Abstract
The serine protease cathepsin (Cat) G dominates the proteolytic processing of the multiple sclerosis (MS)-associated autoantigen myelin basic protein (MBP) in lysosomes from primary human B cells and dendritic cells. This is in contrast to B-lymphoblastoid cell lines, where the asparagine endopeptidase (AEP) is responsible for this task. We have analysed microglia-derived lysosomal proteases for their ability to process MBP in vitro. In lysosomes derived from primary murine microglia, CatD, CatS, AEP and CatG were involved in the processing of MBP. Interestingly, when microglia were treated with interferon-γ to mimic a T helper type 1-biased cytokine milieu in MS, CatG was drastically down-regulated, in contrast to CatS, CatB, CatL, CatD or AEP. This resulted in significantly increased stability of MBP and a selective lack of CatG-derived proteolytic fragments; however, it did not affect the gross pattern of MBP processing. Inhibition of serine proteases eliminated the processing differences between lysosomal extracts from resting microglia compared to interferon-stimulated microglia. Thus, the cytokine environment modulates lysosomal proteases in microglia by a selective down-regulation of CatG, leading to decreased MBP-processing by microglia-derived lysosomal proteases in vitro.
Keywords: antigen processing, cathepsin G, major histocompatibility complex class II, microglia
Introduction
Before major histocompatibility complex class II (MHC II)-mediated activation of antigen-specific T cells can occur, exogenous antigen is internalized by professional antigen-presenting cells (APCs), where it undergoes proteolytic processing. Within the endocytic compartment of APCs, complex protein is processed by a sophisticated proteolytic machinery that serves two essential tasks: it degrades the MHC II-associated invariant chain (Ii) in a stepwise fashion, a process that controls transport, peptide loading and surface display of MHC II molecules, and it converts complex exogenous protein into antigenic peptides suitable for binding to the MHC II peptide-binding groove.1 This pathway not only initializes the specific immune response against exogenous challenges, but is also believed to represent the molecular basis for MHC II-associated autoimmune diseases such as multiple sclerosis (MS). In MS, peptides derived from autoantigens like myelin basic protein (MBP) are presented by central-nervous-system-resident APCs (i.e. microglial cells) that ultimately activate autoagressive T cells, resulting in inflammatory lesions and tissue damage.2,3 Microglia comprise highly differentiated, tissue-macrophage-like central-nervous-system-resident-type cells that are involved in both innate and adaptive immune responses. They are characterized by monocyte-like surface markers such as CD11b4,5 and constitutively express MHC class II and costimulatory molecules. Microglia are capable of priming alloreactive T cells and T-cell lines as well as secreting cytokines such as interleukin-12 (IL-12); they primarily induce a T helper type 1 (Th1) T-cell response (reviewed in ref. 6).
Proteolytic processing in the endocytic compartment of APCs is performed by a set of endocytic proteases most of which belong to the family of cathepsins (Cat).7 The cathepsins are divided into different classes according to the amino acid in their active centre, the aspartate (CatD and CatE),8–10 the cysteine (C1: CatB, CatC, CatF, CatH, CatL, CatS, CatV and CatX, the C13: asparagine-specific endoprotease AEP)11–15 and the serine proteases (CatG).16,17 The endoprotease CatS is required for antigen processing in different APCs and along with CatL in generating the CLIP fragment, which occupies the MHC class II binding groove.13,18–21 This is in contrast to CatB, which is involved in, but not essential to, antigen processing22 and which can act as an exoprotease as well as an endoprotease depending on the pH. Recently it was demonstrated that the carboxypeptidase CatZ was determined to activate T-cell activation via regulation of the integrin β2 lymphocyte function-associated antigen-1 receptor.23
There is growing evidence for a protease-determined model of antigen processing, where intact antigen is ‘unlocked’ by a dominant proteolytic activity in the endocytic compartment. Largely determined by the specificity of this unlocking protease(s), the antigen is converted into a limited number of proteolytic processing intermediates. The intermediates are further processed by the remaining endocytic proteases according to their substrate specificities. In this scenario, the substrate specificity and activity of a dominant protease(s) in the endocytic compartment largely controls the efficiency of T-cell activation by a given antigenic epitope.11,24–26 The strongest evidence for this ‘protease-determined’ model comes from Manoury et al., who not only demonstrated that the asparagine-specific endoprotease AEP, which exclusively cleaves after asparagine residues, initialized and dominated proteolytic processing of the tetanus toxoid C fragment (TTCF) by lysosomal extracts from B lymphoblastoid cells (BLCs) in vitro, but that this observation was transferable to the situation in intact cells, where AEP activity controlled the presentation of TTCF-derived peptides and therefore T-cell activation by TTCF.12 While in this scenario AEP-directed processing of TTCF was required for T-cell activation, the situation was reversed when MBP was processed by the same type of cells: the dominant AEP activity directly cleaved within the immunodominant region of MBP (after N92) when the intact autoantigen was exposed to BLC-derived lysosomal fractions.27 Consequently, AEP activity negatively regulated T-cell activation by the dominant MBP epitope MBP85-99.28 Mainly based on these data, AEP activity is presently regarded as the functionally most important (and so-called ‘unlocking’) protease for antigen processing in the MHC II compartment.
The activity profile of individual endocytic proteases differs between different types of APC29 as well as between primary APCs and immortalized cell lines of the same type of cell.30 We have recently demonstrated that primary human B lymphocytes lack AEP activity, in contrast to BLCs.16 Instead, CatG, a serine protease previously not considered in the context of antigen processing, dominated proteolytic breakdown of MBP by lysosomal fractions derived from primary human B lymphocytes as well as primary dendritic cells (DCs).17 CatG activity quantitatively controlled the turnover of intact MBP and at the same time directly eliminated the immunogenic epitope by cleavage after F90, acting as a dominant protease in MBP processing. These differences in the endocytic proteolytic machinery between BLCs and primary B cells as well as primary DCs and in vitro generated DCs have highlighted the enormous variability of this proteolytic system in the MHC II pathway, where not only different antigens are handled in functionally different ways by a given protease (as demonstrated by the divergent effects of AEP on TTCF and MBP), but where also different types of APC or primary cells and cell lines follow different pathways for the destruction of one particular antigen. In addition, a regulatory influence of exogenous stimuli like cytokines or bacterial products on this proteolytic machinery is likely, although relatively poorly understood: CatS-activity was up-regulated by interferon-γ (IFN-γ) in primary murine and human monocytes31,32 and microglia, as well as by lipopolysaccharide in monocyte-derived DCs. The latter was counteracted by IL-10.29 Thus, to better understand the processing pathways of a given antigen, it is not only important to directly assess primary cells of the relevant type of APC with regard to the proteolytic processing of this particular antigen, but also to take into account local regulatory conditions, like cytokines. Microglia are likely to represent the functionally most important type of central-nervous-system-resident APC33 where they are exposed to a Th1-biased and IFN-γ-dominated cytokine milieu in MS.34 To address this issue, we analysed the proteolytic processing of MBP by lysosomal proteases from primary microglia cultured in the presence or absence of IFN-γ.
Materials and methods
Preparation of mouse microglia
Primary microglial cells were prepared from 1-day-old B10.PL mice as described.5,35 Briefly, mice were quickly immersed in 70% alcohol before decapitation, and their heads were cleaved longitudinally. Frontal cortices were removed from the brain and placed in ice-cold medium. Subsequent to this, the cortices were mechanically dissociated through an 80-μm cell strainer (BD Falcon, Heidelberg, Germany). The cells were seeded into culture flasks, which were precoated with poly l-lysine (Sigma, Taufkirchen, Germany). A cell suspension obtained from two brains was seeded in one 75-cm2 culture flask. The culture medium was Dulbecco's modified Eagle's minimal essential medium (Gibco BRL, Paisley, UK) supplemented with 10% fetal bovine serum (Biochrom, Berlin, Germany), 100 U/ml penicillin/100 μg/ml streptomycin (Sigma), and 2·5 mm l-glutamine (Gibco BRL). The medium was changed after 1 day in culture, then every 3rd or 4th day. The astrocytes reached a confluent monolayer after about 10 days and microglial cells were harvested from the flasks after 12 days. The microglia, which were growing on top of the astrocyte layer, were harvested by shaking the bottles vigorously before medium was collected. The process was repeated with serum-free medium. The purity of the microglia was confirmed as at least 95% by CD11b-phycoerythrin staining (Becton Dickinson, Mountain View, CA). The appropriate animal-use licences were in place.
Protein expression and purification
Human recombinant MBP (18 500 MW, Swiss-Prot: P02686-5 minus methionine at pos.1) was produced in Escherichia coli and purified by ion exchange chromatography, as described elsewhere.27 The purified protein was controlled by MALDI-MS for both purity (>98%) and correct mass; 0·04 μg/μl MBP was used as a standard.
Generation of lysosomal extracts and in vitro processing
Lysosomal extracts were generated by differential centrifugation and characterized as published before.36 In brief, crude endosomal compartments were enriched by differential centrifugation from postnuclear supernatants, followed by a short exposure of the membrane pellet to water, which preferentially disrupts the membrane integrity of lysosomes because of their low osmotic resistance. This yielded virtually pure lysosomal extracts as judged by the distribution of CatD, transferrin receptor and N-acetyl-glucosaminidase. For in vitro processing, substrate solution (0·04 μg/μl MBP, 0·1 m citrate pH 5·0, 2·5 mm dithiothreitol) was incubated with lysosomal fractions at 37° (0·5 μg total protein).
Identification of processing products from MBP
Separation of in vitro processing products was achieved by microbore reverse-phase high-pressure liquid chromatography (RP-HPLC) using a C8 150 × 2 mm column (Wicom, Heppenheim, Germany) as published previously.27 Mass spectrometry was performed using an Esquire3000plus ion trap mass spectrometer (Bruker Daltonics, Bremen, Germany) equipped with a standard electrospray ionisation (ESI) interface (Bruker Daltonics).16 Expected masses were calculated as average masses with statistical isotope distribution. The mass accuracy of the detected molecular ions was in the range of ±200 p.p.m.
Determination of cathepsin and AEP activity
Hydrolysis of the synthetic compound succinyl-alanine-alanine-proline-phenylalanine-aminomethyl-coumarin (Suc-AAPF-AMC) (Bachem, Babendorf, Switzerland), which serves as an established substrate to determine CatG activity,37 was quantified fluorometrically as described.16 AEP activity was quantified as published previously38 using the fluorogenic substrate 45 μm Z-valine-alanine-asparagine-aminomethyl-coumarin (Z-VAN-AMC) in lysosomal fractions taken up into reaction buffer [40 mm citric acid, 120 mm Na2HPO4 pH 5·8, 5 mm mercaptoethanol, 4 mm ethylene diaminetetraacetic acid (EDTA), 10 μm E64, 6 μm aprotinin, 0·01% Triton X-100]. CatD activities were determined using the substrate AMCA-EEKPISFFRLGK (Biotin)-NH2 as described elsewhere.36 Combined CatBLS activities were determined by using the fluorogenic substrate Z-FR-AMC.36
Affinity-labelling of active cysteine proteases
Cell lysates (5 μg) were incubated with reaction buffer (50 mm citrate/phosphate pH 5·0, 1 mm EDTA, 50 mm dithiothreitol) in the presence of DCG-O439 for 1 hr at room temperature. Reactions were terminated by the addition of sodium dodecyl sulfate (SDS) reducing sample buffer and immediate boiling. Samples were resolved by 12·5% SDS–polyacrylamide gel electrophoresis (PAGE), then blotted on a polyvinylidene difluoride (PVDF) membrane and visualized using streptavidin horseradish peroxidase and the enhanced chemiluminescence detection kit40. In addition, the PVDF membrane was stained with Coomassie as described in the manufacturer's manual (SimplyBlue, Invitrogen, Karlsruhe, Germany).
Enzyme-linked immunosorbent assay (ELISA) and reverse transcription–polymerase chain reaction (RT-PCR) for CatG
ELISA and RT-PCR for CatG were performed exactly as described recently.16
Results
IFN-γ results in decreased proteolytic processing of MBP by microglia-derived lysosomes
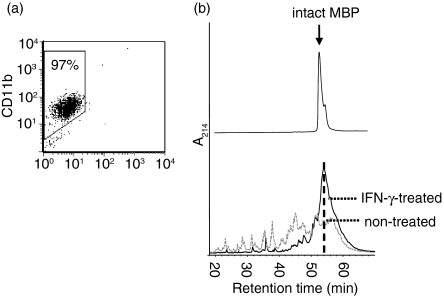
Primary murine microglia were enriched to purities of >95%(Fig 1a), as confirmed by fluorescence-activated cell sorting (FACS) for CD11b,4,5 and were subsequently cocultured with IFN-γ (100 U/ml for 24 hr) before harvesting. Lysosomal extracts were then generated using a two-step fractionation protocol that separates a crude cellular organelle fraction from the postnuclear supernatant by differential centrifugation, followed by a brief exposure to osmotic stress and subsequent ultracentrifugation. This procedure selectively releases lysosomal contents into the supernatant as a result of the high osmotic fragility of dense lysosomes, as previously characterized by us in detail.36
Figure 1.
Effect of IFN-γ on the proteolytic processing of MBP by lysosomal extracts. (a) Characterization of enriched microglia. Single-cell suspensions of murine glia were enriched for microglial cells in vitro and analysed for purity by CD11b expression using FACS. A purity of >95% microglia was regularly achieved. (b) Microglia were treated with IFN-γ (100 U/ml) in vitro for 24 hr (IFN-γ-treated), while control cells remained untreated. Lysosomal extracts were prepared as described, normalized for total protein, and incubated with identical amounts of recombinant MBP in vitro at pH 5·0 for 24 hr. The resulting polypeptides were resolved by RP-HPLC and detected by ultraviolet absorption at 214 nm (A214; black line: IFN-γ-treated microglia; grey line: non-treated microglia). Intact MBP eluted at 54 min is indicated by the hatched line.
Recombinant purified human MBP was then incubated with these lysosomal fractions from IFN-γ-treated/untreated microglia for 24 hr at pH 5, followed by resolution of the emerging proteolytic MBP fragments by RP-HPLC (Fig 1b). While lysosomal enzymes from untreated microglia readily degraded the vast majority of intact MBP (retention time 54 min) into a variety of proteolytic products, incubation with lysosomal material from IFN-γ-exposed microglia resulted in a significantly prolonged stability of intact MBP, and proteolytic intermediates were hence observed in far smaller quantities. In fact, the amount of MBP remaining intact after 24 hr of incubation with lysosomes from resting microglia accounted to only 27% of that detected when lysosomes from IFN-γ-treated cells were assayed. This suggested that the activity of the lysosomal protease(s) that dominate(s) MBP processing by microglial cells was down-regulated by inflammatory stimuli.
Differential processing patterns of MBP by lysosomes from resting versus IFN-γ-stimulated microglia
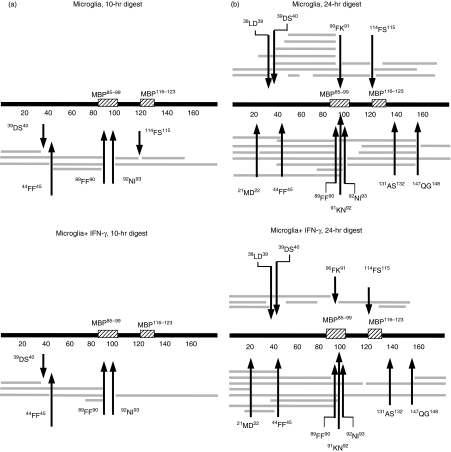
Does stimulation with IFN-γ only prolong the half-life of intact MBP in microglia-derived lysosomal extracts or would it result in qualitative changes in the pathway of MBP processing? To this end we identified the proteolytic fragments that had been generated by turnover of MBP with microglia-derived lysosomal fractions using mass spectrometry (Fig 2 and Table 1). After 10 hr of proteolytic processing with lysosomes from untreated microglia, the proteases involved in the initial steps of processing were CatD (44FF45, 89FF90), CatG (114FS115), AEP (92NI93) and CatS (39DS40) (Fig 2a, Table 1), according to the processing sites observed, which had been previously identified in primary B lymphocytes and BLCs.16,27 An overall similar pattern was observed when lysosomal extracts from IFN-γ-treated microglia were used, except that fragments indicative of CatG-mediated cleavage (90FK91 or 114FS115) were selectively lacking when lysosomes from IFN-γ-treated cells were used. This suggested that CatG was of much lower functional relevance in lysosomes from IFN-γ-treated microglia than in resting control cells, in contrast to CatD, CatS and AEP, where the respective fragments were similarly detected after IFN-γ treatment.
Figure 2.
Proteolytic fragments of MBP after processing with lysosomal extracts. Intact MBP was incubated with lysosomal extracts from resting microglia (microglia; top panel) or IFN-γ-stimulated microglia (microglia + IFN-γ; bottom panel) for 10 hr (a) or 24 hr (b), and the proteolytic fragments obtained were identified by mass spectrometry and displayed along with full-length MBP and the location of the two major immunogenic epitopes (MBP85–99 and MBP116–123). Arrows mark major cleavage sites and the respective flanking amino acids are indicated.
Table 1.
MBP fragments after processing with microglia-derived lysosomes in vitro, as assessed by mass spectrometry
| Retention time | [M + H]+ | Expected mass [M + H]+ | ΔDa | MBP fragment |
|---|---|---|---|---|
| 10-hr lysosomal processing in vitro | ||||
| Microglia | ||||
| 44·0–44·9 | 2238·2 | 2238·2 | 0·0 | 94–114 |
| 45·2–46·3 | 4732·2 | 4732·1 | 0·1 | 45–88 |
| 46·4–47·8 | 4878·6 | 4879·3 | 0·7 | 45–89 |
| 50·3–51·1 | 3471·7 | 3471·7 | 0·0 | 115–147 |
| 53·5–55·0 | 4400·4 | 4401·9 | 1·5 | 1–39 |
| 65·8–67·4 | 4962·1 | 4962·5 | 0·4 | 1–44 |
| 70·2–73·4 | 9822·4 | 9822·7 | 0·3 | 1–89 |
| 73·9–75·5 | 8266·2 | 8266·3 | 0·1 | 93–170 |
| Microglia + IFN-γ | ||||
| 45·8–47·8 | 4879·3 | 4879·2 | 0·1 | 45–89 |
| 48·8–49·8 | 2176·1 | 2176·1 | 0·0 | 71–89 |
| 52·8–55·1 | 4402·0 | 4401·9 | 0·1 | 1–39 |
| 65·2–67·1 | 4962·7 | 4962·5 | 0·2 | 1–44 |
| 69·5–71·9 | 9822·2 | 9822·7 | 0·5 | 1–89 |
| 72·1–75·0 | 8266·4 | 8266·3 | 0·1 | 93–170 |
| 24-hr lysosomal processing in vitro | ||||
| Microglia | ||||
| 20·4–21·4 | 4585·0 | 4584·9 | 0·1 | 46–88 |
| 21·6–22·2 | 601·2 | 601·3 | 0·1 | 52–57 |
| 22·3–23·8 | 1739·1 | 1738·9 | 0·2 | 132–147 |
| 2366·5 | 2366·6 | 0·1 | 73–92 | |
| 4732·3 | 4732·2 | 0·1 | 115–159 | |
| 23·8–24·1 | 2306·6 | 2306·6 | 0·0 | 1–21 |
| 25·7–26·2 | 2458·2 | 2457·7 | 0·5 | 109–131 |
| 27·7–28·3 | 4732·2 | 4732·1 | 0·1 | 45–88 |
| 29·2–29·7 | 5292·8 | 5292·7 | 0·1 | 40–88 |
| 31·3–32·1 | 5407·8 | 5407·8 | 0 | 39–88 |
| 32·3–33·1 | 5121·2 | 5120·7 | 0·5 | 69–114 |
| 34·8–35·3 | 5440·1 | 5439·2 | 0·2 | 40–89 |
| 35·3–36·5 | 4401·8 | 4401·9 | 0·1 | 1–39 |
| 4286·7 | 4286·8 | 0·1 | 1–38 | |
| 37·5–37·9 | 5555·0 | 5555·0 | 0 | 39–89 |
| 37·9–38·9 | 2479·1 | 2478·9 | 0·2 | 148–170 |
| 3473·4 | 3473·7 | 0·3 | 115–147 | |
| 40·6–41·2 | 5830·1 | 5829·3 | 0·8 | 40–92 |
| 42·4–42·8 | 2741·5 | 2742·2 | 0·7 | 90–114 |
| 43·0–43·5 | 7388·6 | 7388·0 | 0·6 | 22–88 |
| 43·7–44·5 | 4962·2 | 4962·5 | 0·3 | 1–44 |
| 4920·0 | 4920·4 | 0·4 | 119–165 | |
| 44·6–45·3 | 5807·4 | 5807·4 | 0 | 93–147 |
| 45·5–46·3 | 9822·9 | 9822·7 | 0·2 | 1–89 |
| 6196·8 | 6196·9 | 0·1 | 90–147 | |
| 46·4–47·1 | 7535·4 | 7535·2 | 0·2 | 22–89 |
| 47·3–48·6 | 10212·5 | 10212·2 | 0·3 | 1–92 |
| 10097·5 | 10098·1 | 0·6 | 1–91 | |
| 51·1–52·6 | 8267·1 | 8266·3 | 0·8 | 93–170 |
| 56·0–59·6 | 18460·6 | 18460·5 | 0·1 | 1–170 |
| Microglia + IFN-γ | ||||
| 23·5–24·3 | 2306·7 | 2306·6 | 0·1 | 1–21 |
| 28·4–29·0 | 2773·1 | 2772·0 | 1·1 | 51–76 |
| 31·7–32·2 | 4417·7 | 4416·9 | 0·8 | 91–132 |
| 32·8–33·3 | 2114·1 | 2114·0 | 0·1 | 127–146 |
| 2762·5 | 2762·1 | 0·4 | 18–42 | |
| 35·7–37·0 | 4401·9 | 4401·9 | 0 | 1–39 |
| 4286·8 | 4286·8 | 0 | 1–38 | |
| 38·1–38·5 | 3473·5 | 3473·7 | 0·2 | 115–147 |
| 38·4–39·2 | 2479·1 | 2478·9 | 0·2 | 148–170 |
| 39·2–39·7 | 5829·6 | 5829·3 | 0·3 | 40–92 |
| 43·4–46·0 | 4962·8 | 4962·5 | 0·3 | 1–44 |
| 9675·1 | 9675·6 | 0·5 | 1–88 | |
| 46·1–47·4 | 9822·5 | 9822·7 | 0·2 | 1–89 |
| 47·6–49·2 | 10212·1 | 10212·2 | 0·1 | 1–92 |
| 50·5–51·1 | 6236·9 | 6236·9 | 0·0 | 113–170 |
| 52·3–52·8 | 12241·8 | 12242·5 | 0·7 | 1–112 |
| 53·5–54·7 | 18460·6 | 18460·5 | 0·1 | 1–170 |
Proteolytic fragments obtained after digesting MBP with lysosomes from non-treated versus IFN-γtreated microglia, as deferred from mass spectrometry. Retention time represents the HPLC retention time; [M + H]+, the protonated mass obtained; expected mass [M + H]+, the mass expected by calculation with stochastic isotope distribution; ΔDa, Δbetween the expected and obtained masses; fragment, MBP fragment deferred from the mass and the retention time. MBP was digested in microglia-derived lysosomal fractions for 10 hr and 24 hr.
Prolonged in vitro processing of MBP for 24 hr (Table 1, Fig 2b) resulted in further degradation and the emergence of additional proteolytic fragments. Of note, we identified two different sets of matching proteolytic fragments after 24 hr for only one cleavage site, suggesting a quantitatively important processing event: lysosomes from resting microglia showed processing at 114FS115 as indicated by both the 69–114 and 90–114 fragments, as well as the 115–147 and 115–159 fragments, in contrast to IFN-γ-stimulated cells, indicative of dominant CatG-mediated cleavage. This is consistent with the observations made after 10 hr of digestion and similarly suggested a dominant role of CatG when MBP is degraded by lysosomal proteases from resting, but not IFN-γ-stimulated microglia.
In addition, sets of two matching proteolytic fragments were identified at position 131AS132 with untreated cells and at 112SR113 with treated cells. The processing sites identified at 38LD39, 112SR113, 131AS132 and 147QG148 have not previously been detected with purified cathepsins or lysosomes from either B cells, BLCs or DCs, and might therefore be the result of as yet unidentified proteases present in microglial cells. Additional MBP cleavage sites were identified after M21, Q147 and H88, as indicated by multiple proteolytic fragments ending at the respective positions. Also, a number of individual fragments were detected at low quantities for which the cleavage sites were only found once.
The major immunodominant region of MBP (85–99 and 116–123) was particularly susceptible for protease attack by microglia-derived proteases, so that more than half of the MBP-derived fragments identified (17 out of 30 fragments, 56%) had been generated by cleavage within this region in resting microglia, in contrast to IFN-γ-treated microglia, where only five out of 17 identified species (29%) matched this region.
In conclusion, the initial steps in MBP proteolytic processing by microglia-derived lysosomes in vitro were consistent with cleavage by AEP, CatS, CatD and CatG. In IFN-γ-stimulated microglia, proteolysis indicative of CatG-mediated cleavage was selectively lost, resulting in a far less efficient proteolytic attack of lysosomal proteases on both intact MBP and the immunodominant MBP peptide.
Stimulation with IFN-γ selectively down-modulates CatG activity
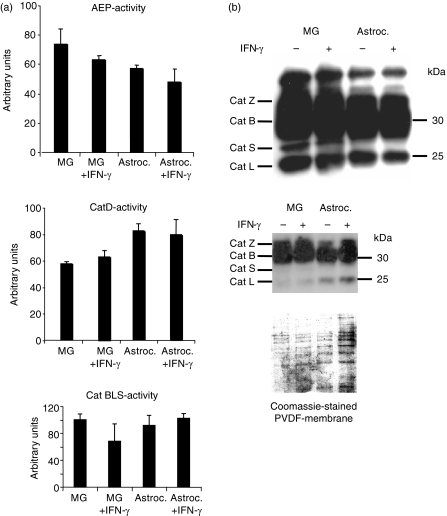
We next directly assessed the impact of IFN-γ-stimulation on the activities of the major lysosomal proteases in microglia and tested the respective activities in lysosomes from resting microglia versus IFN-γ-stimulated microglia as well as astrocytes as a control, using functional assays based on the turnover of fluorogenic substrates (Fig 3a). The activities of AEP and CatD as well as the combined activity of the cathepsins B, L and S showed no significant variability in the presence/absence of IFN-γ in either microglia or astrocytes. To assess the three major cysteine-cathepsins, CatB, L and S, by an independent method that allows discriminating between the individual active species, we incubated lysosomal extracts of both cell types with the activity-based biotinylated synthetic probe DCG-O4. This probe selectively binds to the active site of papain-like cysteine proteases in a covalent fashion, and therefore allows the visualization of individual active cathepsins in a semi-quantitative fashion after SDS–PAGE and streptavidin–horseradish peroxidase blot (Fig. 3b). As in previous work,30,40 the active polypeptides CatZ, CatB, CatS and CatL, were identified as labelled polypeptide species, based on the MW of the respective signals and side by side comparison with lysosomal extracts from monocytes and BLCs, as published41. Active CatS was visualized in lysosomes from both stimulated and unstimulated microglia, with a slightly higher amount of active CatS in resting microglia. Astrocytes, by contrast, lacked the signal from active CatS, as expected.42 No changes were observed in the amounts of active CatL, CatB and CatZ between resting and stimulated microglia. Thus, we did not observe major changes in the activities of CatS, CatD or AEP that were likely to account for the significantly reduced proteolytic processing of both intact MBP as well as the MBP88–93 region in lyosomes from IFN-γ-stimulated microglial cells.
Figure 3.
Effect of IFN-γ on cathepsin and AEP activity in microglia-derived lysosomes. (a) Lysosomal extracts from resting versus IFN-γ-stimulated microglia (MG) were assessed for activity of AEP (upper panel) by turnover of the fluorogenic substrate Z-VAN-AMC and for CatD-activity (middle panel) using AMCA-EEKPISFFRLGK(Biotin)-NH2 as a fluorogenic substrate, as described in the Materials and methods. Astrocytes (Astroc.) served as controls, AEP and CatD activities are expressed in arbitrary units. Mean values and standard deviation of triplicate samples are presented. Lower panel: the combined activity of the cathepsins CatB, CatL, CatS in resting versus IFN-γ-stimulated microglial cells (MG, MG + IFN-γ) and astrocytes (Astroc., Astroc. + IFN-γ), respectively, was compared by measuring the turnover of the fluorogenic substrate Z-FR-AMC in lysosomal extracts normalized for total protein. Results are expressed in arbitrary units, mean values and standard deviation of triplicate samples are presented. (b) Lysosomal fractions from resting versus IFN-γ-stimulated microglia and astrocytes were normalized for total protein content, labelled with the activity-based biotinylated probe DCG-O4, and resolved by SDS–PAGE, followed by semi-quantitative visualization of the active papain-like cysteine proteases by streptavidin-horseradish peroxidase blot. The identity of the activity signals was deduced from side-by-side comparison to lysosomal extracts from human B-lymphoblastoid cells and monocytes, as published. Cathepsins Z, B, S and L are visualized as active species (upper panel). Coomassie staining of a PVDF membrane (lower panel) was performed as a loading control.
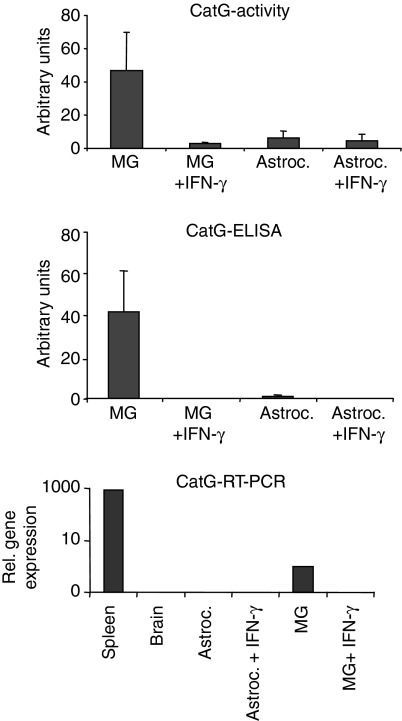
CatG cleaves intact MBP with high efficiency at 90FK91 and 114FS115.16 Since after 10 hr of lysosomal digest, only fragments indicative of cleavage at 114FS115 were present with lysosomes from IFN-γ-treated microglia (see Fig. 2a), differential CatG activity was a likely candidate to explain the differences in processing observed. Indeed, when we measured CatG activity by turnover of the fluorescent peptide Suc-AAPF-AMC, IFN-γ-stimulated microglia lacked more than 90% of their CatG-activity, compared to unstimulated controls (Fig. 4).
Figure 4.
Influence of IFN-γ-treatment on CatG expression and activity in microglia-derived lysosomes. Total amounts of CatG activity (top panel) and CatG protein (middle panel) were compared between equal amounts of protein from lysosomal fractions derived from resting versus IFN-γ-stimulated microglia (MG) or astrocytes (Astroc.), respectively. CatG activity was determined by turnover of the fluorogenic substrate Suc-AAPF-AMC and CatG protein by ELISA, as detailed in the materials and methods, and expressed in arbitrary units. Mean values and standard deviation of three independent experiments are presented. Bottom panel: single-cell suspensions from total spleen and total brain were compared with resting and IFN-γ-stimulated microglia and astrocytes, respectively, with regard to the expression of CatG mRNA, using quantitative PCR, as described.
To assess the presence of CatG protein under both conditions, we used a CatG-specific ELISA (Fig. 4), as previously published.16 While CatG protein was readily detected in resting microglia, no CatG-specific signal was retrieved after IFN-γ treatment of the cells. Astrocytes, by contrast, contained much lower amounts of CatG that were close to the detection limit, independently from the addition of IFN-γ. Quantitative RT-PCR was further used to assess the relative transcription rate of CatG mRNA in resting versus IFN-γ-stimulated microglia in comparison to astrocytes, total murine brain and total spleen. As shown in Fig 4 (lower panel) CatG expression was detected in resting microglia, although in much lower quantities than in total spleen; however, CatG mRNA was absent from IFN-γ-stimulated microglia as well as from astrocytes or total brain, respectively.
Taken together, these results indicated that IFN-γ treatment significantly and selectively down-modulated both CatG protein and CatG activity in lysosomes from primary microglia. They also suggested, that differential CatG activity might account for both the reduced rate and the altered profile of proteolytic processing of MBP in lysosomal extracts from IFN-γ-treated microglia.
Inhibition of serine proteases eliminates differences in lysosomal MBP-processing between resting and IFN-γ-stimulated microglia
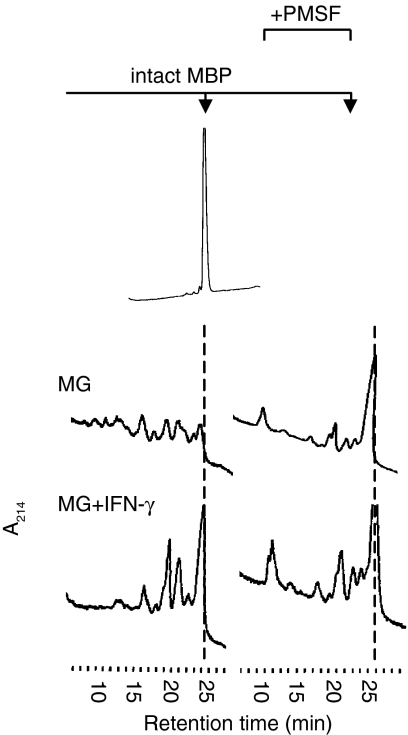
To functionally support the theory that differential CatG activity accounted for the major differences in the efficiency of MBP processing that were observed, we incubated MBP with lysosomal fractions from IFN-γ-treated and untreated microglia in the presence or absence of the serine protease inhibitor phenylmethylsulphonyl fluoride (PMSF). Although this agent is not specific for CatG, it efficiently inhibits CatG activity. By contrast, PMSF does not affect the activity of CatS, CatL, CatD or AEP, which have been shown to be involved in processing of the MBP region 88–93, whose destruction is the rate-limiting step in MBP degradation.27 In the absence of PMSF, lysosomal extracts from IFN-γ-treated microglia showed a processing pattern that was qualitatively distinct from that observed with non-stimulated cells and that still contained a sizeable amount of intact MBP, as expected (Fig 5, left panels). When microglia were treated with PMSF (right panels), however, the HPLC elution profiles of the resulting MBP fragments were virtually identical, irrespective of whether lysosomal enzymes from IFN-γ-treated or untreated microglia had been used. Thus, PMSF-treatment eliminated the differences in MBP processing that had been observed between lysosomes from stimulated and resting microglia, indicating that a serine protease activity accounted for the processing differences observed. Because (1) CatG is the only major serine protease known to be involved in MBP processing, (2) CatG activity is selectively eliminated upon IFN-γ treatment of microglia, (3) the MBP fragments that were selectively lost when lysosomes from IFN-γ-treated cells were tested are consistent with CatG-mediated cleavage, and (4) elimination of serine protease activity in resting microglia eliminated the processing difference observed, we conclude that IFN-γ induces a selective down-regulation of CatG activity in lysosomes from primary microglia, which results in a decreased rate of MBP processing in vitro.
Figure 5.
PMSF treatment eliminates the effect of IFN-γ on MBP processing. Intact MBP was incubated with lysosomal extracts from resting (MG; top panels) or IFN-γ-stimulated (MG + IFN-γ; bottom panels) microglia for 24 hr, either in the absence (left panels) or presence (right panels) of the serine protease inhibitor PMSF (5 mm). Proteolytic fragments obtained are resolved by RP-HPLC and visualized by ultraviolet absorption at 214 nm (A214). The hatched line indicates the elution time of intact MBP.
Discussion
We here demonstrate that both the activity and the protein of the serine protease CatG are practically lost from the lysosomes of primary microglia upon IFN-γ treatment. This effect was selective for CatG, accompanied by the loss of a number of MBP-processing intermediates consistent with CatG-mediated processing, and it resulted in a less efficient proteolytic attack on intact MBP. Because addition of the serine protease inhibitor PMSF eliminated the processing differences observed between resting and IFN-γ-stimulated cells, we conclude that differential CatG activity in IFN-γ-treated versus untreated microglia is likely to be responsible for the qualitative and quantitative differences observed in the degradation of MBP by lysosomal proteases. Recent data demonstrated that treatment with IFN-β inhibits the T-cell activation capacity of central nervous system APCs, probably mediated by less efficient antigen presenting.43 Although entirely speculative at present, modulation of protease activity in glial cells induced by IFN-β exposure could represent one possible molecular basis for this effect.
Microglia probably represent the functionally most important type of central-nervous-system-resident APC.33 While we have some basic knowledge regarding the proteases present as well as the way these process Ii in the MHC II compartment,42,44 information about the proteolytic breakdown of antigen by the endocytic proteases of microglia is essentially lacking. We have therefore established a model system that allows us to biochemically dissect the proteolytic breakdown of autoantigens in vitro by digesting recombinant autoantigens with the protease content of APC-derived lysosomal fractions, followed by mass spectrometry-based identification of the proteolytic products. Antigen presentation relies on the coordinated action of multiple individual factors, which apart from protease activity include the interaction between structural features of the antigen and the human leucocyte antigen (HLA) haplotype45 as well as the evolution and acidification of endocytic compartments as a dynamic system.46 We here aimed to identify major active endocytic proteases in microglia and their regulation by soluble factors such as IFN-γ, as well as their contribution to the degradation of a model antigen in vitro as a first step towards a biochemical characterization of antigen processing by microglia. Binding of MBP to different MHC haplotypes differs fundamentally and influences both MBP epitope selection and presentation. This modifying influence of MHC haplotypes on the processing pathway does not significantly influence the system used here, because MBP is present in large excess in the processing reaction, so that the protease pool interacts predominantly with soluble MBP, as deduced from similar processing experiments with extracts from HLA DRB1*1501 versus DRB1*0401 homozygous B-cell lines, which resulted in identical processing patterns (T. Burster, unpublished results). Also, antigen processing in vivo may be more confined to late endosomal specialized compartments such as MHC class II-enriched compartment (MIIC) rather than the conventional lysosomes analysed here. While this issue is still under discussion, the protease contents in late endosomes and lysosomes differ little in both DCs and BLCs, so that lysosomes can still be considered a valid source of a protease pool that is probably representative also for MIIC compartments. Using an identical approach, we have previously predicted the dominant role of AEP in controlling T-cell activation when MBP-derived epitopes are presented by BLCs,27 which was functionally confirmed by Manoury et al.28 This argues for the validity of the biochemical conclusions drawn from our in vitro processing model, although this cannot a priori be generalized.
We did not observe significant CatG-mediated cleavage at 90FK91. We believe that this is because the cleavage after F89 obviously dominated processing in the MBP88-93 region in both treated or untreated microglia at early time-points. Although CatG is present and active in resting microglia (as demonstrated by the dominant 114FS115 cleavage), CatG cleavage after F90 is most likely not observed here, because the CatD-derived fragments ending at MBP89 do not represent a suitable target for CatG any more (CatG is an endoprotease and would require a higher number of aminoacids N-terminally of the cleavage site of the protein target).
The regulation of the proteolytic activity in the MHC II antigen-processing compartment of primary types of APC is poorly understood. Work with primary DCs, monocytes and microglia showed relatively selective modulation of the activity of one or two major proteases rather than of the entire set of proteolytic enzymes by a given stimulus, supporting specific, non-redundant roles of individual enzymes in the MHC II antigen processing machinery.29,31,32,42 As we show here, the activities of CatL, CatB, AEP and CatD were largely unaffected, whereas CatG activity was eliminated by IFN-γ, accompanied by a modest reduction in CatS activity. Differential CatS activity is unlikely to account for the IFN-γ-induced changes in MBP processing, because the CatS-sensitive cleavages 39DS40 and 91KN92 emerged with similar probabilities under stimulated and non-stimulated conditions. Finally PMSF, which does not affect CatS activity, eliminated the differences in MBP processing between lysosomes from treated and untreated cells.
It is unknown which steps in the proteolytic destruction of a given antigen by primary APCs are altered by such selective modulation of protease activity. Apart from the MBP fragments indicative of CatG activity, which were mostly absent when IFN-γ-treated microglial cells were used, the remaining pattern of processing intermediates was largely identical under resting or stimulated conditions. Thus, the selective down-modulation of a dominant proteolytic enzyme did not result in an alternative, entirely novel pattern of antigen destruction, but rather in a less efficient version of the original proteolytic pathway.
There are conflicting data about the activity and regulation of CatS, which mediates invariant chain processing in microglia. Stimulation of primary mouse microglia as well as of astrocytes with IFN-γ (after 48 hr) neither induced expression of CatS mRNA in astrocytes nor up-regulated its expression in microglia. However, active CatS visualized using activity-based probes was increased when microglia were stimulated with IFN-γ for 48 hr.42 In contrast, stimulation of a cell line derived from mouse microglia caused secretion of CatS and therefore loss of active CatS into the extra plasmatic space.47 In the present work, CatS was not up-regulated by 24 hr of IFN-γ stimulation of primary microglia, which might be explained by the different duration of IFN-γ treatment or different culture techniques to propagate microglia. In primary human monocytes, IFN-γ displayed its maximum effect on CatS activity after 48–72 hr, while no effect was found after 24 hr of incubation, which is consistent with our interpretation.31
CatG has only recently been identified as a functionally dominant protease in the MHC II compartment of primary B cells and DCs. Primary microglia similarly contain active CatG in the lysosomal compartment in significant amounts. CatG might therefore be a dominant endoprotease of the endocytic antigen-processing machinery in primary APCs in a more general sense, implicating that its functional significance for antigen processing could be comparable to that of CatS and AEP. In summary, our findings suggest that CatG is a dominant lysosomal protease in murine primary microglia. CatG activity can be selectively down-modulated by IFN-γ, leading to an increased stability of MBP and its immunodominant epitope in vitro.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (DR378.2-1, DR378.2-3, SFB 685 and BU 1822/3-1) as well as a grant from the Federal Ministry of Education and Research (Fö.01KS9602), the Interdisciplinary Centre of Clinical Research Tübingen (IZKF), Else-Kröner-Fresenius-Stiftung (BOB and TB) and the Marie Curie research training network ‘Drugs for therapy’.
Abbreviations
- AEP
asparagine-specific endopeptidase
- APC
antigen-presenting cell
- BLC
B-lymphoblastoid cells
- Cat
cathepsin
- DC
dendritic cell
- EDTA
ethylenediaminetetraacetic acid
- ELISA
enzyme-linked immunosorbent assay
- HLA
human leucocyte antigen
- MBP
myelin basic protein
- Ii
MHC class II invariant chain
- IFN-γ
interferon-γ
- IL-12
interleukin-12
- MHC
major histocompatibility complex
- MS
multiple sclerosis
- PMSF
phenylmethylsulphonyl fluoride
- PVDF
polyvinylidene difluoride
- RP-HPLC
reverse-phase high-pressure liquid chromatography
- RT-PCR
reverse transcription–polymerase chain reaction
- SDS–PAGE
sodium dodecyl sulphate–polyacrylamide gel electrophoresis
- Th1
T helper type 1
- TTCF
tetanus toxoid C fragment
References
- 1.Wolf PR, Ploegh HL. How MHC class II molecules acquire peptide cargo. Biosynthesis and trafficking through the endocytic pathway. Annu Rev Cell Dev Biol. 1995;11:267–306. doi: 10.1146/annurev.cb.11.110195.001411. [DOI] [PubMed] [Google Scholar]
- 2.Martin R, Sturzebecher CS, McFarland HF. Immunotherapy of multiple sclerosis: where are we? Where should we go? Nat Immunol. 2001;2:785–8. doi: 10.1038/ni0901-785. [DOI] [PubMed] [Google Scholar]
- 3.Steinman L. Multiple sclerosis: a two-stage disease. Nat Immunol. 2001;2:762–4. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- 4.de Groot CJ, Huppes W, Sminia T, Kraal G, Dijkstra CD. Determination of the origin and nature of brain macrophages and microglial cells in mouse central nervous system, using non-radioactive in situ hybridization and immunoperoxidase techniques. Glia. 1992;6:301–9. doi: 10.1002/glia.440060408. [DOI] [PubMed] [Google Scholar]
- 5.Aloisi F, Ria F, Penna G, Adorini L. Microglia are more efficient than astrocytes in antigen processing and in Th1 but not Th2 cell activation. J Immunol. 1998;160:4671–80. [PubMed] [Google Scholar]
- 6.Aloisi F. Immune function of microglia. Glia. 2001;36:165–79. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 7.Chapman HA. Endosomal proteolysis and MHC class II function. Curr Opin Immunol. 1998;10:93–102. doi: 10.1016/s0952-7915(98)80038-1. [DOI] [PubMed] [Google Scholar]
- 8.Moss CX, Villadangos JA, Watts C. Destructive potential of the aspartyl protease cathepsin D in MHC class II-restricted antigen processing. Eur J Immunol. 2005;35:3442–51. doi: 10.1002/eji.200535320. [DOI] [PubMed] [Google Scholar]
- 9.Chain BM, Free P, Medd P, Swetman C, Tabor AB, Terrazzini N. The expression and function of cathepsin E in dendritic cells. J Immunol. 2005;174:1791–800. doi: 10.4049/jimmunol.174.4.1791. [DOI] [PubMed] [Google Scholar]
- 10.Bennett K, Levine T, Ellis JS, Peanasky RJ, Samloff IM, Kay J, Chain BM. Antigen processing for presentation by class II major histocompatibility complex requires cleavage by cathepsin E. Eur J Immunol. 1992;22:1519–24. doi: 10.1002/eji.1830220626. [DOI] [PubMed] [Google Scholar]
- 11.Villadangos JA, Ploegh HL. Proteolysis in MHC class II antigen presentation: who's in charge? Immunity. 2000;12:233–9. doi: 10.1016/s1074-7613(00)80176-4. [DOI] [PubMed] [Google Scholar]
- 12.Manoury B, Hewitt EW, Morrice N, Dando PM, Barrett AJ, Watts C. An asparaginyl endopeptidase processes a microbial antigen for class II MHC presentation. Nature. 1998;396:695–9. doi: 10.1038/25379. [DOI] [PubMed] [Google Scholar]
- 13.Shi GP, Villadangos JA, Dranoff G, et al. Cathepsin S required for normal MHC class II peptide loading and germinal center development. Immunity. 1999;10:197–206. doi: 10.1016/s1074-7613(00)80020-5. [DOI] [PubMed] [Google Scholar]
- 14.Driessen C, Lennon-Dumenil AM, Ploegh HL. Individual cathepsins degrade immune complexes internalized by antigen-presenting cells via Fcγ receptors. Eur J Immunol. 2001;31:1592–601. doi: 10.1002/1521-4141(200105)31:5<1592::AID-IMMU1592>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 15.Tolosa E, Li W, Yasuda Y, et al. Cathepsin V is involved in the degradation of invariant chain in human thymus and is overexpressed in myasthenia gravis. J Clin Invest. 2003;112:517–26. doi: 10.1172/JCI18028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burster T, Beck A, Tolosa E, et al. Cathepsin G, and not the asparagine-specific endoprotease, controls the processing of myelin basic protein in lysosomes from human B lymphocytes. J Immunol. 2004;172:5495–503. doi: 10.4049/jimmunol.172.9.5495. [DOI] [PubMed] [Google Scholar]
- 17.Burster T, Beck A, Tolosa E, et al. Differential processing of autoantigens in lysosomes from human monocyte-derived and peripheral blood dendritic cells. J Immunol. 2005;175:5940–9. doi: 10.4049/jimmunol.175.9.5940. [DOI] [PubMed] [Google Scholar]
- 18.Villadangos JA, Riese RJ, Peters C, Chapman HA, Ploegh HL. Degradation of mouse invariant chain: roles of cathepsins S and D and the influence of major histocompatibility complex polymorphism. J Exp Med. 1997;186:549–60. doi: 10.1084/jem.186.4.549. [published erratum appears in J Exp Med 1997; 186:5945] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riese RJ, Wolf PR, Bromme D, Natkin LR, Villadangos JA, Ploegh HL, Chapman HA. Essential role for cathepsin S in the MHC class II-associated invariant chain processing and peptide loading. Immunity. 1996;4:450–3. doi: 10.1016/s1074-7613(00)80249-6. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa T, Roth W, Wong P, et al. Cathepsin L. critical role in Ii degradation and CD4 T cell selection in the thymus. Science. 1998;280:450–3. doi: 10.1126/science.280.5362.450. [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa TY, Brissette WH, Lira PD, et al. Impaired invariant chain degradation and antigen presentation and diminished collagen-induced arthritis in cathepsin S null mice. Immunity. 1999;10:207–17. doi: 10.1016/s1074-7613(00)80021-7. [DOI] [PubMed] [Google Scholar]
- 22.Chapman HA. Endosomal proteases in antigen presentation. Curr Opin Immunol. 2006;18:78–84. doi: 10.1016/j.coi.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Obermajer N, Premzl A, Zavasnik Bergant T, Turk B, Kos J. Carboxypeptidase cathepsin X mediates β2-integrin-dependent adhesion of differentiated U-937 cells. Exp Cell Res. 2006;312:2515–27. doi: 10.1016/j.yexcr.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Riese RJ, Chapman HA. Cathepsins and compartmentalization in antigen presentation [see comments] Curr Opin Immunol. 2000;12:107–13. doi: 10.1016/s0952-7915(99)00058-8. [DOI] [PubMed] [Google Scholar]
- 25.Lennon-Dumenil AM, Bakker AH, Wolf-Bryant P, Ploegh HL, Lagaudriere-Gesbert C. A closer look at proteolysis and MHC-class-II-restricted antigen presentation. Curr Opin Immunol. 2002;14:15–21. doi: 10.1016/s0952-7915(01)00293-x. [DOI] [PubMed] [Google Scholar]
- 26.Watts C, Moss CX, Mazzeo D, West MA, Matthews SP, Li DN, Manoury B. Creation versus destruction of T cell epitopes in the class II MHC pathway. Ann N Y Acad Sci. 2003;987:9–14. doi: 10.1111/j.1749-6632.2003.tb06028.x. [DOI] [PubMed] [Google Scholar]
- 27.Beck H, Schwarz G, Schroter CJ, et al. Cathepsin S and an asparagine-specific endoprotease dominate the proteolytic processing of human myelin basic protein in vitro. Eur J Immunol. 2001;31:3726–36. doi: 10.1002/1521-4141(200112)31:12<3726::aid-immu3726>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 28.Manoury B, Mazzeo D, Fugger L, et al. Destructive processing by asparagine endopeptidase limits presentation of a dominant T cell epitope in MBP. Nat Immunol. 2002;3:169–74. doi: 10.1038/ni754. [DOI] [PubMed] [Google Scholar]
- 29.Fiebiger E, Meraner P, Weber E, Fang IF, Stingl G, Ploegh H, Maurer D. Cytokines regulate proteolysis in major histocompatibility complex class II-dependent antigen presentation by dendritic cells. J Exp Med. 2001;193:881–92. doi: 10.1084/jem.193.8.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greiner A, Lautwein A, Overkleeft HS, Weber E, Driessen C. Activity and subcellular distribution of cathepsins in primary human monocytes. J Leukoc Biol. 2003;73:235–42. doi: 10.1189/jlb.0802398. [DOI] [PubMed] [Google Scholar]
- 31.Schmid H, Sauerbrei R, Schwarz G, Weber E, Kalbacher H, Driessen C. Modulation of the endosomal and lysosomal distribution of cathepsins B, L and S in human monocytes/macrophages. Biol Chem. 2002;383:1277–83. doi: 10.1515/BC.2002.143. [DOI] [PubMed] [Google Scholar]
- 32.Lennon-Dumenil AM, Bakker AH, Maehr R, Fiebiger E, Overkleeft HS, Rosemblatt M, Ploegh HL, Lagaudriere-Gesbert C. Analysis of protease activity in live antigen-presenting cells shows regulation of the phagosomal proteolytic contents during dendritic cell activation. J Exp Med. 2002;196:529–40. doi: 10.1084/jem.20020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 1999;22:219–40. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- 34.Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 35.Hertz L. Calcium accumulation and calcium effects in astrocytes in primary cultures. Acta Physiol Scand Suppl. 1989;582:33. [PubMed] [Google Scholar]
- 36.Schroter CJ, Braun M, Englert J, Beck H, Schmid H, Kalbacher H. A rapid method to separate endosomes from lysosomal contents using differential centrifugation and hypotonic lysis of lysosomes. J Immunol Meth. 1999;227:161–8. doi: 10.1016/s0022-1759(99)00079-4. [DOI] [PubMed] [Google Scholar]
- 37.Owen CA, Campbell MA, Sannes PL, Boukedes SS, Campbell EJ. Cell surface-bound elastase and cathepsin G on human neutrophils: a novel, non-oxidative mechanism by which neutrophils focus and preserve catalytic activity of serine proteinases. J Cell Biol. 1995;131:775–89. doi: 10.1083/jcb.131.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwarz G, Brandenburg J, Reich M, Burster T, Driessen C, Kalbacher H. Characterization of legumain. Biol Chem. 2002;383:1813–16. doi: 10.1515/BC.2002.203. [DOI] [PubMed] [Google Scholar]
- 39.Greenbaum D, Medzihradszky KF, Burlingame A, Bogyo M. Epoxide electrophiles as activity-dependent cysteine protease profiling and discovery tools. Chem Biol. 2000;7:569–81. doi: 10.1016/s1074-5521(00)00014-4. [DOI] [PubMed] [Google Scholar]
- 40.Lautwein A, Kraus M, Reich M, et al. Human B lymphoblastoid cells contain distinct patterns of cathepsin activity in endocytic compartments and regulate MHC class II transport in a cathepsin S-independent manner. J Leukoc Biol. 2004;75:844–55. doi: 10.1189/jlb.0803367. [DOI] [PubMed] [Google Scholar]
- 41.Lautwein A, Burster T, Lennon-Dumenil AM, Overkleeft HS, Weber E, Kalbacher H, Driessen C. Inflammatory stimuli recruit cathepsin activity to late endosomal compartments in human dendritic cells. Eur J Immunol. 2002;32:3348–57. doi: 10.1002/1521-4141(200212)32:12<3348::AID-IMMU3348>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 42.Gresser O, Weber E, Hellwig A, Riese S, Regnier-Vigouroux A. Immunocompetent astrocytes and microglia display major differences in the processing of the invariant chain and in the expression of active cathepsin L and cathepsin S. Eur J Immunol. 2001;31:1813–24. doi: 10.1002/1521-4141(200106)31:6<1813::aid-immu1813>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 43.Teige I, Liu Y, Issazadeh-Navikas S. IFN-beta inhibits T cell activation capacity of central nervous system APCs. J Immunol. 2006;177:3542–53. doi: 10.4049/jimmunol.177.6.3542. [DOI] [PubMed] [Google Scholar]
- 44.Nishioku T, Hashimoto K, Yamashita K, et al. Involvement of cathepsin E in exogenous antigen processing in primary cultured murine microglia. J Biol Chem. 2002;277:4816–22. doi: 10.1074/jbc.M108382200. [DOI] [PubMed] [Google Scholar]
- 45.Vergelli M, Kalbus M, Rojo SC, et al. T cell response to myelin basic protein in the context of the multiple sclerosis-associated HLA-DR15 haplotype: peptide binding, immunodominance and effector functions of T cells. J Neuroimmunol. 1997;77:195–203. doi: 10.1016/s0165-5728(97)00075-1. [DOI] [PubMed] [Google Scholar]
- 46.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 47.Liuzzo JP, Petanceska SS, Moscatelli D, Devi LA. Inflammatory mediators regulate cathepsin S in macrophages and microglia: a role in attenuating heparan sulfate interactions. Mol Med. 1999;5:320–33. [PMC free article] [PubMed] [Google Scholar]