tumour progression are discussed.
Keywords: cancer, immune escape, immune surveillance, immunoediting
Introduction
Since Ehrlich in 1909 first proposed the idea that nascent transformed cells arise continuously in our bodies and that the immune system scans for and eradicates these transformed cells before they are manifested clinically, immune surveillance has been a controversial topic in tumour immunology.1 In the mid-20th century, experimental evidence that tumours could be repressed by the immune system came from tumour transplantation models. The findings from these models strongly suggested the existence of tumour-associated antigens and formed the basis of immune surveillance, which was postulated by Burnet and Thomas.2 After that the functional role of antigen-presenting cells in cross-priming for T-cell activation was demonstrated, and the cancer immune surveillance model was developed. However, the idea of cancer immune surveillance resisted widespread acceptance until the 1990s when experimental animal models using knockout mice validated the existence of cancer immune surveillance in both chemically induced and spontaneous tumours. The central roles of immune effector cells, such as B, T, natural killer (NK) and natural killer T (NKT) cells, and of type I and II interferons (IFNs), and perforin (pfp) have since been clarified in cancer immune surveillance.3,4
As part of the current concept of cancer immunoediting leading from immune surveillance to immune escape, three essential phases have been proposed:3 (1) elimination; (2) equilibrium; and (3) escape. Nascent transformed cells can be eliminated initially by immune effector cells such as NK cells and by the secreted IFN-γ in an innate immune response. Elimination of transformed cells results in immune selection and immune sculpting, which induce tumour variants that decrease immunogenicity and become resistant to immune effector cells in the equilibrium phase. Eventually, during tumour progression, when the increased tumour size can be detected by imaging diagnosis, tumour-derived soluble factors (TDSFs) can induce several mechanisms for escape from immune attack in the tumour microenvironment.5 In this review, a general overview is provided and the basic principles of immunoediting from immune surveillance to escape, and the central role of immune effector cells in the process of immunoediting are discussed. A better understanding of the mechanisms of immunoediting during tumour progression may provide new insights for improving cancer immunotherapy.
Cancer immune surveillance
Historical background
In the early 20th century, Ehrlich first proposed the existence of immune surveillance for eradicating nascent transformed cells before they are clinically detected.1 In the mid-20th century, 50 years later, Burnet and Thomas postulated that the control of nascent transformed cells may represent an ancient immune system, which played a critical role in surveillance against malignant transformation.2 This idea was supported by experimental results showing strong immune-mediated rejection of transplanted tumours in mice. Although there is excellent evidence to support the belief that immune surveillance mechanisms prevent the outgrowth of tumour cells induced by horizontally transmitted, ubiquitous, potential oncogenic viruses, there is much less evidence for immune surveillance acting against chemically induced tumours in syngeneic mice.6 However using genetically identical mice, tumour-specific protection was generated from methylcholanthrene (MCA) and virally induced tumours.7,8 These results from mouse models strongly suggested the existence of tumour-associated antigens and immune surveillance for protection from transformed cells in the host, as was postulated by Burnet and Thomas.2,9,10 Despite the fact that several lines of evidence from experimental mouse models showed that the immune system played a critical role in dealing with transformed cells, there was no increased incidence of spontaneous or chemically induced tumours in athymic nude mice, as compared to wild-type animals.11,12 This suggested that immune surveillance in mice targeted transforming viruses but not tumours.2 It is now known that athymic nude mice have NK cells and fewer T cells that can contribute to immune surveillance than wild-type mice. Further, they have detectable populations of functional T-cell receptor-αβ (TCR-αβ) -bearing lymphocytes.13,14 Nevertheless, when MCA was injected into nude and control mice with different doses of MCA, nude mice formed more tumours than controls.15 Similarly, tumour formation induced by MCA was greater in immunodeficient (SCID) mice than in wild-type BALB/c mice.16
Experimental evidence for immune surveillance
NK, NKT and γδ T cells
During the mid-1970s to the 1990s, several experimental studies have attempted to demonstrate the immune surveillance concept. The discovery of NK cells provided a considerable stimulus for the possibility that they functioned as the effectors of immune surveillance,17 even though a precise definition and understanding of these cells had not been confirmed. Later, and in the 2000s however, gene-targeted and lymphocyte subset-depleted mice were used to establish the relative importance of NK and NK1.1+ T (natural killer T, NKT) cells in protecting against tumour initiation and metastasis. In these models, CD3+ NK cells were responsible for tumour rejection and protection from metastasis in models where control of major histocompatibility complex (MHC) class I-deficient tumours was independent of interleukin-12 (IL-12).18 C57BL/6 mice that were depleted of both NK and NKT cells by the anti-NK1.1 monoclonal antibody, which can eliminate both NK and NKT cells, were two to three times more susceptible to MCA-induced tumour formation than control mice.19 A similar result was observed in C57BL/6 mice treated with anti-asiaro-GM1, which selectively eliminates NK but not NKT cells, even though anti-asiaro-GM1 can also eliminate activated macrophages. A protective role for NKT cells was only observed when tumour rejection required endogenous IL-12 activity. In particular, studies in TCR Jα281 gene-targeted mice confirmed a critical function for NKT cells in protecting against spontaneous tumours initiated by the chemical carcinogen, MCA. Jα281–/– mice, lacking Vα14Jα281-expressing invariant NKT cells, formed MCA-induced sarcomas at a higher frequency than wild-type mice.18 Another study showed that mice treated with the NKT cell-activating ligand α-galactosylceramide throughout MCA-induced tumorigenesis exhibited a reduced incidence of tumours and displayed a longer latency period to tumour formation than control mice.20
Mice lacking γδ T cells were highly susceptible to multiple regimens of cutaneous carcinogenesis. After exposure to carcinogens, skin cells expressed Rae-1 and H60, MHC-related molecules that structurally resemble human MHC class I chain-related A (MICA). Each of these is a ligand for NKG2D, a receptor expressed by cytolytic T cells and NK cells. In vitro, skin-associated NKG2D+ γδ T cells killed skin carcinoma cells by a mechanism that was sensitive to blocking NKG2D engagement.21 The localization of γδ T cells within epithelia may contribute to the down-regulation of epithelial malignancies.
Interferon-γ
Endogenously produced interferon-γ (IFN-γ) protected the host against transplanted tumours and the formation of chemically induced and spontaneous tumours. When the mice were treated with neutralizing monoclonal antibody to IFN-γ, the growth of immunogenic sarcomas transplanted into mice grew faster than in the control mice.22 Overexpression of the truncated dominant negative form of the murine IFN-γ receptor α-subunit (IFNGR1) in Meth A fibrosarcoma completely abrogated tumour sensitivity to IFN-γ, and the tumours showed enhanced tumorigenicity and reduced immunogenicity when they were transplanted into syngeneic BALB/c mice.22 These results showed that IFN-γ had direct effects on tumour cell immunogenicity and played an important role in promoting tumour cell recognition and elimination. In a study of MCA-induced tumour formation, compared with wild-type mice, mice lacking sensitivity to either IFN-γ (IFNGR-deficient mice) or all IFN family members (Stat1-deficient mice; Stat1 being the transcription factor that is important in mediating IFNGR signalling) developed tumours more rapidly and with greater frequency when challenged with different doses of the chemical carcinogen MCA. In addition, IFN-γ-insensitive mice developed tumours more rapidly than wild-type mice when bred onto a background that was deficient for the p53 tumour-suppressor gene.23 IFN-γ-insensitive p53–/– mice also developed a broader spectrum of tumours compared with mice lacking p53 alone. The importance of this experiment lay in the discovery that certain types of human tumours become selectively unresponsive to IFN-γ. Thus, IFN-γ forms the basis for an extrinsic tumour-suppressor mechanism in immunocompetent hosts. Using experimental (B6, RM-1 prostate carcinoma) and spontaneous (BALB/c, DA3 mammary carcinoma) models of metastatic cancer, mice deficient in both pfp and IFN-γ were significantly less proficient than pfp-deficient or IFN-γ-deficient mice in preventing metastasis of tumour cells to the lung. Both pfp-deficient and IFN-γ-deficient mice were equally as susceptible as mice depleted of NK cells in both tumour metastasis models, and IFN-γ appeared to play an early role in protection from metastasis.24 Further analysis demonstrated that IFN-γ, but not pfp, controlled the growth rate of sarcomas arising in these mice, and that host IFN-γ and direct cytotoxicity mediated by cytotoxic lymphocytes expressing pfp independently contributed antitumour effector functions that together controlled the initiation, growth and spread of tumours in mice. In another study, both IFN-γ and pfp were critical for the suppression of lymphomagenesis, but the level of protection afforded by IFN-γ was strain specific. Lymphomas arising in IFN-γ-deficient mice were very non-immunogenic compared with those derived from pfp-deficient mice, suggesting a comparatively weaker immune selection pressure by IFN-γ.25 A significant incidence of late onset adenocarcinomas observed in both IFN-γ-deficient and pfp-deficient mice indicated that some epithelial tissues were also subject to immune surveillance.
Perforin and Fas/FasL system
Perforin and Fas/Fas ligand (FasL) are the other important factors involved in immune surveillance. In general, cell-mediated cytotoxicity attributed to cytotoxic T lymphocytes (CTLs) and NK cells are derived from either the granule exocytosis pathway or the Fas pathway. The granule exocytosis pathway utilizes pfp to direct the granzymes to appropriate locations in target cells, where they cleave critical substrates that initiate apoptosis. Granzymes A and B induce death via alternate, non-overlapping pathways. The Fas/FasL system is responsible for activation-induced cell death but also plays an important role in lymphocyte-mediated killing under certain circumstances.26 The interplay between these two cytotoxic systems provides opportunities for therapeutic interventions to control malignant disease, but oversuppression of these pathways also leads to decreased tumour cell killing. In fact, C57BL/6 mice lacking pfp (i.e. pfp–/–) were more susceptible for MCA-induced tumour formation. In MCA-induced tumour formation, pfp–/– mice developed significantly more tumours compared with pfp-sufficient mice treated in the same manner.24,25 In addition, a previous study showed that pfp-dependent cytotoxicity is not only a crucial mechanism of both CTL-dependent and NK-dependent resistance to injected tumour cell lines, but also operates during viral and chemical carcinogenesis that were induced by MCA, or 12-O-tetradecanoylphorbol-13-acetate (TPA) plus 7,12-dimethylbenzanthracene (DMBA), or by injection of oncogenic Moloney sarcoma virus in vivo.27 Experiments addressing the role of Fas-dependent cytotoxicity by studying resistance to tumour cell lines that were stably transfected with Fas failed to detect a major role for Fas in tumour control, but cannot exclude a minor contribution of Fas in tumour surveillance.27 Another study showed that pfp–/– mice have a high incidence of malignancy in distinct lymphoid cell lineages (T, B, NKT), indicating a specific requirement for pfp in protection against lymphomagenesis.28 The susceptibility to lymphoma was enhanced by the simultaneous lack of expression of the p53 gene. Mice that were pfp–/– were at least 1000-fold more susceptible to these lymphomas when transplanted, compared with immunocompetent mice, in which tumour rejection was controlled by CD8+ T lymphocytes.28 Taken together, these results indicate that components of the immune system were involved in controlling primary tumour development, and showed the differential role of pfp and IFN-γ in protecting tumour formation between lymphoid and epithelial malignancies.
Lymphocytes
Although evidence had accumulated that the immune surveillance of cancer was dependent on both IFN-γ and lymphocytes, the critical demonstration for the involvement of lymphocytes came from the use of gene-targeted mice lacking the recombination activating gene 1 (Rag1) or Rag2. Homozygous mutants of Rag-2 are viable but fail to produce mature B or T lymphocytes.29 Loss of the Rag2 function in vivo results in a total inability to initiate VDJ rearrangement, leading to a novel SCID phenotype. Rag2 function and VDJ recombinase activity, per se, are not required for the development of cells other than lymphocytes. Since nude mice do not completely lack functional T cells and the two components of the immune system, IFN-γ and pfp, to prevent tumour formation in mice, an elegant study using a Rag2–/– and Stat1–/– mouse model showed for the first time that lymphocytes and IFN-γ collaborate to prevent the formation of carcinogen-induced sarcomas and spontaneous epithelial carcinomas.30 Both the wild-type and Rag2–/– mice had the same genetic background and were injected with MCA and monitored for tumour formation. Rag2–/– mice formed tumours earlier than wild-type mice and with greater frequency. After 160 days, nine out of 15 Rag2–/– mice but only two out of 15 wild-type mice had formed MCA-induced tumours. The increased tumour formation in Rag2–/– mice was comparable to findings in IFN-γ-insensitive mice that lacked either the Ifngr1 (12 out of 20) or Stat1 (17 out of 30) genes versus wild-type mice (11 out of 57). In the collaboration between the lymphocyte- and IFN-γ/Stat1-dependent tumour suppressor mechanisms, mice lacking both genes, i.e. Rag2–/– × Stat1–/– mice (RkSk mice) showed increased susceptibility to MCA-induced tumours with 13 out of 18 mice being susceptible compared to 11 out of 57 wild-type mice. However, they did not show a significantly increased incidence compared to mice that lacked either Rag2 or Stat1. Thus, these findings indicated that T, NKT and/or B cells are essential to suppress the formation of chemically induced tumours, and also indicated the presence of an extensive overlap between lymphocytes and Stat1-dependent IFN-γ-signalling. As for the effect of tumour suppressor mechanisms on spontaneous tumours, nine out of 11 wild-type mice were free of malignant disease, two had adenomas, but none had cancer. In contrast, all 12 Rag2–/– mice showed malignant lesions in the intestinal tract and elsewhere. Half of these mice developed malignant diseases: three caecal adenocarcinoma; one ileocaecal adenocarcinoma; one small intestinal adenocarcinoma; one lung adenocarcinoma. In addition, six out of 11 RkSk mice developed mammary carcinomas including two adenocarcinomas and a distinct adenocarcinoma in the breast and caecum in one mouse. The other five RkSk mice did not show palpable masses but the following were found at necropsy: two caecal adenocarcinomas; one caecal and lung adenocarcinoma; two intestinal adenomas. Overall, 82% of the RkSk mice formed spontaneous cancers. Thus, these findings indicate that the lack of lymphocytes, either alone or in combination with the IFN-γ-signalling defect, causes significantly more susceptibility to spontaneous epithelial tumour formation than is found in their wild-type counterparts. Moreover, RkSk mice form more spontaneous cancers than Rag2–/– mice, suggesting that the overlap of the tumour suppressor mechanisms mediated by lymphocytes and IFN-γ/Stat1-signalling may only be partially effective.30
In another report, the relative contributions of αβ and γδ T cells in blocking tumour formation by chemical carcinogens such as MCA, DMBA and TPA, and the injection of the squamous cell carcinoma cell line PDV in TCR-β–/–, TCR-δ–/– and TCR-β–/– TCR-δ–/– mice, which lack αβ, γδ, and all T cells, were studied.21 Comparing tumour formation using PDV cells between wild-type and TCRδ–/– mice, 41 out of 110 sites developed tumours in TCRδ–/– mice, whereas 13 out of 134 sites developed tumours in wild-type mice. Although tumour latency accounted for a minor reduction, γδ T cells reduced the number of tumours formed. In contrast, in TCRβ–/– and TCRb–/– TCRδ–/– mice, nearly 100% of sites showed tumour formation and the latency was substantially reduced. These findings indicate that αβ T cells and γδ T cells regulate the tumour growth of PDV cells in a distinct fashion, and that the lack of γδ T cells is not compensated by the presence of αβ T cells and NK cells. In addition, as to the role of γδ T cells in the development of MCA-induced sarcomas and spindle cell carcinomas, an increase in the number of tumours formed in TCRδ–/– and TCRβ–/– mice after MCA injection was observed compared to FVB mice,21 In naturally occurring human carcinomas induced by DMBA and TPA, 67% of TCRδ–/– mice showed tumour formation with increased tumour burden compared to 16% of wild-type mice. In contrast, TCRβ–/– and wild-type mice were equally susceptible to DMBA-induced and TPA-induced carcinogenesis. In addition, TCRδ–/– mice also showed a higher incidence of progression of papillomas into carcinomas. These findings indicate a distinct additional contribution to the regulation of tumour growth in γδ T cells and αβ T cells. In turn, it seems that γδ T cells act to inhibit initial tumour formation that converts to malignant progression, whereas αβ T cells directly inhibit tumour progression by using their cytotoxic mechanisms to kill tumour cells. Thus, both the previous and the recent data support the following basic concept of cancer immune surveillance originally proposed by Burnet and Thomas: that the naturally existing immune system can recognize nascent transformed cells and can eliminate primary tumour formation by lymphocytes and secreted cytokines, both of which are important protective mechanisms in the host. The studies that used inbred mouse lines targeting disruptions in genes encoding critical components of the immune system are listed in Table 1, which supports the control of tumour formation by the immune systems of both innate and adaptive immune compartments in cancer immune surveillance.
Table 1.
Experimental studies on the mechanisms of immune surveillance using gene knockout mice
| Target gene | Target/effector cell | Tumour formation | Ref. |
|---|---|---|---|
| TCR J alpha 281 | NKT | MCA-induced sarcoma | 19 |
| TCR delta | gamma delta T | MCA-induced sarcoma DMBA-induced skin tumour | 21 |
| TCR beta TCR beta/TCR delta | alpha beta T T/gamma delta T | MCA-induced sarcoma Reduced latency | 21 |
| IFN-gamma | IFN-gamma | MCA-induced sarcoma Spontaneous lymphoma Lung adenocarcinoma | 24, 25 |
| Stat1 | IFK-gamma R-signaling | MCA-induced sarcoma | 23, 30 |
| Perforin | CTL/NK | MCA-induced sarcoma Spontaneous lymphoma TPA/DMBA-induced sarcoma | 24, 25, 27, 28 |
| RAG-2/Stat1 | T/B/NKT/IFN-signaling | MCA-induced sarcoma Spontaneous epithelial and mammary carcinoma | 30 |
| IFNGR1 or Stat1/p53 | IFN-gamma R-signaling/tumour susceptibility | More rapid tumour formation/wider tumour spectrum | 23 |
| Perforin/p53 | CTL/NK/tumour susceptibility | Enhances susceptibility to lymphoma | 28 |
TCR, T cell receptor; IFN, interferon; Stat1, signal transducers and activators of transcription 1; NKT, natural killer T cell; IFNGR, interferon gamma receptor; CTL, cytotoxic T lymphocyte; NK, natural killer; MCA, methylcholanthrene; TPA, 12-O-tetradecanoylphorbol-13-acetate; DMBA, 7,12-dimethylbenzanthracene.
Type I interferons
Much less is known about the involvement in the cancer immunoediting process of the type I interferons (IFN-α/β), which regulate immunological functions and induce the same biological effects as IFN-γ. Some of the previous studies suggested a potential antitumour function for endogenously produced IFN-α/β. This was demonstrated by showing that neutralization of IFN-α/β using polyclonal antibodies in mice enhanced the growth of transplanted, syngeneic tumour cells in immunocompetent mice,31,32 and the rejection was abrogated in the allografts or tumour xenografts.33 In a recent study on the potential function of endogenously produced IFN-α/β in cancer immunoediting for tumour transplantation and primary tumour formation, endogenously produced IFN-α/β rejected highly immunogenic and syngeneic mouse sarcomas.4 Furthermore, although tumour cell immunogenicity was not influenced by the sensitivity to IFN-α/β, the requirement for IFN-α/β sensitivity in the antitumour immune response for a host-protective effect depends on the level of haematopoietic cells. The host-protective effect of IFN-α/β was not completely overlapped by that of IFN-γ, indicating that IFN-α/β clearly played an important role and was a critical component in the process of cancer immunoediting. In this report, endogenously produced IFN-α/β rejected the tumour formation of highly immunogenic MCA-induced sarcomas and also inhibited the outgrowth of primary carcinogen-induced tumours in immunocompetent mice. Furthermore, MCA-induced sarcomas derived from IFN-α receptor 1-deficient (Ifnar1–/–) mice were rejected in a lymphocyte-dependent manner in wild-type mice. This suggested that tumours formed in the absence of IFN-α/β responsiveness are more immunogenic than those formed in immunocompetent mice, which differs from the poor immunogenicity in tumours derived from Ifngr1–/– mice. Unlike the case of IFN-α/β, this poor immunogenicity can be rendered highly immunogenic and can be rejected when IFN-γ sensitivity is recovered by enforced expression of Ifngr1.23,34 Thus, the finding that the functions of IFN-α/β and IFN-γ for cancer immunoediting do not completely overlap is supported by the differential effects of these cytokines on tumour cell immunogenicity.
Type I interferons are considered to be an important link between innate and adaptive immunity35 and this function acts primarily on several different bone marrow-derived cell subsets for tumour elimination. IFN-α/β has been shown to activate dendritic cells (DCs),36 and to increase the cytotoxic activity of NK cells through the induction of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL).37 In addition, IFN-α-expressing tumour cells can promote antitumour immunity by preventing apoptotic cell death after stimulation of T lymphocytes.38 Also, type I IFNs promote the development of memory-phenotype CD8+ T (but not CD4+) cells through the induction of IL-15.39 These findings indicate that the editing function of the immune system during tumour progression is served not only by lymphocytes and IFN-γ but also by IFN-α/β. Nevertheless, the involvement of the endogenously produced IFN-α/β in a host-protective function for naturally occurring antitumour immune responses to spontaneous tumour formation remains to be elucidated. Previous experimental and clinical studies in which exogenous IFN-α/β was administered showed that it may serve as an important immunostimulator to enhance antitumour immune responses that contribute to tumour reduction. Whether type I interferon is actively and continuously induced during tumour progression by specific cells such as tumours or non-tumorous components in host protection is still not known. Further molecular and cellular analyses to identify type I IFNs and for determining their responsiveness in cancer immunoediting will be needed.
Clinical evidence for immune surveillance
Tumour-infiltrating lymphocytes (TILs)
It is readily accepted that TILs in tumours can attack and eradicate tumour cells in the cancer patient. In fact, the presence of intratumour TILs is important evidence for an immune response between tumour cells and immune effector cells. Several previous studies have shown that the high-grade density of CD8+ T cells in cancer cell nests was correlated with prognosis, and the presence of TILs was able to predict a better survival as an independent prognostic factor in various types of cancers including colon cancer,40,41 oesophageal cancer,42 oral squamous cell carcinoma,43 breast cancer,44 ovarian cancer,45 and malignant melanoma.46 Of importance, the T lymphocytes recruited around the tumour site (peritumour site) do not always contribute to the antitumour immune response but rather intratumour T lymphocytes are important for eradicating tumour cells.40 Other studies have shown a similar positive correlation between NK cell infiltration and the survival for gastric cancer,47 colorectal cancer48 and squamous cell lung cancer.49 Thus, significant evidence has been presented for a link between the presence of TILs and increased survival in cancer patients.
Organ transplant-related cancer
The theory that cancer may arise under conditions of reduced immune capacity is supported by observations in humans with immune deficiencies such as those that occur following organ transplants. Increased relative risk ratios for various types of cancers have been observed in immunosuppressed transplant recipients that have no apparent viral origin. Information on 5692 Nordic recipients of renal transplants in 1964–82 was linked to the national cancer registries in 1964–86 and to population registries.50 Significant overall excess risks of two- to five-fold were seen in both sexes for cancers of the colon, larynx, lung and bladder, and in men for cancers of the prostate and testis. Notable high risks ranging from 10- to 30-fold above expectations, were associated with cancers of the lip, skin (non-melanoma), kidney and endocrine glands, non-Hodgkin's lymphoma, and in women with cancers of the cervix and vulva–vagina.50 Kidney transplantation increases the risk of cancer in the short term and in the long term, consistent with the theory that an impaired immune system allows carcinogenic factors to act. The other study on the development of solid-organ tumours after cardiac transplantation reported that 38 solid tumours were identified in 36 (5·9%) of 608 cardiac transplant recipients who survived more than 30 days. The tumours included the following types: skin, lung, breast, bladder, larynx, liver, parotid, testicle, uterus and melanoma.51 A recent review reported a high frequency of skin cancers and lymphoproliferative diseases in renal transplant recipients.52 However, a survey of the literature showed that the relative frequency of malignancy after renal transplantation varied widely between different geographical regions. The type of malignancy is different in various countries and dependent on genetic and environmental factors. The hypothesis that the action of immunosuppressive drugs is responsible for the increased incidence of cancers in transplant recipients is supported by the observation that patients also develop cancers if they receive immunosuppressive therapy for conditions other than transplantation, e.g. rheumatoid arthritis or systemic lupus erythematosus.
The other possibility for organ transplant-related cancer is the transmission of a tumour via micrometastasis of an undiagnosed malignancy in the donor after transplantation. According to data from the Organ Procurement and Transplantation Network/UNOS, 21 donor-related malignancies were reported out of 108062 transplant recipients.53 Except for 15 tumours that existed in the donor at the time of transplantation, six tumours were de novo donor-derived tumours that developed in transplanted haematogenous or lymphoid cells of the donor. Similar donor-derived tumours have been reported in allografts obtained from donors with breast cancer and malignant melanoma.54 These de novo tumours could be activated by the use of immunosuppressive drugs in the recipient even though the de novo tumours might also be inactivated by immune surveillance in the donor before transplantation.
Non-immunological surveillance
The concept of cancer immune surveillance has been formulated based on the hypothesis that cancer cells are recognized as non-self and are capable of inducing a rejection reaction. The immune system contributes to the surveillance of spontaneously developing tumours as well as of virally induced tumours. Given that the immune system alone is not responsible for protecting primary tumour formation, there is still a need for an intrinsic non-immune surveillance system that regulates the growth of tumour cells. There are two major forms of non-immune surveillance. One is DNA repair as intracellular surveillance, which is observed in the increased incidence of tumours in xeroderma pigmentosum, in which there are several deficiencies of mismatch repair enzymes. The other is intracellular surveillance, which is well documented in apoptotic cell death elicited by DNA damage or the activation of oncogenes. Since the definition of non-immune surveillance is control of tumour cell growth and tumour progression, escape from non-immune surveillance in the early stages and from subsequent immune surveillance in the late stages is associated with an increased resistance to apoptosis. The p53 pathway is a well-known example of genetic surveillance. Upon DNA damage, wild-type p53 is up-regulated and binds DNA to induce growth arrest, allowing DNA repair.55 Since the p53 gene is inactivated in about 50% of human cancers that impair the DNA binding capacity of the protein,56 cell growth can continue despite DNA damage, resulting in tumour development. Inherited mutations in p53 seen in the Li–Fraumeni syndrome are associated with increased susceptibility to malignant diseases.57 The relative contributions of non-immune surveillance compared with immune surveillance remain to be elucidated, but it is likely that they are complementary and not redundant. In fact, p53-deficient mice are susceptible to the formation of spontaneous tumours,58 as demonstrated when mice produced by the crossing of p53-deficient mice and pfp-deficient mice showed disseminated lymphomas, indicating a direct involvement of cytotoxic lymphocytes in cancer immune surveillance.28 Mice lacking p53 and IFN-γ receptor, or p53 and Stat1, showed a wider spectrum of tumours than those lacking only p53.23
As for other types of non-immune surveillance aside from the two major forms already discussed, the existence of intercellular surveillance has been documented. This is elicited by the interaction of cancer cells and surrounding normal cells in the tumour microenvironment that influences the probability of disseminated tumour cell growth.59 In addition, recent studies suggest that there is a genetically determined variation in the stringency of chromatin imprinting. More relaxed imprinting may lead to increased cancer risk, and has been termed epigenetic surveillance. The immune evasion by tumours that is mediated by non-mutational epigenetic events involving chromatin and epigenetics collaborates with mutations in determining tumour progression.60
Cancer immunoediting
Elimination
Elimination is the hallmark of the original concept in cancer immune surveillance for the successful eradication of developing tumour cells, working in concert with the intrinsic tumour suppressor mechanisms of the non-immunogenic surveillance process. The process of elimination includes innate and adaptive immune responses to tumour cells. For the innate immune response, several effector cells such as NK, NKT, and γδ T cells are activated by the inflammatory cytokines, which are released by the growing tumour cells, macrophages and stromal cells surrounding the tumour cells. The secreted cytokines recruit more immune cells, which produce other pro-inflammatory cytokines such as IL-12 and IFN-γ. Perforin-, FasL- and TRAIL-mediated killing of tumour cells by NK cells releases tumour antigens (TAs), which lead to adaptive immune responses.28,61,62 In the cross-talk between NK cells and DCs,63 NK cells promote the maturation of DCs and their migration to tumour-draining lymph nodes (TDLNs), resulting in the enhancement of antigen presentation to naive T cells for clonal expansion of CTLs. The TA-specific T lymphocytes are recruited to the primary tumour site, and directly attack and kill tumour cells with the production of cytotoxic IFN-γ.
The following four phases have been proposed for the elimination process.3 (1) Recognition of tumour cells by innate immune cells and their limited killing: when a solid tumour has grown to more than 2–3 mm, it requires a blood supply and stromal remodelling for tumour progression, which in turn induces pro-inflammatory signals leading to the recruitment of innate immune cells such as NK, NKT, γδ T cells, macrophages and DCs into the tumour site.64,65 The transformed cells can be recognized by infiltrating lymphocytes such as NK, NKT and γδ T cells, which produce IFN-γ.66,67 (2) Maturation and migration of DCs and cross-priming for T cells: IFN-γ exerts a limited cytotoxicity via antiproliferative68 and anti-angiogenic effects,69 and induces apoptosis.70 Some of the chemokines derived from tumours and surrounding non-tumorous tissues block the formation of new blood vessels even while continuing to induce tumour cell death.3,71 Necrotic tumour cells are ingested by immature DCs (iDCs), which have matured under pro-inflammatory conditions, and have migrated to TDLNs. (3) Generation of TA-specific T cells: the recruited tumour-infiltrating NK and macrophages produce IL-12 and IFN-γ, which kill more tumour cells by activating cytotoxic mechanisms such as perforin, TRAIL and reactive oxygen.72,73 In the TDLNs, the migrated DCs present TAs to naive CD4+ T cells that differentiate to CD4+ T cells, which develop TA-specific CD8+ T cells that lead to clonal expansion. (4) Homing of TA-specific T cells to tumour site and elimination of tumour cells. Tumour antigen-specific CD4+ and CD8+ T cells home to the primary tumour site, where the CTLs eliminate the remaining TA-expressing tumour cells; this is enhanced by the secreted IFN-γ, but also selects for tumour cells with reduced immunogenicity.30
Regarding the recognition of tumour cells, how the unmanipulated immune system can be activated in a developing tumour has been controversial, even though tumour-specific antigens may be expressed as distinct recognition molecules on the surface of tumour cells. As a hypothesis of danger theory, it was considered that cellular transformation did not provide sufficient pro-inflammatory signals to activate the immune system in response to a developing tumour. In the absence of such signals, there is often no immune response and tolerance may develop.65 However, recent studies indicate that danger signals such as uric acid,74 the potential toll-like receptor ligands such as heat-shock proteins75 or a ligand transfer molecule in the signalling cascade induced by CpG DNA,76 and extracellular matrix (ECM) derivatives,77 may induce pro-inflammatory responses that activate innate immune responses to foreign pathogens. Danger signals are thought to act by stimulating the maturation of DCs so that they can present foreign antigens and stimulate T lymphocytes. Dying mammalian cells have also been found to release danger signals of unknown identity. Of note, although local limited inflammation may be involved in initiating immune responses, excessive inflammation may promote tumour progression in steady-state conditions.78 This may be in part because of the anti-inflammatory reactions in antigen-presenting cells, which release anti-inflammatory cytokines such as IL-10 and transforming growth factor-β (TGF-β) that inhibits the activation of effector cells.79
Equilibrium
The next step in cancer immunoediting proceeds to the equilibrium phase in which a continuous sculpting of tumour cells produces cells resistant to immune effector cells. This process leads to the immune selection of tumour cells with reduced immunogenicity. These cells are more capable of surviving in an immunocompetent host, which explains the apparent paradox of tumour formation in immunologically intact individuals. Although random gene mutations may occur within tumours that produce more unstable tumours, these tumour cell variants are less immunogenic, and the immune selection pressure also favours the growth of tumour cell clones with a non-immmunogenic phenotype. Several experimental studies using mice with different deficiencies of effector molecules indicated various degrees of immune selection pressure. The lymphomas that formed in pfp-deficient mice were more immunogenic than those in IFN-γ-deficient mice, suggesting that pfp may be more strongly involved in the immune selection of lymphoma cells than IFN-γ.25 In contrast, MCA-induced sarcomas in IFN-γ-receptor-deficient mice are highly immunogenic.30 Furthermore, chemically induced sarcomas in both nude and severe combined immunodeficiency (SCID) mice were more immunogenic than similar tumours from immunocompetent mice.16,18,30,80 These findings suggest that the original tumour cells induced in normal mice and selected by a T-cell-mediated selection process have been adapted to grow in a host with a functional T-cell system, which has eliminated highly immunogenic tumour cells, leaving non-immunogenic tumour cells to grow. There is however, no connection between this loss of immunogenicity and the loss of MHC class I expression. Furthermore, two important issues can be suggested. One is that perforin-mediated cytotoxicity in T cells contributes more to the elimination of lymphoma cells than epithelial tumour cells, whereas IFN-γ-mediated cytotoxicity is directed more to the elimination of mesenchymal tumour cells such as sarcomas. The other is the higher immunogenicity of the tumours derived from immunodeficient mice than those from immnuocompetent mice indicated less immune selection pressure in the tumours derived from immunodeficient mice than in those of immunocompetent mice. Thus, T-cell-mediated elimination has adapted to highly immunogenic tumours, such as chemically and virally induced tumours. On the other hand, the immune selection pressure induces less immunogenic tumour variants that survive and grow in the tumour microenvironment. In cases of spontaneous tumours appearing for a long period of time, the immunogenic sculpting also produces fewer immunogenic tumours than chemically and virally induced tumours.
Since the equilibrium phase involves the continuous elimination of tumour cells and the production of resistant tumour variants by immune selection pressure, it is likely that equilibrium is the longest of the three processes in cancer immunoediting and may occur over a period of many years.81 In this process, lymphocytes and IFN-γ play a critical role in exerting immune selection pressure on tumour cells. During this period of Darwinian selection, many tumour variants from the original are killed but new variants emerge carrying different mutations that increase resistance to immune attack. Since the equilibrium model persists for a long time in the interaction between cancer cells and the host, the transmission of cancer during organ transplantation can be considered. One report described the appearance of metastatic melanoma 1–2 years after transplantation in two patients receiving renal transplants from the same donor. The donor had been previously treated for melanoma 16 years earlier and was considered tumour-free.82 Several similar observations have been reported in recipients of allografts from those considered as healthy donors.83–85 In speculating on the appearance of the transmitted cancer, tumours may have been kept in equilibrium in the donor, and conceivably activated by the continuous administration of immunosuppressive drugs that facilitated the growth of occult cancer.81
Escape
Alterations in signal transduction molecules on effector cells
Given the lack of TA recognition, which is mediated by alterations of effector molecules and which is important for the recognition and activation by the immune system, the loss of signal transducer CD3-ζ chain (CD3-ζ) of TILs has been attributed to immune evasion in the co-operation of immunosuppressive cytokines and local impairment of TILs.86 The loss of CD3-ζ is reported to be correlated with increased levels of IL-10 and TGF-β, and down-regulation of IFN-γ. The CD3-ζ chain is located as a large intracytoplasmic homodimer in the TCR that forms part of the TCR–CD3 complex, which functions as a single transducer upon antigen binding. Since the TCR signal transduction through the formation of the CD3 complex is one of three important signals for initiating a successful immune response as well as the expression of tumour antigen and T helper 1 polarization, any alterations in the CD3-ζ chain that are associated with the absence of p56lck tyrosine kinase, but not CD3-ε, produce the changes in the signalling pathway for T-cell activation. The alterations of TCR-ζ in several types of tumours such as pancreatic cancer,87 uveal malignant melanoma,88 renal cell cancer,89 ovarian cancer90 and oral cancer43 have been shown to be attributed to immune invasion that links to poor prognosis. Tumour-derived macrophages or tumour-derived factors led to a selective loss of TCR-ζ compared with CD3-ε.90 Given that the TCR/CD3-signalling led to lymphocyte proliferation, the poor proliferative responses of TILs could be explained by the defect in TCR-ζ expression. TIL underwent marked spontaneous apoptosis in vitro, which was associated with down-regulation of the anti-apoptotic Bcl-xL and Bcl-2 proteins.91 Furthermore, because TCR-ζ is a substrate of caspase 3 leading to apoptosis,92 tumour cells can trigger caspase-dependent apoptotic cascades in T lymphocytes, which are not effectively protected by Bcl-2.93 In oral squamous cell carcinoma, a high proportion of T cells in the tumour undergo apoptosis, which correlates with FasL expression on tumour cells. FasL-positive microvesicles induced caspase-3 cleavage, cytochrome c release, loss of mitochondrial membrane potential, and reduced TCR-ζ chain expression in target lymphocytes.94
Tumour-derived soluble factors
Tumours evolve mechanisms to escape immune control by a process called immune editing, which provides a selective pressure in the tumour microenvironment that can lead to malignant progression. A variety of tumour-derived soluble factors contribute to the emergence of complex local and regional immunosuppressive networks, including vascular endothelial growth factor (VEGF),95 IL-10,96 TGF-β,97 prostaglandin E2,98 soluble phosphatidylserine,79 soluble Fas,99 soluble FasL100 and soluble MICA.101 Although deposited at the primary tumour site, these secreted factors can extend immunosuppressive effects into local lymph nodes and the spleen, thereby promoting invasion and metastasis.5
VEGF plays a key role in recruiting immature myeloid cells from the bone marrow to enrich the microenvironment as tumour-associated immature DCs (iDCs) and macrophages (TiDCs and TAMs).102 Accumulation of TiDCs may cause roving DCs and T cells to become suppressed through activation of indoleamine 2,3-dioxygenase103 and arginase I104 by tumour-derived growth factors. VEGF prevents DC differentiation and maturation by suppressing the nuclear factor-κB in haematopoietic stem cells.105 Blocking nuclear factor-κB activation in haematopoietic cells by tumour-derived factors is considered to be a mechanism by which tumour cells can directly down-regulate the ability of the immune system to generate an antitumour response.105 In addition, because VEGF can be activated by signal transducers and activators of transcription 3 (Stat3)106 and because DC differentiation requires decreasing activity of Stat3, neutralizing antibody specific for VEGF or dominant-negative Stat3 and its inhibitors can prevent Stat3 activation and promote DC differentiation and function.107,108 The increased serum levels of VEGF in cancers have been reported to be correlated with poor prognosis, which involves not only its angiogenic properties but also its ability to induce immune evasion leading to tumour progression.109,110
Soluble FasL and soluble MICA products also play important roles in immune evasion, by inhibiting Fas-mediated and NKG2D-mediated killing of immune cells.111,112 Soluble phosphatidylserine, another TDSF, acts as an inducer of an anti-inflammatory response to TAMs, resulting in the release of anti-inflammatory mediators—such as IL-10 and TGF-β—that inhibit immune responses of DCs and T cells.79 The altered tumour surface antigen, such as FasL, also causes immune evasion by counterattacking immune cells, resulting in cell death.113 In addition, the soluble forms of FasL and MICA are able to inhibit Fas and the NKG2D-mediated death of immune cells.114,115 Thus it is likely that TDSFs play pivotal roles in constituting immunosuppressive networks that aid tumour progression and metastasis. Indeed, the immunosuppressive networks derived from these TDSFs can be a critical factor in causing unsatisfactory clinical responses that are usually seen in immunotherapy of advanced cancer, and they remain an important obstacle to be overcome in the interaction between tumours and the immune system in the tumour microenvironment.5,116–118
Immunological ignorance and tolerance in tumours
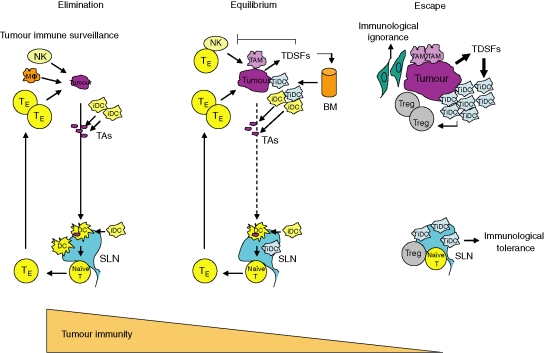
A tumour-specific immune response is regulated by tumour antigen levels and maturation stages of antigen-presenting cells such as DCs. Many solid tumours, such as sarcomas and carcinomas, express tumour-specific antigens that can serve as targets for immune effector T cells. Nevertheless, the overall immune surveillance against such tumours seems relatively inefficient. Tumour cells are capable of inducing a protective cytotoxic T-cell response if transferred as a single-cell suspension. However, if they are transplanted as small tumour pieces, tumours readily grow because the tumour antigen level can be modulated in the tumour microenvironment.119 Thus tumour cells are surrounded by non-tumour cells, including bone marrow-derived cells such as iDCs and non-bone-marrow-derived cells such as fibroblasts, endothelium and ECM. The ECM binds tumour antigen,120 and fibroblasts and endothelial cells compete with DCs for the antigen,121 whereby many tumour antigens are down-regulated, thereby allowing tumour progression.122 Furthermore, these stromal cells increase interstitial fluid pressure in the tumour, resulting in escape from immune attack by effector cells.123 In these situations, insufficient levels of tumour antigen are largely ignored by T cells, even though T-cell function is suppressed by iDCs in the tumour microenvironment. In addition, iDCs stimulate CD4+ CD25+ regulatory T cells, which inhibit T-cell activation.124,125 It is known that sufficient levels of tumour antigen can produce an immune response, which is mediated by mature DCs presenting tumour antigens to T cells by cross-priming. However, even in the presence of sufficient levels of tumour antigen, iDCs inhibit the maturation of DCs and T-cell activation, resulting in immunological tolerance.126 Thus, it is likely that tumour immune evasion is mediated not only by immunological ignorance as a result of decreased levels of tumour antigen but also by immunological tolerance because of inhibition of T-cell activation by iDCs. Many important events and the central roles of effector cells in the process of immunoediting from immune surveillance to escape are summarized in Fig. 1.
Figure 1.
A schematic process for understanding cancer immunoediting from immune surveillance to escape. When nascent transformed cells existed, these cells were easily eradicated by innate and adaptive immune responses. During tumour growth, tumour cells are required for angiogenesis and stromal remodelling, which produce tumour cell variants that have low immunogenicity and are resistant to immune attack, and proceed to the equilibrium phase even though the elimination phase continues through immune selection pressure. Tumour progression leads to the release of tumour-derived soluble factors that are involved in several mechanisms of immune evasion in the escape phase. iDC, immature dendritic cell; Mφ, macrophage; NK, natural killer; TE, effector T cell; TAs, tumour antigens; SLN, sentinel lymph node; TiDC, tumour-associated iDC; TAM, tumour-associated macrophage; TDSFs, tumour-derived soluble factors; Tregs, regulatory T cells; BM, bone marrow.
Concluding remarks
Owing to the abundant experimental and clinical evidence there is no longer any doubt for the existence of cancer immunoediting from immune surveillance to escape. Cancer cells are gradually able to gain several mechanisms of immune evasion during tumour progression, even though they are being pursued by the initial and continuing phases of immune surveillance. Rather, immunological sculpting contributes to immune selection pressure, which produces tumour cell variants that are resistant to immune effector cells because of their low immunogenicity. In advanced cancers, the marked shifting to immunosuppressive conditions as the result of the constitution of the immunosuppressive network in tumours makes it more difficult to provoke an immune activation to eliminate cancer cells. Given that adoptive immunotherapy using peptide vaccine and DC transfer is not sufficient to reduce tumour volume and their elimination by direct priming for T cells in such conditions, indirect cross-priming for T cells, which can be induced by massive cell death in combination with anticancer drugs, will be required. Indeed, not only modulation of anticancer drug-induced cell death, but also activation of antitumour immune responses by using molecular targeting drugs such as antibodies and small molecules may provide remarkable enhancement of chemotherapeutic effects in cancer therapy. Further studies on cellular and molecular mechanisms to contribute to antitumour immune responses will be needed.
Abbreviations
- CTLs
cytotoxic T lymphocytes
- DCs
dendritic cells
- DMBA
7,12-dimethylbenzanthracene
- ECM
extracellular matrix
- FasL
Fas ligand
- iDCs
immature DCs
- IFN
interferon
- IFNAR1
interferon-α receptor 1
- IL
interleukin
- MCA
methylcholanthrene
- MHC
major histocompatibility complex
- MICA
MHC class I chain-related A
- NK
natural killer
- NKT
natural killer T
- pfp
perforin
- TA
tumour antigen
- TCR
T-cell receptor
- TDLNs
tumour-draining lymph nodes
- TDSFs
tumour-derived soluble factors
- TILs
tumour-infiltrating lymphocytes
- TPA
12-O-tetradecanoylphorbol-13-acetate
- TRAIL
tumour necrosis factor-related apoptosis-inducing ligand
References
- 1.Ehrlich P. Ueber den jetzigen stand der karzinomforschung. Ned Tijdschr Geneeskd. 1909;5:73–290. [Google Scholar]
- 2.Burnet FM. Cancer a biological approach. Br Med J. 1957;1:841–7. doi: 10.1136/bmj.1.5023.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 4.Dunn GP, Bruce AT, Sheehan KC, et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6:722–9. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- 5.Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66:5527–36. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- 6.Klein G. Immune surveillance – a powerful mechanism with a limited range. Natl Cancer Inst Monogr. 1976;44:109–13. [PubMed] [Google Scholar]
- 7.Prehn RT, Main JM. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst. 1957;18:769–78. [PubMed] [Google Scholar]
- 8.Old LJ, Boyse EA. Immunology of experimental tumors. Annu Rev Med. 1964;15:167–86. doi: 10.1146/annurev.me.15.020164.001123. [DOI] [PubMed] [Google Scholar]
- 9.Burnet FM. Immunological surveillance in neoplasia. Transplant Rev. 1971;7:3–25. doi: 10.1111/j.1600-065x.1971.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 10.Thomas L. On immunosurveillance in human cancer. Yale J Biol Med. 1982;55:329–33. [PMC free article] [PubMed] [Google Scholar]
- 11.Rygaard J, Povlsen CO. The mouse mutant nude does not develop spontaneous tumours. An argument against immunological surveillance. Acta Pathol Microbiol Scand Microbiol Immunol. 1974;82:99–106. doi: 10.1111/j.1699-0463.1974.tb02299.x. [DOI] [PubMed] [Google Scholar]
- 12.Stutman O. Tumor development after 3-methylcholanthrene in immunologically deficient athymic-nude mice. Science. 1974;183:534–6. doi: 10.1126/science.183.4124.534. [DOI] [PubMed] [Google Scholar]
- 13.Ikehara S, Pahwa RN, Fernandes G, Hansen CT, Good RA. Functional T cells in athymic nude mice. Proc Natl Acad Sci USA. 1984;81:886–8. doi: 10.1073/pnas.81.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maleckar JR, Sherman LA. The composition of the T cell receptor repertoire in nude mice. J Immunol. 1987;138:3873–6. [PubMed] [Google Scholar]
- 15.Engel AM, Svane IM, Mouritsen S, Rygaard J, Clausen J, Werdelin O. Methylcholanthrene-induced sarcomas in nude mice have short induction times and relatively low levels of surface MHC class I expression. APMIS. 1996;104:629–39. doi: 10.1111/j.1699-0463.1996.tb04923.x. [DOI] [PubMed] [Google Scholar]
- 16.Engel AM, Svane IM, Rygaard J, Werdelin O. MCA sarcomas induced in scid mice are more immunogenic than MCA sarcomas induced in congenic, immunocompetent mice. Scand J Immunol. 1997;45:463–70. doi: 10.1046/j.1365-3083.1997.d01-419.x. [DOI] [PubMed] [Google Scholar]
- 17.Herberman RB, Holden HT. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–77. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- 18.Smyth MJ, Thia KY, Street SE, et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–8. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smyth MJ, Crowe NY, Godfrey DI. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol. 2001;13:459–63. doi: 10.1093/intimm/13.4.459. [DOI] [PubMed] [Google Scholar]
- 20.Hayakawa Y, Rovero S, Forni G, Smyth MJ. Alpha-galactosylceramide (KRN7000) suppression of chemical- and oncogene-dependent carcinogenesis. Proc Natl Acad Sci USA. 2003;100:9464–9. doi: 10.1073/pnas.1630663100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girardi M, Oppenheim DE, Steele CR, et al. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–9. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 22.Dighe AS, Richards E, Old LJ, Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1:447–56. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA. 1998;95:7556–61. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97:192–7. doi: 10.1182/blood.v97.1.192. [DOI] [PubMed] [Google Scholar]
- 25.Street SE, Trapani JA, MacGregor D, Smyth MJ. Suppression of lymphoma and epithelial malignancies effected by interferon gamma. J Exp Med. 2002;196:129–34. doi: 10.1084/jem.20020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002;20:323–70. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- 27.van den Broek ME, Kagi D, Ossendorp F, et al. Decreased tumor surveillance in perforin-deficient mice. J Exp Med. 1996;184:1781–90. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med. 2000;192:755–60. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shinkai Y, Rathbun G, Lam KP, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate VDJ rearrangement. Cell. 1992;68:855–67. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 30.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–11. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 31.Gresser I, Bandu MT, Brouty-Boye D. Interferon and cell division. IX. Interferon-resistant L1210 cells. characteristics and origin. J Natl Cancer Inst. 1974;52:553–9. doi: 10.1093/jnci/52.2.553. [DOI] [PubMed] [Google Scholar]
- 32.Affabris E, Romeo G, Federico M, Coccia E, Locardi C, Belardelli F, Rossi GB. Molecular mechanisms of action of interferons in the Friend virus-induced leukemia cell system. Haematologica. 1987;72:76–8. [PubMed] [Google Scholar]
- 33.Gresser I, Maury C, Vignaux F, Haller O, Belardelli F, Tovey MG. Antibody to mouse interferon alpha/beta abrogates resistance to the multiplication of Friend erythroleukemia cells in the livers of allogeneic mice. J Exp Med. 1988;168:1271–91. doi: 10.1084/jem.168.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin Z, Blankenstein T. CD4+ T cell-mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN gamma receptor expression by nonhematopoietic cells. Immunity. 2000;12:677–86. doi: 10.1016/s1074-7613(00)80218-6. [DOI] [PubMed] [Google Scholar]
- 35.Asselin-Paturel C, Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J Exp Med. 2005;202:461–5. doi: 10.1084/jem.20051395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoshino K, Kaisho T, Iwabe T, Takeuchi O, Akira S. Differential involvement of IFN-beta in Toll-like receptor-stimulated dendritic cell activation. Int Immunol. 2002;14:1225–31. doi: 10.1093/intimm/dxf089. [DOI] [PubMed] [Google Scholar]
- 37.Sato K, Hida S, Takayanagi H, et al. Antiviral response by natural killer cells through TRAIL gene induction by IFN-alpha/beta. Eur J Immunol. 2001;31:3138–46. doi: 10.1002/1521-4141(200111)31:11<3138::aid-immu3138>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 38.Hiroishi K, Tuting T, Lotze MT. IFN-alpha-expressing tumor cells enhance generation and promote survival of tumor-specific CTLs. J Immunol. 2000;164:567–72. doi: 10.4049/jimmunol.164.2.567. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–9. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 40.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–4. [PubMed] [Google Scholar]
- 41.Strater J, Hinz U, Hasel C, Bhanot U, Mechtersheimer G, Lehnert T, Moller P. Impaired CD95 expression predisposes for recurrence in curatively resected colon carcinoma. Clinical evidence for immunoselection and CD95L mediated control of minimal residual disease. Gut. 2005;54:661–5. doi: 10.1136/gut.2004.052696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yasunaga M, Tabira Y, Nakano K, Iida S, Ichimaru N, Nagamoto N, Sakaguchi T. Accelerated growth signals and low tumor-infiltrating lymphocyte levels predict poor outcome in T4 esophageal squamous cell carcinoma. Ann Thorac Surg. 2000;70:1634–40. doi: 10.1016/s0003-4975(00)01915-9. [DOI] [PubMed] [Google Scholar]
- 43.Reichert TE, Day R, Wagner EM, Whiteside TL. Absent or low expression of the zeta chain in T cells at the tumor site correlates with poor survival in patients with oral carcinoma. Cancer Res. 1998;58:5344–7. [PubMed] [Google Scholar]
- 44.Yoshimoto M, Sakamoto G, Ohashi Y. Time dependency of the influence of prognostic factors on relapse in breast cancer. Cancer. 1993;72:2993–3001. doi: 10.1002/1097-0142(19931115)72:10<2993::aid-cncr2820721022>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 45.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haanen JB, Baars A, Gomez R, et al. Melanoma-specific tumor-infiltrating lymphocytes but not circulating melanoma-specific T cells may predict survival in resected advanced-stage melanoma patients. Cancer Immunol Immunother. 2006;55:451–8. doi: 10.1007/s00262-005-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishigami S, Natsugoe S, Tokuda K, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88:577–83. [PubMed] [Google Scholar]
- 48.Kondo E, Koda K, Takiguchi N, Oda K, Seike K, Ishizuka M, Miyazaki M. Preoperative natural killer cell activity as a prognostic factor for distant metastasis following surgery for colon cancer. Dig Surg. 2003;20:445–51. doi: 10.1159/000072714. [DOI] [PubMed] [Google Scholar]
- 49.Villegas FR, Coca S, Villarrubia VG, Jimenez R, Chillon MJ, Jareno J, Zuil M, Callol L. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. 2002;35:23–8. doi: 10.1016/s0169-5002(01)00292-6. [DOI] [PubMed] [Google Scholar]
- 50.Birkeland SA, Storm HH, Lamm LU, et al. Cancer risk after renal transplantation in the Nordic countries 1964–86. Int J Cancer. 1995;60:183–9. doi: 10.1002/ijc.2910600209. [DOI] [PubMed] [Google Scholar]
- 51.Pham SM, Kormos RL, Landreneau RJ, Kawai A, Gonzalez-Cancel I, Hardesty RL, Hattler BG, Griffith BP. Solid tumors after heart transplantation: lethality of lung cancer. Ann Thorac Surg. 1995;60:1623–6. doi: 10.1016/0003-4975(95)00120-4. [DOI] [PubMed] [Google Scholar]
- 52.Morath C, Mueller M, Goldschmidt H, Schwenger V, Opelz G, Zeier M. Malignancy in renal transplantation. J Am Soc Nephrol. 2004;15:1582–8. doi: 10.1097/01.asn.0000126194.77004.9b. [DOI] [PubMed] [Google Scholar]
- 53.Myron Kauffman H, McBride MA, Cherikh WS, Spain PC, Marks WH, Roza AM. Transplant tumor registry: donor related malignancies. Transplantation. 2002;74:358–62. doi: 10.1097/00007890-200208150-00011. [DOI] [PubMed] [Google Scholar]
- 54.Zeier M, Hartschuh W, Wiesel M, Lehnert T, Ritz E. Malignancy after renal transplantation. Am J Kidney Dis. 2002;39:E5. doi: 10.1053/ajkd.2002.29926. [DOI] [PubMed] [Google Scholar]
- 55.Meek DW. The p53 response to DNA damage. DNA Repair (Amst) 2004;3:1049–56. doi: 10.1016/j.dnarep.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 56.Soussi T, Lozano G. p53 mutation heterogeneity in cancer. Biochem Biophys Res Commun. 2005;331:834–42. doi: 10.1016/j.bbrc.2005.03.190. [DOI] [PubMed] [Google Scholar]
- 57.Iwakuma T, Lozano G, Flores ER. Li–Fraumeni syndrome: a p53 family affair. Cell Cycle. 2005;4:865–7. doi: 10.4161/cc.4.7.1800. [DOI] [PubMed] [Google Scholar]
- 58.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–21. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 59.Klein G, Klein E. Surveillance against tumors – is it mainly immunological? Immunol Lett. 2005;100:29–33. doi: 10.1016/j.imlet.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 60.Tomasi TB, Magner WJ, Khan AN. Epigenetic regulation of immune escape genes in cancer. Cancer Immunol Immunother. 2006;55:1159–84. doi: 10.1007/s00262-006-0164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mori S, Jewett A, Murakami-Mori K, Cavalcanti M, Bonavida B. The participation of the Fas-mediated cytotoxic pathway by natural killer cells is tumor-cell-dependent. Cancer Immunol Immunother. 1997;44:282–90. doi: 10.1007/s002620050384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takeda K, Hayakawa Y, Smyth MJ, et al. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7:94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- 63.Zitvogel L, Terme M, Borg C, Trinchieri G. Dendritic cell–NK cell cross-talk: regulation and physiopathology. Curr Top Microbiol Immunol. 2006;298:157–74. doi: 10.1007/3-540-27743-9_8. [DOI] [PubMed] [Google Scholar]
- 64.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 65.Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol. 2001;2:293–9. doi: 10.1038/86297. [DOI] [PubMed] [Google Scholar]
- 66.Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol. 2001;1:41–9. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- 67.Street SE, Hayakawa Y, Zhan Y, et al. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and gammadelta T cells. J Exp Med. 2004;199:879–84. doi: 10.1084/jem.20031981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gollob JA, Sciambi CJ, Huang Z, Dressman HK. Gene expression changes and signaling events associated with the direct antimelanoma effect of IFN-gamma. Cancer Res. 2005;65:8869–77. doi: 10.1158/0008-5472.CAN-05-1387. [DOI] [PubMed] [Google Scholar]
- 69.Qin Z, Schwartzkopff J, Pradera F, Kammertoens T, Seliger B, Pircher H, Blankenstein T. A critical requirement of interferon gamma-mediated angiostasis for tumor rejection by CD8+ T cells. Cancer Res. 2003;63:4095–100. [PubMed] [Google Scholar]
- 70.Wall L, Burke F, Barton C, Smyth J, Balkwill F. IFN-gamma induces apoptosis in ovarian cancer cells in vivo and in vitro. Clin Cancer Res. 2003;9:2487–96. [PubMed] [Google Scholar]
- 71.Angiolillo AL, Sgadari C, Taub DD, et al. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995;182:155–62. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 73.Sinha P, Clements VK, Miller S, Ostrand-Rosenberg S. Tumor immunity. A balancing act between T cell activation, macrophage activation and tumor-induced immune suppression. Cancer Immunol Immunother. 2005;54:1137–42. doi: 10.1007/s00262-005-0703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–21. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 75.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–61. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 76.Bandholtz L, Guo Y, Palmberg C, et al. Hsp90 binds CpG oligonucleotides directly: implications for hsp90 as a missing link in CpG signaling and recognition. Cell Mol Life Sci. 2003;60:422–9. doi: 10.1007/s000180300035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Powell JD, Horton MR. Threat matrix: low-molecular-weight hyaluronan (HA) as a danger signal. Immunol Res. 2005;31:207–18. doi: 10.1385/IR:31:3:207. [DOI] [PubMed] [Google Scholar]
- 78.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 79.Kim R, Emi M, Tanabe K. Cancer cell immune escape and tumor progression by exploitation of anti-inflammatory and pro-inflammatory responses. Cancer Biol Ther. 2005;4:924–33. doi: 10.4161/cbt.4.9.2101. [DOI] [PubMed] [Google Scholar]
- 80.Svane IM, Engel AM, Nielsen MB, Ljunggren HG, Rygaard J, Werdelin O. Chemically induced sarcomas from nude mice are more immunogenic than similar sarcomas from congenic normal mice. Eur J Immunol. 1996;26:1844–50. doi: 10.1002/eji.1830260827. [DOI] [PubMed] [Google Scholar]
- 81.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 82.MacKie RM, Reid R, Junor B. Fatal melanoma transferred in a donated kidney 16 years after melanoma surgery. N Engl J Med. 2003;348:567–8. doi: 10.1056/NEJM200302063480620. [DOI] [PubMed] [Google Scholar]
- 83.Cankovic M, Linden MD, Zarbo RJ. Use of microsatellite analysis in detection of tumor lineage as a cause of death in a liver transplant patient. Arch Pathol Laboratory Med. 2006;130:529–32. doi: 10.5858/2006-130-529-UOMAID. [DOI] [PubMed] [Google Scholar]
- 84.Morath C, Rohmeiss P, Schwenger V, et al. Transmission of donor-derived small-cell carcinoma cells by a nontumor-bearing allograft. Transplantation. 2005;80:540–2. doi: 10.1097/01.tp.0000168489.71242.fd. [DOI] [PubMed] [Google Scholar]
- 85.Detry O, De Roover A, de Leval L, Herens C, Delwaide J, Honore P, Meurisse M. Transmission of an undiagnosed sarcoma to recipients of kidney and liver grafts procured in a non-heart beating donor. Liver Transpl. 2005;11:696–9. doi: 10.1002/lt.20457. [DOI] [PubMed] [Google Scholar]
- 86.von Bernstorff W, Voss M, Freichel S, et al. Systemic and local immunosuppression in pancreatic cancer patients. Clin Cancer Res. 2001;7:925s–32s. [PubMed] [Google Scholar]
- 87.Schmielau J, Nalesnik MA, Finn OJ. Suppressed T-cell receptor zeta chain expression and cytokine production in pancreatic cancer patients. Clin Cancer Res. 2001;7:933s–9s. [PubMed] [Google Scholar]
- 88.Staibano S, Mascolo M, Tranfa F, et al. Tumor infiltrating lymphocytes in uveal melanoma: a link with clinical behavior? Int J Immunopathol Pharmacol. 2006;19:171–9. [PubMed] [Google Scholar]
- 89.Riccobon A, Gunelli R, Ridolfi R, et al. Immunosuppression in renal cancer: differential expression of signal transduction molecules in tumor-infiltrating, near-tumor tissue, and peripheral blood lymphocytes. Cancer Invest. 2004;22:871–7. doi: 10.1081/cnv-200039653. [DOI] [PubMed] [Google Scholar]
- 90.Lockhart DC, Chan AK, Mak S, et al. Loss of T-cell receptor-CD3zeta and T-cell function in tumor-infiltrating lymphocytes but not in tumor-associated lymphocytes in ovarian carcinoma. Surgery. 2001;129:749–56. doi: 10.1067/msy.2001.114554. [DOI] [PubMed] [Google Scholar]
- 91.Agrawal S, Marquet J, Delfau-Larue MH, Copie-Bergman C, Jouault H, Reyes F, Bensussan A, Farcet JP. CD3 hyporesponsiveness and in vitro apoptosis are features of T cells from both malignant and nonmalignant secondary lymphoid organs. J Clin Invest. 1998;102:1715–23. doi: 10.1172/JCI3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gastman BR, Johnson DE, Whiteside TL, Rabinowich H. Caspase-mediated degradation of T-cell receptor zeta-chain. Cancer Res. 1999;59:1422–7. [PubMed] [Google Scholar]
- 93.Gastman BR, Johnson DE, Whiteside TL, Rabinowich H. Tumor-induced apoptosis of T lymphocytes: elucidation of intracellular apoptotic events. Blood. 2000;95:2015–23. [PubMed] [Google Scholar]
- 94.Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res. 2005;11:1010–20. [PubMed] [Google Scholar]
- 95.Gabrilovich D, Ishida T, Oyama T, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–66. [PubMed] [Google Scholar]
- 96.Urosevic M, Dummer R. HLA-G and IL-10 expression in human cancer – different stories with the same message. Semin Cancer Biol. 2003;13:337–42. doi: 10.1016/s1044-579x(03)00024-5. [DOI] [PubMed] [Google Scholar]
- 97.Beck C, Schreiber H, Rowley D. Role of TGF-beta in immune-evasion of cancer. Microsc Res Tech. 2001;52:387–95. doi: 10.1002/1097-0029(20010215)52:4<387::AID-JEMT1023>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 98.He X, Stuart JM. Prostaglandin E2 selectively inhibits human CD4+ T cells secreting low amounts of both IL-2 and IL-4. J Immunol. 1999;163:6173–9. [PubMed] [Google Scholar]
- 99.Erdogan B, Uzaslan E, Budak F, et al. The evaluation of soluble Fas and soluble Fas ligand levels of bronchoalveolar lavage fluid in lung cancer patients. Tuberk Toraks. 2005;53:127–31. [PubMed] [Google Scholar]
- 100.Kim R, Emi M, Tanabe K, Uchida Y, Toge T. The role of Fas ligand and transforming growth factor beta in tumor progression: molecular mechanisms of immune privilege via Fas-mediated apoptosis and potential targets for cancer therapy. Cancer. 2004;100:2281–91. doi: 10.1002/cncr.20270. [DOI] [PubMed] [Google Scholar]
- 101.Holdenrieder S, Stieber P, Peterfi A, Nagel D, Steinle A, Salih HR. Soluble MICB in malignant diseases: analysis of diagnostic significance and correlation with soluble MICA. Cancer Immunol Immunother. 2006;55:1584–9. doi: 10.1007/s00262-006-0167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bellamy WT, Richter L, Sirjani D, et al. Vascular endothelial cell growth factor is an autocrine promoter of abnormal localized immature myeloid precursors and leukemia progenitor formation in myelodysplastic syndromes. Blood. 2001;97:1427–34. doi: 10.1182/blood.v97.5.1427. [DOI] [PubMed] [Google Scholar]
- 103.Munn DH, Sharma MD, Lee JR, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–70. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 104.Sinha P, Clements VK, Ostrand-Rosenberg S. Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Res. 2005;65:11743–51. doi: 10.1158/0008-5472.CAN-05-0045. [DOI] [PubMed] [Google Scholar]
- 105.Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, Gabrilovich DI. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J Immunol. 1998;160:1224–32. [PubMed] [Google Scholar]
- 106.Xu Q, Briggs J, Park S, et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24:5552–60. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- 107.Wang T, Niu G, Kortylewski M, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 108.Kortylewski M, Kujawski M, Wang T, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–21. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 109.Suzuki M, Iizasa T, Ko E, et al. Serum endostatin correlates with progression and prognosis of non-small cell lung cancer. Lung Cancer. 2002;35:29–34. doi: 10.1016/s0169-5002(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 110.Bono P, Teerenhovi L, Joensuu H. Elevated serum endostatin is associated with poor outcome in patients with non-Hodgkin lymphoma. Cancer. 2003;97:2767–75. doi: 10.1002/cncr.11399. [DOI] [PubMed] [Google Scholar]
- 111.Webb SD, Sherratt JA, Fish RG. Cells behaving badly. A theoretical model for the Fas/FasL system in tumour immunology. Math Biosci. 2002;179:113–29. doi: 10.1016/s0025-5564(02)00120-7. [DOI] [PubMed] [Google Scholar]
- 112.Doubrovina ES, Doubrovin MM, Vider E, et al. Evasion from NK cell immunity by MHC class I chain-related molecules expressing colon adenocarcinoma. J Immunol. 2003;171:6891–9. doi: 10.4049/jimmunol.171.12.6891. [DOI] [PubMed] [Google Scholar]
- 113.Huber V, Fais S, Iero M, et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology. 2005;128:1796–804. doi: 10.1053/j.gastro.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 114.Song E, Chen J, Ouyang N, Su F, Wang M, Heemann U. Soluble Fas ligand released by colon adenocarcinoma cells induces host lymphocyte apoptosis: an active mode of immune evasion in colon cancer. Br J Cancer. 2001;85:1047–54. doi: 10.1054/bjoc.2001.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 116.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Malmberg KJ. Effective immunotherapy against cancer: a question of overcoming immune suppression and immune escape? Cancer Immunol Immunother. 2004;53:879–92. doi: 10.1007/s00262-004-0577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 119.Ochsenbein AF, Klenerman P, Karrer U, Ludewig B, Pericin M, Hengartner H, Zinkernagel RM. Immune surveillance against a solid tumor fails because of immunological ignorance. Proc Natl Acad Sci USA. 1999;6:2233–8. doi: 10.1073/pnas.96.5.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Juprelle-Soret M, Wattiaux-De Coninck S, Wattiaux R. Subcellular localization of transglutaminase. Effect of collagen. Biochem J. 1988;50:421–7. doi: 10.1042/bj2500421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Savinov AY, Wong FS, Stonebraker AC, Chervonsky AV. Presentation of antigen by endothelial cells and chemoattraction are required for homing of insulin-specific CD8+ T cells. J Exp Med. 2003;97:643–56. doi: 10.1084/jem.20021378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Spiotto MT, Yu P, Rowley DA, Nishimura MI, Meredith SC, Gajewski TF, Fu YX, Schreiber H. Increasing tumor antigen expression overcomes ‘ignorance’ to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity. 2002;7:737–47. doi: 10.1016/s1074-7613(02)00480-6. [DOI] [PubMed] [Google Scholar]
- 123.Pietras K, Ostman A, Sjoquist M, Buchdunger E, Reed RK, Heldin CH, Rubin K. Inhibition of platelet-derived growth factor receptors reduces interstitial hypertension and increases transcapillary transport in tumors. Cancer Res. 2001;1:2929–34. [PubMed] [Google Scholar]
- 124.Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–17. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 125.Ghiringhelli F, Puig PE, Roux S, et al. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–29. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mahnke K, Schmitt E, Bonifaz L, Enk AH, Jonuleit H. Immature, but not inactive: the tolerogenic function of immature dendritic cells. Immunol Cell Biol. 2002;80:477–83. doi: 10.1046/j.1440-1711.2002.01115.x. [DOI] [PubMed] [Google Scholar]