Abstract
Several studies have investigated the interactions between C-reactive protein (CRP) and various complement proteins but none of them took into consideration the different structural forms of CRP. The aim of our study was to investigate whether the different antigenic forms of CRP are able to bind C1q, to trigger activation of the C1 complex and to study the ability of the various CRP forms to bind complement factor H (FH) and C4b-binding protein (C4BP). Interactions between various CRP forms and complement proteins were analysed in enzyme-linked immunosorbent assay and surface plasmon resonance tests and activation of the C1 complex was followed in a reconstituted system using purified C1q, C1r and C1s in the presence of C1-INH. Native, ligand-unbound CRP activated the classical pathway weakly. After binding to phosphocholine, native CRP bound C1q and significantly activated C1. Native CRP complexed to phosphocholine did not bind the complement regulatory proteins FH and C4BP. After disruption of the pentameric structure of CRP, as achieved by urea-treatment or by site-directed mutagenesis, C1q binding and C1 activation further increased and the ability of CRP to bind complement regulatory proteins was revealed. C1q binds to CRP through its globular head domain. The binding sites on CRP for FH and C4BP seemed to be different from that of C1q. In conclusion, in parallel with the increase in the C1-activating ability of different CRP structural variants, the affinity for complement regulatory proteins also increased, providing the biological basis for limitation of excess complement activation.
Keywords: complement, inflammation, C-reactive protein, factor H, C4b-binding protein
Introduction
The classical acute-phase reactant C-reactive protein (CRP) is a pentameric, disc-shaped serum protein.1 Its basic features are the control of inflammation, the stimulation of clearance of damaged cell and tissue components, and the initiation of repair functions.2 CRP shows calcium-dependent affinity for phosphate monoesters, such as phosphatidylcholine, but several other ligands of CRP have been characterized, including damaged cell membranes, small ribonucleoprotein particles, apoptotic cells and fibronectin.3
Native CRP can undergo subunit dissociation into individual monomeric units, for example upon association with negatively charged lipid monolayers.4 This modified form of CRP can be produced in vitro by urea chelation, acid treatment, heating or direct immobilization of native CRP onto polystyrene.5 This modified form of CRP (mCRP) has reduced solubility and exhibits different electrophoretic characteristics as the result of a decrease of isoelectric point (pI) from 6·4 to 5·4.6 The structural changes releasing CRP subunits from the pentamer are correlated with expression of a new antigenic reactivity and formation of neo-epitopes.7 The forms of CRP expressing neo-epitopes are preferentially capable of activating platelets, polymorphonuclear leucocytes, and monocytes in vitro,8 bind various forms of lipoproteins9 and contribute to complement activation.10
CRP activates the classical pathway of complement when bound to appropriate ligands.11 Activation of the complement system by CRP was first demonstrated by Kaplan and Volanakis,12 who reported that addition of pneumococcal C polysaccharide to acute-phase serum caused complement depletion and that this process required the presence of both CRP and C1q. Subsequently, C1q was shown to bind to and agglutinate CRP-coated surfaces.13 Later, various assays were used to analyse the interaction between C1q and CRP and investigate the mechanism of complement activation.14,15 CRP-mediated complement activation is initiated by the binding of one molecule of C1q to two adjacent CRP molecules bound to a ligand.16 Unlike ligand-bound native CRP, mCRP was shown to be unable to induce depletion of haemolytic complement activity.8 CRP efficiently activates the early components of the classical pathway (C1, C4, C2) but generates little C5–C9 consumption.17 Surface-bound CRP decreases the alternative complement pathway C3-convertase and C5-convertase activities, inhibits the alternative amplification loop and reduces deposition of C3b and lysis by the lectin pathway.18–20 An important observation to understand the complex biological role of CRP-induced complement activation was the description of a direct binding of the complement regulatory proteins factor H (FH)21 and C4b-binding protein (C4BP)15 to CRP. These inhibitory effects result from an increased binding of complement regulatory protein FH to CRP-coated surfaces.22 Jarva et al.21 proposed that the function of CRP was to target functionally active FH to injured self tissues. Furthermore, a strong interaction between CRP and the complement regulator C4BP was recently shown, to limit complement activation on CRP.15 Another mechanism through which CRP may limit complement activation is that it up-regulates endothelial cell expression of three complement inhibitory factors (decay accelerating factor, membrane-cofactor protein and protectin).23 Interaction between injured and apoptotic tissues and CRP thus seems to be pivotal in preventing excess complement activation and limiting inflammation by inducing and orchestrating regulatory processes.
Although several studies have aimed at investigating the interactions between CRP and various complement proteins, none of these studies took into consideration the different structural forms of CRP. The aim of the present study was therefore to investigate whether the different antigenic forms of CRP are able to bind C1q, to trigger activation of the C1 complex and to locate the C1q site(s) responsible for CRP binding. In addition, we have investigated the ability of the various CRP forms to bind complement FH and C4BP.
Materials and methods
C-reactive protein and its modified forms
The native, pentameric form of CRP (nCRP) purified from human plasma was purchased from Sigma (St Louis, MO). The modified form of CRP (designated urea-mCRP) was prepared from the native, pentameric preparation by incubation in 8 m urea for 1 hr at 37° followed by extensive dialysis overnight at 4° against 25 mm Tris–HCl (pH 8·3). A recombinant form of modified CRP (r-mCRP) with both its Cys36 and Cys97 residues mutated to alanines and with an added N-terminal formylmethionine residue was expressed in Escherichia coli and was isolated from inclusion bodies to >95% purity (kind gift from L. A. Potempa, ImmTech Inc, Vernon Hills, IL). To enhance its solubility, r-mCRP was acylated with citraconic anhydride. The resulting CRP species is termed Cr-mCRP.24
Complement proteins
The C1q subunit of C1 was purified from human plasma as described previously.25,26 Isolation of the C1s-C1r-C1r-C1s tetramer was performed as described previously.27,28 The concentrations of purified C1q and C1s-C1r-C1r-C1s were determined spectrophotometrically using values of absorbance (1%, 1 cm) at 280 nm (A280) of 6·8 and 13·5, and MW values of 459 300 and 330 000, respectively.
The fragments corresponding to the globular head regions of C1q (C1q GR) were generated by treatment of C1q with Clostridium histolyticum collagenase (C0255; Sigma) (C1q : collagenase ratio, 15 : 1, w/w) for 16 hr at 37° in 250 mm NaCl, 5 mm CaCl2, 50 mm Tris–HCl (pH 7·4), and purification was achieved by high-pressure gel filtration chromatography on a TSK-G2000 SW column (LKB, Rockville, MD). The purified GR were quantified by using an A280 (1%, 1 cm) value of 7·0 and a MW of 48 000. The homogeneity of the purified proteins and fragments was assessed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) under reducing and non-reducing conditions.
Complement FH purified from human plasma was purchased from Quidel (San Diego, CA) and C4BP purified from human plasma was obtained from Hyphen BioMed (Neuville sur Oise, France).
Antibodies and other reagents
Rabbit anti-CRP polyclonal antibody A0073 was obtained from DAKO, Glostrup, Denmark; anti-CRP monoclonal antibody CRP-8, C-1688 was supplied by Sigma; goat anti-human FH A229 was obtained from Quidel; and sheep anti-human C4BP PC026 was from The Binding Site, Birmingham, UK. The following secondary antibodies were used: monoclonal anti-goat/sheep immunoglobulin G (IgG)–peroxidase conjugate (A9452, Sigma), goat anti-mouse IgG–peroxidase conjugate (1030-05, Southern Biotech, Birmingham, AL). The anti-CRP monoclonal antibody panel [clones I-15-1D6 (Isotype: IgG2a, κ), I-26-8D8 (IgG1, κ), II-15-2C10 (IgG2a, κ), III-26-8C10 (IgG1, κ), IV-13-3H12 (IgG1, κ), IV-26-9C9 (IgG1, κ), and IV-13-12D7 (IgG2a, κ)], the phosphatidylcholine conjugated to keyhole-lympet haemocyanin (PC-KLH) were produced as described previously7 and were kindly provided by L. A. Potempa (ImmTech Inc.). Immobilized p-aminophenyl-phosphorylcholine coupled to Sepharose (PC-Sepharose) was purchased from Pierce (Rockford, IL).
Real-time surface plasmon resonance spectroscopy and data evaluation
Surface plasmon resonance analysis of the interaction between CRP and C1q was performed using a BIAcore 3000 instrument. The running buffer for protein immobilization was 145 mm NaCl, 5 mm ethylenediaminetetraacetic acid (EDTA), 10 mm HEPES, pH 7·4. Proteins were diluted to 40–50 μg/ml in 10 mm sodium acetate, pH 5·0 (C1q), 10 mm sodium acetate, pH 4·0 (bovine serum albumin (BSA), CRP) or 10 mm sodium formate, pH 3·0 (mCRP) and immobilized onto the carboxymethylated dextran surface of a CM5 sensor chip (BIAcore AB) using the amine coupling chemistry according to the manufacturer's instructions. Binding of nCRP, Cr-mCRP was measured over immobilized C1q. Binding of C1q and of C1q GR was measured over immobilized nCRP and Cr-mCRP. As controls, all binding tests were also performed over a surface with immobilized BSA (7400 RU) to serve as blank sensorgrams for substraction of the bulk refractive index background. Regeneration of the surfaces was achieved by injection of 5 μl 2-m guanidine–HCl. Sensorgrams were analysed with the BIAevaluation 3·1 software (BIAcore AB). The association and dissociation constants were determined by global fitting of the data using the 1 : 1 Langmuir binding model (A + B ↔ AB). Dissociation constants (KD) were calculated from the ratio of the dissociation and association rate constants (koff/kon). Surface plasmon resonance analyses of the interactions between CRP and C4BP were carried out using a BIAcore X apparatus. Immobilization was performed using 0·01 m HEPES pH 7·4, 0·15 m NaCl, 3 mm EDTA, 0·005% v/v surfactant P20, as flow buffer and a flow rate of 10 μl/min. C4BP was diluted to 50 μg/ml in 10 mm sodium acetate, pH 4·0 and immobilized onto a CM5 sensor chip (BIAcore AB) using the amine coupling chemistry (BIAcore AB amine coupling kit) according to the manufacturer's instructions. Binding of native and modified CRP to C4BP was tested by injecting nCRP or Cr-mCRP to a C4BP-coupled chip and over a surface with immobilized BSA (4845 RU) to serve as blank. Regeneration of the surfaces was achieved by injection of 5 μl 5 mm NaOH. Data were fitted as described above.
C1 activation assay
C1 activation assays were performed as described previously.28–30 Briefly, the C1 complex (0·25 μm) was reconstituted from equimolar amounts of C1q and C1s-C1r-C1r-C1s, and incubated in 50 mm triethanolamine–HCl, 145 mm NaCl, 1 mm CaCl2 (pH 7·4) in the presence of 100 μg/ml nCRP, nCRP + PC-Sepharose, u-mCRP or Cr-mCRP for 90 min at 37° in the presence of 1 μm C1 inhibitor. The reaction mixtures were submitted to SDS–PAGE analysis under reducing conditions using 10% acrylamide gels. The bands corresponding to C1s were revealed by Western blot analysis using a rabbit polyclonal antibody after electrotransfer to a nitrocellulose membrane. Membranes were scanned using a Shimadzu model CS 9000 gel scanner (Tokyo, Japan) and C1 activation was determined from the amounts of the A and B chains of activated C1s relative to that of the proenzyme.
Binding and competitive inhibition assays using enzyme-liked immunosorbent assay
Enzyme-liked immunosorbent assay (ELISA) plates (Greiner Bio-One GmbH, Frickenhausen, Germany Cat. No. 655101) were coated with C1q, the various forms of CRP, or PC-KLH in 0·1 m bicarbonate buffer overnight at 4°. After washing with phosphate-buffered saline (137 mm sodium chloride, 2·7 mm potassium chloride, 100 mm Na2HPO4, and 2 mm KH2PO4; pH 7·4) containing 0·05% Tween-20, plates were incubated with different CRP or complement preparations in Tris-buffered saline (TBS; 154 mm sodium chloride, 25 mm TRIS, pH 7·4) containing 0·5% (w/v) gelatin, 5 mm CaCl2 and 0·05% (w/w) Tween-20 for 1 hr at room temperature. Proteins fixed to the plate were detected with polyclonal or monoclonal antibodies followed by incubation with horseradish peroxidase-conjugated secondary antibodies in TBS (pH 7·4) containing 0·5% (w/v) gelatin and 5 mm calcium, 0·05% (w/w) Tween-20 (each for 1 hr at room temperature). In experiments where C1q was coated onto the plate, 1 m NaCl was added to the buffer to avoid non-specific binding of immunoglobulins. The substrates used were hydrogen peroxide and o-phenylene-diamine (Sigma), the optical density was measured at λ = 490 nm (reference at λ = 620 nm) and the mean values from two to four parallel measurements was calculated.
For competitive inhibition experiments of the CRP–C1q interactions, CRP preparations were preincubated with the different competitors (C1q or C1q GR) in TBS (pH 7·4) containing 0·5% (w/v) gelatin and 5 mm calcium and 0·5% (w/v) Tween-20 for 30 min at room temperature.
Statistical analysis
Data are given as means of parallel measurements with standard deviations. Binding characteristics of different proteins were compared by the analysis of variance method. A P-value <0·05 was considered as significant. GraphPad Prism 3·0 (http://www.graphpad.com) was used for data presentation and statistical analysis.
Results
C-reactive protein undergoes structural changes upon immobilization
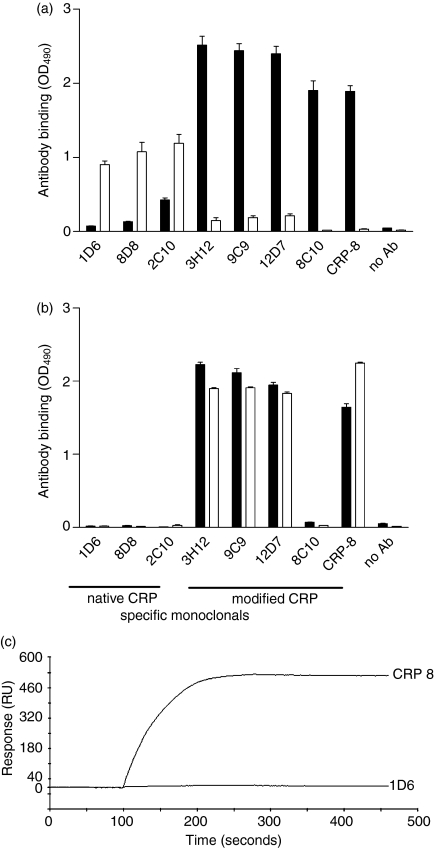
Native CRP circulating in plasma has a disc-shaped form made up from five non-covalently linked identical subunits. Upon interaction with its ligands CRP displays a modified structure. However, various treatments frequently used for in vitro model experiments also modify the structure of the pentameric disc of CRP. Therefore we studied whether direct fixation or adsorption to artificial surfaces, such as coating to a polystyrene ELISA plate or chemical cross-linking to a BIAcore sensor chip, elicits structural modifications of CRP resulting in the emergence of neo-epitopes. Experiments providing support for such modifications are presented in Fig. 1. Thus, direct coating of native CRP (Fig. 1a) to ELISA plates was found to unmask the neo-epitopes recognized by mCRP-specific monoclonal antibodies (3H12, 9C9, 12D7, 8C10, CRP-8). In contrast, indirect coating, through polyclonal anti-CRP antibodies, did not alter the native conformation because in this case the immobilized CRP molecule retained its reactivity only towards the nCRP-specific monoclonal antibodies (1D6, 8D8, 2C10). In contrast with these observations, the monomeric Cr-mCRP molecule produced by site-directed mutagenesis, which is unable to form pentamers, was not reactive with nCRP-specific antibodies, but was recognized by virtually all mCRP-specific antibodies (except 8C10), independently of the method of coating (Fig. 1b).
Figure 1.
Structural modification of C-reactive protein upon immobilization. Polystyrene ELISA plates were coated either directly (at 2 μg/ml, black bars) or indirectly [through anti-CRP polyclonal antibodies (1 : 1000) at 2 μg/ml, open bars] with different C-reactive protein preparations: (a) native CRP; (b) recombinant, modified CRP. A set of anti-CRP monoclonal antibodies (1DH, 8D8, 2C10, 3H12 at 0·1 μg/ml; 3G12, 9C9 at 0·2 μg/ml; 12D7 at 0·9 μg/ml; and 8C10 at 1·6 μg/ml) was used to detect structural modifications. Each value is the mean ± SD of two parallel experiments. (c) Binding of 1D6 and CRP8 anti-CRP antibodies to immobilized native CRP was performed in 0·01 m HEPES, 0·15 m NaCl, 0·67 mm CaCl2, 0·005% v/v surfactant P20, pH 7·4 at a flow rate of 20 μl/min.
Further analyses were performed by surface plasmon resonance spectroscopy. As shown in Fig. 1(c), native CRP covalently cross-linked to the surface of a BIAcore sensor chip also reacted with an mCRP-specific antibody (CRP-8), but lost its reactivity towards an antibody specific for the native conformation (1D6).
Modified CRP but not native CRP interacts with immobilized C1q
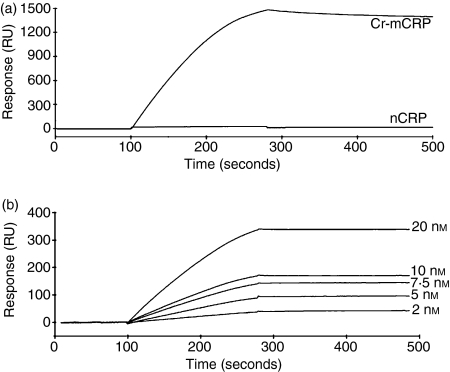
The ability of nCRP and Cr-mCRP to bind to C1q was studied using surface plasmon resonance spectroscopy, using immobilized C1q and CRPs as soluble ligands. At a concentration of 100 nm, Cr-mCRP readily bound to immobilized C1q. In contrast, no interaction was detected under the same conditions using nCRP (Fig. 2a). Non-specific binding to the matrix was eliminated by subtracting the signal obtained on a control surface saturated with BSA.
Figure 2.
Analysis by surface plasmon resonance spectroscopy of the interaction of native CRP and modified CRP with immobilized C1q. Sensorchips were coated with C1q (17 400 RU) as described in the Materials and methods. (a) Sixty microlitres of nCRP or Cr-mCRP (100 nm) was injected in the running buffer (PBS containing 0·005% surfactant P20) at a flow rate of 20 μl/min. Resonance units are indicated as a function of time. (b) Dose–response analysis of the binding of Cr-mCRP to immobilized C1q. Representative sensorgrams (after background subtraction) illustrating the binding of Cr-mCRP at varying concentrations (bottom to top curves: 2, 5, 7·5, 10, 20 nm) to immobilized C1q.
To determine the kinetic parameters of the interaction between C1q and Cr-mCRP, sensorgrams were recorded at varying Cr-mCRP concentrations. Figure 2(b) shows the association and dissociation curves of binding experiments performed at five different concentrations of Cr-mCRP (2–20 nm). The values of kon, koff and of the resulting apparent equilibrium dissociation constant KD were measured and are listed in Table 1. A KD value of 0·28 nm was determined, arising from a very low koff value (6·1 × 10−5 per second) indicative of a very stable interaction between immobilized C1q and soluble Cr-mCRP.
Table 1.
Kinetic and dissociation constants for the interaction of native and recombinant modified CRP with immobilized C1q (17 400 RU) as determined by surface plasmon resonance
Not applicable because of lack of binding.
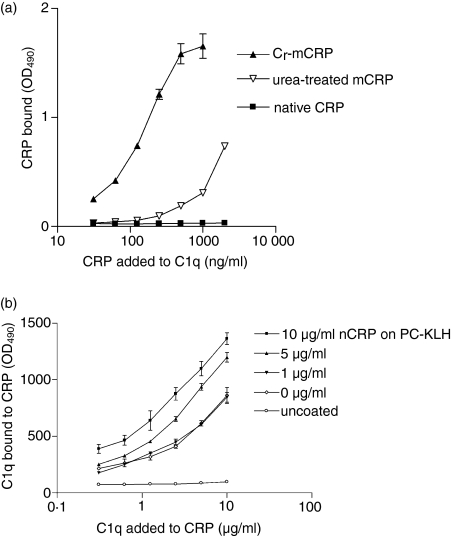
Similar results were obtained by ELISA experiments in which C1q was coated to the plate and binding of native CRP and its modified forms (urea-mCRP and Cr-mCRP) was tested (Fig. 3a). Both Cr-mCRP and urea-mCRP bound reproducibly (P < 0·0001) and in a dose-dependent manner to immobilized C1q. In contrast, no significant interaction was observed using the native CRP preparation. In other experiments, ELISA plates were coated with PC-KLH, nCRP was added at increasing concentrations, and then C1q binding was measured (Fig. 3b). As illustrated in Fig. 3(b), C1q bound to nCRP complexed to PC-KLH in a dose-dependent fashion, indicating that the sites recognized by C1q become available under these conditions. It should be emphasized that PC-KLH itself was recognized by C1q to some extent, because significant binding occurred when nCRP was omitted.
Figure 3.
(a) Binding of different CRP preparations to immobilized C1q in ELISA. C1q (2 μg/ml) was coated onto ELISA plates and incubated with different CRP preparations at varying concentrations. The extent of CRP binding to immobilized C1q was measured by reaction with polyclonal anti-CRP antibodies (1 : 1000) and is expressed as OD490 values. (b) Binding of C1q to ligand-complexed nCRP. ELISA plates were coated with PC-KLH and native CRP was added at increasing concentrations. In the next step varying C1q concentrations were added and the amount of bound C1q was measured by specific antibodies. PC-KLH and uncoated wells were used as controls. The data shown represent the means ± SD of two experiments.
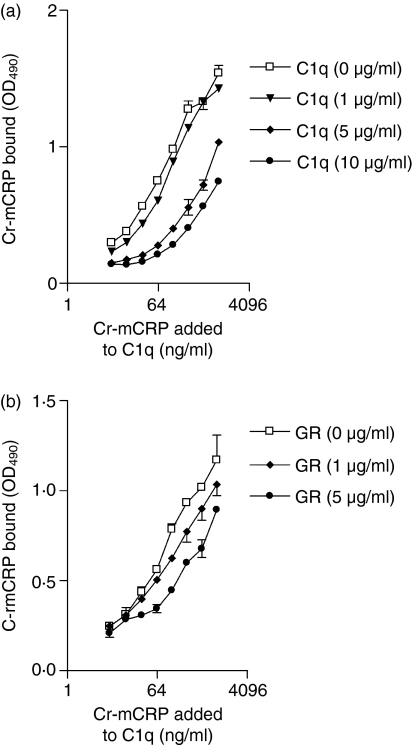
As illustrated in Fig. 4(a), the interaction between Cr-mCRP and C1q coated to an ELISA plate could be inhibited in a dose-dependent manner by C1q added in solution, and significant inhibition was observed at C1q concentrations of 5 and 10 μg/ml (P < 0·0001). Binding of Cr-mCRP to immobilized C1q could also be significantly inhibited by C1q GR at 1 μg/ml (P = 0·0003) and 5 μg/ml (P < 0·0001) (Fig. 4b), indicating an interaction between Cr-mCRP and C1q GR in the fluid phase.
Figure 4.
Competitive inhibition of the binding of Cr-mCRP to immobilized C1q by soluble C1q (a) or its globular regions (GR) (b) C1q (2 μg/ml) was coated onto ELISA plates and incubated with Cr-mCRP at varying concentrations. Before addition to the plate, Cr-mCRP was incubated with C1q (a) or C1q GR (b) at varying concentrations. The extent of Cr-mCRP binding to immobilized C1q was measured by polyclonal anti-CRP antibodies (1 : 1000) and is indicated as OD values. The data shown represent the means ± SD of three experiments.
C1q and C1q GR interact with immobilized nCRP and mCRP
Further analysis by surface plasmon resonance spectroscopy of the interaction between C1q and CRP was carried out in the reverse configuration, ie using C1q or C1q GR in the fluid phase and either nCRP or Cr-mCRP as immobilized ligands. Both C1q and its GR fragment bound to immobilized nCRP and Cr-mCRP. The kinetic parameters of the interactions were determined by recording sensorgrams at different C1q and C1q GR concentrations and data were evaluated by global fitting as described in the Materials and methods. The values of the association (kon) and dissociation (koff) rate constants and of the resulting apparent equilibrium constant KD are listed in Table 2. The binding constants for the interaction of C1q with either native or modified CRP were of the same order, with KD values in the nanomolar range in both cases.
Table 2.
Kinetic and dissociation constants for the interaction of C1q and C1q-GR with immobilized native CRP or modified CRP as determined by surface plasmon resonance
| kon(/m second) | koff(/second) | KD(nm) | Rmax(RU) | |
|---|---|---|---|---|
| Immobilized nCRP (4300 RU) | ||||
| C1q | 7·4 × 105 | 1·1 × 10−3 | 1·45 | 119 |
| C1q-GR | 3·5 × 103 | 2·4 × 10−3 | 680 | 103 |
| Immobilized Cr-mCRP (5500 RU) | ||||
| C1q | 1·5 × 106 | 2·9 × 10−3 | 1·95 | 291 |
| C1q-GR | 5·1 × 104 | 1·6 × 10−3 | 31·6 | 310 |
Binding of C1q and of C1q GR was measured over 4300 and 5500 RU of immobilized nCRP and Cr-mCRP, at a flow rate of 20 μl/min, in 145 mm NaCl, 2 mm CaCl2, 50 mm triethanolamine–HCl containing 0·005% surfactant P20.
Compared to C1q, the KD values determined for C1q GR were much higher, particularly for the interaction with immobilized nCRP, where the KD increased from 1·45 to 680 nm. For both nCRP and Cr-mCRP, the increase in KD resulted essentially from an increase in kon, the koff values remaining practically unchanged (Table 2).
Activation of the C1 complex by CRP
To test the ability of the various CRP species to trigger activation of the classical complement pathway, C1 activation assays were performed in vitro using purified C1 in the presence of excess C1 inhibitor as described in the Materials and methods. Native CRP purified from human plasma only induced weak activation (9%). In contrast, incubation of C1 in the presence of urea-mCRP, PC-Sepharose complexed nCRP, or recombinant modified CRP led to significant activation, with activation levels of 20%, 24% and 48%, respectively (Table 3).
Table 3.
C1 activation by different CRP forms
| Activation % | |
|---|---|
| nCRP (100 μg/ml) | 9·3% |
| uCRP (100 μg/ml) | 20·4% |
| nCRP (100 μg/ml) + PC-Sepharose1 | 23·9% |
| Cr-mCRP (100 μg/ml) | 47·6% |
No significant C1 activation was observed using PC-Sepharose alone as a control.
Interaction of various CRP forms with complement regulatory proteins
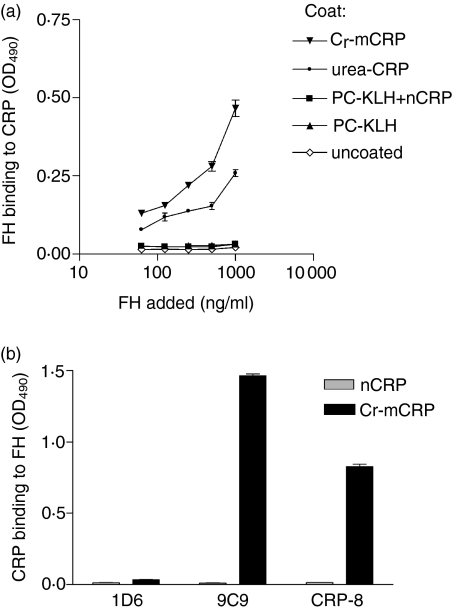
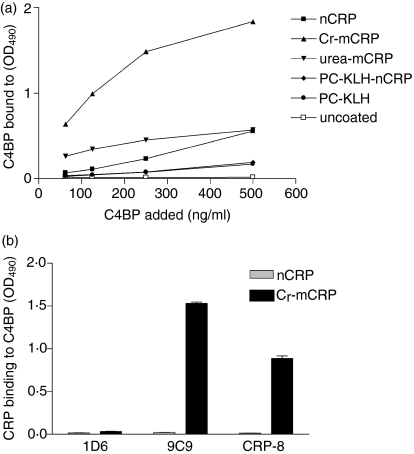
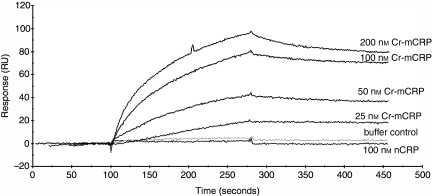
We next investigated the interactions of FH and C4BP with various structural forms of CRP. For this purpose, ELISA plates were coated either directly or indirectly (through PC-KLH) with native or modified CRP and then FH was added. As shown in Fig. 5(a), FH bound readily and in a dose-dependent manner to both Cr-mCRP and urea-mCRP, but no significant binding to native CRP complexed to PC-KLH was observed. Likewise, C4BP bound to modified forms of CRP but not to nCRP complexed to PC-KLH (Fig. 6a). Native CRP, when coated directly to ELISA plates and resulting in structural modification (Fig. 1) bound C4BP (Fig. 6a). When ELISA plates were coated with FH (Fig. 5b) or C4BP (Fig. 6b) Cr-mCRP bound to these complement regulatory proteins, but native CRP did not. These observations were corroborated by surface plasmon resonance analyses, indicating that Cr-mCRP bound to immobilized C4BP in a dose-dependent manner (Fig. 7). In contrast, no interaction was observed using native CRP. Table 4 shows the kinetic parameters of the interaction between C4BP and Cr-mCRP, which were determined by recording sensorgrams at different protein concentrations. The KD value is in the nanomolar range, indicating high affinity.
Figure 5.
Binding of various CRP forms to human complement FH. (a) ELISA plates were coated directly with Cr-mCRP or urea-mCRP, or indirectly (through PC-KLH) with native CRP at 2 μg/ml concentration. Uncoated wells and wells coated with PC-KLH only were used as controls. Increasing amounts of purified human FH were added and detected by anti-FH antibodies. (b) ELISA plates were coated with human complement FH (2 μg/ml) and incubated with either nCRP or Cr-mCRP (10 μg/ml). Monoclonal antibodies specific for the native (1D6) or modified (9C9 and CRP-8) CRP conformation were used to determine the amounts of CRP bound to the plate. The data shown represent the means ± SD of two experiments.
Figure 6.
Binding of various CRP forms to human CD binding protein (C4BP). (a) ELISA plates were coated directly with nCRP, Cr-mCRP, urea-mCRP or with nCRP complexed to PC-KLH at 2 μg/ml concentration. PC-KLH-coated wells and uncoated wells were used as controls. Increasing amounts of purified human C4BP were added and detected by anti-C4BP antibodies. (b) ELISA plates were coated with human C4BP (2 μg/ml) and incubated with either nCRP or Cr-mCRP (10 μg/ml). Monoclonal antibodies specific of the native (1D6) or modified (9C9 and CRP-8) CRP conformation were used to determine the amounts of CRP bound to the plate. The data shown represent the means ± SD of two experiments.
Figure 7.
Analysis by surface plasmon resonance spectroscopy of the interaction between native CRP or modified CRP and immobilized C4BP. Sensor chips were coated with C4BP (5230 RU) as described in the Materials and methods. Sixty microlitres of nCRP (100 nm), or Cr-mCRP (25–200 nm) were injected in the running buffer (0·01 m HEPES, 0·15 m NaCl, 0·67 mm CaCl2, 0·005% v/v surfactant P20, pH 7·4) at a flow rate of 20 μl/min. Resonance units are indicated as a function of time.
Table 4.
Kinetic and dissociation constants for the interaction of native CRP or modified CRP with immobilized C4BP (5230 RU) as determined by surface plasmon resonance
Not applicable because of the lack of binding.
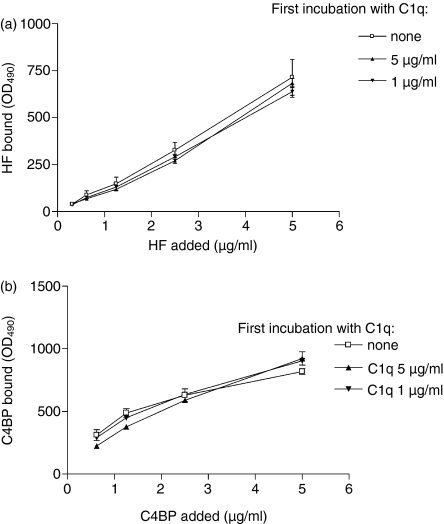
Cross-competition experiments were carried out to test whether the binding sites for C1q and complement regulatory proteins FH and C4BP overlap on CRP. For this purpose, ELISA plates were coated with Cr-mCRP, first incubated with C1q and then with increasing concentrations of FH (Fig. 8a) or C4BP (Fig. 8b). As shown in Fig. 8, preincubation of immobilized Cr-mCRP with C1q had no significant effect on subsequent binding of FH or C4BP, indicating that the binding sites for these proteins on CRP do not overlap with those of C1q.
Figure 8.
Mapping of the binding sites for C1q, FH and C4BP on CRP by competitive ELISA experiments. ELISA plates coated with Cr-mCRP (2 μg/ml) were incubated with C1q and then allowed to interact with complement regulatory proteins FH and C4BP. Binding was detected by specific antibodies and secondary antibodies as described in the Materials and methods. The observed differences were not statistically significant. The data shown represent the means ± SD of three experiments.
Discussion
In the present study, we have systematically tested the various forms of CRP for their interaction with C1q, their C1-activating ability and their binding to two complement regulatory proteins, FH and C4BP. We provide evidence that CRP chemically cross-linked to a sensor chip surface undergoes structural changes resulting in the appearance of neo-epitopes and the formation of new ligand-binding sites. Furthermore, our observation on the structural modification of CRP coated to ELISA plates is in complete agreement with Potempa et al.6 These forms are therefore different from the native, pentameric state but have nevertheless been frequently used as native CRP preparations in previous experiments, without taking into account these structural modifications. As documented here, native CRP in solution does not bind to surface-coated C1q and only activates the C1 complex to a very small extent. In contrast, when complexed to its ligand phosphocholine, native CRP binds C1q and significantly activates C1. Interestingly, native CRP complexed to phosphocholine does not bind complement regulatory proteins FH and C4BP. After disruption of the pentameric structure of CRP as achieved by urea-treatment or by site-directed mutagenesis, C1q binding and C1 activation further increase and the ability of CRP to bind complement regulatory proteins is revealed. In this respect, our results fully agree with the report by Sjöberg et al.15 underlying the importance of surface-immobilized forms of CRP in inducing complement regulation. Similar structural modifications unmasking the binding sites for C1q, FH and C4BP take place in vitro after coating or chemical linkage of native CRP on a surface.
Based on these observations, it is essential to differentiate between the different forms of CRP when testing the various biological activities of this protein. After tissue damage during the acute-phase reaction, the serum concentration of native, pentameric CRP increases while the monomeric tissue form of CRP may also appear. Indeed, mCRP has been shown to be present in the kidneys of diabetic patients in tubular localization31 and in atherosclerotic plaques in colocalization with complement proteins.32,33 Once CRP becomes surface-bound to its ligands the binding site for C1q opens on the disc, resulting in activation of the classical complement pathway. This structural form does not display neo-epitopes and binding sites for complement regulatory proteins. In contrast, after disruption of the pentameric disc and appearance of neo-epitopes in the tissues the molecule readily activates C1 and also acquires the ability to bind complement regulatory molecules. As known from the studies of Giannakis et al.34 and Ji et al.10 the result of these processes is the activation of the classical pathway up to the C3 cleavage stage, without significant activation of the terminal lytic pathway. The results obtained in different animal models, such as coronary ligation35 or cerebral ischaemia,36 show that the size of the lesions increases substantially with increasing CRP levels, indicating that the balance between stimulation and control of complement activation by CRP is very sensitive. This may be explained by the fact, that serum concentrations of CRP may raise 100-fold to 500-fold during the acute-phase reaction, whereas those of FH and C4BP only raise by 33–100%37 resulting in depletion of complement regulation and emergence of dysbalance.
With respect to the precise mechanism of the C1q–CRP interaction, our results support the view of McGrath et al.14 because the globular head region of C1q was also shown by us to be the interaction site for CRP. In the same way, the results of our C1 activation assays indicate a gradual increase in the extent of C1 activation when fluid-phase CRP, ligand-bound CRP or monomeric CRP was used as the C1 activator. Most probably, concomitantly with the structural modification of CRP, the shape of the C1q binding sites also changed, resulting in a higher affinity interaction with C1q and hence a more efficient C1 activation.
The binding site(s) for FH and C4BP appear(s) to be different from that for C1q on CRP. We used a competition assay where the proteins were added in sequential order to mimic the in vivo situation. In this assay, CRP was first incubated with various amounts of C1q (the complement activation step) and thereafter with the FH or C4BP (the regulatory step). In this assay C1q was not able to inhibit binding of FH or C4BP to CRP which, with regard to C4BP, is in agreement with Sjöberg et al.15 As for the interaction of CRP with FH, Jarva et al. located the Ca2+-dependent binding sites to SCR7 and SCR8–SCR11 on FH.21 These sites are in close vicinity on the elongated FH molecule, making it likely that after structural rearrangement of the pentameric CRP on the injured membranes one FH molecule may bind to two monomeric CRP units.
CRP was previously found to colocalize with classical pathway components on the surface of infarcted myocardium38 and in liver and kidney infarction sites in a patient who died from multiple complications after cholecystectomy.39 Likewise, complement proteins colocalize with monomeric CRP in atherosclerotic lesions.32 The results of Oksjoki et al. indicate that in early lesions (American Heart Association types II and III), FH is found in the superficial proteoglycan-rich layer in association with numerous macrophages and C3d, whereas C5b-9 is found deeper in the intima, where FH is virtually absent.40 Taken together, these data strongly support the hypothesis that once complement activation is induced at sites of cellular damage, CRP might actively promote inflammation and further complement activation if a dysbalance between complement activation and regulation exists. It is therefore essential to understand better the nature of the interaction of CRP with its many ligands and with complement proteins. Further clinical studies are therefore necessary to analyse why the activity of complement regulatory proteins decreases in acute and chronic inflammatory states rendering CRP a proinflammatory, complement-activating molecule.
In conclusion, in this study we report on two novel observations. First, different structural forms of CRP induce the activation of a classical complement pathway through C1q. Second, regulation of both classical and alternative pathways by modified CRP is achieved by the binding of soluble regulatory proteins. Finally, in parallel with the increase in the C1-activating ability of different CRP structural variants the affinity for complement regulatory proteins also increases, providing the biological basis for limitation of excess complement activation. This dual role of CRP in the regulation of the extent of inflammation after tissue damage makes CRP a keystone molecule in maintenance and repair processes under pathological circumstances.
Acknowledgments
We thank László Patthy and Mária Trexler for access to BIAcore X system instrument, and Larry A Potempa for providing monoclonal antibodies to CRP and the Cr-mCRP molecule. This study was supported by the following grants: EU QLG1-CT-2002–90397 (ATHERNET), and National Research Fund (OTKA T46837). The work of Z.R. was carried out within the framework of Hungarian Research Movement Action (http://www.kutdiak.hu). The skilful technical assistance of Szigeti Antalné is acknowledged with many thanks.
Abbreviations
- C1q GR
globular head regions of C1q
- C4BP
C4b-binding protein
- CRP
C-reactive protein
- FH
factor H
- IgG
immunoglobulin G
- mCRP
modified form of CRP
- nCRP
native CRP
- PC-KLH
phosphatidylcholine conjugated to keyhole-limpet haemocyanin
- r-mCRP
recombinant form of modified CRP
- RU
resonance unit
- SPR
surface plasmon resonance
References
- 1.Volanakis JE. Human C-reactive protein. Expression, structure and function. Mol Immunol. 2001;38:189–97. doi: 10.1016/s0161-5890(01)00042-6. [DOI] [PubMed] [Google Scholar]
- 2.Pepys MB. C-reactive protein fifty years on. Lancet. 1981;1(8221):653–7. doi: 10.1016/s0140-6736(81)91565-8. [DOI] [PubMed] [Google Scholar]
- 3.Du Clos TW, Mold C. C-reactive protein: an activator of innate immunity and a modulator of adaptive immunity. Immunol Res. 2004;30:261–77. doi: 10.1385/IR:30:3:261. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y, Wang HW, Ji SR, Sui SF. Two-dimensional crystallization of rabbit C-reactive protein monomeric subunits. Acta Crystallogr D Biol Crystallogr. 2003;59:922–6. doi: 10.1107/s0907444903004414. [DOI] [PubMed] [Google Scholar]
- 5.Kresl JJ, Potempa LA, Anderson BE. Conversion of native oligomeric to a modified monomeric form of human C-reactive protein. Int J Biochem Cell Biol. 1998;30:1415–26. doi: 10.1016/s1357-2725(98)00078-8. [DOI] [PubMed] [Google Scholar]
- 6.Potempa LA, Maldonado BA, Laurent P, Zemel ES, Gewurz H. Antigenic, electrophoretic and binding alterations of human C-reactive protein modified selectively in the absence of calcium. Mol Immunol. 1983;20:1165–75. doi: 10.1016/0161-5890(83)90140-2. [DOI] [PubMed] [Google Scholar]
- 7.Ying SC, Gewurz H, Kinoshita CM, Potempa LA, Siegel JN. Identification and partial characterization of multiple native and neoantigenic epitopes of human C-reactive protein by using monoclonal antibodies. J Immunol. 1989;143:221–8. [PubMed] [Google Scholar]
- 8.Potempa LA, Zeller JM, Fiedel BA, Kinoshita CM, Gewurz H. Stimulation of human neutrophils, monocytes, and platelets by modified C-reactive protein (CRP) expressing a neoantigenic specificity. Inflammation. 1988;12:391–405. doi: 10.1007/BF00915774. [DOI] [PubMed] [Google Scholar]
- 9.Ji SR, Wu Y, Potempa LA, Qiu Q, Zhao J. Interactions of C-reactive protein with low-density lipoproteins: implications for an active role of modified C-reactive protein in atherosclerosis. Int J Biochem Cell Biol. 2006;38:648–61. doi: 10.1016/j.biocel.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Ji SR, Wu Y, Potempa LA, Liang YH, Zhao J. Effect of modified C-reactive protein on complement activation: a possible complement regulatory role of modified or monomeric C-reactive protein in atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2006;26:935–41. doi: 10.1161/01.ATV.0000206211.21895.73. [DOI] [PubMed] [Google Scholar]
- 11.Gewurz H, Mold C, Siegel J, Fiedel B. C-reactive protein and the acute phase response. Adv Intern Med. 1982;27:345–72. [PubMed] [Google Scholar]
- 12.Kaplan MH, Volanakis JE. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin and sphingomyelin. J Immunol. 1974;112:2135–47. [PubMed] [Google Scholar]
- 13.Volanakis JE, Kaplan MH. Interaction of C-reactive protein complexes with the complement system. II. Consumption of guinea pig complement by CRP complexes: requirement for human C1q. J Immunol. 1974;113:9–17. [PubMed] [Google Scholar]
- 14.McGrath FD, Brouwer MC, Arlaud GJ, Daha MR, Hack CE, Roos A. Evidence that complement protein C1q interacts with C-reactive protein through its globular head region. J Immunol. 2006;176:2950–7. doi: 10.4049/jimmunol.176.5.2950. [DOI] [PubMed] [Google Scholar]
- 15.Sjoberg AP, Trouw LA, McGrath FD, Hack CE, Blom AM. Regulation of complement activation by C-reactive protein: targeting of the inhibitory activity of c4b-binding protein. J Immunol. 2006;176:7612–20. doi: 10.4049/jimmunol.176.12.7612. [DOI] [PubMed] [Google Scholar]
- 16.Claus DR, Siegel J, Petras K, Osmand AP, Gewurz H. Interactions of C-reactive protein with the first component of human complement. J Immunol. 1977;119:187–92. [PubMed] [Google Scholar]
- 17.Berman S, Gewurz H, Mold C. Binding of C-reactive protein to nucleated cells leads to complement activation without cytolysis. J Immunol. 1986;136:1354–9. [PubMed] [Google Scholar]
- 18.Mold C, Gewurz H. Inhibitory effect of C-reactive protein on alternative C pathway activation by liposomes and Streptococcus pneumoniae. J Immunol. 1981;127:2089–92. [PubMed] [Google Scholar]
- 19.Mold C, Kingzette M, Gewurz H. C-reactive protein inhibits pneumococcal activation of the alternative pathway by increasing the interaction between factor H and C3b. J Immunol. 1984;133:882–5. [PubMed] [Google Scholar]
- 20.Suankratay C, Mold C, Zhang Y, Potempa LA, Lint TF, Gewurz H. Complement regulation in innate immunity and the acute-phase response: inhibition of mannan-binding lectin-initiated complement cytolysis by C-reactive protein (CRP) Clin Exp Immunol. 1998;113:353–9. doi: 10.1046/j.1365-2249.1998.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarva H, Jokiranta TS, Hellwage J, Zipfel PF, Meri S. Regulation of complement activation by C-reactive protein: targeting the complement inhibitory activity of factor H by an interaction with short consensus repeat domains 7 and 8–11. J Immunol. 1999;163:3957–62. [PubMed] [Google Scholar]
- 22.Mold C, Gewurz H, Du Clos TW. Regulation of complement activation by C-reactive protein. Immunopharmacology. 1999;42:23–30. doi: 10.1016/s0162-3109(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 23.Li SH, Szmitko PE, Weisel RD, Wang CH, Fedak PW, Li RK, Mickle DA, Verma S. C-reactive protein upregulates complement-inhibitory factors in endothelial cells. Circulation. 2004;109:833–6. doi: 10.1161/01.CIR.0000117087.27524.0E. [DOI] [PubMed] [Google Scholar]
- 24.Khreiss T, Jozsef L, Hossain S, Chan JS, Potempa LA, Filep JG. Loss of pentameric symmetry of C-reactive protein is associated with delayed apoptosis of human neutrophils. J Biol Chem. 2002;277:40775–81. doi: 10.1074/jbc.M205378200. [DOI] [PubMed] [Google Scholar]
- 25.Arlaud GJ, Sim RB, Duplaa AM, Colomb MG. Differential elution of Clq, Clr and Cls from human Cl bound to immune aggregates. Use in the rapid purification of Cl subcomponents. Mol Immunol. 1979;16:445–50. doi: 10.1016/0161-5890(79)90069-5. [DOI] [PubMed] [Google Scholar]
- 26.Tenner AJ, Lesavre PH, Cooper NR. Purification and radiolabeling of human C1q. J Immunol. 1981;127:648–53. [PubMed] [Google Scholar]
- 27.Arlaud GJ, Villiers CL, Chesne S, Colomb MG. Purified proenzyme C1r. Some characteristics of its activation and subsequent proteolytic cleavage. Biochim Biophys Acta. 1980;616:116–29. doi: 10.1016/0005-2744(80)90269-7. [DOI] [PubMed] [Google Scholar]
- 28.Tacnet-Delorme P, Chevallier S, Arlaud GJ. Beta-amyloid fibrils activate the C1 complex of complement under physiological conditions: evidence for a binding site for A beta on the C1q globular regions. J Immunol. 2001;167:6374–81. doi: 10.4049/jimmunol.167.11.6374. [DOI] [PubMed] [Google Scholar]
- 29.Rossi V, Bally I, Thielens NM, Esser AF, Arlaud GJ. Baculovirus-mediated expression of truncated modular fragments from the catalytic region of human complement serine protease C1s. Evidence for the involvement of both complement control protein modules in the recognition of the C4 protein substrate. J Biol Chem. 1998;273:1232–9. doi: 10.1074/jbc.273.2.1232. [DOI] [PubMed] [Google Scholar]
- 30.Bíró A, Thielens NM, Cervenák L, Prohászka Z, Füst G, Arlaud GJ. Modified low density lipoproteins differentially bind and activate the C1 complex of complement. Mol Immunol. 2007;44:1169–77. doi: 10.1016/j.molimm.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Schwedler SB, Guderian F, Dammrich J, Potempa LA, Wanner C. Tubular staining of modified C-reactive protein in diabetic chronic kidney disease. Nephrol Dial Transplant. 2003;18:2300–7. doi: 10.1093/ndt/gfg407. [DOI] [PubMed] [Google Scholar]
- 32.Torzewski J, Torzewski M, Bowyer DE, Frohlich M, Koenig W, Waltenberger J, Fitzsimmons C, Hombach V. C-reactive protein frequently colocalizes with the terminal complement complex in the intima of early atherosclerotic lesions of human coronary arteries. Arterioscler Thromb Vasc Biol. 1998;18:1386–92. doi: 10.1161/01.atv.18.9.1386. [DOI] [PubMed] [Google Scholar]
- 33.Vaith P, Potempa LA. Deposition of modified or native C-reactive protein in atherosclerotic arteries? Arterioscler Thromb Vasc Biol. 2000;20:1173–4. doi: 10.1161/01.atv.20.4.1173. [DOI] [PubMed] [Google Scholar]
- 34.Giannakis E, Male DA, Ormsby RJ, Mold C, Jokiranta TS, Ranganathan S, Gordon DL. Multiple ligand binding sites on domain seven of human complement factor H. Int Immunopharmacol. 2001;1:433–43. doi: 10.1016/s1567-5769(00)00040-0. [DOI] [PubMed] [Google Scholar]
- 35.Griselli M, Herbert J, Hutchinson WL, Taylor KM, Sohail M, Krausz T, Pepys MB. C-reactive protein and complement are important mediators of tissue damage in acute myocardial infarction. J Exp Med. 1999;190:1733–40. doi: 10.1084/jem.190.12.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gill R, Kemp JA, Sabin C, Pepys MB. Human C-reactive protein increases cerebral infarct size after middle cerebral artery occlusion in adult rats. J Cereb Blood Flow Metab. 2004;24:1214–8. doi: 10.1097/01.WCB.0000136517.61642.99. [DOI] [PubMed] [Google Scholar]
- 37.Barnum SR, Dahlback B. C4b-binding protein, a regulatory component of the classical pathway of complement, is an acute-phase protein and is elevated in systemic lupus erythematosus. Complement Inflamm. 1990;7:71–7. doi: 10.1159/000463131. [DOI] [PubMed] [Google Scholar]
- 38.Lagrand WK, Niessen HW, Wolbink GJ, Jaspars LH, Visser CA, Verheugt FW, Meijer CJ, Hack CE. C-reactive protein colocalizes with complement in human hearts during acute myocardial infarction. Circulation. 1997;95:97–103. doi: 10.1161/01.cir.95.1.97. [DOI] [PubMed] [Google Scholar]
- 39.Baidoshvili A, Nijmeijer R, Lagrand WK, Hack CE, Niessen HW. Localisation of C reactive protein in infarcted tissue sites of multiple organs during sepsis. J Clin Pathol. 2002;55:152–3. doi: 10.1136/jcp.55.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oksjoki R, Jarva H, Kovanen PT, Laine P, Meri S, Pentikainen MO. Association between complement factor H and proteoglycans in early human coronary atherosclerotic lesions: implications for local regulation of complement activation. Arterioscler Thromb Vasc Biol. 2003;23:630–6. doi: 10.1161/01.ATV.0000057808.91263.A4. [DOI] [PubMed] [Google Scholar]