Abstract
Retrocyclins are cyclic antimicrobial peptides that exhibit potent activity towards a broad range of primary and laboratory-adapted strains of human immunodeficiency virus type 1 (HIV-1) in vitro. The current study shows that RC-101, an analogue of retrocyclin, prevented HIV-1 infection in an organ-like construct of human cervicovaginal tissue and retained full activity in the presence of vaginal fluid. The peptide remained within the cervicovaginal tissues throughout the 9-day incubation period without altering tissue viability, inducing damage or inducing the release of inflammatory cytokines. Collectively, these data support the potential development of RC-101 as a topical microbicide to prevent HIV-1 infection and transmission.
Keywords: antimicrobial peptide, human immunodeficiency virus, acquired immunodeficieny syndrome, microbicide, θ-defensin, vagina
Introduction
Cationic antimicrobial peptides and proteins are integral components of the natural anti-human immunodeficiency virus type 1 (HIV-1) armamentarium of the human cervicovagina.1,2 The activities of many of these cationic (poly)peptides have been at least partially attributed to their ability to bind membranes or membrane components and subsequently disrupt microbial integrity or fusion of the viral and host cell membranes.3,4 Defensins constitute a family of cationic antimicrobial peptides that has three subfamilies in primates: α, β, and θ. The θ-defensins are unique tri-disulphide-bonded octadecapeptides rendered macrocyclic by head-to-tail ligation of the peptide backbone.5 While all primates are believed to express α-defensins and β-defensins, only certain non-human primates express θ-defensin peptides. In contrast, the genomes of humans, bonobos, chimpanzees and gorillas all contain θ-defensin pseudogenes that may be transcribed, but cannot be translated because of a premature stop codon within the signal sequence.6
Retrocyclins are ancestral human θ-defensins that we have recreated from their genetic blueprint through solid-phase peptide synthesis.7 Retrocyclins, and other θ-defensins, show activity against X4 and R5 strains of HIV-1 in vitro.7 They operate by preventing six-helix bundle formation of gp41 (a 41 000 MW glycoprotein)3,4 and are promising antiretroviral preventatives. RC-101, a non-haemolytic and minimally cytotoxic analogue of retrocyclin-1, can inhibit infection in vitro by over two dozen primary isolates of HIV-1 representing most known viral clades and subtypes.8
In the current study, we examined the ability of RC-101 to prevent HIV-1 infection of a biologically relevant organotypic model of cervicovaginal epithelia. We revealed that topical application of RC-101 inhibited HIV-1 infection of immunocompetent cervicovaginal tissues and that the peptide was sequestered for extended periods of time (9 days) within the tissue. RC-101 did not alter cervicovaginal tissue viability or induce inflammation, and retained complete anti-HIV-1 activity in the presence of human vaginal fluid. Collectively, these studies support the further development of RC-101 as a topical microbicide to prevent the heterosexual transmission of HIV-1.
Materials and methods
Preparation of peptide and viral stocks
The 18-amino-acid peptide RC-101 was synthesized in our laboratory as previously described.3,7 HIV-1 BaL, an R5 strain, was acquired from the National Institutes of Health AIDS Research and Reference Reagent Program (Dr Jay Levy), and stocks were prepared in our laboratory by infecting PM1 cells.3
Cell culture
The human cell lines TZM-bl (Dr John C. Kappes, Dr Xiaoyun Wu and Tranzyme Inc.) and PM1 (Dr Marvin Reitz), were acquired from the National Institutes of Health AIDS Research and Reference Reagent Program. TZM-bl cells were grown in Dulbecco's modified Eagle's medium (4·5 g/l glucose) containing penicillin, streptomycin and 10% (v/v) fetal bovine serum. PM1 promyelocytic cells were maintained in RPMI-1640 supplemented with penicillin, streptomycin, 10 mm HEPES, and 20% fetal bovine serum.
Cervicovaginal tissue model to assess RC-101 anti-HIV-1 activity
EpiVaginal™ tissues were purchased from MatTek Corp., Ashland, MA and maintained with proprietary growth medium according to the company's instructions. The in vitro reconstituted tissue model is comprised of a complete, stratified vaginal–ectocervical epithelial layer mixed with Langerhans cells and maintained atop microporous membrane cell culture inserts to allow growth at the air–liquid interface. Each batch was treated in triplicate or quadruplicate with 100 μl topically applied phosphate-buffered saline (PBS; control), PBS containing 25 ng p24/tissue HIV-1 BaL, or PBS containing HIV-1 BaL and RC-101 (0·2 or 2 μg/tissue) for 24 hr. HIV-1 BaL-infected PM1 cells (106/5 ml underlay) were added to the underlay (basal surface) media for 3 days to help sustain the initial viral infection. The apical surface of each tissue was washed twice with PBS and re-applied with 50 μl PBS or PBS containing RC-101 (0·1 or 1 μg/tissue) on days 1, 3 and 6 postinfection. Underlay media (5 ml/tissue) was changed every other day, and tissues were harvested on the 9th day postinfection. The recently available full thickness vaginal tissue model is almost identical to the tissue reconstituted in vitro, yet it contains an additional fibroblast-containing lamina propria. The full-thickness tissues were infected with HIV-1 BaL and 1–2 μg RC-101 in the same manner as the in vitro reconstituted tissues with the following exceptions: no infected PM1 cells were added to the underlays, underlay medium (2 ml/tissue) was changed daily, and tissues were harvested on the 6th day postinfection. For all tissue batches, harvested tissues were processed for immunohistochemistry, DNA isolation (DNA Micro kit, Qiagen, Valencia, CA), or total protein isolation. Apical washings and underlay media were collected and stored at −80° before analysis.
Assays for tissue viability and inflammation
The effect of increasing doses of RC-101 (0–32 μg/tissue) on vaginal tissue viability was measured using an MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay kit (MatTek Corp., Ashland, MA). In brief, metabolically active cells reduce MTT to an insoluble blue formazan dye; thus MTT reduction is correlated with viability.9,10 Cytotoxicity was also monitored by measuring lactate dehydrogenase (LDH) activity (Promega Corp., Madison, WI) in the tissue underlay media collected on days 1, 3 and 6 post-treatment. Separate aliquots of the same underlain media samples were assayed for various cytokines known to be markers of inflammation, including interleukin-6 (IL-6), IL-8, tumour necrosis factor-α, IL-1β, and interferon-γ. These enzyme-linked immunosorbent assay (ELISA) kits were purchased from BD Pharmingen, San Diego, CA.
Detection of RC-101 by quantitative immuno-dotblot
Tissues, apical washes and underlay media were extracted by adding 5 volumes of 10% acetic acid and vortexing for 20 min. Extracts were then centrifuged (20 800 g, 10 min) to pellet the insoluble material. Supernatants were transferred to fresh tubes, dried to near completion (ThermoSavant SpeedVac; ThermoSavant, Waltham, MA), and resuspended to the original volume in 0·1% acetic acid. Extracts (4 μl/dot) and standards (0–8 ng RC-101 peptide per 4-μl dot) were dotted onto a polyvinylidene fluoride (Immobilon-P) membrane that had been activated with methanol and presoaked in Tris-buffered saline (TBS; 20 mm Tris–HCl pH 7·5, 500 mm NaCl). The dotted membrane was fixed with 0·05% glutaraldehyde in TBS for 20 min at room temperature, blocked for 30 min at 37° using Superblock (Pierce, Rockford, IL), and incubated overnight with anti-RC-101 polyclonal antisera (Invitrogen custom antibody service, Carlsbad, CA) diluted 1 : 1000 in antibody buffer (1 : 3 Superblock in TBS containing 0·05% Tween-20 and 0·01% thimerosal). The membrane was then washed thrice, blocked for an additional 15 min (37°), and incubated with peroxidase-conjugated anti-rabbit immunoglobulin G for 1 hr before washing and development with Immun-Star HRP (Bio-Rad, Hercules, CA). Images were captured and analysed using the ChemiDoc XRS with quantity one software (Bio-Rad).
Immunohistochemical analysis of RC-101
Tissues were fixed in 4% paraformaldehyde and then haematoxylin & eosin (H&E) -stained slides, as well as unstained slides, were prepared by Mass Histology (Worcester, MA). Unstained tissue sections were deparaffinized, washed in TBS, and then incubated overnight with anti-RC-101 or preimmune serum, each diluted in TBS containing 1% gelatin, 0·05% Tween-20 and 0·01% thimerosal (TBS-T). Following three 20-min washes in TBS-T, alkaline phosphatase-conjugated secondary antibody was applied at 1 : 2000 and the sections were left overnight at room temperature. The next day, slides were washed thrice for 20 min in TBS-T, developed with Fast Red (Sigma, St. Louis, MO), and counterstained with Harris haematoxylin.
Real-time polymerase chain reaction to quantify HIV-1 infection in cervicovaginal tissues
Total DNA was isolated from each tissue, and a 200-ng DNA template was used for real-time polymerase chain reaction analysis, using primers specific for HIV-1 BaL proviral DNA or cellular β-actin DNA (control). Three different primer sets for BaL were tested, covering conserved regions of 5′ long terminal repeat (LTR), gag and env. The 5′ LTR primers were reported previously to detect proviral BaL DNA in human cervical tissues.11 HIV-1 BaL gag and env primers were designed using Beacon Designer 4·0 with GenBank accession number M68893. Relative changes in HIV-1 BaL copy number were measured by comparing cycle threshold differences between treatment groups and normalizing the data to β-actin levels. To confirm our findings, vaginal tissues were analysed for HIV-1 proviral copy numbers by the method of J. Zack and colleagues,12–14 which utilizes primers specific for full-length LTR-gag reverse transcripts and β-globin primers for normalization. Copy number standards were created by cloning target sequences into pCR4-TOPO (Invitrogen) and making 10-fold serial dilutions of linearized plasmids in the range of 300 000 to 30 copies. Saccharomyces cerevisiae genomic DNA (100 ng/reaction) was added to the standards so that input DNA levels of each standard remained consistent with the unknowns. In our hands, the presence of this background DNA did not change the slopes of the standard curves. Data are presented as HIV-1 copies per 10 000 copies of β-globin.
Measurement of anti-HIV-1 activity of RC-101 using a luciferase-based infection assay
TZM-bl cells, containing a luciferase construct under the control of the HIV-1 promoter15 were seeded in 96-well dishes (4000 cells/well) and left to grow overnight. The next day, the medium was removed and the cells were incubated with 50 μl of either plain medium, pooled vaginal fluid diluted 1 : 10 in plain medium, or pooled diluted vaginal fluid in combination with 2 μg/ml RC-101, and then immediately infected with 50 μl medium containing HIV-1 BaL (5 ng p24/well) for 24 hr. Cells were then washed and lysed, and luciferase was measured using Promega's BrightGlo luciferase assay system. Whole vaginal fluid was collected from eight donors and pooled as described by our laboratory previously,2 according to IRB guidelines of the University of Central Florida.
Results and discussion
RC-101 effectively inhibits HIV-1 infection of organotypic cervicovaginal tissues
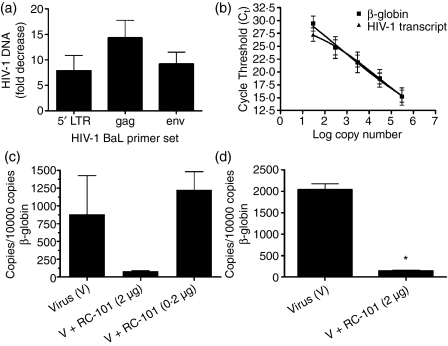
We have previously revealed that retrocyclins prevent infection of CD4+ peripheral blood mononuclear cells and lymphocytic cell lines by both primary and laboratory-adapted strains of HIV-1. To pursue the development of retrocyclins as microbicides to be applied topically to the human vagina, we examined the role of RC-101 in preventing HIV-1 infection using a biologically relevant organotypic model of cervicovaginal epithelial tissue. Tissues were treated apically with HIV-1 BaL and RC-101 (2 μg in 100 μl PBS) and maintained for 9 days as described in the Materials and methods. An eightfold to 14-fold decrease in HIV-1 DNA levels was observed by quantitatively measuring 5′ LTR, gag and env of HIV-1 BaL (Fig. 1a). The 5′ LTR primers were reported previously to detect proviral BaL DNA in human cervical tissues.11 Note that proviral DNA was not detected in tissues treated with heat-inactivated HIV-1 BaL (not shown). To gain an accurate assessment of tissue infection, we next determined HIV-1 copy number in the presence and absence of RC-101 by quantifying full-length LTR-gag reverse transcripts for both in vitro reconstituted (Fig. 1c) and full-thickness (Fig. 1d) cervicovaginal tissues normalized to β-globin (standard curves for LTR-gag and β-globin are shown in Fig. 1b). RC-101 significantly inhibited the formation of viral reverse transcripts in the full thickness tissues (n = 4; P < 0·001), while a similar trend was observed for the in vitro reconstituted tissues.
Figure 1.
RC-101 inhibits HIV-1 infection of vaginal tissues. (a) Vaginal tissues were treated with either HIV-1 BaL alone or with HIV-1 BaL in the presence of RC-101 (2 μg in 100 μl PBS) and then maintained for 9 days as described in the Materials and methods. Tissue DNA (200 ng) served as templates for real-time polymerase chain reaction using primers specific for HIV-1 BaL 5′ LTR, gag, or env, or for cellular β-actin (control). Results are presented as fold-decrease in HIV-1 BaL DNA for RC-101 versus vehicle-treated vaginal tissues (mean ± SEM, n = 3). (b–d) Quantitative analysis of HIV-1 reverse transcripts in RC-101 versus vehicle-treated vaginal tissues. Standard curves for HIV-1 transcript and β-globin levels were generated by plotting cycle threshold (Ct) values for known numbers of input templates (b), and Ct values for tissue DNA samples were fit to the standard curves and presented as copies of HIV-1 transcript per 10 000 copies of β-globin. (c) Vaginal tissues were treated with either 2 μg or 0·2 μg RC-101 at the time of HIV-1 BaL infection and then maintained for 9 days as described in the Materials and methods. (d) Levels of HIV-1 transcript in full-thickness tissues treated with HIV-1 BaL alone or in the presence of 2 μg RC-101. Bars represent mean ± SEM, n = 4. *P < 0·01.
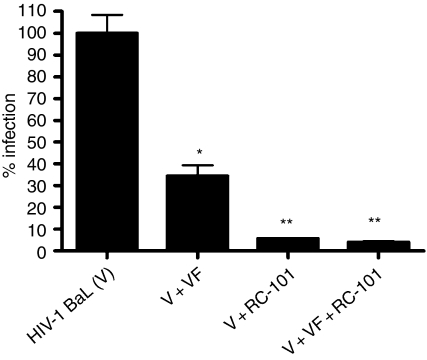
In an effort to determine the efficacy of RC-101 in the presence of human vaginal fluid, we performed anti-HIV-1 assays of RC-101 in the presence or absence of vaginal fluid (Fig. 2). Human vaginal fluid (1 : 20 final dilution) exhibited modest intrinsic anti-HIV-1 activity. RC-101 alone was potently antiviral, and this activity was not changed in the presence of vaginal fluid (Fig. 2). The endogenous cationic (poly)peptides within the fluid2 might act in concert with RC-101. Likewise, in a single trial assessing HIV-1 infection of cervicovaginal tissues, RC-101 co-incubated with human vaginal fluid was as active as RC-101 alone (data not shown). Together, these data suggest that apically applied RC-101 can effectively inhibit HIV-1 infection of immunocompetent cervicovaginal epithelia, that the activity is retained in normal human vaginal fluid, and that RC-101 enhances the intrinsic protective activity of normal human vaginal fluid.
Figure 2.
RC-101 retains anti-HIV-1 activity while in the presence of vaginal fluid. HIV-1 entry indicator cells, TZM-bl, were infected either with HIV-1 BaL virus alone (V), virus in combination with pooled human vaginal fluid (VF, 1 : 20 final dilution), virus in combination with RC-101 (2 μg/ml), or virus combined with VF and RC-101 for 24 hr before luciferase measurement. Data are expressed as per cent infection and bars represent mean ± SEM, n = 4–6 for each treatment condition. *P < 0·001 versus V. **P < 0·01 versus V + VF.
Topically applied RC-101 is retained within cervicovaginal tissues
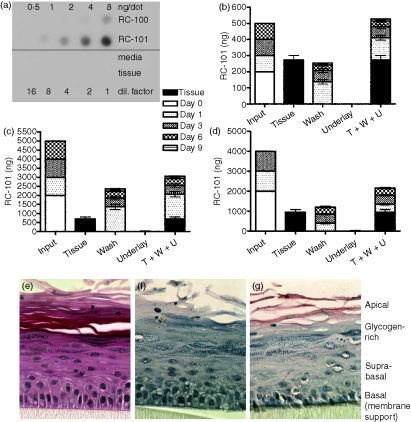
We next applied RC-101 to the apical surface of cervicovaginal tissues and examined the level retained in the tissue following 9 days of culture (Fig. 3). RC-101 was applied at days 0, 1, 3 and 6, and levels of RC-101 were quantified by immuno-dotblot from the apical wash, underlying media, and cervicovaginal tissue at time-points from 1 to 9 days (peptide concentrations and time-points are given in Fig. 3b–d). When a total of 500 ng RC-101 was applied to the tissue surface (‘input’; Fig. 3b), the entire amount was recovered from the tissue and apical washes. However, when higher concentrations of RC-101 were applied (4–5 μg; Fig. 3c,d), approximately 60% of the RC-101 was recovered from the tissue and washes, suggesting tissue saturation leading to binding of excess peptide to the plastic inserts that support the tissue. Immunohistochemical analysis confirmed that while RC-101 was present in all layers of the stratified epithelia at day 9, the majority was localized to the apical and suprabasal and basal layers (Fig. 3g). RC-101 was not detected in the underlying medium of either the in vitro reconstituted (Fig. 3b,c) or the full-thickness (Fig. 3d) tissues, signifying that the peptide did not cross the epithelium into the lamina propria. This would suggest that should an RC-101-containing microbicidal product be applied topically to the vagina, minimal systemic absorption would occur.
Figure 3.
Topically applied RC-101 is retained within or at the apical surface of vaginal tissues. (a) Quantitative dot blot demonstrating that rabbit polyclonal anti-RC-101 recognized RC-101 peptide but not the tissue maintenance media or untreated vaginal tissue. (b,c) Vaginal tissues were infected (day 0) with HIV-1 BaL in the presence of 200 ng RC-101 in 100 μl PBS (b) or 2000 ng RC-101 in 100 μl PBS (c) and then tissues were washed and re-applied with RC-101 at days 1, 3 and 6 postinfection (Input), as described in the Materials and methods. Collected apical surface wash fluids (Wash, W) and underlay media (Underlay, U), as well as harvested tissues (Tissue, T) were then assayed for RC-101 by dot blot. Values are displayed as stacked bars correlating to harvested tissue or collection day number. (d) Full-thickness vaginal tissues were treated and maintained over 6 days as described in the Materials and methods. Bars represent mean ± SEM, n = 4–6. (e–g) Tissues infected with HIV-1 BaL (25 ng/tissue) and treated with two applications of 400 ng RC-101 (in 50 μl PBS). Tissue sections were subjected to H&E staining (e) and immunohistochemistry with preimmune (f) and anti-RC-101 polyclonal antisera (g) Note the immuno-localization of RC-101 (red stain) primarily in the apical, suprabasal and basal layers.
RC-101 does not adversely affect tissue viability or induce inflammation
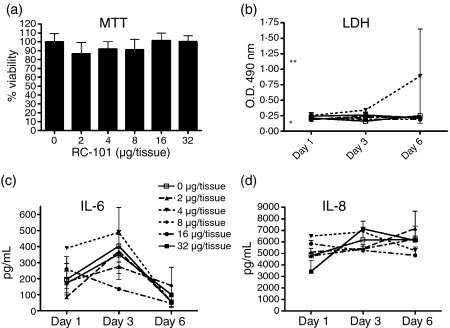
To assess potential deleterious effects of RC-101 on full-thickness cervicovaginal epithelia, we tested the ability of the peptide to affect tissue viability and protein expression of inflammatory markers during 6 days of culture. As measured by a tetrazolium-based MTT assay, RC-101 at the highest concentration measured (32 μg in 100 μl PBS) did not adversely affect tissue viability (Fig. 4a). Similarly LDH activity, which is another measure of epithelial cytotoxicity, was unchanged between the RC-101-treated tissues and untreated tissues (Fig. 4b). As measured by quantitative ELISA, RC-101 did not induce significant expression of several markers of epithelial inflammation. Data for IL-6 and IL-8 appear in Fig. 4(c,d), respectively, while the protein levels of tumour necrosis factor-α, IL-1β, and interferon-γ remained below the level of detection for every condition tested (data not shown).
Figure 4.
RC-101 does not alter the viability or inflammatory profiles of human vaginal tissue. Full-thickness vaginal tissues were treated with 100 μl PBS (control) or PBS containing 2–32 μg/tissue RC-101 on day 0 and then washed and re-applied with 50 μl PBS or PBS containing half-doses of RC-101 (1–16 μg/tissue, respectively) at days 1 and 3 postinfection. Tissue viability was assessed by measuring MTT reduction at day 6 post-treatment (a) and LDH activity in the underlay media collected on days 1, 3 and 6 post-treatment (b) Media alone (negative control) and LDH-spiked media (positive control) values are indicated by a single and double asterisk, respectively (b). Underlay media collected on days 1, 3 and 6 post-treatment were also assayed for IL-6 (c) and IL-8 (d) by ELISA. Data are presented as mean ± SEM, n = 4.
Overall, these experiments demonstrate the ability of RC-101 to inhibit HIV-1 infection of a biologically relevant human cervicovaginal tissue model without causing appreciable toxicity. The absence of inflammatory cytokine stimulation and the ability of RC-101 to be retained within the cervicovaginal tissue further validate its potential as a topical microbicide. While toxicity and efficacy studies in non-human primate models remain to be done, the current study validates RC-101 as a candidate topical microbicide to prevent heterosexual transmission of HIV-1.
Acknowledgments
This work has been supported by grants from the National Institutes of Health: AI052017, AI065430, and AI060753 (to A.M.C). We thank Nitya Venkataraman for expert collection of vaginal fluid.
Abbreviations
- ELISA
enzyme-linked immunosorbent assay
- H&E
haematoxylin and eosin
- HIV-1
human immunodeficiency virus type 1
- IL
interleukin
- LDH
lactate dehydrogenase
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- PBS
phosphate-buffered saline
- RC
retrocyclin
- TBS
Tris-buffered saline
References
- 1.Valore EV, Park CH, Igreti SL, Ganz T. Antimicrobial components of vaginal fluid. Am J Obstet Gynecol. 2002;187:561–8. doi: 10.1067/mob.2002.125280. [DOI] [PubMed] [Google Scholar]
- 2.Venkataraman N, Cole AL, Svoboda P, Pohl J, Cole AM. Cationic polypeptides are required for anti-HIV-1 activity of human vaginal fluid. J Immunol. 2005;175:7560–7. doi: 10.4049/jimmunol.175.11.7560. [DOI] [PubMed] [Google Scholar]
- 3.Cole AL, Yang OO, Warren AD, Waring AJ, Lehrer RI, Cole AM. HIV-1 adapts to a retrocyclin with cationic amino acid substitutions that reduce fusion efficiency of gp41. J Immunol. 2006;176:6900–5. doi: 10.4049/jimmunol.176.11.6900. [DOI] [PubMed] [Google Scholar]
- 4.Gallo SA, Wang W, Rawat SS, et al. Theta-defensins prevent HIV-1 Env-mediated fusion by binding gp41 and blocking 6-helix bundle formation. J Biol Chem. 2006;281:18787–92. doi: 10.1074/jbc.M602422200. [DOI] [PubMed] [Google Scholar]
- 5.Tang YQ, Yuan J, Osapay G, Osapay K, Tran D, Miller CJ, Ouellette AJ, Selsted ME. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science. 1999;286(5439):498–502. doi: 10.1126/science.286.5439.498. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen TX, Cole AM, Lehrer RI. Evolution of primate theta-defensins: a serpentine path to a sweet tooth. Peptides. 2003;24:1647–54. doi: 10.1016/j.peptides.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Cole AM, Hong T, Boo LM, et al. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc Natl Acad Sci USA. 2002;99:1813–18. doi: 10.1073/pnas.052706399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owen SM, Rudolph D, Wang W, Cole AM, Waring AJ, Lal RB, Lehrer RI. RC-101, a retrocyclin-1 analogue with enhanced activity against primary HIV-1 isolates. AIDS Res Hum Retroviruses. 2004;20:1157–65. doi: 10.1089/aid.2004.20.1157. [DOI] [PubMed] [Google Scholar]
- 9.Holt PS, Buckley S, Deloach JR. Detection of the lethal effects of T-2 mycotoxin on cells using a rapid colorimetric viability assay. Toxicol Lett. 1987;39:301–12. doi: 10.1016/0378-4274(87)90246-3. [DOI] [PubMed] [Google Scholar]
- 10.Levitz SM, Diamond RD. A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J Infect Dis. 1985;152:938–45. doi: 10.1093/infdis/152.5.938. [DOI] [PubMed] [Google Scholar]
- 11.Greenhead P, Hayes P, Watts PS, Laing KG, Griffin GE, Shattock RJ. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. Jvirol. 2000;74:5577–86. doi: 10.1128/jvi.74.12.5577-5586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole SW, Naliboff BD, Kemeny ME, Griswold MP, Fahey JL, Zack JA. Impaired response to HAART in HIV-infected individuals with high autonomic nervous system activity. Proc Natl Acad Sci USA. 2001;98:12695–700. doi: 10.1073/pnas.221134198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorry PR, Bristol G, Zack JA, et al. Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity. J Virol. 2001;75:10073–89. doi: 10.1128/JVI.75.21.10073-10089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zack JA, Arrigo SJ, Weitsman SR, Go AS, Haislip A, Chen IS. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–22. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 15.Wei X, Decker JM, Liu H, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46:1896–905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]