Abstract
Respiratory syncytial virus (RSV) is the major causative agent of severe lower respiratory tract disease and death in infants worldwide. The epithelial cells of the airways are the target cells for RSV infection and the site of the majority of the inflammation associated with the disease. However, despite five decades of intensive RSV research there exist neither an effective active vaccine nor a promising antiviral and anti-inflammatory therapy. Recently, peroxisome proliferator-activated receptor-γ (PPAR-γ), a member of the nuclear hormone receptor superfamily, has been shown to possess anti-inflammatory properties. Therefore, we hypothesized whether the detrimental increase of intercellular adhesion molecule-1 (ICAM-1) on RSV-infected lung epithelial cells (A549 and primary normal human bronchial epithelial cells (NHBE)) might be modulated by natural and synthetic PPAR-γ agonists (15d-PGJ2, ciglitazone, troglitazone, Fmoc-Leu). Our data show that all PPAR-γ agonists under study significantly down-regulated the RSV-induced expression of ICAM-1 on A549- and NHBE cells in a dose-dependent manner resulting in a reduced β2 integrin-mediated adhesion of monocytic effector cells (U937) to RSV-infected A549 cell monolayers. In contrast, the PPAR-α agonist bezafibrate had no impact on the RSV-induced ICAM-1 expression. The reduced ICAM-1 expression was associated with a diminished ICAM-1 mRNA level and binding activity of nuclear factor-κB (p65/p50) in A549 cells. These findings suggest that PPARγ agonists have beneficial effects in the suppression of the inflammatory response during RSV infection and therefore might have clinical efficacy in the course of severe RSV-infection.
Keywords: RSV, PPARγ, ICAM-1, viral, adhesion molecule, inflammation, A549, NF-κB
Introduction
Respiratory syncytial virus (RSV) is worldwide the single most important respiratory pathogen in infancy and early childhood.1 Currently, despite five decades of intensive RSV research, there exist neither an effective active vaccination nor a promising antiviral or anti-inflammatory therapy.2,3 In its most severe form, the infection of the lower respiratory tract is characterized by peribronchiolar cellular infiltrates that are accompanied by submucosal oedema and bronchorrhoea.4 Additionally, polymorphonuclear neutrophils (PMN) chemotactically recruited into the broncho-alveolar space, the enhanced production of mucus, and the cellular debris from RSV-destroyed lung epithelial cells lead to bronchiolar obstruction and compromised oxygen transfer.5 The RSV infection induces an intense inflammatory response in human lung epithelial cells.6 Especially, the increased cell surface expression of intercellular adhesion molecule-1 (ICAM-1) on RSV-infected human airway epithelial cells is responsible for an enhanced adhesion of the recruited immune effector cells resulting in an increased cytotoxicity.7,8 Thereby, ICAM-1 serves as a counter receptor for the β2 leucocyte integrins expressed on leucocytes, i.e. leucocyte function-associated-1 (LFA-1) (CD11a/CD18) and Mac-1 (CD11b/CD18). Thus, down-regulation of the RSV-induced expression of ICAM-1 on infected lung epithelial cells seems to be a promising anti-inflammatory therapeutic strategy.
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors which form a subfamily of the nuclear receptor gene family consisting of three isotypes: PPAR-α, PPAR-β and PPAR-γ.9 They were first identified as regulators of the lipid and glucose metabolism.10,11 However, during the last decade data accumulated showing that PPARs may also be involved in the complex regulation of immune and inflammatory processes.12,13 The activation of PPAR-α and PPAR-γ correlated with the inhibition of inflammatory cell responses in a variety of cell types. For example, PPAR-γ agonists have been shown to diminish the synthesis of proinflammatory cytokines in activated macrophages, endothelial cells, T-cell lines, and epithelial cells.14–17 Moreover, the viral-induced expression of proinflammatory cytokines was inhibited in the course of RSV and human immunodeficiency virus infection.18,19 In an inflammatory murine model it was shown that rosiglitazone reduced the ICAM-1 expression in the lungs of carrageenan-treated rats.20 However, it is not known whether PPAR-γ agonists are able to modulate the expression of ICAM-1 on human lung epithelial cells. The present study was designed to clarify the inhibitory effect of PPAR-γ agonists on RSV-induced ICAM-1 expression on human lung epithelial cells. The following PPAR-γ-specific ligands were used in our study: the naturally occurring compound 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2), a metabolite of prostaglandin D2, the synthetic antidiabetic thiazolidinedione (TZD) derivatives ciglitazone and troglitazone, and a N-protected leucine analogue designated as Fmoc-Leu.21 Two molecules of Fmoc-Leu interact with one PPAR-γ molecule in a highly specific manner. In addition, bezafibrate known to be a specific PPAR-α agonist was also analysed. Our data presented herein show that treatment of lung epithelial cells with natural and synthetic PPAR-γ ligands: (i) inhibited the RSV-induced up-regulation of ICAM-1 on human lung epithelial cells; (ii) reduced the RSV-induced cellular amount of ICAM-1 mRNA; (iii) down-regulated the RSV-induced activity of nuclear factor (NF)-κB (p65/50); and (iv) significantly diminished the adhesion of monocytic cells to RSV-infected epithelial cells.
Materials and methods
Materials
The human epithelial cell lines A549 and HEp-2, the human monocytic cell line U937, and the Long strain of RSV were obtained from ATCC (Rockville, MD). Frozen vials of primary normal human bronchial epithelial cells (NHBE), the complete bronchial epithelial cell growth medium (BEGM Bulletkit), Hank's balanced salt solution (HBSS), and phosphate-buffered saline (PBS) were obtained from Cambrex/Clonetics (Verviers, Belgium). Dulbecco's modified Eagle's minimal essential medium (DMEM), RPMI-1640, glutamine, and antibiotics were obtained from Gibco BRL (Karlsruhe, Germany). Fetal calf serum (FCS) was obtained from Biochrom AG seromed (Berlin, Germany). Cell culture plastic material was used from Greiner Labortechnik (Frickenhausen, Germany). Ciglitazone, troglitazone, Fmoc-Leu, bezafibrate, calcein-acetyloxymethylester (calcein-AM), and plurionic F-127 detergent were supplied by Merck Biosciences (Schwalbach, Germany). 5d-PGJ2 was purchased from Biomol (Hamburg, Germany). Enzyme-linked immunosorbent assay (ELISA) specific for human interleukin-1α (IL-1α), IL-6, and tumour necrosis factor-α (TNF-α), blocking antibodies specific for IL-1α (clone 4414.141), IL-6 (clone 6708.111), TNF-α (clone 1825.12), ICAM-1 (clone BBIG-I1), CD11a (clone 38), and unspecific immunoglobulin G1 (IgG1) (clone 11711.11) and IgG2a (clone 20102) isotype controls were supplied by R & D Systems (Wiesbaden-Nordenstadt, Germany). Neutralizing antibodies specific for CD11b (clone 44) and CD18 (clone 685A5) were supplied by Leinco Technologies (St. Louis, MO). Phycoerythrin (PE)-labelled mouse anti-human ICAM-1 antibody (clone HA58), and PE-labelled mouse IgG1 isotype control antibody were obtained from Becton Dickinson (BD) Biosciences (Heidelberg, Germany). Mouse anti-RSV P protein antibody (clone 3C4) was a generous gift from Dr H. Werchau (Department of Medical Virology, Ruhr-Universität Bochum, Germany). The secondary horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG antibody was obtained from DAKO (Hamburg, Germany). Both NF-κB-binding activity assay kit and nuclear extraction kit were obtained from Active Motif Europe (Rixensart, Belgium). Moloney murine leukaemia virus reverse transcriptase (MLV-RT), MLV RT-buffer, deoxyribonucleoside triphosphate (dNTP)-set, and oligo d(T)(12−18) were obtained from Invitrogen (Karlsruhe, Germany). RNAase inhibitor was supplied by Applied Biosystems (Foster City, CA). RNA Biorobot™ kit, QuantiTect™ Probe PCR Master Mix, Taq DNA polymerase, primer sets and FAM-labelled probes (human glyceraldehyde 3-phosphate dehydrogenase and ICAM-1) for Taqman real-time RT–polymerase chain reaction (PCR) were obtained from Qiagen (Düsseldorf, Germany). T4 polynucleotide kinase was obtained from Fermentas (Vilnius, Lithuania). Mycoplasma detection kit, and a modified MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, designated as WST-1 assay, were obtained from Roche Diagnostics (Mannheim, Germany). [γ-32P]-ATP was supplied by Amersham Biosciences Europe (Freiburg, Germany). Fibronectin, dimethylsulphoxide (DMSO), human AB serum, bovine serum albumin (BSA), and all fine chemicals were supplied by Sigma (Deisenhofen, Germany).
Cell culture
Human A549 pulmonary type II epithelial cells (passages 4–20) were cultured in DMEM (4500 mg/l d-glucose), supplemented with 2 mm glutamine, 5% (v/v) inactivated FCS, streptomycin (100 µg/ml), and penicillin (100 IU/ml). The NHBE cells were cultured in complete BEGM medium (5 µg/ml insulin, 0·5 µg/ml hydrocortisone, 10 µg/ml transferrin, 6·5 ng/ml triiodothyronine, 0·5 µg/ml epinephrine, 0·5 ng/ml human epidermal growth factor, 0·1 ng/ml retinoid acid, 50 µg/ml gentamicin, and 52 µg/ml bovine pituitary extract). NHBE cells were cultured and expanded according to the instructions of the manufacturer. To support cell attachment and growth of NHBE cells, tissue culture flasks and plastic plates were precoated with fibronectin (10 µg/ml) for 30 min at 37°. Cells of passages 3–7 were seeded on 24-well plates and cultured until confluency in complete medium. U937 cells were maintained in RPMI-1640 (10% FCS, streptomycin (100 µg/ml), penicillin (100 IU/ml)). All cell cultures and prepared virus stocks were free of mycoplasmic contamination routinely verified by a commercially available mycoplasma detection kit.
Virus growth and preparation
The Long strain of RSV was propagated and titrated in HEp-2 cells. The cells were cultured in DMEM (5% FCS, 2 mm glutamine, streptomycin (100 µg/ml), penicillin (100 U/ml). For virus propagation confluent monolayers were infected with RSV at a multiplicity of infection (m.o.i) of 0·1 (for 3 hr, in DMEM, without FCS). The monolayers were washed, overlayed with DMEM (0·5% FCS) and incubated at 37° in 5% CO2 atmosphere until cytopathic effect reached ∼80%. Thereafter, the supernatants were harvested and cellular debris was removed by centrifugation (5000 g, 10 min). RSV was concentrated by polyethylene glycol precipitation (10%) and purified by means of discontinuous sucrose gradient centrifugation.22 To stabilize the purified virus particles they were resolved in 20% sucrose/NT-buffer (150 mm NaCl, 50 mm Tris-HCl, pH 7·5) and stored at −80°.
RSV titration
For virus titration pepared RSV stock solutions were serially tenfold diluted onto confluent HEp-2 monolayers cultured in 96-well flat-bottomed plates. The virus titre was quantified using the microplaque immunoperoxidase method.23 Briefly, infected cells were cultured under methyl cellulose. After 48 hr the monolayers were fixed with paraformaldehyde (2%) and stained with mouse anti-P protein monoclonal antibody (mAb). Bound antibody was visualized by a second staining with HRP-conjugated rabbit anti-mouse immunoglobulins. The RSV plaques were identified by colorimetric staining and counted microscopically. The stock titre of the used virus pool was 108 plaque forming units (p.f.u.)/ml.
Cell experiments
Epithelial cell monolayer grown in 24-well culture plates were washed with basal medium and PPAR-γ agonists were added 30 min prior to RSV-infection. Compounds were stored in DMSO at −80°. At the day of experiment they were freshly diluted in basal medium (DMEM or BEGM) and added to the cells with a final DMSO concentration of 0·05–0·25% (v/v). The cells were pretreated with varying doses of ciglitazone (5–50 µm), troglitazone (5–50 µm), 15d-PGJ2 (5–20 µm), Fmoc-Leu (50–300 µm), and solvent (DMSO; 0·1–0·3% (v/v)), respectively, in a volume of 1 ml. By using these PPAR-γ agonist concentrations the PPAR-γ receptors are fully activated but agonists still lack any cell cytotoxicity. Thereafter, the medium was reduced to a volume of 200 µl and cells were infected with RSV (m.o.i. = 3) for 2 hr still in the presence of the used PPAR-γ agonists. Then, cells were washed and incubated with fresh medium (DMEM (2% FCS) or supplemented BEGM in case of NHBE cells) in the presence of freshly supplied agonists for another 20 hr to 36 hr. Thereafter, cells were detached and harvested as previously described.7 Determined by WST-1 assay, the added agonists induced no significant cell death (>3%) at the concentrations used.
Cell cytotoxicity studies
Approximately 5 × 104 cells placed in 96-well flat-bottomed plates were incubated with the agonists for 48 hr. The final concentration of the vehicle DMSO was maximal 0·3% (v/v). Cell viability was measured on a microplate SpectraFluorPlus Reader (Tecan, Crailsheim, Germany) using a modified MTT assay, designated as WST-1 assay. The assay was performed according to the instructions of the manufacturer (Roche Diagnostics).
Flow cytometry
Harvested cells were immediately stained for fluorescence-activated cell sorting (FACS) analysis. The cell surface expression of ICAM-1 was determined with a FACSCalibur (BD Biosciences, Heidelberg, Germany) as previously described.24 The mean fluorescence intensity (MFI) of 10 000 cells was determined and corrected by subtraction of background fluorescence of isotype control.
Quantification of cytokines
The amount of IL-1α, TNF-α, and IL-6 was determined in the harvested cell supernatants by cytokine-specific ELISAs. The ELISAs had a sensitivity of 1·0 pg/ml, 1·6 pg/ml, and 0·7 pg/ml for IL-1α, TNF-α, and IL-6, respectively, and were performed according to the manufacturer's instructions (R & D Systems).
Adhesion assay
Adhesion of U937 cells to confluent A549 cell monolayers was determined by measuring the fluorescence signal of adherent U937 cells fluorescently labelled with the acetyloxymethylester of calcein (calcein-AM). Briefly, 50 µg calcein-AM was suspended in 5 µl DMSO, 5 µl 250 mg/ml plurionic F-127 detergent, and 60 µl heat-inactivated FCS. This labelling solution was sufficient for staining of 5 × 107 U937 cells in a volume of 5 ml HBSS. The cells were incubated at room temperature in the dark and rocked for 60 min. Thereafter, cells were washed two times with RPMI-1640 (5% BSA), counted again, and resuspended at a density of 8 × 106 cells/ml. Confluent A549 monolayers grown in 96-well flat-bottomed plates were infected with RSV (m.o.i. = 3) and cultured in the presence of PPAR-γ agonists for another 36 hr. Preliminary performed experiments revealed an optimum of adherent cells at that time point. Thereafter, the monolayers were washed and overlaid with PMA-preactivated (50 ng/ml, 12 hr), calcein-AM-labelled U937 cells (4 × 105) in a volume of 50 µl medium. In case of blocking experiments monolayers were preincubated with a-ICAM-1 (20 µg/ml) antibody or unspecific IgG1 (20 µg/ml) for 30 min and Fcγ-receptors on cocultured U937 cells were additionally blocked by prior incubation with 50% human AB serum for 15 min. Furthermore, β2 integrins expressed on U937 cells were blocked by preincubation with a-CD11a (20 µg/ml)-, a-CD11b (20 µg/ml)-, and a-CD18 (20 µg/ml) antibody for 30 min. Matched isotype control antibodies served as control. To analyse IL-1α-, IL-6-, and TNF-α-dependent up-regulation of ICAM-1 on RSV-infected epithelial cells, the cells were cultured in the presence of specific cytokine blocking antibodies (10 µg/ml) and matched isotype control antibodies, respectively, for 36 hr. Thereafter, washed monolayers and U937 cells were cocultured to allow to attach for 30 min at 37°. Unbound cells were removed by flicking and washing the plates two times. Residual cell bound fluorescence was measured using a SpectraFluorPlus Reader. To determine the linearity between cell number and fluorescence signal, standard curves were pipetted in 96-well plates at the day of the experiment. Linearity of the fluorescence signal was always given up to 4 × 105 cells, i.e. the amount of the added U937 cells.
NF-κB-binding activity
Nuclear extracts were prepared from A549 cells by means of a commercially available kit and performed according to the instructions of the manufacturer (Active motif). The binding activities of the NF-κB subunits Rel A (p65) and NF-κB1 (p50) were measured by using specific Trans-AM™ transcription factor assay kits (Active Motif) as previously described.25 Briefly, oligonucleotides encoding for the human NF-κB consensus binding site were bound to microtitre plates. The binding of activated NF-κB-heterodimers, in the prepared nuclear extracts, to the immobilized DNA was revealed by incubation with p65- and p50-specific antibodies using ELISA technology. Optical density was determined at 450 nm.
RNA extraction and real-time RT–PCR
The total cellular RNA from uninfected and RSV-infected epithelial cells (5 × 105) was extracted using the QiAmp 96 viral RNA Biorobot™ kit on the Biorobot 3000 System from Qiagen. RT–PCR was performed with M-MLV RT-buffer components. Prepared total RNA (2 µg) was added to 50 µl PCR reaction mix consisting of 50 mm Tris-HCl buffer (pH 8·3), 3 mm MgCl2, 75 mm KCl, 10 mm DTT, 500 µm dNTPs, 2 ng/µl oligo d(T)(12−18), 1 U/µl RNAase inhibitor, and 4 U/µl M-MLV. The synthesis of cDNA was performed at 37° for 60 min. The cDNA was stored at −20°. The amount of ICAM-1 mRNA was quantified by Taqman real-time RT-PCR. Briefly, using the Gene Amp 5700 Sequence Detector (Applied Biosystems) 2·5 µl cDNA (100 ng RNA) was amplified in a volume of 25 µl by means of TaqMan Universal PCR Master Mix containing specific primers for ICAM-1 and a fluorescently labelled probe synthesized by Qiagen (ICAM-1 primers: forward, 5′-GACCATCTACAGCTTTCC-3′; reverse, 5′-CTCACACTTCACTGTCACCTC-3′; probe, 5′-FAM-CCAACGTGATFAMTCTGAC-TAMRA-3′. The primer/probe set for the housekeeping gene human GAPDH was purchased as a predeveloped kit from Qiagen. The reaction components were mixed and the amplification profile settled according to the instructions of the manufacturer. Negative controls were carried out with water instead of cDNA. cDNA prepared from RSV-infected cells and diluted up to 105 served as positive control validating that the efficiencies of ICAM-1- and GAPDH PCR were approximately equal. Expression of GAPDH gene was not significantly altered during the time of incubation with RSV, drugs and vehicle. Therefore, the relative mRNA expression of each gene was normalized to the level of GAPDH in the same RNA preparation, i.e. the comparative CT method was used to analyse the relative quantities of ICAM-1 in cell samples. The relative RNA amount was calculated by using the following equation: 2–ΔΔCT were ΔΔCT = ΔCT,q − CT,cb. CT is defined as the CT value for ICAM-1 minus the CT value for GAPDH for a given sample, q is the unknown sample, and cb is the calibrator (non-infected sample).
Electrophoretic mobility shift assay (EMSA) and supershift EMSA
Prior to RSV infection (m.o.i. = 3) A549 cells were pretreated with PPAR-γ agonists for 30 min. Thereafter, cells were washed and incubated with agonists for another 20 hr. Nuclear extracts were prepared as previously described.26 The NF-κB oligonucleotide (forward: 5′-AGCTTGACCAAGAGGGATTTCCCCTAAATC-3′/reverse: 5′-AGCTTGATTTAGGGGAAATCCCTCTTGGTCA-3′) spanning the consensus NF-κB site in the murine IL-2 promoter was end-labelled with [γ-32P]ATP (10 µCi) by T4 polynucleotide kinase and purified by native polyacrylamide gel electrophoresis (PAGE, 15%). Nuclear extracts (4 µg) were incubated with the labelled NF-κB oligonucleotide as previously described.26 In supershift EMSA, nuclear extracts were incubated with 1 µl of the indicated Rel protein-specific antibodies after addition of the labelled probes.27 The DNA–protein complexes were electrophoretically separated on a 6% non-denaturating PAGE. Specificity was determined by addition of an excess (25 ng/reaction) of unlabelled NF-κB oligonucleotides to the nuclear extracts before formation of DNA-protein complexes.
Statistics
If not stated otherwise, the results are presented as means and SEM (MFI ± SEM). Statistical significance analysis of data was performed using Student's t-test (two-sided).
Results
Effect of PPAR-γ agonists on RSV-induced ICAM-1 expression
Previously, we and others observed a prominent up-regulation of ICAM-1 on RSV-infected A549 cells.6,7 To assess whether this induced cell surface expression might be modulated by PPAR-γ agonists we infected and cultured A549 cells in the presence of specific PPAR-γ agonists. As shown in Fig. 1(a), RSV increased the constitutive ICAM-1 expression on A549 cells from MFI 149 ± 61 to MFI 1186 ± 91 36 hr postinfection in a significant manner. However, no RSV-induced cytotoxicity was observed at that incubation time. Pretreatment of A549 cells with the TZD ciglitazone (10–50 µm) and troglitazone (5–50 µm), respectively, resulted in a significant inhibition of the RSV-induced ICAM-1 expression. Both glitazones reduced the ICAM-1 expression in a dose-dependent manner. Also, the natural PPAR-γ ligand 15d-PGJ2 down-regulated the RSV-induced ICAM-1 expression dose-dependently and had its maximal effect at a concentration of 20 µm. However, at higher concentrations of 15d-PGJ2 (>30 µm) we observed a slightly increased cytotoxicity in RSV-infected A549 cells (data not shown). Also, the synthetic PPAR-γ ligand Fmoc-Leu, structurally different from the glitazones, significantly reduced the RSV-induced expression of ICAM-1 at a concentration of 100 µm (Fig. 1b). Similar results were obtained when all PPAR-γ agonists under study were added post RSV infection suggesting that the agonists did not interfere with the primary infection process (data not shown). By contrast, the PPAR-α-specific agonist bezafibrate (100–300 µm) and the vehicle control (DMSO: 0·1% and 0·3% ((v/v)) did not modulate the RSV-induced expression of ICAM-1 (Fig. 1b).
Figure 1.
PPAR-γ but not PPAR-α agonists inhibit cell surface expression of ICAM-1 on RSV-infected A549 cells. (a) Cells were pretreated with ciglitazone (5–50 µm), troglitazone (5–50 µm), 15d-PGJ2 (5–20 µm) or medium (control) for 30 min. (b) Cells were pretreated with Fmoc-Leu (50–100 µm) or vehicle (DMSO; 0·1–0·3% (v/v)) for 30 min. Thereafter, cells were infected with RSV (m.o.i. = 3) and cultured in the presence of the agonists for 36 hr. The cell surface amount of ICAM-1 was determined by FACS analysis. In case error bars are not visible they are smaller than symbols shown. Results are means ± SEM (n = 4). Significant differences from ICAM-1 expression on RSV-infected cells (MFI = 1186 ± 91) are indicated by *P < 0·01.
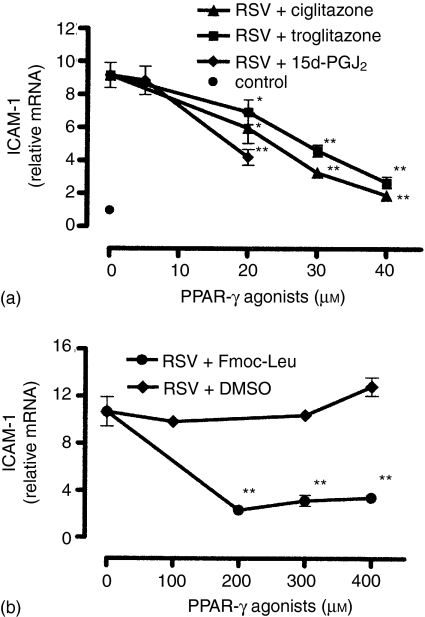
The up-regulation of ICAM-1 on RSV-infected A549 cells correlates with an enhanced ICAM-1 gene expression resulting in an elevated cellular amount of ICAM-1 mRNA.28 To assess whether the observed down-regulation of ICAM-1 by PPAR-γ agonists might be at least partly mediated via an altered gene expression level, we analysed the cellular amount of ICAM-1 mRNA by real-time RT–PCR. Preliminary experiments revealed an optimal ICAM-1 mRNA amount in RSV-infected cells 24 hr postinfection (data not shown). Therefore, the cellular ICAM-1 mRNA amount of RSV-infected cells cultured in the presence of agonists was determined after 24 hr. As shown in Fig. 2(a, b), the reduced cell surface expression of ICAM-1 on RSV-infected cells, observed following pretreatment with PPAR-γ agonists, was associated with a diminished cellular amount of ICAM-1 mRNA. All PPAR-γ agonists under study reduced the steady state ICAM-1 mRNA amount dose-dependently. Comparable concentrations of the agonists were needed for both inhibition of ICAM-1 cell surface expression and the ICAM-1 mRNA level. Again, DMSO had no impact on the analysed ICAM-1 mRNA level (Fig. 2b).
Figure 2.
PPAR-γ agonists inhibit ICAM-1 mRNA expression in RSV-infected A549 cells. The cellular amount of mRNA encoding for ICAM-1 was quantitatively determined by real-time RT–PCR 24 hr postinfection. (a) Cells were pretreated with ciglitazone (20–40 µm), troglitazone (20–40 µm), 15d-PGJ2 (5–20 µm) or medium (control) for 30 min. (b) Cells were pretreated with Fmoc-Leu (200–400 µm) or vehicle (DMSO, 0·1–0·3% (v/v)) for 30 min. Then, cells were infected with RSV (m.o.i. = 3) and cultured in the presence of the agonists for 24 hr. Results are means ± SEM (n = 6); *P < 0·05 versus non-treated RSV-infected cells; **P < 0·01 versus non-treated RSV-infected cells.
Next, we asked whether PPAR-γ agonists are also able to inhibit the RSV-induced ICAM-1 expression on NHBE cells. For this purpose, NHBE cells were treated with ciglitazone, troglitazone, and the natural ligand 15d-PGJ2 in an identical manner as A549 cells. NHBE cells, prepared from several donors, showed remarkably different ICAM-1 expression levels following RSV-infection. In order to be able to compare the data, we normalized them to the expression level of RSV-infected NHBE cells, which was adjusted to 100%. Similar to A549 cells, we observed that the PPAR-γ agonists ciglitazone, troglitazone, and 15d-PGJ2, respectively, markedly reduced the RSV-induced cell surface expression of ICAM-1 on NHBE cells (Fig. 3).
Figure 3.
PPAR-γ agonists inhibit RSV-induced cell surface expression of ICAM-1 on NHBE cells. The cells were pretreated with agonists (ciglitazone (20 µm), troglitazone (20 µm), 15d-PGJ2 (20 µm)) for 30 min, infected with RSV (m.o.i. = 3), and cultured in the presence of the agonists for another 36 hr. The expression of ICAM-1 was determined by FACS analysis. The mean fluorescent intensity of ICAM-1 on RSV-infected NHBE cells was designated as 100% and data were calculated as a percentage of this value. The bars represent normalized mean fluorescent intensity with standard deviations for three independent experiments; *P < 0·01 versus non-treated, RSV-infected cells.
PPAR-γ agonists inhibit the RSV-induced activation of NF-κB
It has been shown that the activation of the transcription factor NF-κB plays an important role in the RSV-induced synthesis of ICAM-1.28 Further evidence accumulated that activated PPAR-γ may interfere with the activity of NF-κB.29,30 Thus, we analysed whether the observed anti-inflammatory effects of PPAR-γ agonists might be associated with a down-regulation of NF-κB activity. EMSAs were performed using nuclear extracts from A549 cells and radiolabelled oligonucleotide probes corresponding to a consensus NF-κB binding site. The lung epithelial cells were incubated for 20 hr because NF-κB is maximally activated by RSV at 20–24 hr postinfection. The nuclear extracts were prepared from RSV-infected A549 cells cultured with or without PPAR-γ agonists. The EMSAs demonstrated that the PPAR-γ ligands ciglitazone (Fig. 4a, b), troglitazone, Fmoc-Leu, and the natural ligand 15d-PGJ2 (Fig. 4b) markedly reduced the amount of gel-retarded NF-κB complexes in RSV-infected A549 cells. In contrast, the vehicle control DMSO had no impact on the NF-κB binding activity. Supershift EMSAs performed with p65- and p50-specific antibodies demonstrated that the binding complex consisted of p65 heterodimers, mainly p65/p50 dimers (Fig. 4a). Experiments with both antibodies resulted in strong supershift signals. However, application of the p50-specific antibody could not completely shift the specific binding complex indicating that other Rel proteins might also interact with p65 (Fig. 4a). Although no supershift signal was detectable using the c-Rel-specific antibody, a reduction of the signal intensity of the binding complex was notable, which is indicative for a supershift without supershift signal. No such effect was seen in a supershift EMSA using the RelB-specific antibody (Fig. 4a). Specificity of complex formation was analysed by the addition of unlabelled oligonucleotides (Fig. 4a, lane 4, cold probe). To substantiate our obtained NF-κB data we determined the binding activity of the NF-κB heterodimer p65/p50 by means of a commercially available quantitative NF-κB binding assay. As shown, the RSV-infection led to a significantly increased binding activity of p65 and p50 containing NF-κB heterodimers (Fig. 5). Similar to the obtained EMSA data we observed a down-regulation of NF-κB p65 and p50 binding activity in A549 cells pretreated with the PPAR-γ agonists under study and cultured for 24 hr. Similar results were obtained after 20 hr postinfection (data not shown). Both, p65 (Fig. 5a) and p50 (Fig. 5b) showed a comparable reduction of DNA-binding activity in RSV-infected cells pretreated with the agonists suggesting that especially p65/p50 heterodimers activated in RSV-infected epithelial cells are down-regulated by PPAR-γ agonists. For comparison, the p65 and p50 binding activities in nuclear extracts prepared from IL-1α-activated A549 cells are shown (Fig. 5a,b).
Figure 4.
PPAR-γ agonists inhibit RSV-induced activation of NF-κB. (a) EMSA of untreated A549 cells (lanes 1, 2, 4–8) or cells pretreated with ciglitazone (20 µm) for 30 min (lane 3). Non-infected cells (lane 1) and RSV-infected cells (m.o.i. = 3) (lanes 2–8) were cultured for 20 hr. Specificity was determined by addition of 25 ng unlabelled NF-κB oligonucleotide (cold probe, lane 4). Supershift assays were performed with antip65/(lane 5), anti-cRel (lane 6), anti-RelB (lane 7), and anti-p50 (lane 8) antibodies. (b) Nuclear extracts were prepared from untreated A549 cells (lanes 1, 2) or cells pretreated with ciglitazone (20 µm) (lane 3), troglitazone (20 µm) (lane 4), 15d-PGJ2 (10 µm) (lane 5), Fmoc-Leu (100 µm) (lane 6), or DMSO (0·2% (v/v)) (lane 7) for 30 min. Cells were infected with RSV (m.o.i. = 3) (lanes 2–7) and cultured for 20 hr. The arrows show NF-κB binding and supershifted bands. The bottom panels show laser densitometry analysis of the specific signals of p65/p50 heterodimers. Representative results out of two independent experiments are shown.
Figure 5.
PPAR-γ agonists inhibit RSV-induced activation of NF-κB. The binding activity of the NF-κB subunits p65 (Rel A) and p50 (NF-κB1) in nuclear extracts prepared from A549 cells was quantitatively determined by using the p65- and p50-specific Trans-AM™ transcription factor assay kit. Cells were pretreated with the agonists for 30 min, infected with RSV (m.o.i. = 3), and cultured in the presence of the agonists for another 24 hr. For control, cells were stimulated with IL-1α (20 ng/ml) alone. Prepared nuclear extracts were assayed for p65- (a) and p50 binding activity (b) Data are mean values of two independent experiments.
PPAR-γ agonists diminish the RSV-induced adhesion of U937 cells to A549 cells
A cell adhesion assay was performed to investigate whether the PPAR-γ agonist-mediated down-regulation of RSV-induced ICAM-1 expression on A549 cells might affect the adhesion of cocultured monocytic cells. For this purpose, RSV-infected A549 cell monolayers cultured in the presence or absence of agonists were cocultured with PMA-activated U937 cells. As shown in Fig. 6(a), the infection of A549 cells with RSV strongly increased the adhesion of U937 cells. However, RSV-infected A549 cell monolayers cultured in the presence of ciglitazone, troglitazone, Fmoc-Leu, and 15d-PGJ2, respectively, showed a markedly diminished adhesion rate of U937 cells. The vehicle control DMSO, which did not modulate the RSV-induced ICAM-1 expression, had no impact on cell adhesion. To demonstrate that the increased adhesion of U937 cells to the RSV-infected epithelial cell monolayer was functionally mediated by the up-regulated ICAM-1 expression, U937 cells were cocultured in the presence of blocking mAbs specific for ICAM-1, CD11b, CD11a, CD18 or isotype control IgG antibodies, respectively. As shown in Fig. 6(b), preincubation of the monolayers with specific ICAM-1 blocking mAbs diminished the adhesion rate of U937 cells. Additionally, blocking the β2 integrins expressed on U937 cells by means of neutralizing antibodies specific for CD11a, CD11b, and CD18, led to a similar reduction of U937 cell adhesion verifying that ICAM-1/β2 integrin interactions are mainly responsible for the adhesion of U937 cells to the RSV-infected A549 cell monolayers.
Figure 6.
PPAR-γ agonists inhibit the adhesion of monocytic cells (U937) to RSV-infected epithelial cell monolayers (A549). (a) Following RSV infection (m.o.i. = 1) the monolayers were incubated with the following PPAR-γ agonists: ciglitazone (20 µm), troglitazone (20 µm), Fmoc-Leu (200 µm),15d-PGJ2 (10 µm), DMSO (0·1% v/v)), or medium for 36 hr. (b) Role of ICAM-1 and β2 integrins in the adhesion process of U937 cells to A549 cell monolayers. Cells were preincubated with blocking antibodies specific for ICAM-1 (20 µg/ml), CD11a (20 µg/ml), CD11b (20 µg/ml), CD18 (20 µg/ml) or IgG isotype controls (20 µg/ml IgG1 + 20 µg/ml IgG2a). The adhesion of calcein-AM-labelled U937 cells to A549 monolayers was determined by measuring cell-bound fluorescence. The fluorescence signal of U937 cells adherent to RSV-infected monolayer was adjusted to 100% and data are expressed as percent of this value. Results are means ± SEM (n = 3, performed in quadruplicate); *P < 0·01 versus non-treated, RSV-infected cells.
PPAR-γ agonist-diminished release of IL-1α and TNF-α from RSV-infected A549 cells is associated with down-regulation of ICAM-1 and leucocyte adherence
It is known that the up-regulation of ICAM-1 on RSV-infected epithelial cells is partly mediated by IL-1α released from infected epithelial cells themselves.31 Moreover, the proinflammatory cytokine TNF-α, which is also able to up-regulate the ICAM-1 expression on A549 cells,7 is released from RSV-infected epithelial cells. Quite recently, we observed that PPAR-γ agonists significantly inhibited the RSV-induced release of IL-1α (300 ± 29 pg/ml), TNF-α (157 ± 41 pg/ml), and IL-6 (150 ± 18 pg/ml), respectively, from human A549 epithelial cells 36 hr postinfection.18
To analyse whether this cytokine-inhibiting potential of the PPAR-γ agonists might be associated with our observed ICAM-1-reducing activity, we determined the ICAM-1 expression pattern dependent on IL-1α, TNF-α, and IL-6 secreted by the RSV-infected epithelial cells. As can be seen from our cytokine blocking experiments presented in Fig. 7(a), both IL-1α and TNF-α released from the infected epithelial cells possess a comparable ICAM-1-inducing capacity. Most intriguingly, both cytokines increased the ICAM-1 expression on RSV-infected cells in a synergistic manner. In contrast, IL-6 also secreted in an autocrine manner from RSV-infected cells had no impact on the ICAM-1 cell surface expression. The performed adhesion experiments verified that the down-regulated expression of ICAM-1 on RSV-infected cells cultured in the presence of the IL-1α- and TNF-α neutralizing antibodies was associated with a similar reduced adhesion rate of cocultured monocytic effector cells (Fig. 7b).
Figure 7.
ICAM-1 expression on A549 cells and adhesion of leucocytic cells is dependent on autocrine released IL-1α and TNF-α. Cells were infected with RSV (m.o.i. = 3) and cultured for 36 hr in the presence of blocking antibodies specific for IL-1α (10 µg/ml), TNF-α (10 µg/ml), IL-6 (10 µg/ml), IL-1α + TNF-α (10 + 10 µg/ml), IgG isotype controls (10 µg/ml IgG1 + 10 µg/ml IgG2a) or medium alone. (a) The cell surface amount of ICAM-1 was determined by FACS analysis. Results are means ± SEM (n = 3). Significant differences from ICAM-1 expression on RSV-infected cells are indicated by *P < 0·01. (b) The adhesion of calcein-AM-labelled U937 cells to A549 monolayers was determined by measuring cell-bound fluorescence. The fluorescence signal of U937 cells adherent to RSV-infected monolayer was adjusted to 100% and data are expressed as percentage of this value. Results are means ± SEM (n = 3, performed in quadruplicate); *P < 0·01 versus non-treated, RSV-infected cells.
Discussion
In the course of RSV-induced bronchiolitis and pneumonia the inflammatory host response as well as direct viral cytopathic effects contribute to the disease process. Therefore, efforts directed to attenuate the RSV-induced inflammation might be of therapeutical value.
Our in vitro data presented herein supply evidence that the PPAR-γ agonists ciglitazone, troglitazone, 15d-PGJ2, and Fmoc-Leu, repectively, are able to significantly reduce the RSV-induced up-regulation of ICAM-1 on human lung epithelial cells. We observed a reduced ICAM-1/β2 integrin-dependent adhesion of monocytic cells (U937) to RSV-infected epithelial cell monolayers treated with the PPAR-γ agonists under study.
Immune effector cells (PMN, eosinophils, NK cells and monocytes) are chemotactically recruited into the broncho-alveolar lumen as well as lung tissue of the RSV-infected lung.4,5 Subsequently, a variety of proinflammatory mediators are released by these cells into the lumen of the RSV-infected lung.32–34 The close cell–cell contact between immune effector cells and RSV-infected lung epithelial cells is mainly mediated by an increased ICAM-1 expression on the virus-infected epithelial cell.35 Thereafter, these adherence-activated effector cells secrete a variety of prestored as well as newly generated cytotoxins and inflammatory mediators into the microenvironment of the RSV-infected lung epithelium. Because most compounds are short-lived and are active in a dose-dependent manner, they damage the RSV-infected epithelium most effectively when released directly onto the epithelial cell surface.36 Therefore, our observation that treatment of RSV-infected epithelial cells with PPAR-γ agonists led to a lowered adhesion of monocytic cells suggests that immune-mediated cytotoxicity should be reduced. In addition, the RSV-induced expression of major histocompatibility complex (MHC) class I on A549- and NHBE cells was also significantly diminished by PPAR-γ agonists (data not shown) suggesting that the cytolytic activity of NK cells might not be impaired by the viral induced MHC class I cell surface expression. However, we were not able to demonstrate a reduced innate immune response mediated cytotoxicity in our in vitro infection model because PPAR-γ agonists by themselves had a protective effect on RSV-infected lung epithelial cells by inhibiting the replication of RSV.37 Taken together, we assume that pretreatment with PPAR-γ agonists protects the RSV-infected epithelium by both down-regulation of cell-mediated cytotoxicity and inhibition of viral replication. Moreover, PPAR-γ agonists might directly interfere with the activation state of the recruited immune effector cells.14,38,39 Therefore, the combined protective effect of PPAR-γ agonists remains to be determined in a suitable in vivo RSV-infection model.40
The transcription factor NF-κB is one of the pivotal regulators of proinflammatory gene expression, i.e. it induces the transcription of proinflammatory cytokines, chemokines and adhesion molecules.41 NF-κB is a collective name for a family of dimeric transcription factors composed of five Rel proteins. In this study, we confirm that RSV infection of human lung epihelial cells leads predominantly to an activation of NF-κB complexes consisting of RelA/NF-κB1 (p65/p50).25,28 All PPAR-γ agonists under study counter-regulated the RSV-induced binding activity of RelA/NF-κB1 (p65/p50) heterodimers and reduced the cellular ICAM-1 mRNA amount. Because the RSV-induced ICAM-1 expression is highly dependent on NF-κB activation these results suggest that the decreased expression of ICAM-1 might be at least partly mediated at the gene transcription level.
Quite recently we reported that PPAR-γ agonists inhibited the RSV-induced release of IL-1α and TNF-α from human lung epithelial cells.18 It is known that the up-regulation of ICAM-1 on RSV-infected human lung epithelial cells is mainly mediated by IL-1α released by recruited immune effector cells or by the RSV-infected epithelial cells themselves.7,31 Our data showing that the blockade of IL-1α released from RSV-infected lung epithelial cells leads to a significantly reduced cell surface ICAM-1 expression pattern are in line with these published results. Intriguingly, we observed that the pro-inflammatory cytokine TNF-α released from the RSV-infected epithelial cell possess a similar ICAM-1-inducing capacity. Both cytokines acted synergistically in the up-regulation of ICAM-1 on RSV-infected A549 epithelial cells. Therefore, our previous published data that the PPAR-γ ligands inhibited the release of IL-1α and TNF-α from RSV-infected A549 cells18 suggest that the diminished ICAM-1 expression following treatment with PPAR-γ agonists is mainly mediated by the interruption of these autocrine feedback loops.
Evidence accumulated that the exogenous addition of PPAR-γ ligands dampens inflammatory processes in the lung.42,43 Genovese and coworkers reported that neutrophil infiltration of the lung was significantly reduced by PPAR-γ agonists in the course of bleomycin-induced mouse lung injury.44 Also, in a murine in vivo model of LPS-induced airway inflammation it was shown by Birrell and colleagues that the TZD rosiglitazone but not dexamethasone reduced the neutrophil number in the lung tissue when administered after the LPS insult.45 Interestingly, when we added PPAR-γ agonists directly or 12 hr post-RSV-infection we still observed a down-regulation of ICAM-1 on RSV-infected A549 cells similar to the data presented in this study (data not shown). Therefore, one may hypothesize that PPAR-γ agonists have an impact on the RSV-induced inflammatory lung response also in a more clinical setting, i.e. when agonists are applied following infection. We conclude that PPAR-γ agonists might have therapeutical value in down-regulating the inflammatory response in the course of severe primary RSV infection.
Acknowledgments
The authors wish to thank Ms. Bettina Polte for her excellent technical assistance.
Abbreviations
- 15d-PGJ2
15-deoxy-Δ12,14-prostaglandin J2
- EMSA
electrophoresis mobility shifting assay
- ICAM-1
intercellular adhesion molecule-1
- m.o.i.
multiplicity of infection
- NHBE
normal human bronchial epithelial
- RT–PCR
reverse transcription–polymerase chain reaction
- PGD2
prostaglandin D2
- PMN
polymorphonuclear neutrophils
- PPAR
peroxisome proliferator-activated receptor
- RSV
respiratory syncytial virus
- TZD
thiazolidinedione
References
- 1.Hall CB, McCarthy CA. Respiratory syncytial virus. In: Mandell GL, Benett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. Philadelphia: Churchill Livingstone; 2000. pp. 1782–801. [Google Scholar]
- 2.Kimpen JLL. Management of respiratory syncytial virus infection. Curr Opin Infect Dis. 2001;14:323–8. doi: 10.1097/00001432-200106000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Kneyber MCJ, Moll HA, de Groot R. Treatment and prevention of respiratory syncytial virus infection. Eur J Pediatr. 2000;159:399–411. doi: 10.1007/s004310051296. [DOI] [PubMed] [Google Scholar]
- 4.Aherne W, Bird T, Court S, Gardner P, McQuillin J. Pathological changes in virus infections of the lower respiratory tract in children. J Clin Pathol. 1970;23:7–18. doi: 10.1136/jcp.23.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Everard ML, Swarbrick A, Wrightham M, McIntyre J, Dunkley C, James PD, Sewell HF, Milner AD. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch Dis Child. 1994;7:428–32. doi: 10.1136/adc.71.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garofalo RP, Haeberle H. Epithelial regulation of innate immunity to respiratory syncytial virus. Am J Respir Cell Mol Biol. 2000;23:581–5. doi: 10.1165/ajrcmb.23.5.f204. [DOI] [PubMed] [Google Scholar]
- 7.Arnold R, König W. ICAM-1 expression and low molecular weight G protein activation of human bronchial epithelial cells (A549) infected with RSV. J Leukoc Biol. 1996;60:766–71. doi: 10.1002/jlb.60.6.766. [DOI] [PubMed] [Google Scholar]
- 8.Wang SZ, Forsyth KD. The interaction of neutrophils with respiratory epithelial cells in viral infection. Respirology. 2000;5:1–9. doi: 10.1046/j.1440-1843.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- 9.Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;140:889–95. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee CH, Olson P, Evans RM. Minireview: Lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology. 2003;144:2201–3. doi: 10.1210/en.2003-0288. [DOI] [PubMed] [Google Scholar]
- 11.Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:1–7. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 12.Daynes RA, Jones DJ. Emerging roles of PPARs in inflammation and immunity. Nat Rev. 2002;2:748–59. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 13.Clark RB. The role of PPARs in inflammation and immunity. J Leukoc Biol. 2002;71:388–400. [PubMed] [Google Scholar]
- 14.Jiang C, Ting AT, Seed B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–6. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 15.Marx N, Mach F, Sauty A, et al. Peroxisome-proliferator-activated receptor-γ activators inhibit IFN-γ-induced expression of the T cell-active CXC chemokines IP-10, Mig, and I-TAC in human endothelial cells. J Immunol. 2000;164:6503–8. doi: 10.4049/jimmunol.164.12.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunard R, Eto Y, Muljadi JT, Glass CK, Kelly CJ, Ricote M. Repression of IFN-γ expression by peroxisome-proliferator-activated receptor γ. J Immunol. 2004;172:7530–6. doi: 10.4049/jimmunol.172.12.7530. [DOI] [PubMed] [Google Scholar]
- 17.Wang ACC, Dai X, Luu B, Conrad DJ. Peroxisome proliferator-activated receptor-γ regulates airway epithelial cell activation. Am J Respir Cell Mol Biol. 2001;24:688–93. doi: 10.1165/ajrcmb.24.6.4376. [DOI] [PubMed] [Google Scholar]
- 18.Arnold R, König W. Peroxisome proliferator-activated receptor-γ agonists inhibit the release of proinflammatory cytokines from RSV-infected epithelial cells. Virology. 2006;346:427–39. doi: 10.1016/j.virol.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Skolnik PR, Rabbi MF, Mathys JM, Greenberg AS. Stimulation of peroxisome proliferator-activated receptors alpha and gamma blocks HIV-1 replication and TNFalpha production in acutely infected primary blood cells, chronically infected U1 cells, and alveolar macrophages from HIV-infected subjects. J Acquir Immune Defic Syndr. 2002;31:1–10. doi: 10.1097/00126334-200209010-00001. [DOI] [PubMed] [Google Scholar]
- 20.Cuzzocrea S, Pisano B, Dugo L, et al. Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-γ, reduces acute inflammation. Eur J Pharmacol. 2004;483:79–93. doi: 10.1016/j.ejphar.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 21.Rocchi S, Picard F, Vamecq J, et al. A unique PPARγ ligand with potent insulin-sensitizing yet weak adipogenic activity. Mol Cell. 2001;8:737–47. doi: 10.1016/s1097-2765(01)00353-7. [DOI] [PubMed] [Google Scholar]
- 22.Ueba O. Respiratory syncytial virus. I. Concentration and purification of the infectious virus. Acta Med Okayama. 1978;32:265–72. [PubMed] [Google Scholar]
- 23.Cannon MJ. Microplaque immunoperoxidase detection of infectious respiratory syncytial virus in the lungs of infected mice. J Virol Meth. 1987;16:293–301. doi: 10.1016/0166-0934(87)90014-0. [DOI] [PubMed] [Google Scholar]
- 24.Arnold R, König W. Respiratory syncytial virus infection of human lung endothelial cells enhances selectively ICAM-1 expression. J Immunol. 2005;174:7359–67. doi: 10.4049/jimmunol.174.11.7359. [DOI] [PubMed] [Google Scholar]
- 25.Arnold R, König B, Werchau H, König W. RSV deficient in soluble G protein induced an increased proinflammatory response in human lung epithelial cells. Virology. 2004;330:384–97. doi: 10.1016/j.virol.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Neumann M, Grieshammer T, Chuvpilo S, Kneitz B, Lohoff M, Schimpl A, Franza BR, Jr, Serfling E. RelA/p65 is a molecular target for the immunosuppressive action of protein kinase A. EMBO J. 1995;14:1991–2004. doi: 10.1002/j.1460-2075.1995.tb07191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marienfeld R, Berberich-Siebelt F, Berberich I, Denk A, Serfling E, Neumann M. Signal-specific and phosphorylation-dependent RelB degradation. a potential mechanism of NF-κB control. Oncogene. 2001;20:8142–7. doi: 10.1038/sj.onc.1204884. [DOI] [PubMed] [Google Scholar]
- 28.Chini BA, Fiedler MA, Milligan L, Hopkins T, Stark JM. Essential roles of NF-κB and C/EBP in the regulation of intercellular adhesion molecule-1 after respiratory syncytial virus infection of human respiratory epithelial cell cultures. J Virol. 1998;72:1623–6. doi: 10.1128/jvi.72.2.1623-1626.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruan H, Pownall HJ, Lodish HF. Troglitazone antagonizes tumor necrosis factor-α- induced reprogramming of adipocyte gene expression by inhibiting the transcriptional regulatory functions of NF-κB. J Biol Chem. 2003;278:28181–92. doi: 10.1074/jbc.M303141200. [DOI] [PubMed] [Google Scholar]
- 30.Straus DS, Pascual G, Li M, et al. 15-Deoxy-Δ12,14-prostaglandin J2 inhibits multiple steps in the NF-κB signaling pathway. Proc Natl Acad Sci USA. 2000;97:4844–9. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel JA, Kunimoto M, Sim TC, et al. Interleukin-1α mediates the enhanced expression of intercellular adhesion molecule-1 in pulmonary epithelial cells infected with respiratory syncytial virus. Am J Respir Cell Mol Biol. 1995;13:602–9. doi: 10.1165/ajrcmb.13.5.7576697. [DOI] [PubMed] [Google Scholar]
- 32.Jaovisidha P, Peeples ME, Brees AA, Carpenter LR, Moy JN. Respiratory syncytial virus stimulates neutrophil degranulation and chemokine release. J Immunol. 1999;163:2816–20. [PubMed] [Google Scholar]
- 33.Abu-Harb M, Bell F, Finn A, Rao AW, Nixon L, Shale D, Everard ML. IL-8 and neutrophil elastase levels in the respiratory tract of infants with RSV bronchiolitis. Eur Respir J. 1999;14:139–43. doi: 10.1034/j.1399-3003.1999.14a23.x. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg HF, Domachowske JB. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J Leukoc Biol. 2001;70:691–8. [PubMed] [Google Scholar]
- 35.Stark JM, Godding V, Sedgwick JB, Busse WW. Respiratory syncytial virus infection enhances neutrophil and eosinophil adhesion to cultured respiratory epithelial cells. J Immunol. 1996;156:4774–82. [PubMed] [Google Scholar]
- 36.Wang SZ, Xu H, Wraith A, Bowden JJ, Alpers JH, Forsyth KD. Neutrophils induce damage to respiratory epithelial cells infected with respiratory syncytial virus. Eur Respir J. 1998;12:612–8. doi: 10.1183/09031936.98.12030612. [DOI] [PubMed] [Google Scholar]
- 37.Arnold R, König W. Peroxisome proliferator-activated receptor-γ agonists inhibit the replication of respiratory syncytial virus (RSV) in human lung epithelial cells. Virology. 2006;350:335–46. doi: 10.1016/j.virol.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Imamoto E, Yoshida N, Uchiyama K, et al. Inhibitory effect of pioglitazone on expression of adhesion molecules on neutrophils and endothelial cells. Biofactors. 2004;20:37–47. doi: 10.1002/biof.5520200104. [DOI] [PubMed] [Google Scholar]
- 39.Woerly G, Honda K, Loyens M, Papin JP, Auwerx J, Staels B, Capron M, Dombrowicz D. Peroxisome-proliferator-activated receptors alpha and gamma downregulate allergic inflammation and eosinophil activation. J Exp Med. 2003;198:411–21. doi: 10.1084/jem.20021384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jafri HS, Chávez-Bueno S, Mejías A, et al. Respiratory syncytial virus induces pneumonia, cytokine responses, airway obstruction, and chronic inflammatory infiltrates associated with long-term airway hyperresponsiveness in mice. J Inf Dis. 2004;189:1856–65. doi: 10.1086/386372. [DOI] [PubMed] [Google Scholar]
- 41.Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Standiford TJ, Keshamouni VG, Reddy RC. Peroxisome proliferator-activated receptor-γ as a regulator of lung inflammation and repair. Proc Am Thorac Soc. 2005;2:226–31. doi: 10.1513/pats.200501-010AC. [DOI] [PubMed] [Google Scholar]
- 43.Denning GM, Stoll LL. Peroxisome proliferator-activated receptors: potential therapeutic targets in lung disease? Pediatr Pulmonol. 2006;41:23–34. doi: 10.1002/ppul.20338. [DOI] [PubMed] [Google Scholar]
- 44.Genovese T, Cuzzocrea S, Di Paola Mazzon E, et al. Effect of rosiglitazone and deoxy-Δ12,14-prostaglandin J2 on bleomycin-induced lung injury. Eur Respir J. 2005;25:225–34. doi: 10.1183/09031936.05.00049704. [DOI] [PubMed] [Google Scholar]
- 45.Birrell MA, Patel HJ, McCluskie K, Wong S, Leonard T, Yacoub MH, Belvisi MG. PPAR-γ agonists as therapy for diseases involving airway neutrophilia. Eur Respir J. 2004;24:18–23. doi: 10.1183/09031936.04.00098303. [DOI] [PubMed] [Google Scholar]