Abstract
The role of regulatory T cells (Tregs) in maintaining self tolerance has been intensively researched and there is a growing consensus that a decline in Treg function is an important step towards the development of autoimmune diseases, including diabetes. Although we show here that CD25+ cells delay diabetes onset in non-obese diabetic (NOD) mice, we found, in contrast to previous reports, neither an age-related decline nor a decline following onset of diabetes in the frequency of CD4+ CD25+ Foxp3+ regulatory T cells. Furthermore, we demonstrate that CD4+ CD25+ cells from both the spleen and pancreatic draining lymph nodes of diabetic and non-diabetic NOD mice are able to suppress the proliferation of CD4+ CD25– cells to a similar extent in vitro. We also found that pretreatment of NOD mice with anti-CD25 antibody allowed T cells with a known reactivity to islet antigen to proliferate more in the pancreatic draining lymph nodes of NOD mice, regardless of age. In addition, we demonstrated that onset of diabetes in NOD.scid mice is faster when recipients are co-administered splenocytes from diabetic NOD donors and anti-CD25. Finally, we found that although diabetic CD4+ CD25+ T cells are not as suppressive in cotransfers with effectors into NOD.scid recipients, this may not indicate a decline in Treg function in diabetic mice because over 10% of CD4+ CD25+ T cells are non-Foxp3 and the phenotype of the CD25– contaminating population significantly differs in non-diabetic and diabetic mice. This work questions whether onset of diabetes in NOD mice is associated with a decline in Treg function.
Keywords: CD25, diabetes, Foxp3, non-obese diabetic (NOD) mice, regulatory T cells
Introduction
There has been great interest in the role of regulatory T cells (Tregs) in controlling a range of immune responses including autoimmunity.1–4 Several different populations of Tregs have been described, including naturally arising thymus-derived Tregs and peripherally induced Tregs.1–4 Naturally arising Tregs have been characterized by the expression of a range of molecular markers including CD25 and the transcription factor Foxp3. The relevance of this population to the maintenance of self tolerance and to the control of immune responses in general is readily demonstrated by the induction of autoimmunity following depletion of CD4+ CD25+ cells in adoptive transfer systems and by their ability to suppress CD4+ CD25– proliferation and cytokine production in cocultures.4 The identification of Foxp3 as a definitive marker of natural Tregs was made following observations that the single gene defect of Foxp3 that caused widespread autoimmunity in both humans and mice was similar to the clinical syndromes in experimental models in which Tregs were selectively depleted.5–7 The importance of Foxp3 as a key gene for the development of Tregs was further supported by experiments where retroviral gene transfer of Foxp3 converted naturally occurring naive T cells to develop a regulatory phenotype and where mice that over-expressed Foxp3 had increased numbers of Tregs in the periphery.8–10
Dysregulation of Treg function has been implicated as an important event in the development of autoimmunity in animal models and in spontaneous autoimmune diseases in humans.4,11 Particular attention has been focused on quantitative and qualitative changes in Tregs in the widely used, spontaneous model of diabetes, the non-obese diabetic (NOD) mouse.12 For example, Tregs from 16-week-old NOD mice have been shown to be less suppressive in vitro and in vivo than Tregs from 8-week-old mice and this decline in Treg function has been implicated as a crucial step in the development of diabetes.13 Interestingly, there is also evidence that therapies that can successfully treat diabetes in NOD mice do so by restoring the functional capabilities of Tregs and the efficacy of anti-CD3 in human trials has led to speculation that a similar effect may occur in humans.14–16 It is particularly noteworthy that qualitative changes in Tregs have also been found in studies of humans with type 1 diabetes.17–19 Defects in Treg function have also been observed in other autoimmune diseases, including rheumatoid arthritis, myasthenia gravis, multiple sclerosis and autoimmune polyglandular syndrome type II.20–23 In addition, reversal of compromised Treg function has been demonstrated in humans with rheumatoid arthritis who experienced clinical improvement following anti-tumour necrosis factor-α therapy.20
In light of these observations, we evaluated the frequency and function of Tregs in our NOD mouse colony. The recent availability of antibodies to Foxp3 enabled us to enumerate the number of cells expressing this transcription factor. We found that there was no age-related decline in the proportion of CD4+ cells expressing CD25 or Foxp3 in the spleen, the pancreatic draining lymph nodes (PLNs) or pancreas. We also found no decrease in the frequency or absolute number of these cells when the mice became overtly diabetic. In contrast to previous studies, we provide data that demonstrate no qualitative changes in the in vitro ability of CD4+ CD25+ T cells from diabetic NOD mice to suppress the proliferation of CD4+ CD25– effector T cells compared to CD4+ CD25+ cells from non-diabetic NOD mice. We also describe experiments that show that anti-CD25 treatment of both 8-week-old and 18-week-old non-diabetic NOD mice can result in enhanced proliferation of transferred T cells with known islet-reactive BDC2.5NOD T cells,24 implying that even in older mice, Tregs play a role in suppressing autoreactivity in vivo. In addition, we demonstrate that the onset of diabetes in NOD.scid mice is faster when recipients are given diabetic splenocytes and anti-CD25. Finally, we found that although diabetic CD4+ CD25+ T cells are not as suppressive in cotransfers with effectors into NOD.scid recipients, this may not indicate a decline in Treg function in diabetic mice because >10% of CD4+ CD25+ T cells are non-Foxp3+ and the phenotype of the CD25– contaminating population differs in non-diabetic and diabetic mice. This study demonstrates that diabetes can be present in NOD mice with no quantitative abnormalities in regulatory T-cell function and questions the growing assumption that abnormal regulatory T-cell function is an essential step in the development of autoimmune disease.
Materials and methods
Mice
NOD, BDC2.5NOD and NOD.scid mice were bred and maintained under barrier conditions in the Biological Services facility of the Department of Pathology, University of Cambridge, Cambridge, UK. They received standard laboratory food and water ad libitum. NOD.scid mice were maintained in microisolator cages with filtered air and were handled under sterile conditions in a laminar flow hood. All animal experiments were approved by the Ethical Review Committee of the University of Cambridge.
Antibodies and reagents
Commercially available monoclonal antibodies used in this study were fluorescein isothiocyanate, phycoerythrin or biotin conjugates raised against CD3 (145-2C11), CD4 (RM4-5), CD11b (M1/70), CD49b (DX5), Vβ4 (KT4), major histocompatibility complex class II (OX6), CD44 (IM7) and CD62L (MEL-14) and were obtained from BD Biosciences, San Diego, CA or Serotec, Oxford, UK. Antibodies for in vivo treatment were anti-CD25 (PC61) (ATCC No. TIB222) and the isotype control (MAC221; rat immunoglobulin G1) supplied by Dr Geoff Butcher, Babraham, UK. These hybridomas were grown in our own laboratory in hollow fibre cartridges. Antibodies were purified by precipitation with 50% saturated ammonium sulphate and dialysed extensively against phosphate-buffered saline (PBS). An estimate of total protein was determined from the optical density at 280 nm (OD280). Antibody concentrations were determined by an anti-rat immunoglobulin enzyme-linked immunosorbent assay. The endotoxin levels were <1 EU/mg protein and the preparations were stored at −20° until use. The presence of the transcription factor Foxp3 was evaluated using a Foxp3 staining set (eBioscience, San Diego). A peptide mimotope was used to activate BDC2.5 T cells in vitro (acetyl-RTRPLWVRME-NH2, Southampton Polypeptide, Southampton, UK).25
Flow cytometric analysis
Single-cell suspensions were made from PLNs and spleens of NOD mice. Cells were washed and re-suspended in staining buffer (PBS containing 2% bovine serum albumin and 0·05% NaN3). Cells were stained with the appropriate monoclonal antibodies and then washed and analysed using a FACScan flow cytometer and CellQuest software (Becton Dickinson Europe, Erembodegem, Belgium).
CD4+ CD25+ T-cell in vitro assay
In vitro suppression assays were used to evaluate the ability of CD4+ CD25+ T cells from NOD mice to modify the proliferative response of CD4+ CD25– T cells in response to anti-CD3 and antigen-presenting cells (APCs). CD4+ CD25– T cells and CD4+ CD25+ T cells were separated from a single suspension of NOD splenocytes using microbeads according to the manufacturer's instructions (Miltenyi Biotech, Auburn, CA). CD4+ CD25– T cells were suspended at 5 × 107/ml in PBS with 5 μm 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) and incubated at 37° for 20 min. Cells were washed with PBS and then resuspended in complete medium [Iscove's modified Dulbecco's medium (Gibco, Paisley, UK) supplemented with 10% fetal calf serum (Harlan Sera Lab Ltd., Crawley Down, UK), 100 μg/ml streptomycin (Sigma-Aldrich Company Ltd., Poole, UK) and 60 μg/ml penicillin (Sigma)]. Triplicate cultures of the stated number of CFSE-labelled CD4+ CD25– T cells were incubated for 72 hr in round-bottom 96-well plates (Falcon, Becton Dickinson, San Jose, CA) with the stated number of irradiated, CD4+ T-cell-depleted, red-cell-lysed splenocytes, 1 μg/ml anti-CD3 and the stated number of CD4+ CD25+ T cells. Cells from each well were harvested after 72 hr, stained for CD4 and analysed by flow cytometry. As well as assessing the proliferation of effector cells by CFSE dilution, proliferation was also assessed by adding 1 μCi [3H]thymidine (Amersham Biosciences UK Ltd, Little Chalfont, UK) to the cultures for the final 16 hr. Incorporation of [3H]thymidine was measured by liquid scintillation spectrometry. Results are expressed as the mean counts/min (c.p.m.) ± standard error of triplicate wells.
A second suppression assay was performed which investigated the ability of NOD CD4+ CD25+ T cells to suppress the proliferation of BDC2.5NOD splenocytes cocultured with a peptide mimotope.24–26 Splenocytes (5 × 104) from a BDC2.5NOD mouse were cultured with 2·5 × 104 CD4+ CD25+ T cells from either an 8-week-old, a 16-week-old or a diabetic NOD mouse in the presence of 3 μg/ml of a BDC2.5NOD mimotope peptide25 for 72 hr. Proliferation was assessed by adding thymidine to the cells for the last 16 hr of the cocultures as previously described.
T-cell isolation for in vivo CFSE proliferation studies
T cells from BDC2.5NOD mice were isolated by negative selection. Single-cell suspensions from pooled spleens were incubated with an antibody-depletion cocktail (CD11b, CD45R, OX6, DX5) which targeted cells expressing non-T-cell surface markers. Cells were then incubated with GAR beads (goat anti-rat immunoglobulin G beads, Polysciences Inc, Warrington, PA) following the manufacturer's instructions. Cells that were non-adherent to the GAR beads were then collected and their purity was assessed by flow cytometric analysis. The BDC2.5NOD cells were re-suspended at 5 × 107/ml in PBS with 5 μm CFSE and incubated at 37° for 20 min. Cells were washed with PBS and then resuspended in PBS.
In vivo assessment of proliferation of BDC2.5NOD T cells in NOD mice pretreated with either anti-CD25 antibody or a control antibody
Non-diabetic, female NOD mice were injected intraperitoneally with 2 mg anti-CD25 (PC61) or 2 mg control antibody (MAC221). Seven days later purified T cells were isolated by negative selection and CFSE labelled as described above. Then, 1 × 107 cells were injected into the lateral tail vein of each NOD mouse. After 72 hr, mice were killed and PLNs were harvested. Single-cell suspensions were prepared, stained for Vβ4 and analysed by flow cytometry.
Cell transfers from diabetic NOD mice into NOD.scidrecipients
Single-cell suspensions from pooled spleens from diabetic female NOD mice were prepared and red blood cells were lysed in ammonium chloride buffer. The cells were resuspended in PBS and 2 × 107 were injected into the lateral tail vein of each NOD.scid mouse together with 2 mg anti-CD25 (PC61) or 2 mg control antibody (MAC221) or 200 μl PBS. Recipient NOD.scid mice were tested for the presence of urinary glucose using Diastix (Bayer, Newbury, UK).
Cotransfer of splenocytes from diabetic NOD mice with Tregs from NOD mice into NOD.scid recipients
CD4+ CD25+ and CD4+ CD25– T cells were separated from a single suspension of NOD splenocytes using microbeads according to the manufacturer's instructions (Miltenyi Biotech). 5 × 105 CD4+ CD25+, T cells, either 5 × 105 CD4+ CD25– or 1 × 106 CD4+ CD25+, from 6-week-old or diabetic NOD mice were cotransferred into 8-week-old, male NOD.scid recipients together with 1 × 107 spleen cells from diabetic female NOD mice. In addition, a control group received 1 × 107 of these splenocytes alone. Recipient NOD.scid mice were tested for the presence of urinary glucose.
Statistics
Data were analysed using the GraphPad Prism computer package. Student's t-test and Mann–Whitney U-test were used to assess differences between parametric and non-parametric data groups, respectively. Analysis of variance (anova) was used to compare multiple parametric groups. Spearman rank correlation was used to assess correlation between non-parametric variables. Log rank analysis was used to compare time of onset of diabetes between the two treatment regimens. Results were considered to be significant if P < 0·05.
Results
Regulatory T cells control diabetes development in young NOD mice
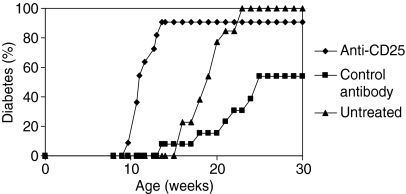
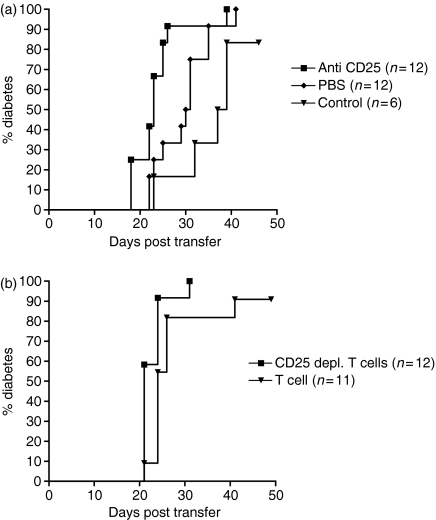
As it has been suggested that diabetes development in NOD mice may arise as the result of the progressive loss of Treg function we determined whether administration of anti-CD25 antibody could be shown to accelerate diabetes onset. Anti-CD25 antibody was given during the first 2 weeks of life to female NOD mice. This treatment resulted in a significantly faster onset of diabetes compared to mice treated with the isotype control antibody. Interestingly, administration of the isotype control antibody slightly reduced the incidence of diabetes in NOD female mice compared to untreated female NOD mice. Nevertheless, the effects of anti-CD25 treatment on diabetes incidence were still statistically significant when compared to untreated mice and this suggests that Tregs play an important role in regulating diabetes onset in NOD mice (Fig. 1).
Figure 1.
CD25+ T cells play a role in diabetes prevention in NOD mice. Female NOD mice were treated i.p. when 8, 11 and 15 days old with 1 mg of anti-CD25 or with the isotype control antibody. The incidence of diabetes in anti-CD25-treated mice (n = 11) was significantly higher than in either control antibody-treated (n = 13) (P < 0·0005, log rank analysis) or untreated female NOD mice (n =13) (P < 0·05, log rank analysis). Administration of isotype control antibody significantly reduced the incidence of diabetes compared to untreated NOD mice (P < 0·005, log rank analysis).
There is no age-related decline in the proportion of CD4+ T cells expressing either CD25 or Foxp3
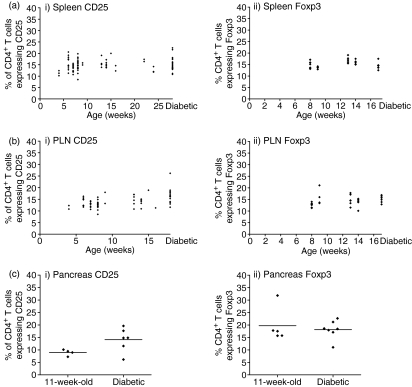
To further explore the role of Tregs in the prevention of diabetes in NOD mice we evaluated the expression of CD25, a cell surface marker that is known to be expressed on regulatory T cells. We costained for expression of CD4 and CD25 in the spleen, PLNs and pancreas. Because a number of cell types other than regulatory T cells can express CD25, most notably activated T cells, we also assessed the proportion of CD4 cells in NOD mice that expressed the transcription factor Foxp3, which is regarded as a definitive marker of regulatory T cells. There was no correlation between the age of non-diabetic mice and the proportion of splenic CD4+ T cells expressing CD25. The proportion of CD4+ T cells expressing CD25 in the spleen was not lower in diabetic mice compared to non-diabetic mice (Fig. 2a part i). There was no significant decrease with age in the proportion of CD4+ cells expressing Foxp3 in the spleens of non-diabetic mice and there was no difference in the proportion of splenic CD4+ cells expressing Foxp3 in diabetic mice compared to all the non-diabetic NOD mice (Fig. 2a part ii).
Figure 2.
There is no age-related decline in the proportion of Tregs in the spleen, PLNs or pancreas. Single-cell suspensions from the spleen and PLNs of female NOD mice were costained for CD4 and CD25 and/or CD4 and Foxp3. The proportions of CD4+ T cells which expressed CD25 (i) and expressed Foxp3 (ii) were calculated as a percentage for the spleen (a), PLNs (b) and pancreas (c). (a) There was no significant correlation between age and proportion of CD4+ T cells expressing CD25 and expressing Foxp3 in non-diabetic NOD mice in the spleen (CD25 r = 0·14, P = 0·23; Foxp3 r = 0·27, P = 0·19, Spearman rank correlation). The proportion of CD4+ cells that expressed CD25 in diabetic mice was not significantly different to that in non-diabetic mice (Mann–Whitney U-test, P = 0·64). Similarly, the proportion of CD4+ cells that expressed Foxp3 was not significantly different in diabetic NOD mice compared to non-diabetic NOD mice (Mann–Whitney U-test, P = 0·15). (b) There was no significant correlation between age and proportion of CD4+ T cells expressing CD25 in non-diabetic NOD mice in the PLNs (r = 0·24, P = 0·09, Spearman rank correlation). There was a small but significant positive correlation between age and the proportion of CD4+ T cells that expressed Foxp3 in the PLNs of non-diabetic mice (r = 0·48, P < 0·05, Spearman rank correlation). A significantly higher proportion of CD4+ cells expressed CD25 in diabetic mice compared to in all non-diabetic mice (Mann–Whitney U-test, P < 0·001). The proportion of CD4+ T cells that expressed Foxp3 was not significantly different in diabetic NOD mice compared to non-diabetic NOD mice (Mann–Whitney U-test, P =0·08). (c) The proportion of CD4+ T cells expressing CD25 in 11-week-old, non-diabetic mice was not significantly different from that in diabetic NOD mice (Mann–Whitney U-test, P = 0·11). There was no significant difference between the proportion of CD4+ T cells expressing Foxp3 in 11-week-old non-diabetic mice compared to diabetic NOD mice (Mann–Whitney U-test, P = 0·58).
To assess whether there may be localized differences in CD25 expression around the diseased organ, we assessed CD25 and Foxp3 expression in CD4+ cells from the PLNs. Again, there was no correlation between the age of non-diabetic mice and the proportion of PLN CD4+ T cells expressing CD25 and, in fact, the proportion of PLN CD4+ T cells expressing CD25 in diabetic mice was actually higher compared to that in non-diabetic NOD mice (Fig. 2b part i). There was a small increase with age in the proportion of CD4+ cells expressing Foxp3 from the PLNs of non-diabetic mice. In addition, there was no difference in the proportion of PLN CD4+ cells expressing Foxp3 in diabetic mice compared to all the non-diabetic NOD mice (Fig. 2b part ii).
We also assessed CD25 and Foxp3 expression in CD4+ T cells isolated from the pancreas itself. We found that there was no significant difference between the proportion of CD4+ T cells that expressed CD25 in 11-week-old and diabetic NOD mice (Fig. 2c part i). Furthermore, there was no significant decline in the proportion of CD4+ T cells isolated from the pancreas that expressed Foxp3 in 11-week-old and diabetic NOD mice (Fig. 2c part ii). These data indicate that even in diseased tissue, the frequency of Foxp3+ Tregs was similar to that in non-diabetic mice.
We additionally assessed the absolute number of CD4+Foxp3+ T cells in the spleens of 8-week-old (n = 7), 11-week-old (n = 7) and diabetic (n = 7) NOD mice. There was no significant decline in the absolute number of CD4+ Foxp3+ T cells with age or following onset of diabetes (data not shown).
CD4+ CD25+ T cells from diabetic mice are able to suppress the proliferation of CD4+ CD25– T cells in vitro
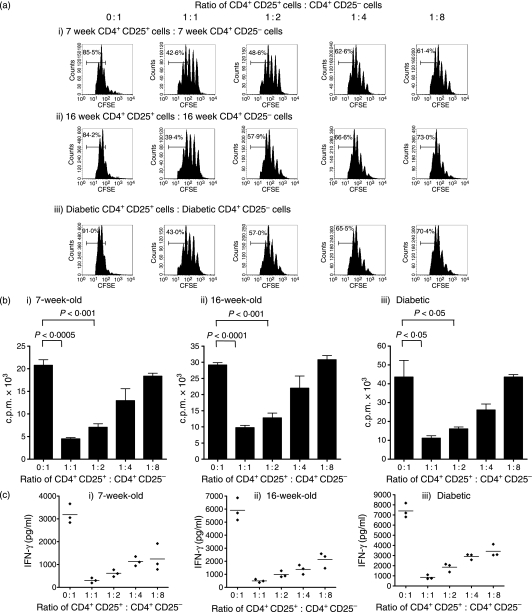
The above observations exclude the possibility of a quantitative decline in frequency of regulatory T cells in diabetic NOD mice but they do not exclude the possibility of a qualitative decline in regulatory T-cell function. To assess the qualitative function of regulatory T cells in young (5- to 8-week-old), old non-diabetic (16- to 18-week-old) and diabetic NOD mice, we used in vitro assays, which assessed the ability of CD4+ CD25+ T cells to suppress the proliferation of CD4+ CD25– effector T cells when cocultured with irradiated splenocytes and anti-CD3. When using CFSE dilution of effector cells as a measure of proliferation, we found that CD4+ CD25+ cells from young, old or diabetic NOD mice could markedly reduce the proliferation of their own effector cells (Fig. 3a). The observation that regulatory T cells from non-diabetic and diabetic NOD mice were able to suppress proliferation of their own effector cells was confirmed using a thymidine incorporation assay (Fig. 3b). In addition, CD4+ CD25+ T cells from either young, old or diabetic NOD mice were able to suppress the production of the pathogenic cytokine interferon-γ when cocultured with their own CD4+ CD25– T cells, irradiated APCs and anti-CD3 (Fig. 3c).
Figure 3.
CD4+ CD25+ T cells from the spleens of either 7-week-old, 16-week-old or diabetic female NOD mice can suppress proliferation of their own splenic CD4+ CD25– T cells in vitro. (a) 1 × 105 CFSE-labelled splenic CD4+ CD25– T effector cells together with 5 × 105 irradiated, CD4-depleted splenocytes and anti-CD3 (1 μg/ml) were cultured with either no CD4+ CD25+ cells (0 : 1 ratio) or with various numbers of splenic CD4+ CD25+ T cells from the same mouse ranging from 1 × 105 (1 : 1 ratio) to 1·25 × 104 (1 : 8 ratio) for 72 hr. Representative FACS histogram plots from triplicate wells of CFSE and CD4+ cells show a marked reduction of effector proliferation in 7-week (i), 16-week (ii) and diabetic (iii) NOD mice when regulatory T cells are added at a 1 : 1 ratio compared to effectors alone. The suppressive effect of the CD4+ CD25+ T cells was reduced as the ratio of CD4+ CD25+ T cells to CD4+ CD25– T cells decreased. (b) The ability of NOD CD4+ CD25+ T cells from both diabetic and non-diabetic mice to suppress the proliferation of CD4+ CD25– T cells from the same individual was also demonstrated by repeating the above assay using thymidine incorporation as the measure of proliferation. 5 × 104 splenic CD4+ CD25– T effector cells together with 2·5 × 105 irradiated, CD4-depleted splenocytes and anti-CD3 (1 μg/ml) were cultured with either no CD4+ CD25+ cells (0 : 1 ratio) or various numbers of splenic CD4+ CD25+ T cells from the same mouse ranging from 5 × 104 (1 : 1 ratio) to 6·25 × 103 (1 : 8 ratio) for 72 hr. The addition of CD4+ CD25+ T cells at Treg : T effector ratios of 1 : 1 and 1 : 2 caused a significant reduction in proliferation in cocultures of Tregs and T effectors from 7-week-old non-diabetic mice (i), 16-week-old non-diabetic mice (ii) and diabetic NOD mice (iii). Thymidine incorporation was less than 5·0 × 102 c.p.m. in all triplicates of 5 × 104 CD4+ CD25– T cells cultured alone and in wells with irradiated splenocytes with anti-CD3 alone. (c) IFN-γ was measured in the supernatants from the experiment described in (a). The addition of CD4+ CD25+ cells to their own CD4+ CD25– cells caused a significant reduction in IFN-γ production in 7-week-old (i), 16-week-old (ii) and diabetic (iii) NOD mice at all four dilutions of Tregs compared to T effectors cultured with irradiated APCs and anti-CD3 (Student t-test, P < 0·05 in all cases).
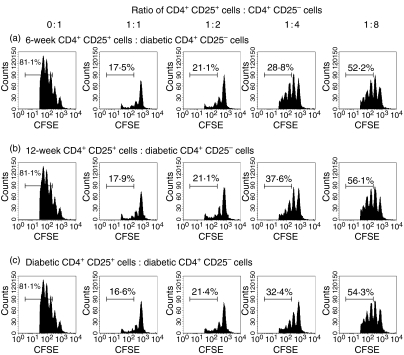
The ability of CD4+ CD25+ T cells from diabetic or non-diabetic mice to control the proliferation of their own effectors may, in part, be the result of differences in the proliferative capacity of their own CD4+ CD25– T cells. To explore this possibility, we repeated the above assay using CD4+ CD25– T cells from diabetic mice and cocultured them with irradiated splenocytes and anti-CD3 together with CD4+ CD25+ T cells from young, old or diabetic NOD mice. The CD4+ CD25+ cells from young, old or diabetic NOD mice were able to suppress the proliferation of the standardized effectors from a diabetic NOD mouse to a similar extent (Fig. 4).
Figure 4.
CD4+ CD25+ T cells from the spleens of both 6-week, 12-week and diabetic female NOD mice can suppress CD4+ CD25– T cells from diabetic NOD female mice in vitro. 1 × 105 CFSE-labelled splenic CD4+ CD25– T effector cells from a diabetic donor together with 5 × 105 irradiated, CD4-depleted splenocytes from the same diabetic donor and anti-CD3 (1 μg/ml) were cultured with either no CD4+ CD25+ cells (0 : 1 ratio) or with various numbers of splenic CD4+ CD25+ cells ranging from 1 × 105 (1 : 1 ratio) to 1·25 × 104 (1 : 8 ratio) from either a 6-week-old (a), 12-week-old (b) or diabetic (c) NOD donor. Representative FACS histogram plots from triplicate wells of CFSE and CD4+ cells show a marked reduction of diabetic effector T-cell proliferation when CD4+ CD25+ T cells are added at a 1 : 1 ratio compared to effectors alone irrespective of the source of the CD4+ CD25+ T cells. The suppressive effect of the CD4+ CD25+ T cells was gradually reduced as the ratio of CD4+ CD25+ T cells to CD4+ CD25– T cells decreased in all three regulatory T-cell groups.
We also performed a suppression assay similar to that described by Du et al.25 BDC2.5NOD splenocytes were cultured with BDC2.5NOD peptide mimotope either alone or with CD4+ CD25+ T cells from an 8-week-old, 16-week-old or diabetic NOD mouse. There was no significant reduction in proliferation when CD4+ CD25+ T cells from any of the three groups of NOD donors were added. In addition, there was no significant difference in the thymidine incorporation values between the three groups of CD4+ CD25+ T cells that were cocultured with BDC2.5NOD splenocytes plus peptide (data not shown).
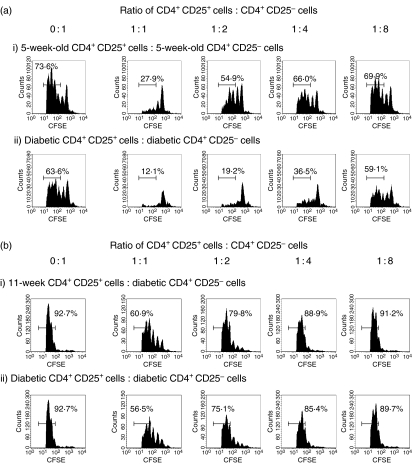
Although we had established that there were no qualitative differences in regulatory splenic T-cell function in diabetic mice compared to non-diabetic NOD mice, we had not eliminated the possibility of a more localized defect in qualitative regulatory T-cell function in the PLNs. We evaluated Treg function in the PLNs by repeating the above assay using CD4+ CD25+ T cells and CD4+ CD25– T cells isolated from pooled PLNs of young or diabetic NOD mice. Again, we found that CD4+CD25+ T cells from both young mice and diabetic mice could markedly suppress the proliferation of their own CD4+ CD25– T cells (Fig. 5a). In addition, we also found that CD4+ CD25+ T cells from PLNs of both 11-week-old and diabetic NOD mice could suppress the proliferation of CD4+ CD25– T cells from a diabetic NOD mouse in vitro (Fig. 5b).
Figure 5.
CD4+ CD25+ T cells from the PLNs of 5-week-old female NOD mice and diabetic female NOD mice can suppress the proliferation of CD4+ CD25– T cells from PLNs in vitro. (a) 2·5 × 104 CFSE-labelled CD4+ CD25– T effector cells from a pool of PLNs from either 5-week-old (i) or diabetic (ii) donor NOD donors together with 1·25 × 105 of irradiated, CD4-depleted splenocytes and anti-CD3 (1 μg/ml) were cultured with either no CD4+ CD25+ cells (0 : 1 ratio) or various numbers of their own PLN CD4+ CD25+ cells ranging from 2·5 × 104 (1 : 1 ratio) to 3·125 × 103 (1 : 8 ratio). Representative FACS histogram plots from triplicate wells of CFSE and CD4+ cells show a marked reduction of effector T-cell proliferation when regulatory T cells are added at a 1 : 1 ratio compared to effectors alone. The suppressive effect of the CD4+ CD25+ T cells was gradually reduced as the ratio of CD4+ CD25+ T cells to CD4+ CD25– T cells decreased in both regulatory T-cell groups. (b) 1 × 105 CFSE-labelled CD4+ CD25– T effector cells from a pool of PLNs from diabetic NOD donors together with 5 × 105 irradiated, CD4+-depleted diabetic splenocytes and anti-CD3 (1 μg/ml) were cultured with either no CD4+ CD25+ cells (0 : 1 ratio) or various numbers of PLNs CD4+ CD25+ cells ranging from 1 × 105 (1 : 1 ratio) to 1·25 × 104 (1 : 8 ratio) from either 11-week-old (i) or diabetic NOD (ii) mice. Representative FACS histogram plots from triplicate wells of CFSE and CD4+ cells show a marked reduction of effector T-cell proliferation when CD4+ CD25+ T cells are added at a 1 : 1 ratio compared to effectors alone. The suppressive effect of the CD4+ CD25+ T cells was gradually reduced as the ratio of CD4+ CD25+ T cells to CD4+ CD25– T cells decreased in both regulatory T-cell groups.
Pretreatment with anti-CD25 antibody allows T cells with reactivity to islet antigen to proliferate more in the pancreatic lymph nodes of both 8-week-old and 18-week-old NOD mice
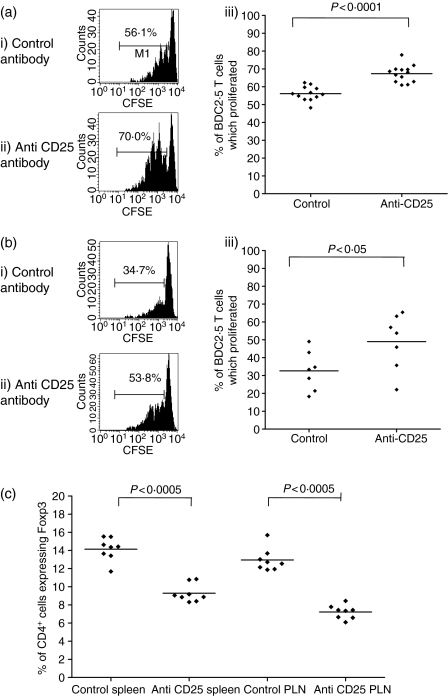
The above observations demonstrate that there is no quantitative change in the frequency of Tregs in vivo and that there is no age-related decline in qualitative regulatory T-cell function in vitro. Furthermore, Tregs from overtly diabetic mice were still able to suppress proliferation of effectors in vitro. However, they did not demonstrate that regulatory T cells could suppress effector cells in vivo in old or diabetic NOD mice. To evaluate the role of regulatory T cells in old NOD mice in vivo, we pretreated 18-week-old female, non-diabetic NOD mice with anti-CD25 antibody or isotype control antibody and then 7 days later transferred CFSE-labelled T cells from BDC2.5NOD mice. Seventy-two hours later, proliferation of the transgenic T cells in the PLNs was assessed. Single cell suspensions were made from the PLNs and stained for Vβ4. There was a significant increase in BDC2.5NOD T-cell proliferation in mice pretreated with anti-CD25 compared to isotype control antibody-treated mice in both young and old NOD mice (Fig. 6a,b) indicating that even in old, pre-diabetic NOD mice regulatory T cells could suppress the proliferation of T cells with known reactivity to islets in vivo. The increased proliferation of BDC2.5NOD T cells could potentially be explained by the anti-CD25 treatment inducing a lymphopenic state and reducing the homeostatic control of autoreactive T cells. We therefore assessed both CD25 and Foxp3 expression in NOD mice following anti-CD25 and isotype control treatment. Although CD25+ cells were not detectable following this treatment (data not shown), Tregs were still present, as shown by the presence of Foxp3-expressing cells (Fig. 6c).27,28 This suggests that the enhanced proliferation of BDC2.5NOD T cells in mice pretreated with anti-CD25 was not simply the result of the absolute elimination of Tregs.
Figure 6.
Pretreatment with anti-CD25 antibody increases BDC2.5 NOD T-cell proliferation in the PLNs of both 7-week-old and 18-week-old NOD mice. The 7- or 18-week-old NOD mice were injected with 2 mg anti-CD25 or isotype control antibody i.p. and 7 days later 1 × 107 CFSE-labelled BDC2.5 NOD T cells were transferred i.v. Pancreatic lymph nodes were harvested 72 hr later and BDC2.5 NOD T-cell proliferation was measured by assessing CFSE dilution on Vβ4+ cells. (a) Representative histograms show CFSE staining gated on Vβ4+ T cells in NOD PLNs pretreated with (i) isotype control antibody and (ii) anti-CD25 antibody. (iii) There is a significant difference between the percentage of BDC2.5 T cells which have divided in the PLNs of NOD mice pretreated with control antibody (n = 12) compared to the PLNs of NOD mice pretreated with anti-CD25 antibody (n = 13) (P < 0·0001 Mann–Whitney U-test). (b) The same experiment was repeated using non-diabetic 18-week-old NOD female mice. Representative histograms show CFSE staining gated on Vβ4+ T cells in the PLNs of NOD mice pretreated with (i) the control antibody and (ii) the anti-CD25 antibody. (iii) There is a significant difference between the percentage of BDC2.5 NOD T cells which have divided in the PLNs of NOD mice pretreated with isotype control antibody (n = 7) compared to the PLNs of NOD mice pretreated with anti-CD25 antibody (n = 7) (P < 0·05, Mann–Whitney U-test). (c) NOD mice were injected with 2 mg anti-CD25 antibody or control antibody and the proportion of CD4+ T cells in the spleen and PLNs which express Foxp3 was assessed by FACS analysis. Treatment with anti-CD25 caused a significant, although not absolute, decrease in the proportion of CD4+ T cells expressing Foxp3.
Onset of diabetes in NOD.scid recipients is faster when recipients are given anti-CD25 together with splenocytes from diabetic mice
To explore the role of regulatory T cells in diabetic mice, we transferred splenocytes from diabetic NOD mice into NOD.scid mice and concurrently treated the recipient mice with either anti-CD25 antibody or a control antibody. NOD.scid recipient mice treated with anti-CD25 antibody developed diabetes significantly earlier than mice treated with isotype control antibody, indicating that even in diabetic mice, CD25+ cells were still regulating effector cells in vivo(Fig. 7a). CD25-depleted T cells from diabetic donors transferred disease significantly faster into NOD.scid recipients compared to non-CD25-depleted T cells, further emphasizing the role of CD25+ T cells in slowing the adoptive transfer of disease (Fig. 7b).
Figure 7.
Onset of diabetes in NOD.scid mice is faster when recipients are given splenocytes from diabetic NOD donors and anti-CD25. (a) 2 × 107 splenocytes from diabetic female NOD donors were injected i.v. into 7-week-old male NOD.scid mice together with either 2 mg anti-CD25 antibody i.p., 2 mg isotype control antibody i.p. or 200 μl PBS i.p. Recipient NOD.scid mice were tested for the presence of urinary glucose. Onset of diabetes was faster in recipients treated with anti-CD25 compared with isotype control antibody or PBS (log rank analysis, P < 0·05 in both cases). There was no significant difference in the incidence of diabetes between PBS-treated and control antibody-treated recipients (log rank analysis, P = 0·06). (b) 5 × 106 T cells or 5 × 106 T cells depleted of CD25+ cells from diabetic females NOD mice were injected i.v. into 8-week-old male NOD.scid mice. Onset of diabetes was faster in recipients given CD25-depleted T cells than in those given non-depleted T cells (log rank analysis, P < 0·05).
CD4+ CD25+ T cells from non-diabetic but not diabetic NOD donors can slow the adoptive transfer of disease by splenocytes from diabetic NOD mice into NOD.scid recipients
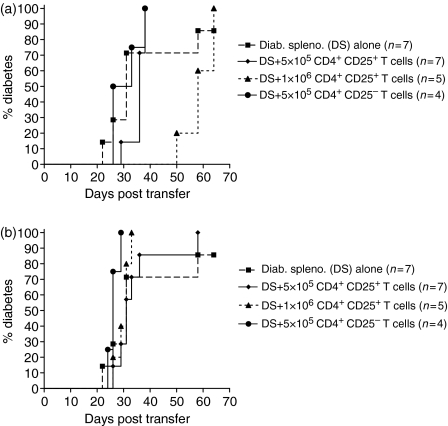
The adoptive transfer data presented so far indicate that T cells from diabetic NOD mice contained a population of CD25+ cells, which can slow the transfer of disease into NOD.scid recipients. These observations suggest that even diabetic mice have CD25+ T cells, which can regulate effector cells. To further explore this, we transferred CD4+ CD25+ cells from either non-diabetic or diabetic NOD mice together with spleen cells from diabetic mice to male NOD.scid recipients. When 1 × 106 CD4+ CD25+ T cells from 6-week-old NOD mice were cotransferred with 1 × 107 splenocytes from diabetic NOD donors, the transfer of disease was delayed, although not completely prevented, compared with 1 × 107 splenocytes from diabetic NOD donors (Fig. 8a). In contrast, neither 5 × 105 nor 1 × 106 CD4+ CD25+ T cells from diabetic donors was able to delay the transfer of disease (Fig. 8b). These experiments suggest that Tregs from diabetic mice were less able to regulate effector cells than Tregs from non-diabetic NOD mice.
Figure 8.
CD4+ CD25+ cells from 6-week-old female NOD mice but not from diabetic NOD mice can delay, although not prevent, the transfer of disease when cotransferred with splenocytes from diabetic NOD donors into NOD.scid recipients. (a) 1 × 107 splenocytes from diabetic NOD donors were cotransferred into NOD.scid recipients either alone or with 5 × 105 CD4+ CD25+ T cells, 1 × 106 CD4+ CD25+ T cells or 5 × 105 CD4+ CD25– T cells from 6-week-old female, non-diabetic NOD donors. At day 48 post transfer, there was a significant delay in the onset of diabetes between the NOD.scid recipients administered diabetic splenocytes alone compared to recipients given diabetic splenocytes and 1 × 106 CD4+ CD25+ T cells (log rank analysis, P < 0·05). However, by day 70 there was no statistically significant difference in the onset of diabetes between the diabetic splenocytes alone and 5 × 105 CD4+ CD25+ T cells (log rank analysis, P = 0·86), diabetic splenocytes and 1 × 106 CD4+ CD25+ T cells (log rank analysis, P = 0·30) or diabetic splenocytes and 5 × 105 CD4+ CD25– T cells (log rank analysis, P = 0·56). (b) 1 × 107 diabetic splenocytes were cotransferred into NOD.scid recipients either alone or with 5 × 105 CD4+ CD25+ T cells, 1 × 106 CD4+ CD25+ T cells or 5 × 105 CD4+ CD25– T cells from diabetic female NOD donors. There was no statistically significant difference in the onset of diabetes between the diabetic splenocytes alone and 5 × 105 CD4+ CD25+ T cells (log rank analysis, P = 0·69), diabetic splenocytes and 1 × 106 CD4+ CD25+ T cells (log rank analysis, P = 0·43). The onset of diabetes was faster in NOD.scid recipients given diabetic splenocytes and 5 × 105 CD4+ CD25– T cells compared to diabetic splenocytes alone (log rank analysis, P < 0·05).
Purified CD4+ CD25+ T cells contain a minority of cells that are Foxp3–; the phenotype of the CD25– population is different in diabetic mice
Since CD25 is not a definitive marker of Tregs, it is possible that the CD4+ CD25+ population from diabetic mice may contain fewer Foxp3+ cells and more activated CD25+ T cells. If this is the case, it cannot be assumed that the experiments described above demonstrate an in vivo decline in the suppressive capacity of diabetic Tregs. To investigate this further, CD4+ CD25+ T cells were purified by magnetic antibody cell sorting (MACS) columns and the proportion of cells that were CD25+ or Foxp3+ was assessed by fluorescence-activated cell sorting (FACS). There was no significant difference in the proportion of cells from 8-week-old and diabetic NOD mice that were CD25+ or Foxp3+, indicating that MACS purification of CD4+ CD25+ produced a similar yield of Foxp3+ cells from both non-diabetic and diabetic NOD mice (Fig. 9a parts i, ii).
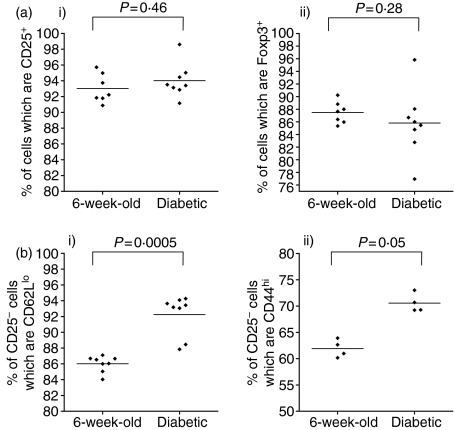
Figure 9.
When using CD4 and CD25 expression to purify T regs, the phenotype of the contaminating CD25– population is different in diabetic NOD mice. (a) CD4+ CD25+ T cells from individual NOD mice which were either 6 weeks old or diabetic were purified on MACS columns and the proportion of cells which were CD25+ or Foxp3+ post purification was analysed by FACS. There was no significant difference in the proportion of cells expressing CD25 (i) or Foxp3 (ii) post MACS CD4+ CD25+ purification from 6-week-old mice compared to diabetic NOD mice. (b) As the MACS purification method could not achieve a totally pure population of CD25+ T cells, the phenotype of the contaminating CD25– population was analysed by costaining with CD44 or CD62L. The proportion of CD25– cells which were CD62Llo (i) and CD44hi (ii) was significantly higher in diabetic mice.
However, it was also clear that MACS purification of CD4+ CD25+ T cells yielded a minority of cells which were not CD25+ from both non-diabetic and diabetic NOD mice and it is possible that the contaminating population of non-CD25+ cells had a different phenotype in diabetic mice compared to non-diabetic mice. To explore this potential further, CD44 and CD62L expression was compared on CD25– cells after CD4+ CD25+ MACS purification. The proportion of CD44hi and CD62Llo cells was higher in the CD25– population in diabetic mice, indicating that the number of memory T cells was higher in the CD25– contaminating cells in diabetic mice. These data highlight the fact that in vivo cotransfer data should be interpreted with caution when they are based on Treg purification by CD25 expression (Fig. 9b parts i, ii).
Discussion
This study attempted to clarify two key questions about regulatory T cells in the NOD mouse; first, does the frequency of regulatory T cells decline in an age-dependent manner or following the onset of diabetes and second, are there alterations in the qualitative function of the regulatory T cells with age or with the onset of diabetes? Regarding the initial question, there is currently no clear consensus on whether the frequency of regulatory T cells in the NOD mouse is abnormal. Several studies have argued that there are no abnormalities in regulatory T-cell frequency in the NOD mouse, including the study by Berzins et al.29 that found that the proportion of CD4+ T cells expressing CD25 in the spleen, lymph nodes and PLNs in NOD mice was similar to that in three other non-autoimmune-prone mouse strains. Gregori et al. reported no differences in the frequency and in the total number of CD4+ CD25+ cells in the spleen and PLNs of 8-week-old NOD mice compared to 16-week-old NOD mice.13 Additional support for the notion that diabetic mice do not have a decline in the frequency of regulatory T cells comes from the observation made by You et al. who found that CD4+ CD25+ T cells from diabetic NOD mice expressed higher levels of Foxp3 mRNA compared to those from 6-week-old NOD mice.30 In contrast, other studies have shown both an age-related decline in regulatory T cells in NOD mice and a reduced total number of regulatory T cells compared to non-autoimmune-prone strains of mice. For example, Pop et al. demonstrated that the frequency of Foxp3-expressing CD25+ CD62Lhi cells declined with age in the PLNs of NOD but not C57BL/6 mice and Wu et al. noted that the total number of splenic CD4+ CD25+ cells was lower in NOD mice compared to age-matched BALB/c mice.31,32 In addition, Alard et al. found that the percentage of CD4+ CD25+ T cells was lower in the spleens and lymph nodes of 9-week-old, female NOD mice compared to age-matched C57BL/6 mice.33
The recent availability of antibodies to Foxp3 has allowed us to examine the proportion of CD4+ cells that express the only currently known exclusive marker of regulatory T cells. Most of the previous studies have used CD25 as a marker of Tregs, which is an imprecise method of quantifying regulatory T cells because CD25 is also expressed on a range of other cell types. Consequently, our observation of no age-related or onset-of-disease-related decline in the proportion of CD4+ cells expressing Foxp3 in either the spleen, PLNs or the pancreas is the most definitive evidence to date that this population of regulatory T cells in the NOD mouse does not undergo changes in function or frequency as the mice age or become diabetic. This is particularly relevant because previous studies have relied on measurement of Foxp3 mRNA when quantifying the numbers of regulatory T cells.31
Regarding the second key question, there is a general consensus that there are defects in the qualitative function of regulatory T cells, both in vitro and in vivo, which are dependent on age or onset of disease. A number of investigators have used in vitro suppression assays to study changes in Treg function over time or following onset of disease. For example, Gregori et al. used an alloantigen driven in vitro assay to investigate whether regulatory T-cell function declines with age in NOD mice.13 They found that CD4+ CD25+ T cells isolated from 16-week-old NOD mice were less able to suppress the proliferation of 16-week-old NOD CD4+ CD25– T cells in response to coculture with C57BL/6 splenocytes than 8-week-old NOD CD4+ CD25+ T cells. Belghith et al. studied the effect of Treg cell function following CD3-specific antibody treatment and found that CD4+ CD25+ T cells isolated using MACS beads from untreated diabetic NOD mice were ineffective at suppressing the proliferation of CD4+ CD25– cells when cultured in vitro with autologous T-cell-depleted spleen cells. However, they also demonstrated that splenic CD4+ CD25hi cells purified using FACS, which allows gating of CD4+ CD25hi cells among the CD4+ CD25+ subset, showed substantially higher inhibition indices independent of age of the mouse.14 You et al. found a progressive decline in the ability of CD4+ CD25+ cells from 6-week-old, 8-week-old and diabetic NOD mice to control the proliferation of their own CD4+ CD25– cells when cultured in vitro with APCs and anti-CD3. The low level of suppression exerted by CD4+ CD25+ T cells from diabetic NOD mice was not enhanced when the ratio of CD4+ CD25+ T cells to CD4+ CD25– T cells was increased. Furthermore, they showed that CD4+ CD25+ T cells from diabetic NOD mice were unable to suppress the proliferation of CD4+ CD25– T cells from 6-week-old NOD mice as effectively as CD4+ CD25+ cells from 6-week-old mice when cultured in vitro with APCs and anti-CD3.30 Further support for the notion that there is an age-related decline in regulatory T-cell function was presented by Pop et al., who showed that CD4+ CD25+ CD62Lhi T cells from the PLNs of 16-week-old NOD mice were less suppressive in vitro than CD4+ CD25+ CD62Lhi T cells from 4-week-old NOD mice.31 In contrast to other studies, this report is the first to demonstrate no age-related decline of CD4+ CD25+ NOD T cells in vitro.
The failure of CD4+ CD25+ T cells from either diabetic or non-diabetic NOD mice to suppress the proliferation of BDC2.5NOD cells when cocultured with the peptide mimotope was also observed by Du et al.26 It is not absolutely clear why both studies found that NOD Tregs were unable to suppress BDC2.5NOD proliferation in response to the mimotope. However, because all BDC2.5NOD T cells are potentially able to respond to the mimotope peptide but only a small number of NOD CD4+ CD25+ T cells could be expected to respond to this peptide, it seems most likely that the few antigen-specific NOD Tregs would be insufficient to suppress the proliferation of this large number of BDC2.5NOD T cells in vitro.
Arguably, the most objective way to assess the in vivo function of Tregs is in cotransfers with a standardized dose of effector cells into NOD.scid recipients and this approach has been adopted by a number of investigators. For example, Gregori et al. found that CD4+ CD25+ T cells from 8-week-old but not 16-week-old NOD mice could suppress the transfer of disease by 16-week-old CD25-depleted splenocytes into NOD.scid recipients.13 In addition, Gregg et al. reported that CD4+ CD25+ cells from 6-week-old but not 8-week-old NOD mice could inhibit the transfer of disease by diabetic splenocytes into NOD.scid recipients.34 Using BDC2.5NOD CD4+ T cells as effectors, Pop et al. found that CD4+ CD25+ T cells isolated from the PLNs of 4-week-old but not 8-week-old or 16-week-old NOD mice were able to suppress the transfer of disease to NOD.scid recipients.31
As illustrated above, most studies that have documented an age-related decline in the suppressive function of Tregs in NOD mice in vivo have used cotransfer systems that rely on the purification of Tregs by CD25 expression.13,14,34 The two important assumptions of this approach are first, that using CD25 as a marker of Tregs gives a similar yield of Foxp3-expressing cells irrespective of the age or disease status and second, that the non-CD25-contaminating population has a similar phenotype between different age groups and disease statuses. We have shown that in our experience the first assumption is reasonable because the proportion of purified cells that were Foxp3+ from diabetic and non-diabetic mice was not significantly different. However, we have also shown that over 10% of cells purified by CD4+ CD25+ expression using MACS columns are Foxp3– and that the phenotype of the CD25– cells is significantly different in diabetic mice compared to non-diabetic mice. The exact influence of this contaminating population on cotransfer studies is difficult to establish; however, our results do highlight the difficulties of interpreting data from cotransfer studies as any apparent changes in Treg function in cotransfer studies may not be the result of changes in Treg function but may instead be because of changes in the phenotype of the contaminating CD25– cells. Ultimately, assessing changes in Treg function based on cotransfer studies will remain difficult until a marker has been identified which enables Tregs to be more accurately purified, thereby circumventing concerns about the potential influence of the contaminating cells from mice of different age groups or disease status.
In an effort to further clarify the age-related changes in Treg function in NOD mice, we adopted a different approach by investigating the proliferation of BDC2.5 NOD T cells in 7-week-old and 18-week-old NOD mice that had been pretreated with anti CD25 antibody. We found that BDC2.5 NOD T cells, which have a known reactivity to islet antigen, were able to proliferate more in the PLNs of NOD mice pretreated with anti-CD25 antibody compared to NOD mice pretreated with a control antibody in both age groups. This demonstrates that the lack of an age-related decline in regulatory T-cell function observed in vitro is mirrored in the NOD mouse itself in vivo. We feel that this observation cannot be explained simply by the anti-CD25 antibody treatment creating a lymphopenic environment and reducing the homeostatic control of autoreactive T cells35,36 because CD4+ Foxp3+ T cells were not completely eliminated following anti-CD25 treatment.27,28 Nevertheless, the decrease in Tregs may be sufficient to enable autoreactive BDC2.5NOD T cells to escape regulatory control.
The reasons for the disparities between studies on regulatory T cells in the NOD mouse is unclear; our work indicates no age-related decline in the frequency or function of regulatory T cells in our NOD mouse colony and the lack of consensus indicates that regulatory T-cell function does vary between NOD colonies. This discrepancy is perhaps to be expected given that both the incidence and speed of onset can vary widely between NOD colonies and that the environmental conditions can have a marked impact on the incidence of diabetes in NOD mice.37–40 In our NOD colony 85–100% of female NOD mice became diabetic by 25 weeks of age (Fig. 1) yet we found no age-related changes in regulatory T-cell frequency or function. We believe that the onset of diabetes is more likely to be associated with changes in the frequency and function of pathogenic T cells rather than a qualitative or quantitative decline in regulatory T cells. Evidence in favour of this argument comes from recent observations that 6-week-old but not 4-week-old NOD splenocytes depleted of CD25+ and CD62L+ cells are able to transfer disease to NOD.scid mice, suggesting a temporal change in the diabetogenic capacity of NOD effector cells.30 This is further supported by our own observations that CD25-depleted splenocytes from 14-week-old non-diabetic NOD mice are able to transfer diabetes more rapidly in NOD.scid mice than CD25+-depleted splenocytes from 7-week-old NOD mice (data not shown).
In conclusion, we have shown that although Tregs undoubtedly play a role in governing the onset of diabetes, the frequency of regulatory T cells does not decline in an age-related or a disease-onset-related manner in the NOD mouse. We have also demonstrated no in vitro decline in the suppressive capacity of NOD CD4+ CD25+ T cells irrespective of age or disease state. Furthermore, we have shown that regulatory T cells still exist in 18-week-old, prediabetic NOD mice and these are capable of suppressing the in vivo proliferation of autoreactive T cells. We have presented data that question the validity of relying on CD25 expression to purify Tregs for cotransfer studies. These observations have important implications for the growing consensus that a decline in regulatory T-cell function underlies diabetes and that regulatory T cells may have great potential as an organ-specific therapy for diabetes.41,42
Acknowledgments
This work was supported by the Wellcome Trust. The authors are very grateful to all members of the Cooke laboratory for helpful discussion, in particular Tim Raine. R.J.M. is supported by a Wellcome Trust Research Training Fellowship and T.D.C.T. is supported by the Jean Shanks Foundation and The James Baird Fund.
Abbreviations
- PLN
pancreatic draining lymph node
- Treg
regulatory T cell
References
- 1.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–60. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 2.Nomura T, Sakaguchi S. Naturally arising CD25+CD4+ regulatory T cells in tumor immunity. Curr Top Microbiol Immunol. 2005;293:287–302. doi: 10.1007/3-540-27702-1_13. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 4.Fehervari Z, Sakaguchi S. CD4+ Tregs and immune control. J Clin Invest. 2004;114:1209–17. doi: 10.1172/JCI23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 6.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 7.Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 8.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 9.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 10.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 11.Paust S, Cantor H. Regulatory T cells and autoimmune disease. Immunol Rev. 2005;204:195–207. doi: 10.1111/j.0105-2896.2005.00247.x. [DOI] [PubMed] [Google Scholar]
- 12.Chatenoud L, Bach JF. Regulatory T cells in the control of autoimmune diabetes. The case of the NOD mouse. Int Rev Immunol. 2005;24:247–67. doi: 10.1080/08830180590934994. [DOI] [PubMed] [Google Scholar]
- 13.Gregori S, Giarratana N, Smiroldo S, Adorini L. Dynamics of pathogenic and suppressor T cells in autoimmune diabetes development. J Immunol. 2003;171:4040–7. doi: 10.4049/jimmunol.171.8.4040. [DOI] [PubMed] [Google Scholar]
- 14.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–8. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 15.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 16.Herold KC, Gitelman SE, Masharani U, et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1 (Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763–9. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brusko TM, Wasserfall CH, Clare-Salzler MJ, Schatz DA, Atkinson MA. Functional defects and the influence of age on the frequency of CD4+ CD25+ T-cells in type 1 diabetes. Diabetes. 2005;54:1407–14. doi: 10.2337/diabetes.54.5.1407. [DOI] [PubMed] [Google Scholar]
- 18.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+) CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–9. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 19.Kukreja A, Cost G, Marker J, et al. Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest. 2002;109:131–40. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–85. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kriegel MA, Lohmann T, Gabler C, Blank N, Kalden JR, Lorenz HM. Defective suppressor function of human CD4+ CD25+ regulatory T cells in autoimmune polyglandular syndrome type II. J Exp Med. 2004;199:1285–91. doi: 10.1084/jem.20032158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balandina A, Lecart S, Dartevelle P, Saoudi A, Berrih-Aknin S. Functional defect of regulatory CD4(+) CD25+ T cells in the thymus of patients with autoimmune myasthenia gravis. Blood. 2005;105:735–41. doi: 10.1182/blood-2003-11-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haskins K, McDuffie M. Acceleration of diabetes in young NOD mice with a CD4+ islet-specific T cell clone. Science. 1990;249(4975):1433–6. doi: 10.1126/science.2205920. [DOI] [PubMed] [Google Scholar]
- 25.Judkowski V, Pinilla C, Schroder K, Tucker L, Sarvetnick N, Wilson DB. Identification of MHC class II-restricted peptide ligands, including a glutamic acid decarboxylase 65 sequence, that stimulate diabetogenic T cells from transgenic BDC2.5 nonobese diabetic mice. J Immunol. 2001;166:908–17. doi: 10.4049/jimmunol.166.2.908. [DOI] [PubMed] [Google Scholar]
- 26.Du W, Wong FS, Li MO, Peng J, Qi H, Flavell RA, Sherwin R, Wen L. TGF-beta signaling is required for the function of insulin-reactive T regulatory cells. J Clin Invest. 2006;116:1360–70. doi: 10.1172/JCI27030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephens LA, Gray D, Anderton SM. CD4+CD25+ regulatory T cells limit the risk of autoimmune disease arising from T cell receptor crossreactivity. Proc Natl Acad Sci USA. 2005;102(48):17418–23. doi: 10.1073/pnas.0507454102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohm AP, McMahon JS, Podojil JR, Begolka WS, DeGutes M, Kasprowicz DJ, Ziegler SF, Miller SD. Cutting Edge. Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J Immunol. 2006;176:3301–5. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- 29.Berzins SP, Venanzi ES, Benoist C, Mathis D. T-cell compartments of prediabetic NOD mice. Diabetes. 2003;52:327–34. doi: 10.2337/diabetes.52.2.327. [DOI] [PubMed] [Google Scholar]
- 30.You S, Belghith M, Cobbold S, et al. Autoimmune diabetes onset results from qualitative rather than quantitative age-dependent changes in pathogenic T-cells. Diabetes. 2005;54:1415–22. doi: 10.2337/diabetes.54.5.1415. [DOI] [PubMed] [Google Scholar]
- 31.Pop SM, Wong CP, Culton DA, Clarke SH, Tisch R. Single cell analysis shows decreasing FoxP3 and TGFbeta1 coexpressing CD4+CD25+ regulatory T cells during autoimmune diabetes. J Exp Med. 2005;201:1333–46. doi: 10.1084/jem.20042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu AJ, Hua H, Munson SH, McDevitt HO. Tumor necrosis factor-alpha regulation of CD4+CD25+ T cell levels in NOD mice. Proc Natl Acad Sci U S A. 2002;99:12287–92. doi: 10.1073/pnas.172382999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alard P, Manirarora JN, Parnell SA, Hudkins JL, Clark SL, Kosiewicz MM. Deficiency in NOD antigen-presenting cell function may be responsible for suboptimal CD4+CD25+ T-cell-mediated regulation and type 1 diabetes development in NOD mice. Diabetes. 2006;55:2098–105. doi: 10.2337/db05-0810. [DOI] [PubMed] [Google Scholar]
- 34.Gregg RK, Jain R, Schoenleber SJ, Divekar R, Bell JJ, Lee HHYuP, Zaghouani H. A sudden decline in active membrane-bound TGF-beta impairs both T regulatory cell function and protection against autoimmune diabetes. J Immunol. 2004;173:7308–16. doi: 10.4049/jimmunol.173.12.7308. [DOI] [PubMed] [Google Scholar]
- 35.Stockinger B, Kassiotis G, Bourgeois C. Homeostasis and T cell regulation. Curr Opin Immunol. 2004;16:775–9. doi: 10.1016/j.coi.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 36.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–77. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 37.Cooke A, Zaccone P, Raine T, Phillips JM, Dunne DW. Infection and autoimmunity: are we winning the war, only to lose the peace? Trends Parasitol. 2004;20:316–21. doi: 10.1016/j.pt.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 38.David T, Thomas C, Zaccone P, Dunne DW, Cooke A. The impact of infection on the incidence of autoimmune disease. Curr Top Med Chem. 2004;4:521–9. doi: 10.2174/1568026043451258. [DOI] [PubMed] [Google Scholar]
- 39.Zaccone P, Raine T, Sidobre S, Kronenberg M, Mastroeni P, Cooke A. Salmonella typhimurium infection halts development of type 1 diabetes in NOD mice. Eur J Immunol. 2004;34:3246–56. doi: 10.1002/eji.200425285. [DOI] [PubMed] [Google Scholar]
- 40.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–20. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 41.Masteller EL, Warner MR, Tang Q, Tarbell KV, McDevitt H, Bluestone JA. Expansion of functional endogenous antigen-specific CD4+CD25+ regulatory T cells from nonobese diabetic mice. J Immunol. 2005;175:3053–9. doi: 10.4049/jimmunol.175.5.3053. [DOI] [PubMed] [Google Scholar]
- 42.Bluestone JA, Tang Q. Therapeutic vaccination using CD4+CD25+ antigen-specific regulatory T cells. Proc Natl Acad Sci USA. 2004;101(Suppl. 2):14622–6. doi: 10.1073/pnas.0405234101. [DOI] [PMC free article] [PubMed] [Google Scholar]