Abstract
Cationic liposomes are being used increasingly as efficient adjuvants for subunit vaccines but their precise mechanism of action is still unknown. Here, we investigated the adjuvant mechanism of cationic liposomes based on the synthetic amphiphile dimethyldioctadecylammonium (DDA). The liposomes did not have an effect on the maturation of murine bone-marrow-derived dendritic cells (BM-DCs) related to the surface expression of major histocompatibility complex (MHC) class II, CD40, CD80 and CD86. We found that ovalbumin (OVA) readily associated with the liposomes (> 90%) when mixed in equal concentrations. This efficient adsorption onto the liposomes led to an enhanced uptake of OVA by BM-DCs as assessed by flow cytometry and confocal fluorescence laser-scanning microscopy. This was an active process, which was arrested at 4° and by an inhibitor of actin-dependent endocytosis, cytochalasin D. In vivo studies confirmed the observed effect because adsorption of OVA onto DDA liposomes enhanced the uptake of the antigen by peritoneal exudate cells after intraperitoneal injection. The liposomes targeted antigen preferentially to antigen-presenting cells because we only observed a minimal uptake by T cells in mixed splenocyte cultures. The adsorption of antigen onto the liposomes increased the efficiency of antigen presentation more than 100 times in a responder assay with MHC class II-restricted OVA-specific T-cell receptor transgenic DO11.10 T cells. Our data therefore suggest that the primary adjuvant mechanism of cationic DDA liposomes is to target the cell membrane of antigen-presenting cells, which subsequently leads to enhanced uptake and presentation of antigen.
Keywords: adjuvants, antigen presentation, dendritic cells
Introduction
Cationic liposomes have been used extensively in both drug delivery and vaccine research. However, cationic liposomes can be based on different types of lipids, which may vary in their number of cationic charges and have different numbers and compositions of fatty acid chains which may also vary in their degree of saturation.1,2 The characteristics of cationic liposomes are hence highly dependent on the type of lipid on which they are based.3 In addition, cationic liposomes can be composed of mixtures of lipids, often including neutral helper lipids such as dioleoylphosphoethanolamine or cholesterol. Consequently, the literature reports contradictory statements regarding the immunogenicity of cationic liposomes and a general consensus regarding their precise mechanism of action and their effect on the immune system is lacking.4–7 Furthermore, cationic liposome carriers are used experimentally to enhance transfection efficiency in gene therapy but the underlying mechanism is, also in this case, not fully elucidated.1,8 It was originally suggested that it involved a passive process whereby cationic liposomes bind to the cell surface and fuse directly with the cell membrane releasing the entrapped nucleic acids into the cytosol.1,9,10 However, more recent evidence points to an active process whereby the liposomes are first taken up by endocytosis followed by disruption or fusion with internal cellular membranes.11–15
As part of subunit vaccines, liposomes can be used as delivery vehicles for antigen. This is based on the assumption that liposomes are able to deliver the antigen to antigen-presenting cells (APCs) and thus enhance antigen-specific immune responses.16 In contrast to the strict need for cytosolic delivery of nucleic acids in gene therapy, subunit vaccine antigens have to be degraded in the endosomal compartment to be presented in the context of major histocompatibility complex (MHC) class II molecules on the surfaces of APCs.17 In this context, dendritic cells (DCs) are important for their ability to efficiently stimulate primary immune responses and to establish immunological memory.18 In their immature state, DCs reside in the periphery where they continuously sample proteins from their surroundings. Upon maturation DCs up-regulate functions associated with antigen processing, antigen presentation and T-cell activation including the increased expression of MHC class II and the costimulatory molecules CD40, CD80 and CD86.17 Maturation also induces the surface expression of homing receptors, such as C-C chemokine receptor 7 (CCR7, CD197), which will guide the DCs to regional lymphoid organs where they can interact with and activate naive antigen-specific T cells.19 The ability to prime T cells, and thus initiate specific immune responses, makes DCs obvious targets for subunit vaccines.
The cationic liposome-forming synthetic amphiphile dimethyldioctadecylammonium (DDA) is known to induce cell-mediated immunity and delayed-type hypersensitivity.20 Liposomes based on DDA have previously been evaluated as carriers for drugs,21 as antimicrobial agents22 and as adjuvants for a range of vaccines for both parenteral and mucosal delivery.20,23–28 In recent years they have been used as part of more complex adjuvant systems for experimental subunit vaccines.29–32 In addition, DDA-based stable liposome formulations, including the immunostimulatory glycolipid trehalose dibehenate, have been improved pharmaceutically33 and are now due to enter phase I clinical trials (Michael Theisen, personal communication). Here, we have evaluated the effect of DDA-based cationic liposomes and we suggest that their primary adjuvant mechanism is to target antigen to the membrane of APCs and to induce the uptake and increased presentation of antigen.
Materials and methods
Animals
Female BALB/c mice were purchased from Harlan (Horst, the Netherlands), and female OVA-specific T-cell receptor transgenic DO11.10 mice were purchased from Charles River Laboratories (Kisslegg, Germany). The animal experiments were approved by the Danish Council for Animal Experiments.
Reagents
All reagents and antibodies were purchased from the manufacturers indicated below: AbD Serotec (Hamar, Norway): rat anti-mouse F4/80-phycoerythrin (PE); Amersham Biosciences (GE Healthcare, Hillerød, Denmark): [3H]thymidine; Avanti Polar Lipids (Alabaster, AL): dimethyldioctadecylammonium bromide (DDA); BD Biosciences Pharmingen (Brøndby, Denmark): anti-CD3-allophycocyanin, anti-CD4-PE, anti-CD11c-allophycocyanin/PE, purified anti-CD16/CD32, anti-CD19-allophycocyanin, anti-CD40-fluorescein isothiocyanate (FITC), anti-CD80-FITC, anti-CD86-FITC, anti-I-A/I-E-FITC, purified anti-interferon-γ (IFN-γ), anti-IFN-γ-biotin, rat-immunoglobulin G2κ (IgG2aκ)-FITC, hamster-IgG2κ-FITC; Bie & Berntsen (Rødovre, Denmark): 10% formalin buffer; CALTAG Laboratories (San Francisco, CA): anti-DO11.10 T-cell receptor-allophycocyanin (KJ1-26); DakoCytomation (Glostrup, Denmark): fluorescent mounting medium; Invitrogen/Molecular Probes (Taastrup, Denmark): fluorescein-conjugated ovalbumin (OVA), AlexaFluor488-conjugated OVA, goat anti-rat AlexaFluor568, TO-PRO-3 iodide; Kem-En-Tec (Taastrup, Denmark): TMB Plus Ready-to-use; Leo Pharma (Ballerup, Denmark): Heparin; PeproTech (London, UK): recombinant granulocyte–macrophage colony-stimulating factor (GM-CSF); Sigma-Aldrich (Brøndby, Denmark): chicken egg OVA, trypan blue, tetramethylrhodamine B isothiocyanate-conjugated phalloidin, cytochalasin D; Vector Laboratories (Peterborough, UK): Vectashield mounting medium; Zymed (San Francisco, CA): horseradish peroxidase-conjugated streptavidin.
Buffers
Complete RPMI was used, which was RPMI-1640 (Gibco Invitrogen, Taastrup, Denmark) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 5 × 10−6 mβ-mercaptoethanol, 1% (v/v) penicillin–streptomycin, 1% sodium pyruvate, 1 mm l-glutamine, 10 mm HEPES and 100 μg/ml gentamicin. Ethanoic acid buffer comprised 0·2 m ethanoic acid, 0·5 m NaCl in sterile distilled water.
Preparation of liposomes
DDA liposomes were prepared using the aqueous heat method. DDA was suspended in sterile distilled water at 2·5–5 mg/ml and heated to 80° for 20 min with continuous stirring. For incorporation of antigen, OVA was mixed with DDA liposomes in sterile distilled water and left for 1 hr at room temperature with intermittent mixing.
Incorporation of antigen into liposomes
Mixtures of DDA and/or OVA were ultra-centrifuged at 100 000 g for 1 hr at room temperature to pellet the liposomes. The supernatant was removed and filtered through a 0·2 μm polyvinylidene fluoride filter (Millipore, Hundested, Denmark) to avoid contamination with pellet remnants. The pellet was redissolved in the original volume of sterile distilled water by heating to 80° for 20 min. The concentration of OVA in the supernatants was quantified by the Micro BCA Protein Assay (Pierce Biotechnology, Rockford, IL) according to the manufacturer's protocol. The amount of OVA in supernatants and pellets was semi-quantified by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) followed by silver staining according to the protocols described by Laemmli34 and Blum et al.35
Bone-marrow-derived dendritic cells
Bone-marrow-derived dendritic cells (BM-DCs) were generated by growing 2 × 106 bone marrow precursor cells from the tibia and femur of naive female BALB/c mice in 10 ml complete RPMI with 10% GM-CSF-containing supernatant from X-63 cells. (These were a kind gift from J. Brewer, University of Glasgow, Glasgow, UK but were originally generated by B. Stockinger, Division of Molecular Immunology, National Institute for Medical Research, London, UK) or 10 ng/ml recombinant GM-CSF in 10 mm Ultra Low Attachment Culture Dishes (Corning, Biotech Line, Slangerup, Denmark) for 7 days. Every 3 days 5 ml complete RPMI supplemented with 4 mm l-glutamine and 20% X-63 supernatant or 20 ng/ml recombinant GM-CSF was added. BM-DCs were classified as immature by an intermediate expression of MHC class II and a low expression of CD40, CD80 and CD86.
Stimulation of cells
Cells (2 × 106 cells/ml) were pulsed with OVA and/or DDA in 24-well Ultra-Low Attachment Plates (Costar, Biotech Line) in a humidified atmosphere at 5% CO2 and 37° or at 4°. Fluorescein- and AlexaFluor488-conjugated OVA were used interchangeably and 5 μg/ml DDA liposomes was used to avoid excessive cell death. For brief stimulation of single-cell suspensions of BALB/c splenocytes or BM-DCs, the cells were cultured in tubes. To inhibit endocytosis the cells in some experiments were pretreated for 60 min with 5 μm cytochalasin D. To investigate the effect of heparin on the degree of OVA association, cells were pretreated for 45 min with 250 IU/ml heparin. After stimulation the cells were kept in the dark on ice and washed three times in cold phosphate-buffered saline (PBS) or twice in ice-cold ethanoic acid buffer followed by twice in cold PBS. Ethanoic acid removes surface-associated protein but it also kills the cells. This method had a minor effect on the background fluorescence signal of unstimulated cells but it was considered negligible because the fluorescence distribution profile of stimulated cells changed in an entirely different manner.
Antigen-presentation assay
B cells and T cells were purified from the spleens of BALB/c mice by negative selection using the magnetic antibody cell sorting (MACS) B-cell Isolation and MACS Pan T cell Isolation kits according to the manufacturer's protocols (Miltenyi Biotec, Biotech Line). Cells pulsed with OVA and/or DDA were set up in 200 μl complete RPMI in flat-bottom 96-well Nunclon plates (Nunc, Roskilde, Denmark) (1 × 105 cells/well) with purified T cells from naive female DO11.10 mice (2 × 105 cells/well). Purified DO11.10 T cells were >95% CD3+ and 80–90% KJ1-26+. After 48 hr, 100 μl supernatant was collected for cytokine analysis, medium was topped up and the cells were pulsed with 1 μCi/well [3H]thymidine for 20 hr. Radioactively labelled cells were harvested using a Skatron Micro Cell Harvester (Skatron, Lier, Norway) and counts per minute (c.p.m.) were measured in a 1205 Betaplate Liquid Scintillation Counter (Wallac, Perkin Elmer, Hvidovre, Denmark). IFN-γ was measured in supernatants from 48-hr cultures by enzyme-linked immunosorbent assay (ELISA) using purified anti-mouse IFN-γ, biotin-conjugated anti-mouse IFN-γ, horseradish peroxidase-conjugated streptavidin, and TMB Plus Ready-to-use substrate. The reaction was stopped with 0·2 m H2SO4 and absorbance was read at 450 nm.
Flow cytometry
After stimulation the cells were washed and Fc receptors were blocked with anti-CD16/CD32. The cells were kept at 4° during staining. TO-PRO-3 iodide was added at 100 nm immediately before acquisition on a FACSCalibur (BD Biosciences) to exclude dead cells except when the cells had been washed in ethanoic acid buffer. All analyses were carried out using the FCS Express Software v2 (DeNovo Software, Thornhill, Canada): cells were gated on the forward scatter–side scatter plot, then TO-PRO-3+ cells were excluded and finally cells were gated according to the specific stain. Maturation status and degree of OVA uptake are expressed as the geometric mean fluorescence intensity (MFI).
Confocal fluorescence laser-scanning microscopy
Staining was performed in the dark at room temperature. Cells were washed in cold PBS and fixed in formalin buffer for 15 min before being mounted onto slides. Cells were stained for 45 min with CD11c-PE in 1% FBS/PBS. Slides were analysed sequentially using 488/543-nm lasers and BP505-530/LP585 filters through a Plan-Apochromat 63×/1·4 Oil DIC objective of a Zeiss LSM510 connected to a Zeiss Axiovert 100M microscope (Carl Zeiss Ltd, Birkerød, Denmark). Alternatively, pulsed cells were adhered to four-well LabTek Chamber Slides (Nunc, Denmark) in complete RPMI for 75 min in a humidified atmosphere at 5% CO2 and 37°. Cells were fixed and rendered permeable as above and stained for F-actin with 0·1 μg/ml phalloidin-tetramethylrhodamine B isothiocyanate in 1% FBS/PBS. Slides were analysed on a Leica SP2 Laser Scanning Confocal Microscope (Leica Microsystems, Herlev, Denmark).
Animal experiments
Naive female BALB/c mice were injected once intraperitoneally (i.p.) with various doses of OVA-AlexaFluor488 alone or adsorbed to 250 μg DDA liposomes. The formulations were adjusted with sterile saline to a final volume of 200 μl per dose. The mice were killed 2 hr later by cervical dislocation and peritoneal exudate cells were harvested by flushing the peritoneal cavity with complete RPMI. The cells were subsequently washed in ethanoic acid buffer as described above and analysed by flow cytometry. Statistically significant differences between the two treatments were assessed by the t-test.
Results
Maturation effect on dendritic cells
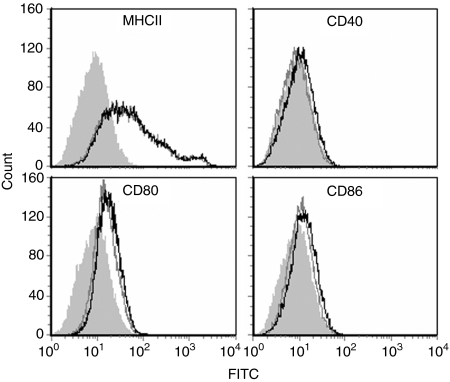
We investigated whether the adjuvant activity of DDA liposomes was associated with the ability to induce maturation of DCs. Immature murine BM-DCs were exposed to the maximum tolerable concentration of DDA (5 μg/ml ∼8 μm; data not shown) for 22 hr and the effect on maturation was evaluated by flow cytometry (Fig. 1). The BM-DCs had a distinct immature profile, which was not affected by stimulation with DDA liposomes because the surface expression of MHC class II, CD40, CD80 and CD86 was unchanged. The absence of a phenotypical maturation effect was repeatedly observed.
Figure 1.
Effect of DDA liposomes on the maturation status of BM-DCs. Expression of MHC class II (MHCII), CD40, CD80 and CD86 on the surfaces of live CD11c+ cells was determined by flow cytometry after stimulation for 22 hr with DDA (black line). Unstimulated BM-DCs (dark grey line) and the corresponding isotype controls (shaded light grey area) are shown for comparison. Data are representative of nine experiments.
Adsorption of antigen to liposomes
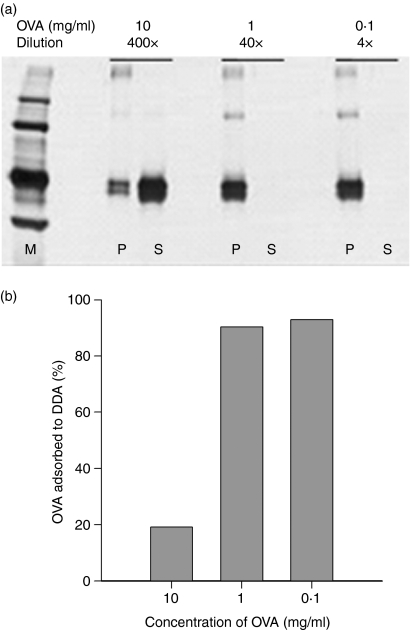
The interaction of DDA liposomes with antigen and its effect on antigen uptake were investigated next. Efficient liposome delivery is believed to be influenced by the extent to which the protein to be delivered is associated with the liposomes, either by vesicular entrapment or by surface adsorption. The degree of adsorption of various concentrations of OVA to a standard DDA liposome formulation was investigated. We evaluated the adsorption after incubation for 1 hr by SDS–PAGE and silver staining following ultra-centrifugation. The majority of the protein was found to be associated with the pellet as a result of adsorption to liposomes at concentrations of OVA up to 1 mg/ml (Fig. 2a). At a 10 times higher concentration of OVA the majority was found in the supernatant. This was confirmed when we quantified the amount of free OVA in the supernatants: DDA liposomes had a binding capacity of 90% of soluble OVA at concentrations below 1 mg/ml (Fig. 2b). At higher concentrations, the binding capacity of the liposomes became saturated and a large fraction of antigen was found free in solution. Ultra-centrifugation of OVA alone showed no precipitation or aggregation (data not shown).
Figure 2.
Adsorption of OVA onto DDA liposomes. DDA (1 mg/ml) was incubated with different concentrations of OVA for 1 hr and ultra-centrifuged. (a) Pellets (P) and supernatants (S) were analysed for OVA by SDS–PAGE and silver staining. Samples were diluted pairwise before loading onto the gel. M is the molecular weight marker. (b) Concentration of OVA in the supernatants was determined by the Micro BCA Protein Assay and the fraction of OVA adsorbed to DDA liposomes was calculated. Bars show the mean values of duplicate serial determinations. Data are representative of two experiments.
Cellular acquisition of antigen
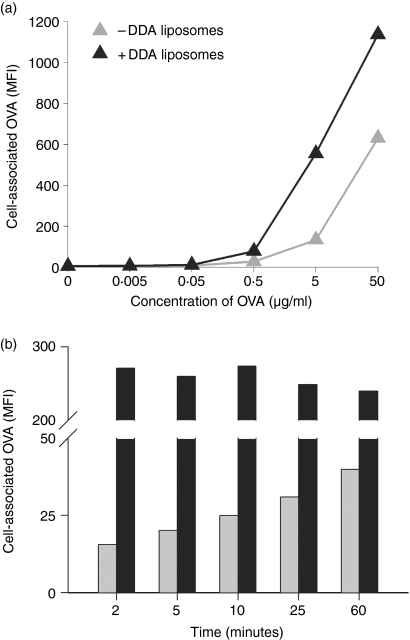
Immature BM-DCs were stimulated for 1 hr with various concentrations of fluorescently labelled OVA to investigate if adsorption onto DDA liposomes would enhance cellular acquisition of antigen. OVA was acquired in a dose-dependent manner and an increase in geometric MFI was observed at concentrations from 0·5 to 50 μg/ml (Fig. 3a, grey symbols). Within this range of concentrations cellular acquisition of OVA was enhanced by adsorption to the liposomes (Fig. 3a, black symbols). Next, the potential influence of DDA on the kinetics of antigen uptake was investigated. At a fixed OVA concentration (5 μg/ml) adsorption onto DDA liposomes resulted in maximum levels of cell-associated OVA as early as 2 min into the incubation period with no further increase during the following hour (Fig. 3b, black bars). In the absence of liposomes, the degree of OVA acquisition was delayed and a time-dependent increase was observed (Fig. 3b, grey bars).
Figure 3.
Liposome–mediated association of OVA to BM-DCs. (a) OVA acquisition is concentration-dependent: BM-DCs were cultured for 1 hr with different concentrations of OVA-fluorescein with (+DDA) or without (–DDA) DDA liposomes. (b) DDA liposomes enhance the rate of OVA acquisition: varying incubation time, BM-DCs were pulsed with 5 μg/ml OVA with (black columns) or without (grey columns) liposomes. The amount of OVA associated with live CD11c+ cells was determined by flow cytometry. Data are representative of two experiments each.
Active uptake of antigen by dendritic cells
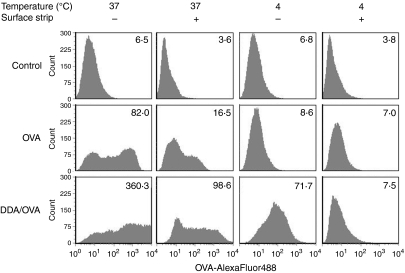
To distinguish between surface-bound and internalized antigen, we attempted to use trypan blue to quench surface-associated OVA. However, the combination of trypan blue and DDA led to inconsistent quenching and caused interference with the fluorescence signal from the TO-PRO-3 iodide stain used for dead cell exclusion. Consequently, trypan-blue quenching could not be used reliably in this assay. As an alternative to quenching, surface-associated antigen could be removed by treatment with ethanoic acid. Stripping the surface by this method diminished the fluorescent signal from stimulated cells (Fig. 4, compare the two left-hand columns). This indicated that not all of the OVA had been internalized by the cells during the 30-min stimulation and some of the antigen was still present at the cell surface. It also showed that DDA liposomes were able to enhance not only surface adsorption but also actual uptake of antigen. In addition, we performed the experiment at 4°, to stop active cellular processes and so investigate if endocytosis was involved (Fig. 4, two right-hand columns). Only DDA-adsorbed OVA associated with the cells at this temperature, although none of it was internalized because surface stripping reduced the fluorescent signal to background levels.
Figure 4.
Liposome-mediated uptake of OVA by BM-DCs. BM-DCs were cultured for 30 min at 37° or 4° with 5 μg/ml OVA-AlexaFluor488 alone (middle panel) or adsorbed to DDA liposomes (lower panel). Cells were washed in either PBS or ethanoic acid buffer to remove surface-associated OVA. The amount of OVA associated with CD11c+ cells was determined by flow cytometry. The corresponding geometric MFI values are given in the top right-hand corner of each histogram. The top panel shows the unstimulated control. Data are representative of three experiments.
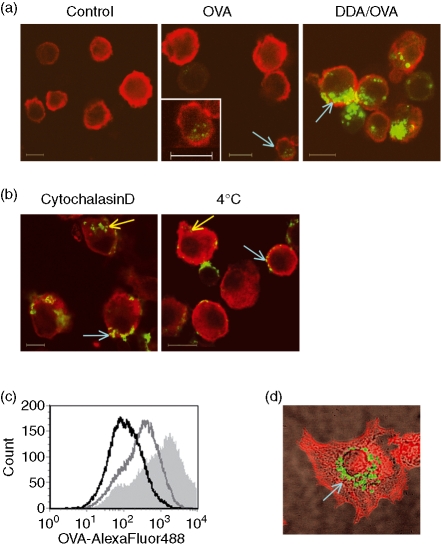
Confocal fluorescence laser-scanning microscopy confirmed that DDA liposomes enhance internalization of OVA (Fig. 5a). OVA was found in distinct vesicles inside the cells when adsorbed to the liposomes (Fig. 5a, right panel, arrow). The fluorescence level of OVA was hardly detectable in the absence of liposomes when the photomultiplier detector gain of the microscope was set to avoid saturation of the signal from the highly OVA+ cells observed in the presence of DDA (Fig. 5a, middle panel). However, adjusting the brightness of the photomicrograph revealed some OVA inside a minor proportion of these cells (Fig. 5a, middle panel, insert). We found the majority of OVA aggregating at the cell surface after treatment with cytochalasin D (Fig. 5b, left panel, blue arrow). Cytochalasin D is an inhibitor of actin polymerization and hence inhibits actin-dependent endocytosis and intracellular trafficking. At 4° in the absence of endocytic inhibitors, OVA was mainly found at the cell surface but with a more diffuse distribution pattern (Fig. 5b, right panel, blue arrow). A flow cytometric analysis of the same cells confirmed that the level of OVA acquisition was reduced after the different treatments (Fig. 5c). That only a small amount of OVA was found inside cells when stimulated in the presence of cytochalasin D (Fig. 5b, yellow arrow; Fig. 5c) indicates that, although several routes of entry may contribute to liposome-mediated uptake of OVA, active actin-dependent endocytosis is the most predominant one. Finally, the presence of OVA in distinct intracellular vesicles was demonstrated when cells were stimulated for 1 hr with DDA-adsorbed OVA, then washed to remove unbound antigen and subsequently allowed to adhere to a microscope slide for another 75 min (Fig. 5d, arrow).
Figure 5.
Visualization of liposome-mediated uptake of OVA by BM-DCs. (a) confocal microscopy images from a single confocal plane (0·7 μm) acquired through the centre of cells stimulated for 1 hr at 37° with 5 μg/ml OVA-AlexaFluor488 (green) with or without DDA liposomes. Cells were costained with CD11c-PE (red). The control shown is unstimulated cells. In the middle image the cell indicated with the blue arrow has been magnified and the colours adjusted to reveal the presence of internalized OVA. (b) As in (a) but shown is only cells stimulated with OVA adsorbed to DDA liposomes at 37° in the presence of Cytochalasin D or cells stimulated at 4°. (c) Distribution of OVA acquisition by CD11c+ cells stimulated with OVA and DDA liposomes for 1 hr at 4° (black line) or at 37° in the absence (shaded light grey area) or presence of Cytochalasin D (dark grey line) as determined by flow cytometry. (d) Confocal microscopy maximum intensity projection showing vesicular distribution of OVA after 1 hr of stimulation with DDA-adsorbed OVA-fluorescein (green) in a cell subsequently allowed to adhere for another 75 min and then stained for F-actin (red). The image has been overlaid with the corresponding transmission photomicrograph. Scale bars represent 10 μm. Data are representative of two to five experiments.
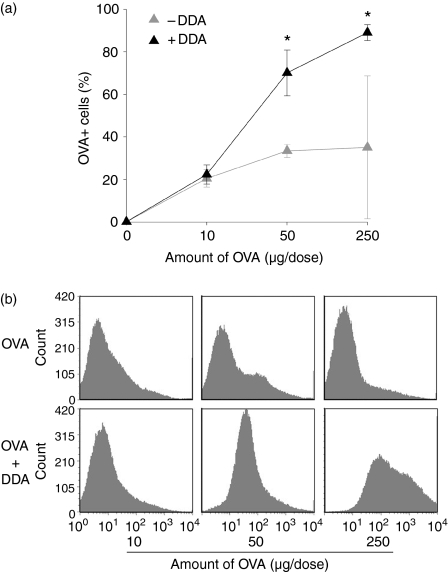
Antigen uptake in vivo
To ascertain if the ability of the DDA liposomes to enhance antigen uptake in vitro was also applicable in vivo we injected OVA in different doses into the peritoneal cavities of mice and monitored the uptake of antigen by peritoneal exudate cells 2 hr later. Consistent with the results obtained in vitro we found that adsorption to DDA liposomes significantly enhanced antigen uptake except at the lowest concentration of antigen (Fig. 6). This was the case both in terms of the percentage of cells that had internalized the antigen (Fig. 6a) and the amount internalized by the cells (Fig. 6b). The peritoneal exudate cells consisted primarily of B cells and neutrophilic granulocytes although the composition differed markedly depending on the presence of the liposomes (data not shown). A more detailed analysis of the cell types being targeted by the liposomes was therefore performed in vitro.
Figure 6.
Liposome-mediated uptake of OVA by peritoneal exudate cells. (a) Mice were injected with the indicated dose of OVA-AlexaFluor488 alone (–DDA) or adsorbed to 250 μg DDA liposomes (+DDA) and peritoneal exudate cells were harvested 2 hr later. Cells were washed in ethanoic acid buffer to remove surface-associated OVA. The percentage of cells with internalized OVA was determined by flow cytometry. Bars show the means of three individual mice per group and the error bars represent the standard error of the means (SEM). (b) The distribution of OVA uptake by the cells from one representative mouse from each group. The top panel is OVA injected alone, the lower panel is OVA adsorbed to liposomes. Where adsorption onto the liposomes statistically significantly enhanced the uptake of OVA, this has been indicated: *P < 0·05 and **P < 0·01. Data are representative of two experiments.
Selective uptake of antigen by APCs
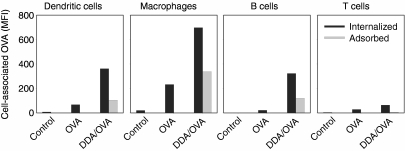
Having shown that DDA liposomes enhanced the adherence and uptake of antigen by BM-DCs in vitro and also peritoneal exudate cells in vivo, we investigated whether this effect was specific or a more generalized mechanism applying equally to different cellular subsets. We stimulated a single-cell suspension of splenocytes for 30 min with a high concentration of OVA (20 μg/ml) alone or adsorbed to liposomes and used flow cytometry to measure the level of cell-associated OVA in different cell subsets (DCs, macrophages, B cells and CD4+ T cells). Liposome-delivery predominantly targeted OVA to the APC subsets that had both internalized (Fig. 7, black bars) and adsorbed (Fig. 7, grey bars) much higher levels of OVA compared to the T cells.
Figure 7.
Liposome-mediated OVA uptake by APCs. Uptake (black columns) and adsorption (grey columns) of OVA by different cell populations in the spleen determined by flow cytometry after stimulation with 20 μg/ml OVA-AlexaFluor488 with or without DDA liposomes for 30 min at 37°. Adsorption was determined as the difference in MFI values between cells washed in PBS and ethanoic acid buffer. Cell subsets were defined according to their forward-side scatter profile and their expression of CD11c+ (Dendritic cells), F4/80+ CD11c− (Macrophages), CD19+ (B cells) or CD3+ CD4+ (T cells). Data are representative of four experiments.
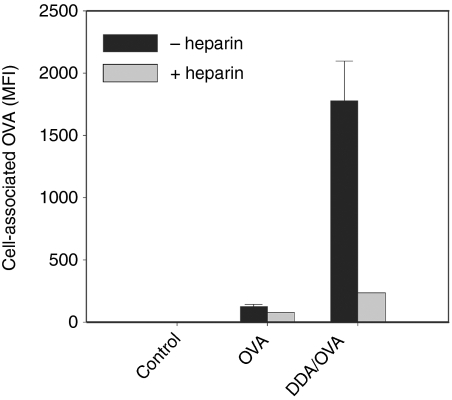
The nature of the liposome-enhanced cellular association of OVA was further investigated by pretreatment with heparin, which binds to heparan sulphate, a negatively charged proteoglycan that is expressed on the cell surface. Pretreatment of BM-DCs with heparin decreased the ability of DDA liposomes to enhance cellular association of antigen by almost an order of magnitude, whereas the effect was minimal in the absence of liposomes (Fig. 8).
Figure 8.
DDA-mediated targeting of OVA to BM-DCs is inhibited by heparin. BM-DCs were pretreated for 45 min at 37° with or without 250 IU/ml heparin before stimulation for 30 min with OVA-AlexaFluor488 alone or adsorbed to DDA liposomes. Unstimulated cells are shown as control. The amount of OVA associated with live CD11c+ cells was determined by flow cytometry. Bars show the mean and error bars represent the standard deviation of triplicate determinations. The triplicate determinations of the heparin-treated groups were so similar that the error bars fall within the edge of the bars.
Antigen presentation
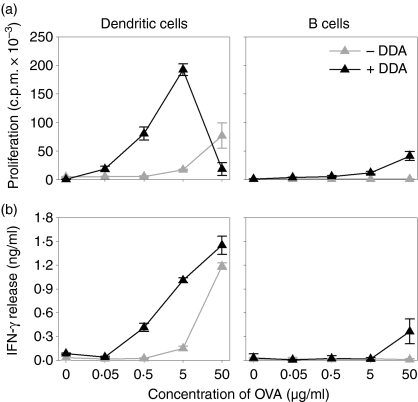
To test if liposome-mediated OVA uptake was associated with a functional enhancement of the APCs, we performed an antigen presentation assay. As presenter cells we used murine BM-DCs and purified B cells from spleens of BALB/c mice. As responder cells we used purified autologous DO11.10 T cells transgenic for a T-cell receptor recognizing an MHC class II-restricted OVA-epitope (OVA323−339). The combination of antigen-specific proliferation and cytokine release was used to assess the antigen-presenting ability of the different presenter cells. To ensure a high degree of antigen-loading even in the absence of liposomes, the presenter cells were pulsed for 4 hr with various concentrations of OVA alone or adsorbed to DDA liposomes; after this they were washed and cocultured with the responder cells. BM-DCs efficiently presented the OVA epitope and induced both a strong proliferative response and high levels of IFN-γ release from the DO11.10 responder cells (Fig. 9, left panels, grey symbols). Detectable responses were only observed for OVA alone at concentrations above 5 μg/ml. Delivering OVA in DDA liposomes amplified the presentation of antigen by the BM-DCs, resulting in increased responder-cell activation (Fig. 9, left panels, black symbols). Adsorption onto liposomes increased the efficiency of antigen presentation by approximately two orders of magnitude except at the highest concentration. Here, we repeatedly observed, although to varying degrees, attenuated proliferation while the release of IFN-γ remained high. This is presumably because the responder T cells were unable to proliferate further at the onset of the radioactive pulse because they had become exhausted by overstimulation. B cells were much less efficient antigen presenters. DDA enhanced the ability of B cells to present antigen to the responder cells, i.e. induce their proliferation and IFN-γ release, only at the highest concentration of OVA (Fig. 9, right panels).
Figure 9.
Liposome-mediated presentation of OVA by APCs. Antigen-specific responses expressed as (a) proliferation of and (b) IFN-γ release from purified DO11.10 T cells cocultured with BM-DCs (Dendritic cells) or B cells from the spleens of BALB/c mice. Presenter cells were pulsed with different concentrations of OVA with (+DDA) or without (–DDA) DDA liposomes for 4 hr before culture with the DO11.10 responder cells. Error bars represent the standard deviation of triplicate determinations. Data are representative of four experiments.
Discussion
The effect of cationic liposomes on DCs is not well characterized in the literature. It is, however, well known that cationic amphiphiles can be used as liposome carriers for nucleic acids and thus enhance the transfection efficiency of cells in general.8,36 DDA has been tested experimentally20,26,31,37 and also clinically in its chloride form38 as an adjuvant that induces cell-mediated immunity and delayed-type hypersensitivity. The mechanism for this adjuvant activity has so far been largely undefined, although the ability of DDA liposomes to form a depot at the injection site has been suggested.31 Consistent with electrostatic forces between the net negatively charged protein and the positively charged liposomes, we demonstrate that DDA-based cationic liposomes readily adsorbed OVA and bound avidly to murine BM-DCs on a time-scale of less than a few minutes and thus mediated enhanced cellular adsorption and uptake of antigen. This finding is consistent with the results from Carmona-Ribeiro et al. who found that DDA vesicles interact rapidly with cells with very high affinity.21
Huth et al. have recently shown that pH-sensitive liposomes composed of the lipids dioleoylphosphatidylethanolamine and cholesteryl hemisuccinate utilize several different endocytic pathways in parallel, including clathrin-dependent and caveolae endocytosis.39 In addition, they found that distinct cell types internalize liposomes differently. Consistent with recent data suggesting that cationic liposomes are taken up by active endocytosis,11,13,14 we found that cationic DDA liposomes enhanced the ability of peritoneal exudate cells in vivo and murine BM-DCs in vitro to internalize OVA. This was primarily via an active actin-dependent pathway because treatment with cytochalasin D and stimulation of the cells at 4° both had a strong inhibitory effect on antigen-uptake. Cytochalasin D depolymerizes the actin filaments of the cytoskeleton and it thus inhibits intracellular trafficking and actin-dependent endocytosis including macropinocytosis and phagocytosis. It has also been shown to block the internalization of caveolae while increasing their lateral mobility and clustering in the plasma membrane.40 This would explain the observed accumulation of OVA at distinct areas of the cell surface after treatment with cytochalasin D. As a result of the polydispersity of DDA formulations (the average diameter of the particles is 846 ± 130 nm33) it is likely that multiple cellular entry pathways are involved.41,42 This issue is currently being further investigated. Delivery of antigen to cells by immediate contact with the cell surface via electrostatic interaction followed by the induction of active uptake seems to be one mechanism behind the ability of DDA liposomes to act as an adjuvant.
DDA liposomes predominantly targeted antigen to APC subsets as we observed a clearly enhanced uptake of OVA by splenic DCs, macrophages and B cells but not by T cells. As binding, and not just uptake, of OVA to the cell surface also appeared to be APC-specific, the increased uptake cannot be explained solely by differences in endocytic capacity. Variations in the expression of cell surface receptors and molecules, such as the proteoglycan heparan sulphate, are likely to be involved because they affect the affinity of cells for cationic entities and thus influence their uptake.43–45 This is consistent with the observation that pretreatment of DCs with heparin markedly reduced the adsorption and uptake of liposome-adsorbed OVA. In addition, preliminary experiments with liposomes comprised of DDA and the neutral lipid DSPC (distearoylglycerophosphocholine) showed that OVA acquisition by BM-DCs decreased if the molar concentration of DDA, and hence the positive charge of the liposomes, was reduced (data not shown). That macrophages seemed to acquire more OVA than DCs may be an effect of the differential expression of cell surface molecules involved in the binding of OVA and DDA liposomes and the fact that splenic DCs are mature and hence have a reduced ability to acquire antigens; unlike the immature BM-DCs used elsewhere in this study.
The functional relevance of the enhanced active uptake of antigen was underlined by the ability of the DDA liposomes to mediate MHC class II-restricted antigen presentation to OVA-specific CD4+ T cells using the well-established DO11.10 transgenic mouse system. The DDA liposomes clearly enhanced the presentation of OVA by murine BM-DCs and, to a lesser extent, by B cells, leading to significantly increased proliferation of IFN-γ release from the OVA-specific responder cells. Here, we did not investigate the ability of DDA liposomes to enhance MHC class I-restricted antigen presentation but based on studies with a DDA-based adjuvant in vivo46 and other types of liposomes in vitro47 cross-presentation is not likely to occur because the fatty acid chains of DDA are fully saturated. Theoretically cross-presentation may, however, be achieved by the addition of ligands for Toll-like receptor 3 or 9.48 Recently, Cuit et al. have found that cholesterol-containing cationic liposomes mature DCs.49 Unfortunately, this effect could not be distinguished from changes in apoptotic cells and subsequent bystander activation because the study did not control for liposome-induced cytotoxicity or exclude dead cells from the analyses. In a more recent study by the same group a range of different liposomes was used to show that cationic liposomes might be immunostimulating if they had shorter fatty acid chains (12–14 carbon atoms), preferentially unsaturated, and the right structure of the cationic head group.3 They suggest the presence of a specific cationic lipid receptor and the study clearly emphasizes that cationic liposomes are highly heterogeneous and that care should be taken when comparing results from different studies. In our experiments the ability of DCs to activate DO11.10 T cells was not associated with an adjuvant-induced up-regulated surface expression of maturation markers despite the fact that activation of naive T cells requires costimulation. The incubation of DCs with OVA and the extra washing and replating associated with the antigen presentation assay did not lead to DC maturation either (data not shown). The transgenic DO11.10 T cells do not, however, behave exactly like wild-type cells and they may not have the same strict requirement for costimulation.50,51 Another explanation could be that up-regulation of CD40 ligand (CD40L) on the T cells is induced by T-cell receptor ligation at high antigen concentrations as the result of increased peptide–MHC densities leading to subsequent APC maturation through CD40 ligation.52
The relevance of our results is underlined by recent data clearly demonstrating a marked benefit of adding immunostimulatory components such as monophosphoryl lipid-A,29 mycobacterial cord factor,23 its synthetic analogue,31 and mycobacterial lipid extracts32 to DDA-based adjuvants. These immunomodulators enhance the effect of the DDA liposomes and the overall efficacy of the vaccine in vivo. Based on this and the present findings we therefore suggest that DDA liposomes function primarily as a delivery vehicle to mediate initial contact with the cell surface; a step that facilitates subsequent active antigen uptake, thereby efficiently augmenting antigen presentation to T cells.
Acknowledgments
We would like to thank Maria Nørtoft, Linda Christensen, Anniezette Amentorp Lander and Annette Hansen for excellent technical assistance, Michael Hansen (The Royal Veterinary and Agricultural University, Denmark) for his kind and skilled assistance when using the Leica SP2 laser scanning confocal microscope, and Thomas Lindenstrøm for critical revision of the manuscript. This work was supported by the European Commission contract no. LSHP-CT-2003–503367. K.S.K. is supported by the Danish Council for Developmental Research.
Abbreviations
- APC
antigen-presenting cell
- BM-DC
bone-marrow-derived dendritic cell
- c.p.m.
counts per minute
- DC
dendritic cell
- DDA
dimethyldioctadecylammonium
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- IFN
interferon
- IgG
immunoglobulin G
- MFI
mean fluorescence intensity
- MHC
major histocompatibility complex
- OVA
ovalbumin
- PBS
phosphate-buffered saline
- PE
phycoerythrin
- SDS–PAGE
sodium dodecyl sulphate–polyacrylamide gel electrophoresis
References
- 1.Felgner PL, Gadek TR, Holm M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA. 1987;84:7413–17. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Audouy SA, de Leij LF, Hoekstra D, Molema G. In vivo characteristics of cationic liposomes as delivery vectors for gene therapy. Pharm Res. 2002;19:1599–605. doi: 10.1023/a:1020989709019. [DOI] [PubMed] [Google Scholar]
- 3.Vangasseri DP, Cui Z, Chen W, Hokey DA, Falo LD, Huang L. Immunostimulation of dendritic cells by cationic liposomes. Mol Membr Biol. 2006;23:385–95. doi: 10.1080/09687860600790537. [DOI] [PubMed] [Google Scholar]
- 4.Freimark BD, Blezinger HP, Florack VJ, Nordstrom JL, Long SD, Deshpande DS, Nochumson S, Petrak KL. Cationic lipids enhance cytokine and cell influx levels in the lung following administration of plasmid: cationic lipid complexes. J Immunol. 1998;160:4580–6. [PubMed] [Google Scholar]
- 5.Miller AD. The problem with cationic liposome/micelle-based non-viral vector systems for gene therapy. Curr Med Chem. 2003;10:1195–211. doi: 10.2174/0929867033457485. [DOI] [PubMed] [Google Scholar]
- 6.Carmona-Ribeiro AM. Interactions between cationic liposomes and drugs or biomolecules. An Acad Bras Cienc. 2000;72:39–43. doi: 10.1590/s0001-37652000000100005. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad A, Evans HM, Ewert K, George CX, Samuel CE, Safinya CR. New multivalent cationic lipids reveal bell curve for transfection efficiency versus membrane charge density: lipid–DNA complexes for gene delivery. J Gene Med. 2005;7:739–48. doi: 10.1002/jgm.717. [DOI] [PubMed] [Google Scholar]
- 8.Dass CR. Lipoplex-mediated delivery of nucleic acids: factors affecting in vivo transfection. J Mol Med. 2004;82:579–91. doi: 10.1007/s00109-004-0558-8. [DOI] [PubMed] [Google Scholar]
- 9.Pantazatos DP, Pantazatos SP, MacDonald RC. Bilayer mixing, fusion, and lysis following the interaction of populations of cationic and anionic phospholipid bilayer vesicles. J Membr Biol. 2003;194:129–39. doi: 10.1007/s00232-003-2031-y. [DOI] [PubMed] [Google Scholar]
- 10.Stamatatos L, Leventis R, Zuckermann MJ, Silvius JR. Interactions of cationic lipid vesicles with negatively charged phospholipid vesicles and biological membranes. Biochemistry. 1988;27:3917–25. doi: 10.1021/bi00411a005. [DOI] [PubMed] [Google Scholar]
- 11.Friend DS, Papahadjopoulos D, Debs RJ. Endocytosis and intracellular processing accompanying transfection mediated by cationic liposomes. Biochim Biophys Acta. 1996;1278:41–50. doi: 10.1016/0005-2736(95)00219-7. [DOI] [PubMed] [Google Scholar]
- 12.Hafez IM, Maurer N, Cullis PR. On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Ther. 2001;8:1188–96. doi: 10.1038/sj.gt.3301506. [DOI] [PubMed] [Google Scholar]
- 13.Wrobel I, Collins D. Fusion of cationic liposomes with mammalian cells occurs after endocytosis. Biochim Biophys Acta. 1995;1235:296–304. doi: 10.1016/0005-2736(95)80017-a. [DOI] [PubMed] [Google Scholar]
- 14.Lin AJ, Slack NL, Ahmad A, George CX, Samuel CE, Safinya CR. Three-dimensional imaging of lipid gene-carriers: membrane charge density controls universal transfection behavior in lamellar cationic liposome–DNA complexes. Biophys J. 2003;84:3307–16. doi: 10.1016/S0006-3495(03)70055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Ouahabi A, Thiry M, Pector V, Fuks R, Ruysschaert JM, Vandenbranden M. The role of endosome destabilizing activity in the gene transfer process mediated by cationic lipids. FEBS Lett. 1997;414:187–92. doi: 10.1016/s0014-5793(97)00973-3. [DOI] [PubMed] [Google Scholar]
- 16.Cox JC, Coulter AR. Adjuvants – a classification and review of their modes of action. Vaccine. 1997;15:248–56. doi: 10.1016/s0264-410x(96)00183-1. [DOI] [PubMed] [Google Scholar]
- 17.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 18.Steinman RM, Pope M. Exploiting dendritic cells to improve vaccine efficacy. J Clin Invest. 2002;109:1519–26. doi: 10.1172/JCI15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 20.Hilgers LA, Snippe H. DDA as an immunological adjuvant. Res Immunol. 1992;143:494–503. doi: 10.1016/0923-2494(92)80060-x. discussion 74–6. [DOI] [PubMed] [Google Scholar]
- 21.Carmona-Ribeiro AM, Ortis F, Schumacher RI, Armelin MCS. Interactions between cationic vesicles and cultured mammalian cells. Langmuir. 1997;13:2215–18. [Google Scholar]
- 22.Lincopan N, Mamizuka EM, Carmona-Ribeiro AM. In vivo activity of a novel amphotericin B formulation with synthetic cationic bilayer fragments. J Antimicrob Chemother. 2003;52:412–18. doi: 10.1093/jac/dkg383. [DOI] [PubMed] [Google Scholar]
- 23.Dzata GK, Wyckoff JHd, Confer AW. Immunopotentiation of cattle vaccinated with a soluble Brucella abortus antigen with low LPS content: an analysis of cellular and humoral immune responses. Vet Microbiol. 1991;29:15–26. doi: 10.1016/0378-1135(91)90107-q. [DOI] [PubMed] [Google Scholar]
- 24.Hilgers LA, Snippe H, Jansze M, Willers JM. Immunomodulating properties of two synthetic adjuvants. Dependence upon type of antigen, dose, and time of administration. Cell Immunol. 1984;86:393–401. doi: 10.1016/0008-8749(84)90394-0. [DOI] [PubMed] [Google Scholar]
- 25.Klinguer C, Beck A, De-Lys P, et al. Lipophilic quaternary ammonium salt acts as a mucosal adjuvant when co-administered by the nasal route with vaccine antigens. Vaccine. 2001;19:4236–44. doi: 10.1016/s0264-410x(01)00156-6. [DOI] [PubMed] [Google Scholar]
- 26.Snippe H, Belder M, Willers JM. Dimethyl diotadecyl ammonium bromide as adjuvant for delayed hypersensitivity in mice. Immunology. 1977;33:931–6. [PMC free article] [PubMed] [Google Scholar]
- 27.Lima KM, Bonato VL, Faccioli LH, Brandao IT, dos Santos SA, Coelho-Castelo AA, Leao SC, Silva CL. Comparison of different delivery systems of vaccination for the induction of protection against tuberculosis in mice. Vaccine. 2001;19:3518–25. doi: 10.1016/s0264-410x(01)00042-1. [DOI] [PubMed] [Google Scholar]
- 28.Tsuruta LR, Quintilio W, Costa MH, Carmona-Ribeiro AM. Interactions between cationic liposomes and an antigenic protein: the physical chemistry of the immunoadjuvant action. J Lipid Res. 1997;38:2003–11. [PubMed] [Google Scholar]
- 29.Brandt L, Elhay M, Rosenkrands I, Lindblad EB, Andersen P. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect Immun. 2000;68:791–5. doi: 10.1128/iai.68.2.791-795.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindblad EB, Elhay MJ, Silva R, Appelberg R, Andersen P. Adjuvant modulation of immune responses to tuberculosis subunit vaccines. Infect Immun. 1997;65:623–9. doi: 10.1128/iai.65.2.623-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holten-Andersen L, Doherty TM, Korsholm KS, Andersen P. Combination of the cationic surfactant dimethyl dioctadecyl ammonium bromide and synthetic mycobacterial cord factor as an efficient adjuvant for tuberculosis subunit vaccines. Infect Immun. 2004;72:1608–17. doi: 10.1128/IAI.72.3.1608-1617.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenkrands I, Agger EM, Olsen AW, Korsholm KS, Andersen CS, Jensen KT, Andersen P. Cationic liposomes containing mycobacterial lipids: a new powerful Th1 adjuvant system. Infect Immun. 2005;73:5817–26. doi: 10.1128/IAI.73.9.5817-5826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davidsen J, Rosenkrands I, Christensen D, Vangala A, Kirby D, Perrie Y, Agger EM, Andersen P. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′-dibehenate) – a novel adjuvant inducing both strong CMI and antibody responses. Biochim Biophys Acta. 2005;1718:22–31. doi: 10.1016/j.bbamem.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 35.Blum H, Beier H, Gross HJ. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–9. [Google Scholar]
- 36.Audouy S, Hoekstra D. Cationic lipid-mediated transfection in vitro and in vivo (review) Mol Membr Biol. 2001;18:129–43. [PubMed] [Google Scholar]
- 37.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–44. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanfield JP, Gall D, Bracken PM. Single-dose antenatal tetanus immunisation. Lancet. 1973;1:215–19. doi: 10.1016/s0140-6736(73)90062-7. [DOI] [PubMed] [Google Scholar]
- 39.Huth US, Schubert R, Peschka-Suss R. Investigating the uptake and intracellular fate of pH-sensitive liposomes by flow cytometry and spectral bio-imaging. J Control Release. 2006;110:490–504. doi: 10.1016/j.jconrel.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 40.Thomsen P, Roepstorff K, Stahlhut M, van Deurs B. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol Biol Cell. 2002;13:238–50. doi: 10.1091/mbc.01-06-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004;377:159–69. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm. 2005;298:315–22. doi: 10.1016/j.ijpharm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 43.Mislick KA, Baldeschwieler JD. Evidence for the role of proteoglycans in cation-mediated gene transfer. Proc Natl Acad Sci USA. 1996;93:12349–54. doi: 10.1073/pnas.93.22.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mounkes LC, Zhong W, Cipres-Palacin G, Heath TD, Debs RJ. Proteoglycans mediate cationic liposome-DNA complex-based gene delivery in vitro and in vivo. J Biol Chem. 1998;273:26164–70. doi: 10.1074/jbc.273.40.26164. [DOI] [PubMed] [Google Scholar]
- 45.Ruponen M, Honkakoski P, Tammi M, Urtti A. Cell-surface glycosaminoglycans inhibit cation-mediated gene transfer. J Gene Med. 2004;6:405–14. doi: 10.1002/jgm.522. [DOI] [PubMed] [Google Scholar]
- 46.Bennekov T, Dietrich J, Rosenkrands I, Stryhn A, Doherty TM, Andersen P. Alteration of epitope recognition pattern in Ag85B and ESAT-6 has a profound influence on vaccine-induced protection against Mycobacterium tuberculosis. Eur J Immunol. 2006;36:3346–55. doi: 10.1002/eji.200636128. [DOI] [PubMed] [Google Scholar]
- 47.Taneichi M, Ishida H, Kajino K, et al. Antigen chemically coupled to the surface of liposomes are cross-presented to CD8+ T cells and induce potent antitumor immunity. J Immunol. 2006;177:2324–30. doi: 10.4049/jimmunol.177.4.2324. [DOI] [PubMed] [Google Scholar]
- 48.Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A, Bosio C, Dow S. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006;176:7335–45. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]
- 49.Cui Z, Han SJ, Vangasseri DP, Huang L. Immunostimulation mechanism of LPD nanoparticle as a vaccine carrier. Mol Pharm. 2005;2:22–8. doi: 10.1021/mp049907k. [DOI] [PubMed] [Google Scholar]
- 50.Schweitzer AN, Borriello F, Wong RC, Abbas AK, Sharpe AH. Role of costimulators in T cell differentiation. Studies using antigen-presenting cells lacking expression of CD80 or CD86. J Immunol. 1997;158:2713–22. [PubMed] [Google Scholar]
- 51.Schuhbauer D, Muller B, Mitchison A. Unrepresentative behavior of T cell receptor-transgenic CD4+ T cells upon adoptive transfer: lack of need for priming and an extended booster dose–response. Immunobiology. 1996;195:152–9. doi: 10.1016/S0171-2985(96)80035-0. [DOI] [PubMed] [Google Scholar]
- 52.Ruedl C, Bachmann MF, Kopf M. The antigen dose determines T helper subset development by regulation of CD40 ligand. Eur J Immunol. 2000;30:2056–64. doi: 10.1002/1521-4141(200007)30:7<2056::AID-IMMU2056>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]