Abstract
Vaccines that comprise attenuated viral vectors encoding antigens from target pathogens generate potent T-cell responses. One such pathogen is malaria, and in particular the liver stage of its life cycle. Immunogenicity and efficacy studies in animals and humans have revealed the generation of memory T cells of both the central and effector phenotypes, depending on the viral vectors used in the malaria vaccination regime (viral species and serotype, combination and sequence for prime–boost) and suggest a divergence in their protective role. Being able to influence the memory T-cell make-up in a rational manner may allow us to develop more efficacious vaccines.
Keywords: malaria, memory T cells, vaccines, viral vectors
Introduction
Although an essential feature of the adaptive immune response, immunological memory remains a subject of some debate, despite intense investigation. T-cell memory, a focus of this article, has been described with semantic descriptions chiefly based on the possession of surface molecules and cytokine secretion. There are also differences between observations in humans and animal models (e.g. the use of T-cell-receptor transgenic mice, differing molecule expression, different tissues examined) that may preclude over-generalization. Furthermore, there may be some differences in the behaviour of CD4+ and CD8+ T cells. Immunological memory lies at the heart of the mechanism of action of vaccination and immunotherapies, and so increased understanding of it is essential in such endeavours. Clarification of the description of the types of T cells that comprise T-cell immunological memory help us to describe, in this article, how such cells may be generated when using recombinant viral vectors as vaccines, and allow a perspective to be offered.
Malaria provides an ideal reason and model for the investigation of T-cell memory responses. As it remains a major cause of illness and mortality in endemic areas, novel and effective vaccination strategies are highly sought after.1 Vaccines comprising antigen-encoding viral vectors (such as attenuated vaccinia, fowlpox and adenovirus strains, sometimes in regimes in combination with antigen-encoding DNA vaccines) offer one such strategy. Similarly to standard vaccine regimens, the use of recombinant viruses may require a sequence of priming and boosting immunizations to achieve a sufficient level of effective immunity. But, in order to avoid the generation of antivector immunity, which may depress immunity against the vectored antigen, the sequential delivery of differing vectors, heterologous prime–boosting, is being pursued. An array of malarial target antigens exist, depending on the phase in the life cycle, with the liver stage offering an opportunity for T cells to eliminate infected hepatocytes before they are able to release their merozoite progeny, thus breaking the life cycle and producing sterile immunity (reviewed in ref. 2). Malaria is one of a few infections that can be safely modelled in both animals and humans.3 Therefore, modifications to the molecular design of the vectors (the choice of vector, antigen and other stimulatory additions), and to their combinations and timings during sequential immunization, allow the measurement and characterization of resulting memory T-cell responses, as well as correlation with protective efficacy.
Viral vector vaccines
Vaccines have classically comprised either attenuated/inactivated pathogenic organisms, or antigens derived from them, delivered in such a way as to provide a ‘depot’ of antigen together with an immunological adjuvant that will initiate an immune response via innate pathways. Viral vector vaccines act in somewhat similar ways, with the vector acting as the attenuated pathogen with intrinsic innate-stimulatory activity, and encoding an antigen of choice against which an immune response is desired. Viral vectors express the encoded antigen as though it were their own protein (i.e. within the infected cell cytoplasm), from which the antigen has access to the endogenous pathway of antigen presentation. Thus, epitopes can be presented on major histocompatibility complex (MHC) class I molecules to CD8+ T cells. Priming of naïve T cells requires presentation by professional antigen-presenting cells (APCs), chiefly dendritic cells (DCs), and DCs are able to acquire the antigen by direct infection with the virus or through a process of cross-priming when antigen is taken up from other infected and dying cells and debris. The latter process is also amenable to uptake by APCs for entry into the exogenous antigen-processing pathway where antigen is presented by MHC class II on APCs to CD4+ T cells.
Viruses contain pathogen-associated molecular patterns (PAMPs), such as double-stranded (ds)RNA (a by-product of symmetrical transcription in DNA viruses) and viral DNA, capable of being recognized by pattern recognition receptors (PRRs) such as TLR3/RIG-I and TLR7/9, respectively, of particular APCs (reviewed by Kawai & Akira).4 Inflammatory cytokine secretion can, in this way, be initiated, with type I interferons (IFN-α and -β) being particularly prominent. Thus, viruses are able to activate and modulate DCs to programme an effective type of T-cell response [i.e. cytotoxic T lymphocytes (CTL) and T helper 1 (Th1) cell-mediated immunity].
Poxviruses
Poxviruses, and in particular vaccinia, were the first viruses to be investigated as antigen-encoding vectors. This stems from the fact that vaccinia was found to be a highly immunogenic vector for generating cell-mediated immunity in the form of T cells.5 Following vaccination with vaccinia, the migration of infected APCs to draining lymph nodes is a rapid process, peaking at 6 hr and declining rapidly thereafter.6 Therefore, it was concluded that early antigen expression is essential, but is sufficient as CD8+ T cells (CTL) need only a brief contact with antigen to proliferate.7 Infected macrophages may also provide a source of antigen for cross-priming of CD8+ T cell responses6 and for generating CD4+ T-cell responses. Thus, poxviruses are able to elicit the main cells of the adaptive cell-mediated immune response.
An attenuated version of vaccinia, modified vaccinia Ankara (MVA), developed as a vaccine for smallpox and found to be very safe to administer, has since been utilized as a vaccine for a range of intracellular infections [such as tuberculosis,8 human immunodeficiency virus (HIV)9 and malaria10] and for cancer.11 The passage-attenuation of the virus resulted in ≈31 kb of genomic deletions. Thus, MVA has a large recombinant DNA capacity to accommodate large antigens, such as those from malaria, or combinations thereof. Furthermore, MVA is easily produced and does not persist in the host. Compared with vaccinia, MVA is less cytopathic and lacks certain immune-suppressive signalling molecules and soluble receptors, making it potentially more immunogenic.12 MVA retains the ability to induce type I IFN expression in infected cells and this may have a profound contribution to an enhanced generation of memory T cells by direct cytokine action.13 Although infecting immature DCs more efficiently than mature DCs, as measured by transgene expression, MVA induced increased apoptosis in immature DCs, together with reduced maturation because of shut down of cellular protein synthesis,14 emphasizing the requirement for early antigen expression. Despite reducing their MHC class I expression and T-cell-stimulatory capacity in vitro, and causing cell death, MVA-infected murine DCs retained their ability to generate a CTL response in vivo,15 perhaps mainly by serving as a source of immunogenic antigen for cross-priming. Indeed, mice administered MVA showed an overall enhanced T-cell-stimulatory capacity of their DCs. Furthermore, co-administration of MVA with antigen was shown to generate enhanced T-cell and antibody responses, suggesting that the virus has potent adjuvant activity.16
The FP9 strain of fowlpox virus was attenuated by passage, similarly to MVA, giving rise to 25 kb of genomic deletions. Murine DCs infected with fowlpox vectors were shown to be potent at stimulating transgene-specific CD8+ T cells for up to 3 days and also up-regulated MHC and costimulatory molecules.17 Human DCs were able to express the transgene for up to 20 days.17 This suggests that fowlpox may be less cytopathic than MVA and allow for longer transgene, and hence antigen, expression.
The further characterization of the innate immune responses generated by infection of cells by pox viral vectors is the subject of current investigation in our laboratory.
Adenoviruses
Adenoviruses are being used in a similar way to poxviruses, as viral-vector vaccines for numerous diseases.18 They are species-specific [derived from human or simian (chimpanzee) sources], but can be used in rodent models as well as in humans, and comprise numerous serotypes. Adenoviruses can be produced in high titres (although higher vaccine titres may be needed compared with poxviruses), and can accommodate up to 1·8 kb of recombinant DNA when the E1 region is deleted, and up to 3·5 kb if the E3 region is excised. E1 and E3 deletions, as well as rendering virus replication incompetent, also reduce viral immune evasion because they interfere with IFN signalling and MHC class I peptide presentation, respectively. Adenoviruses of serotypes such as human (Hu) 2,5 and chimpanzee (C) 68, attach to the Coxsackie-Adenovirus receptor (CAR) on target cells via the knob domain of their fibre proteins. CAR is expressed on epithelial cells, endothelial cells and hepatocytes, but weakly by DCs. Further viral attachment occurs to the αv integrin on target cells via the RGD motifs on the viral penton base protein, possibly allowing tropism for other cell types. Adenoviruses of serotype B, such as Hu34 and 35, amongst others, attach via a different receptor, CD46, that belongs to a family of complement activation regulatory proteins expressed on all nucleated cells in humans, whereas in the mouse, CD46 is not widespread, precluding their use. Adenoviruses have been shown to stimulate in vivo secretion by the mouse innate immune system of interleukin (IL)-6, IL-12 and tumour necrosis factor-α (TNF-α),19 although they were less efficient than poxviruses at adjuvanting T-cell responses to co-administered proteins.16 Human DCs can be matured by high titres of adenovirus Hu2 or Hu5 via the fibre knob and nuclear factor-κB (NF-κB) signalling,20,21 or by a TNF-α autocrine loop following penton base recognition.22 E1 deletion, by virtue of reduced cell death of infected cells, allows sustained expression of recombinant antigen. Indeed, adenovirus Hu5-transduced cells took 7–10 days to be cleared by CD8+ CTL in mice.23 Together with potent cytomegalovirus (CMV) promoters controlling antigen expression, this suggests that adenoviruses express high levels of transgene for considerable periods of time, and that this differs somewhat from poxviruses. The use of simian adenoviruses, for possible human use, avoids the potential inhibitory effects of neutralizing pre-existing immunity, mainly antibodies against human adenoviruses. When administered in vivo, simian adenoviruses elicited the production of high amounts of type I IFN compared with adenovirus Hu5.24 They were also superior at generating CD8+ responses against HIVgag in mice25 and macaques, and can be used in heterologous prime–boost regimens to potentiate the immune response.26 Human DCs can be activated by Hu5, but often only with a very high multiplicity of infection (MOI) (> 1000).27 Most recently, adenovirus Hu5, at a MOI of 500, was shown to infect slowly (5 days) and activate human DCs, and induce IL-6, IL-12 and type I IFN secretion, although some suppressive effect may also result via indoleamine dioxygenase.28
Memory T-cell responses
There is some debate regarding the description of T cells that circulate through the blood and lymphoid system, owing to the semantics of their nomenclature. Nevertheless, this nomenclature is useful in giving a framework by which to think about and work with these cells (Fig. 1), and refers broadly to both CD4+ and CD8+ T cells. When naïve T cells are stimulated within lymphoid tissue by DCs that present antigen which their T-cell receptors recognize, together with appropriate costimulatory ligations and cytokine milieu they undergo a programme of proliferation, differentiation and activation into T cells that are able to perform a function when they recognize the antigen again, either in the near or distant future. After the peak of proliferation, 7–10 days following initiation, a programmed contraction phase follows where >90% of the resulting cells die, whilst the remainder are maintained by homeostatic cytokines.29,30 The term memory, in its simplest interpretation, describes T cells that remain once antigen that was involved in their priming has been cleared from the system. However, the latter is difficult to be sure of and may not always be the case. These T cells, at the centre of the adaptive immune response, are able to respond vigorously when they again come into contact with the antigen (at an appropriate concentration and context). The type of response produced by the memory T cells, and other characteristics, has suggested a logical subdivision into effector-memory (TEM) and central-memory (TCM) T cells.31 TEM generally exist during and shortly after the expansion/contraction phase and have rapid effector functions upon antigen recognition, such as IFN-γ and perforin secretion, but proliferate poorly. These cells are sometimes referred to as ‘effector T cells’, but this is not entirely accurate because they are not actually releasing their cytopathic effector molecules when circulating, but rather when they recognize antigen presented by infected cells or local APCs. It is neither resource efficient nor safe to maintain large numbers of such poised cells unless they are potentially required to combat an infection that occurs extremely rapidly and in high density, and/or is persistent. In humans, TEM may be more long-lived than in mice for this reason, particularly to contain persistent viruses. These cells are excluded from lymph nodes (LN) because they lack receptors that allow access (CD62L LN homing receptor, and CCR7 LN chemokine receptor), and so they access peripheral tissues where the majority of infections occur and therefore where they are needed. TCM are established later after contraction (perhaps up to 40 days) and do possess the above-mentioned molecules, enabling access to LN. TCM are able to proliferate vigorously and produce more proliferation-inducing IL-2, compared with TEM, upon antigen recognition within LN, where they also have a greater requirement for costimulation and possess high levels of CD28 for this purpose. They also possess receptors for homeostatic cytokines, such as IL-7 (CD127) and IL-15, and anti-apoptotic molecules, such as bcl-XL. Thus, their proliferation is tightly controlled and accompanied by acquiring features of TEM (i.e. access to tissues through loss of the above molecules and the ability to express and secrete effector molecules required to fight the ‘new’ infection). Therefore, TCM are believed to provide a reservoir of T cells that can survive in the absence of antigen for a long period of time but can proliferate rapidly, when required, to produce a large population of TEM.32 The expression of the LN homing receptors, and the capacity to produce certain effector molecules, however, is not a clear-cut differentiation, in that there is a broad spectrum of expression of these molecules, and so there appears to be considerable overlap or blurring between TEM and TCM. Such intermediates appear appropriate as flexibility in the system is required, and as long as protection from infection and ultimately survival of the host, is provided. Describing a specific T-cell pool as ‘good’ memory T cells, capable of persisting and able to undergo vigorous secondary (or tertiary, etc.) expansion upon restimulation, may be a useful descriptive compromise.33
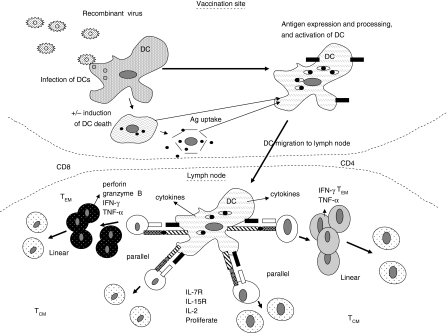
Figure 1.
T-cell priming associated with viral vector immunization. Potential pathways leading to T-cell responses [effector-memory T cells (TEM) and central-memory T cells (TCM)] are generated by viral vector vaccines. Viruses may infect dendritic cells (DCs) for direct priming of T cells, or infected DCs may die and provide antigens and activation signals for cross-priming via other DCs. DCs migrate to local lymph nodes (LNs) and can prime TEM and TCM cells simultaneously in the parallel pathway, or can prime TEM cells, which differentiate into TCM cells in the linear pathway. TEM rapidly produce effector molecules. TCM possess receptors for homeostatic proliferation. Ag, antigen; IFN-γ, interferon-γ; IL, interleukin; TNF-α, tumour necrosis factor-α.
The process by which TEM and TCM are generated is also one where opinion is divided. However, in vaccine design it is important to consider how these subsets are generated (Fig. 1), and if protective immunity is provided by one subset rather than another. A few theories have been proposed. The linear hypothesis suggests that TEM are generated from naïve T cells and then differentiate, over time, into TCM,34 possibly following multiple cell divisions. Another theory proposes parallel generation, where either TEM or TCM are generated from naïve T cells,35 depending on the strength of the priming signal received by the T cells. Stronger signals give rise to TEM, whereas weaker TCM, and cells receiving either extreme of signal, fail to survive. A more recent suggestion is that naïve T cells become intermediates between TEM and TCM before they become either.36 Regarding protection from infection, the Leishmania model suggests that both T-cell types are capable of protection,37 whereas the lymphocytic choriomeningitis virus (LCMV) model favours IL-127high cells (i.e. long-term memory).38 It seems that the precise nature of the challenge may determine whether TEM or TCM are more efficacious. Infectious challenges that give rise to high antigen concentrations, together with innate-activating PAMPs, are probably able to cause, via APCs, the activation and mobilization of TCM and their conversion to TEM. Infections that provide little antigen, and ‘danger signals’, such as certain viral and parasitic infections (e.g. liver stage of malaria), and similarly for cancer, are more likely to require TEM, poised for molecule release upon recognition of antigen on infected tissue cells (often non-APC), and in sufficient numbers to eliminate the infected cells.
Being able to define and reduce the time interval between prime and boost vaccinations would be highly desirable in vaccine design. This depends somewhat on the rate of generation of memory T cells. DCs pulsed with peptide have been shown to generate CD8+ T cells with a ‘good’ memory phenotype in only 4–6 days.39 Furthermore, the presence of inflammatory cytokines, such as IFN-γ, during T-cell priming, causes rapid contraction of the T-cell population.29,39 The memory T cells can be rapidly boosted with various forms of vaccine, and within only a short period of time (e.g. 6 days) after priming, to produce high numbers of TEM and TCM that are protective against Listeria challenge. The further benefits of a prime–boost regime have been emphasized by the findings that memory T cells generated after a secondary vaccination retain the TEM phenotype for longer and are more protective than T cells generated by priming alone.40
Vaccines that elicit protective memory T-cell responses against malaria
Our laboratory has recently carried out a number of studies investigating the generation, in mice and humans, of memory T cells against the liver stage of malaria, and associated protection from infectious challenge. The antigens encoded by the pox and adenovirus vectors are the pre-erythrocytic/liver stage circumsporozoite (CS) and thrombospondin-related adhesion protein antigens. In mice, using the immunodominant Pb9 CD8+ epitope from CS as the antigen, antimalarial efficacy has been demonstrated against the murine malaria Plasmodium berghei, using combinations of DNA, MVA, FP9 and adenovirus. This protection was associated with strong IFN-γ responses against the Pb9 peptide, as measured by ex vivo enzyme-linked immunosorbent spot-forming cell assay (ELISPOT) shortly after the final booster vaccination (Table 1).
Table 1.
Immunogenicity and protective efficacy against malaria sporozoite challenge from representative studies carried out in our laboratory
| Vaccination regime | |||||||
|---|---|---|---|---|---|---|---|
| Mouse | Human | ||||||
| DF | DM | FM | MF | AM1 | D(D)DM | FFM | |
| IFN-γ spot-forming cells/106 spleen cells (mouse) or PBMC (human) | 750 | 1200 | 3200 | 3100 | 1500 | 1200 CD4 | 400–500 CD4/CD8 |
| Percentage of individuals protected from challenge with sporozoites | 12·5 | 17·5 | 67·5 | 37·5 | 100 | ||
| Percentage reduction in infected liver cells | 80 | 92 | |||||
Separate study.
A, adenovirus priming; D, DNA priming; F, FP9 priming; IFN-γ, interferon-γ; M, modified vaccinia Ankara (MVA) boosting; PBMC, peripheral blood mononuclear cells.
Priming was multiple in the human studies.
AdHu5-CSP has been an effective vaccine for generating T cells and protection against P. yoelii (another murine malaria) challenge in mice,41 and this has also been shown for AdHu35-CSP.42 We have recently demonstrated, using Pb9 peptide stimulation and intracellular cytokine staining combined with surface phenotyping, that single and multiple vaccinations with different vectors are able to generate differing memory T-cell profiles.43 Poxviruses tended to generate responses that are of the TCM type (CD62L+ IL-2high CD127+), peaking at 7 days, whereas adenoviruses generated overall higher T-cell numbers, with a greater proportion of TEM responses (CD62Lnull/low CD127+ IL-2low in combination with the CD43 activation marker and the granzyme B cytotoxic molecule), which peaked at 20 days. This difference could be a result of the antigen persistence associated with these vectors, as shown for other systems where antigen causes the conversion from TCM to TEM or a preponderance of TEM.44 Single vaccinations with poxviruses, in particular MVA, may give rise to a rapid expression and clearance of antigen, promoting the generation of memory responses. Indeed, FP9 gave somewhat more of a TEM memory response than MVA, perhaps reflecting its longer persistence. Furthermore, evidence suggests that MVA, in particular, is highly effective at boosting previously primed T-cell responses generated by DNA, FP9 or adenovirus (Table 1), and we have also seen this with other adenovirus serotypes following pox priming (A. Reyes-Sandoval, unpublished). The antigen persistence associated with adenoviruses (single vaccination) favours more the generation of TEM at the time at which the cells were tested. It is a possibility that a viral vector which persists for some time may allow not only priming of a T-cell response, but also boosting within the same vaccination inoculation. This process may also reduce memory cell generation through IFN-γ feedback from generated TEM signalling, which enhances contraction in cell numbers. Overall, greater protection from malarial sporozoite challenge was observed with the adenovirus vector vaccines with single vaccination.
In humans it appears that CD25+ FoxP3+ regulatory T cells may be generated during infection with malaria.45 Furthermore, a role of regulatory T cells that express CD25 in controlling vaccination with viral vectors against malaria was demonstrated in depletion experiments in mice.46 This indicates further scope for improvement in vaccination regimes, especially where malaria is endemic.
In our human studies, immunogenicity and efficacy of prime–boost regimens have also been demonstrated, with FP9/FP9/MVA (FFM) being the most effective to date (Table 1), the double prime with FP9 being particularly important (Fig. 2). Because we were working with relatively fewer T cells than in mice, we had to utilize a cell-expansion technique – cultured ELISPOT – to detect TCM cells and only following multiple prime–boost vaccinations. We propose that in broad terms, and in our human system, the cultured ELISPOT technique allows the proliferation and measurement of TCM (which differentiate into IFN-γ-secreting TEM, and which can be removed by preculture CCR7+ cell depletion), whereas ex vivo ELISPOT measures TEM. This may differ from mouse systems where both TEM and TCM clearly secrete IFN-γ. We found that human TCM responses against the thrombospondin-related adhesion protein antigen, following prime–boost regimes with poxviruses and at the time of challenge, were associated with protection against sporozoite challenge, whereas TEM, as measured by ex vivo ELISPOT, were less clearly associated.47 TCM may have a greater capacity for expansion upon challenge compared with TEM and could play more of a role in P. falciparum challenge in humans where the liver stage lasts for 6–8 days. Conversely, TEM might be more important at reducing murine infection with P. berghei because the liver stage lasts for only 2 days. TEM and TCM may also have differential access to the site of infection, namely the liver.48 Recent studies in humans, from our laboratory, suggest that vaccination regimes, involving alternating viral vectors, may be more effective at generating long-term memory responses,49 perhaps by reduced interference by antivector T-cell immunity, which may also be causing IFN-γ-induced contraction of the specific T-cell pool. The latter study was in a malaria-endemic area, where levels of immunity already exist, which may or may not be advantageous for vaccination. The large capacity of poxviruses to accommodate recombinant genes has allowed the generation of multiantigen multistage vaccines, such as NYVAC-Pf7 and pox L3-SEPTL, the former of which generated some protection,50 and the latter of which51 is currently in clinical trials. Striving to express the protozoan antigens appropriately in mammalian cells remains of importance.
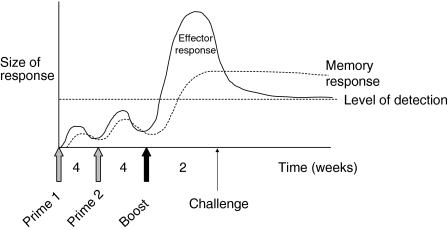
Figure 2.
Possible kinetics of vaccine regimen-induced immune response in humans. Two prime immunizations with vaccines such as recombinant DNA or FP9 allow the generation of sufficient T cells to then be boosted significantly by modified vaccinia Ankara (MVA), such that effector T-cell responses are easily detected and are potentially protective from infectious challenge.
Conclusions
The generation of memory T cells is at the heart of numerous vaccination strategies against intracellular pathogens and cancer. However, there are very few successfully deployed T-cell-inducing vaccines for humans as a result of the complicated nature of generating T-cell responses. A prime–boost vaccination regime, using recombinant viral vectors, may provide one avenue against infections such as the liver stage of malaria. Evidence suggests that effective future regimens may include a prime with a vaccine which generates ‘good’ memory responses, followed by a boost with a vaccine that generates a strong secondary TEM and TCM (such as combinations of adenovirus and MVA). As well as using adenovirus vectors from non-human sources (simian) to avoid neutralizing anti-adenoviral antibodies, weaker anti-adenoviral T-cell responses may reduce the IFN-γ milieu and avoid overt contraction of the T-cell population. Alternating vectors, as well as using heterologous vectors, should also generate improved immune responses. Overall, preclinical studies in models need to be carried out in parallel with clinical trials in humans, with either side learning from each other, if progress is to be made within as short a space of time as possible. Malaria certainly warrants the latter.
Acknowledgments
We are grateful to Adrian Hill for comments on the manuscript and for his support. We acknowledge funding support from the Welcome Trust, Medical Research Council, and European Malaria Vaccine Initiative. We apologise to those groups whose work we have been unable to describe because of space limitations.
References
- 1.Hill AV. Pre-erythrocytic malaria vaccines: towards greater efficacy. Nat Rev Immunol. 2006;6:21–32. doi: 10.1038/nri1746. [DOI] [PubMed] [Google Scholar]
- 2.Todryk SM, Walther M. Building better T cell-inducing malaria vaccines. Immunology. 2005;115:163–9. doi: 10.1111/j.1365-2567.2005.02154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Church LW, Le TP, Bryan JP, et al. Clinical manifestations of Plasmodium falciparum malaria experimentally induced by mosquito challenge. J Infect Dis. 1997;175:915–20. doi: 10.1086/513990. [DOI] [PubMed] [Google Scholar]
- 4.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–7. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 5.Moss B. Vaccinia virus: a tool for research and vaccine development. Science. 1991;252:1662–7. doi: 10.1126/science.2047875. [DOI] [PubMed] [Google Scholar]
- 6.Norbury CC, Malide D, Gibbs JS, Bennink JR, Yewdell JW. Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo. Nat Immunol. 2002;3:265–71. doi: 10.1038/ni762. [DOI] [PubMed] [Google Scholar]
- 7.van Stipdonk MJ, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4:361–5. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 8.McShane H, Pathan AA, Sander CR, Keating SM, Gilbert SC, Huygen K, Fletcher HA, Hill AV. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med. 2004;10:1240–4. doi: 10.1038/nm1128. [DOI] [PubMed] [Google Scholar]
- 9.Dorrell L, Yang H, Ondondo B, et al. Expansion and diversification of virus-specific T cells following immunization of human immunodeficiency virus type 1 (HIV-1)-infected individuals with a recombinant modified vaccinia virus Ankara/HIV-1 Gag vaccine. J Virol. 2006;80:4705–16. doi: 10.1128/JVI.80.10.4705-4716.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McConkey SJ, Reece WH, Moorthy VS, et al. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat Med. 2003;9:729–35. doi: 10.1038/nm881. [DOI] [PubMed] [Google Scholar]
- 11.Smith CL, Dunbar PR, Mirza F, et al. Recombinant modified vaccinia Ankara primes functionally activated CTL specific for a melanoma tumor antigen epitope in melanoma patients with a high risk of disease recurrence. Int J Cancer. 2005;113:259–66. doi: 10.1002/ijc.20569. [DOI] [PubMed] [Google Scholar]
- 12.Blanchard TJ, Alcami A, Andrea P, Smith GL. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J Gen Virol. 1998;79:1159–67. doi: 10.1099/0022-1317-79-5-1159. [DOI] [PubMed] [Google Scholar]
- 13.Le Bon A, Durand V, Kamphuis E, Thompson C, Bulfone-Paus S, Rossmann C, Kalinke U, Tough DF. Direct stimulation of T cells by type I IFN enhances the CD8+ T cell response during cross-priming. J Immunol. 2006;176:4682–9. doi: 10.4049/jimmunol.176.8.4682. [DOI] [PubMed] [Google Scholar]
- 14.Kastenmuller W, Drexler I, Ludwig H, Erfle V, Peschel C, Bernhard H, Sutter G. Infection of human dendritic cells with recombinant vaccinia virus MVA reveals general persistence of viral early transcription but distinct maturation-dependent cytopathogenicity. Virology. 2006;350:276–88. doi: 10.1016/j.virol.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 15.Behboudi S, Moore A, Gilbert SC, Nicoll CL, Hill AV. Dendritic cells infected by recombinant modified vaccinia virus Ankara retain immunogenicity in vivo despite in vitro dysfunction. Vaccine. 2004;22:4326–31. doi: 10.1016/j.vaccine.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Hutchings CL, Gilbert SC, Hill AV, Moore AC. Novel protein and poxvirus-based vaccine combinations for simultaneous induction of humoral and cell-mediated immunity. J Immunol. 2005;175:599–606. doi: 10.4049/jimmunol.175.1.599. [DOI] [PubMed] [Google Scholar]
- 17.Brown M, Zhang Y, Dermine S, et al. Dendritic cells infected with recombinant fowlpox virus vectors are potent and long-acting stimulators of transgene-specific class I restricted T lymphocyte activity. Gene Ther. 2000;7:1680–9. doi: 10.1038/sj.gt.3301288. [DOI] [PubMed] [Google Scholar]
- 18.Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther. 2004;10:616–29. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Chirmule N, Gao GP, Qian R, Croyle M, Joshi B, Tazelaar J, Wilson JM. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol Ther. 2001;3:697–707. doi: 10.1006/mthe.2001.0329. [DOI] [PubMed] [Google Scholar]
- 20.Morelli AE, Larregina AT, Ganster RW, et al. Recombinant adenovirus induces maturation of dendritic cells via an NF-kappaB-dependent pathway. J Virol. 2000;74:9617–28. doi: 10.1128/jvi.74.20.9617-9628.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molinier-Frenkel V, Prevost-Blondel A, Hong SS, Lengagne R, Boudaly S, Magnusson MK, Boulanger P, Guillet JG. The maturation of murine dendritic cells induced by human adenovirus is mediated by the fiber knob domain. J Biol Chem. 2003;278:37175–82. doi: 10.1074/jbc.M303496200. [DOI] [PubMed] [Google Scholar]
- 22.Philpott NJ, Nociari M, Elkon KB, Falck-Pedersen E. Adenovirus-induced maturation of dendritic cells through a PI3 kinase-mediated TNF-alpha induction pathway. Proc Natl Acad Sci USA. 2004;101:6200–5. doi: 10.1073/pnas.0308368101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y, Ertl HC, Wilson JM. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–42. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 24.Varnavski AN, Calcedo R, Bove M, Gao G, Wilson JM. Evaluation of toxicity from high-dose systemic administration of recombinant adenovirus vector in vector-naive and pre-immunized mice. Gene Ther. 2005;12:427–36. doi: 10.1038/sj.gt.3302347. [DOI] [PubMed] [Google Scholar]
- 25.Fitzgerald JC, Gao GP, Reyes-Sandoval A, et al. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J Immunol. 2003;170:1416–22. doi: 10.4049/jimmunol.170.3.1416. [DOI] [PubMed] [Google Scholar]
- 26.Reyes-Sandoval A, Fitzgerald JC, Grant R, et al. Human immunodeficiency virus type 1-specific immune responses in primates upon sequential immunization with adenoviral vaccine carriers of human and simian serotypes. J Virol. 2004;78:7392–9. doi: 10.1128/JVI.78.14.7392-7399.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rea D, Schagen FH, Hoeben RC, Mehtali M, Havenga MJ, Toes RE, Melief CJ, Offringa R. Adenoviruses activate human dendritic cells without polarization toward a T-helper type 1-inducing subset. J Virol. 1999;73:10245–53. doi: 10.1128/jvi.73.12.10245-10253.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan PH, Beutelspacher SC, Xue SA, et al. Modulation of human dendritic-cell function following transduction with viral vectors: implications for gene therapy. Blood. 2005;105:3824–32. doi: 10.1182/blood-2004-10-3880. [DOI] [PubMed] [Google Scholar]
- 29.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3:619–26. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 30.Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat Immunol. 2004;5:809–17. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- 31.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 32.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 33.Badovinac VP, Harty JT. Programming, demarcating, and manipulating CD8+ T-cell memory. Immunol Rev. 2006;211:67–80. doi: 10.1111/j.0105-2896.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 34.Opferman JT, Ober BT, Ashton-Rickardt PG. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science. 1999;283:1745–8. doi: 10.1126/science.283.5408.1745. [DOI] [PubMed] [Google Scholar]
- 35.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–7. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 36.Moulton VR, Farber DL. Committed to memory: lineage choices for activated T cells. Trends Immunol. 2006;27:261–7. doi: 10.1016/j.it.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Scott P, Artis D, Uzonna J, Zaph C. The development of effector and memory T cells in cutaneous leishmaniasis: the implications for vaccine development. Immunol Rev. 2004;201:318–38. doi: 10.1111/j.0105-2896.2004.00198.x. [DOI] [PubMed] [Google Scholar]
- 38.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–34. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 39.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med. 2005;11:748–56. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 40.Jabbari A, Harty JT. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J Exp Med. 2006;203:919–32. doi: 10.1084/jem.20052237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodrigues EG, Zavala F, Eichinger D, Wilson JM, Tsuji M. Single immunizing dose of recombinant adenovirus efficiently induces CD8+ T cell-mediated protective immunity against malaria. J Immunol. 1997;158:1268–74. [PubMed] [Google Scholar]
- 42.Ophorst OJ, Radosevic K, Havenga MJ, Pau MG, Holterman L, Berkhout B, Goudsmit J, Tsuji M. Immunogenicity and protection of a recombinant human adenovirus serotype 35-based malaria vaccine against Plasmodium yoelii in mice. Infect Immun. 2006;74:313–20. doi: 10.1128/IAI.74.1.313-320.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reyes-Sandoval A, Sridar S, Moore AC, Gilbert SC, Gao GP, Wilson JM, Hill AV. Simian adenoviral vectors as malaria vaccines: single dose immunogenicity and protective efficacy against P. berghei. Proc Natl Acad Sci USA. 2007 in press. [Google Scholar]
- 44.Harari A, Vallelian F, Meylan PR, Pantaleo G. Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. J Immunol. 2005;174:1037–45. doi: 10.4049/jimmunol.174.2.1037. [DOI] [PubMed] [Google Scholar]
- 45.Walther M, Tongren JE, Andrews L, et al. Upregulation of TGF-beta, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity. 2005;23:287–96. doi: 10.1016/j.immuni.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 46.Moore AC, Gallimore A, Draper SJ, Watkins KR, Gilbert SC, Hill AV. Anti-CD25 antibody enhancement of vaccine-induced immunogenicity: increased durable cellular immunity with reduced immunodominance. J Immunol. 2005;175:7264–73. doi: 10.4049/jimmunol.175.11.7264. [DOI] [PubMed] [Google Scholar]
- 47.Keating SM, Bejon P, Berthoud T, et al. Durable human memory T cells quantifiable by cultured enzyme-linked immunospot assays are induced by heterologous prime boost immunization and correlate with protection against malaria. J Immunol. 2005;175:5675–80. doi: 10.4049/jimmunol.175.9.5675. [DOI] [PubMed] [Google Scholar]
- 48.Heydtmann M, Hardie D, Shields PL, Faint J, Buckley CD, Campbell JJ, Salmon M, Adams DH. Detailed analysis of intrahepatic CD8 T cells in the normal and hepatitis C-infected liver reveals differences in specific populations of memory cells with distinct homing phenotypes. J Immunol. 2006;177:729–38. doi: 10.4049/jimmunol.177.1.729. [DOI] [PubMed] [Google Scholar]
- 49.Bejon P, Kai OK, Mwacharo J, et al. Alternating vector immunizations encoding pre-erythrocytic malaria antigens enhance memory responses in a malaria endemic area. Eur J Immunol. 2006;36:2264–72. doi: 10.1002/eji.200636187. [DOI] [PubMed] [Google Scholar]
- 50.Ockenhouse CF, Sun PF, Lanar DE, et al. Phase I/IIa safety, immunogenicity, and efficacy trial of NYVAC-Pf7, a pox-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. J Infect Dis. 1998;177:1664–73. doi: 10.1086/515331. [DOI] [PubMed] [Google Scholar]
- 51.Prieur E, Gilbert SC, Schneider J, Moore AC, Sheu EG, Goonetilleke N, Robson KJ, Hill AV. A Plasmodium falciparum candidate vaccine based on a six-antigen polyprotein encoded by recombinant poxviruses. Proc Natl Acad Sci USA. 2004;101:290–5. doi: 10.1073/pnas.0307158101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson RJ, Hannan CM, Gilbert SC, et al. Enhanced CD8+ T cell immune responses and protection elicited against Plasmodium berghei malaria by prime boost immunization regimens using a novel attenuated fowlpox virus. J Immunol. 2004;172:3094–100. doi: 10.4049/jimmunol.172.5.3094. [DOI] [PubMed] [Google Scholar]
- 53.Gilbert SC, Schneider J, Hannan CM, Hu JT, Plebanski M, Sinden R, Hill AV. Enhanced CD8 T cell immunogenicity and protective efficacy in a mouse malaria model using a recombinant adenoviral vaccine in heterologous prime-boost immunisation regimes. Vaccine. 2002;20:1039–45. doi: 10.1016/s0264-410x(01)00450-9. [DOI] [PubMed] [Google Scholar]
- 54.Vuola JM, Keating S, Webster DP, Berthoud T, Dunachie S, Gilbert SC, Hill AV. Differential immunogenicity of various heterologous prime-boost vaccine regimens using DNA and viral vectors in healthy volunteers. J Immunol. 2005;174:449–55. doi: 10.4049/jimmunol.174.1.449. [DOI] [PubMed] [Google Scholar]
- 55.Webster DP, Dunachie S, Vuola JM, et al. Enhanced T cell-mediated protection against malaria in human challenges by using the recombinant poxviruses FP9 and modified vaccinia virus Ankara. Proc Natl Acad Sci USA. 2005;102:4836–41. doi: 10.1073/pnas.0406381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bejon P, Andrews L, Andersen RF, et al. Calculation of liver-to-blood inocula, parasite growth rates, and preerythrocytic vaccine efficacy, from serial quantitative polymerase chain reaction studies of volunteers challenged with malaria sporozoites. J Infect Dis. 2005;191:619–26. doi: 10.1086/427243. [DOI] [PubMed] [Google Scholar]