Abstract
Human natural killer (NK) cells form a circulating population in a state of dynamic homeostasis. We investigated NK cell homeostasis by labelling dividing cells in vivo using deuterium-enriched glucose in young and elderly healthy subjects and patients with viral infection. Following a 24-hr intravenous infusion of 6,6-D2-glucose, CD3– CD16+ NK cells sorted from peripheral blood mononuclear cells (PBMC) by fluorescence-activated cell sorter (FACS) were analysed for DNA deuterium content by gas chromatography mass spectrometry to yield minimum estimates for proliferation rate (p). In healthy young adults (n = 5), deuterium enrichment was maximal ∼10 days after labelling, consistent with postmitotic maturation preceding circulation. The mean (± standard deviation) proliferation rate was 4·3 ± 2·4%/day (equivalent to a doubling time of 16 days) and the total production rate was 15 ± 7·6 × 106 cells/l/day. Labelled cells disappeared from the circulation at a similar rate [6·9 ± 4·0%/day; half-life (T½) <10 days]. Healthy elderly subjects (n = 8) had lower proliferation and production rates (P = 2·5 ± 1·0%/day and 7·3 ± 3·7 × 106 cells/l/day, respectively; P = 0·04). Similar rates were seen in patients chronically infected with human T-cell lymphotropic virus type I (HTLV-I) (P = 3·2 ± 1·9%/day). In acute infectious mononucleosis (n = 5), NK cell numbers were increased but kinetics were unaffected (P = 2·8 ± 1·0%/day) a mean of 12 days after symptom onset. Human NK cells have a turnover time in blood of about 2 weeks. Proliferation rates appear to fall with ageing, remain unperturbed by chronic HTLV-I infection and normalize rapidly following acute Epstein–Barr virus infection.
Keywords: natural killer cells, ageing, acute infectious mononucleosis, Epstein–Barr virus, human T-cell lymphotropic virus type I
Introduction
Natural killer (NK) cells are bone-marrow-derived lymphocytes that form part of the innate immune system.1,2 They provide early non-specific defence against viral infections, particularly in the early phase before the adaptive immune system has been activated,3 and have a role in tumour cell detection and elimination.4 In humans, the importance of NK cells in protection against viral infections is demonstrated by the susceptibility of individuals with NK cell deficiencies to infections such as cytomegalovirus, Epstein–Barr virus (EBV), varicella-zoster virus (VZV) and herpes simplex virus.5 An extreme case occurs with fatal childhood primary EBV infection in X-linked proliferative disease, where a defect in the gene encoding a protein pivotal in NK cell activation (signalling lymphocytic activating molecule (SLAM)-associated protein (SAP)) results in the inability of NK cells to kill EBV-infected cells.6 In mice, depletion of NK cells results in enhanced susceptibility to viral infections such as murine cytomegalovirus (MCMV) infection.7 Thus the maintenance of adequate numbers of functional NK cells is crucial in maintaining defence against viral infections.
NK cells account for about 10–20% of peripheral blood lymphocytes at any one time. Most (∼ 90%) express high levels of FCγRIII (CD16) and are CD56dim, although a CD16dim/neg CD56bright subpopulation with distinct functional attributes has also been described. NK functional responses occur in tissues and depend on the balance of activating and inhibitory signals. Inhibition, primarily signalled through interaction of major histocompatibility complex (MHC)-1 with inhibitory NK receptors on the NK cell surface, prevents autoaggression. Once activated, NK cells are cytolytic and they thus show functional similarities with cytotoxic T lymphocytes (CTLs). However, unlike CTLs, they do not progress through a prior activation and proliferation stage before acquiring cytolytic potential. For NK cell populations, proliferation and expansion mostly precede activation, whereas for CD8+ T lymphocytes most (clonal) expansion follows activation (although, of course, generation of naïve CD8+ T-cell populations depends upon proliferation of immature cells.) Thus, in NK cell physiology, regulation of proliferation may be considered a key ‘upstream’ event, the role of which is to maintain sufficient NK cells in the circulation and in tissues to provide for rapid non-specific responses.
Proliferation of NK cells primarily occurs in the bone marrow from the same common progenitor cells as T lymphocytes.8,9 Immature cells undergo a serial maturation process with further significant expansion during the latter stages of this process. Finally, functional capabilities are acquired [associated with expression of Mac-1 and CD43 in the mouse] before cells are released into the circulation.1 Further division of mature cells may occur on stimulation, although this only occurs at relatively low levels; in mice, according to bromodeoxyuridine (BrdU) studies, only 1–3% of splenic NK cells are dividing.10
Like other lymphocyte populations, NK cells show apparent homeostasis. When transferred to NK-deficient mice, both mature and immature NK cells undergo proliferation, but such cells do not proliferate when transferred into a host with an endogenous NK cell pool.11 Thus it appears that factors such as ‘space’ (the availability of physiological niches), the availability of growth-promoting factors or the presence of cytokines that limit proliferation operate to regulate NK cell numbers.11,12
Homeostasis is the consequence not just of proliferation but also of cellular loss. NK cells disappear from blood either by entering tissues, predominantly the spleen and the liver,13,14 or through cell death. Relatively few NK cells are found in lymph nodes and there is little evidence for recirculation, although cells from the small CD56bright subpopulation of NK cells are found in parafollicular T-cell areas of lymph nodes, where they may play a role in interactions between innate and adaptive immune responses.14 Survival of mature NK cells is cytokine dependent; in mice, interleukin (IL)-15 appears to prolong survival via the antiapoptotic factor Bcl-2.15 Adoptive transfer experiments and long-term BrdU studies in mice have demonstrated short circulating half-lives of about 7–10 days for mature NK cells.16 In mice, therefore, the NK cell pool is more dynamic than the T-lymphocyte pool, but little is known of the parallel kinetics in humans.
NK cell function and dynamics may be affected by pathophysiological states such as ageing and viral infection. Ageing impairs human NK activity. Several studies have shown age-related reductions in NK-cell-mediated cytotoxic activity (reviewed by Mocchegiani and Malavolta17) which appear to be clinically relevant as they are associated with increased risk of infection or death in cohorts of elderly subjects.17,18 However, this functional impairment is not related to a reduction in NK cell numbers, as NK cell numbers tend to be well maintained or to increase with age.17,19–21 The disparity between NK cells, which are increased, and T cells, which tend to decline in number with ageing, may be related to better preservation of telomere length in NK cells compared with T cells.22 It has been suggested that the increase in NK cell numbers with age, which is the consequence of expansion of the CD56dim (mature) rather than the CD56bright subset,23 may be a compensatory mechanism to offset the reduction in per-cell cytotoxicity.17
Although mature NK cells do not normally divide frequently in vivo, acute viral infections may be associated with substantial proliferation. In the mouse, MCMV infection induces proliferation in two phases: an initial non-specific phase, analogous to ‘bystander proliferation’ in T cells, is generally followed by specific proliferation of Ly49h+ cells.1 Down-regulation of proliferation occurs early (day 6 in MCMV infection) and is associated with the onset of an adaptive response.10 Parallel data in humans are lacking and the cytokine responsiveness of precursors and mature NK cells differs between humans and mice. In patients with acute EBV infection, numbers of CD3– CD56+ CD244+ NK cells in blood are increased, with an increased proportion of CD56bright cells.24 Whether this elevation represents peripheral expansion (by analogy with MCMV infection1), increased bone marrow production from NK precursors, or redistribution between fixed tissue and circulating NK cells remains unclear.
Although there are some murine data on NK cell kinetics, there are no in vivo data on human NK kinetics. We therefore set out to provide preliminary data on human NK cell dynamics using stable (non-radioactive) in vivo labelling with deuterium-labelled glucose.25 As glucose is a precursor for nucleotide pentose ring synthesis, new DNA synthesis can be labelled using this tracer. This approach has been successfully applied to investigate T- and B-lymphocyte kinetics in human studies in health and disease.25–29 Firstly, we set out to investigate the kinetics of normal NK cells in terms of the lag between mitosis and appearance in the circulation, the production rate of new cells, and their disappearance rate from the circulation. Secondly, we investigated the effects of ageing on NK cell dynamics. Thirdly, we explored the effects of acute and chronic viral infection on NK cell dynamics by investigating the impact of acute EBV infection and chronic HTLV-I infection.
Materials and methods
Subjects
In order to study NK cell kinetics in health, to assess the impact of ageing and to investigate the impact of acute and chronic infection, subjects were recruited from four groups: firstly, young healthy subjects [n = 5; three male and two female subjects; median age (range) 22 (19–34) years]; secondly, healthy elderly subjects [n = 8; two male and six female subjects; median age (range) 78 (65–85) years] as previously described;29 thirdly, subjects with chronic HTLV-I infection [n = 5; one male and four female subjects; median age (range) 48 (39–72) years]; and fourthly, five subjects with acute infectious mononucleosis (AIM) as a result of acute EBV infection [n = 5; four male and one female subject; median age (range) 19 (18–21) years, as previously described30]. All subjects with AIM had been previously healthy; all had a positive monospot test and EBV-specific immunoglobulin M (IgM) in the context of fever, pharyngitis and fatigue. Investigation was performed 4–14 days from the onset of symptoms and 0–7 days from maximal symptoms, as judged by the subject. All made a prompt complete clinical recovery subsequent to investigation. All subjects gave written informed consent under protocols approved by the Local Research Ethics Committee and executed in accordance with the declaration of Helsinki.
Labelling
Subjects received ∼1 g/kg deuterium-labelled glucose (6,6-D2-glucose; Cambridge Isotopes, Cambridge, MA) as a primed 24-hr intravenous (i.v.) infusion, as previously described.25 To estimate 2H-enrichment in cellular DNA, 50 ml of heparinized blood was taken on days 3, 4, 10 and 20 or 21 postinfusion in control and acute EBV-infected subjects. In elderly subjects, day 3 samples were omitted to minimize blood-taking and in HTLV-I-infected subjects days 3, 4, 7 and 10 were sampled, or the closest days possible, throughout.
Cell sorting
Peripheral blood mononuclear cells (PBMC) were isolated from blood by Ficoll-Paque (Pharmacia, St Albans, UK) density gradient centrifugation and stained [1 × 107 cells/ml in phosphate-buffered saline (PBS) + 0·2% bovine serum albumin (BSA)] with CD3-RPE (Serotec Ltd, Oxford, UK) before sorting (Moflow flow cytometer; Cytomation, Fort Colins, CO). CD3– cells were further stained with CD16 Cy-Chrome (PharMingen, San Diego, CA) or CD16-Quantum Red (Sigma, St Louis, MO) and sorted into CD3– CD16+ cells; population purities were typically greater than 90%.
Analysis of deuterium enrichment and modelling of proliferation
Enrichment of deuterium in DNA was assayed essentially as previously described25,27 Briefly, the deuterium content of DNA was estimated from analysis of the aldononitrile triacetate derivative of deoxyadenosine, extracted and digested enzymatically by gas chromatography mass spectrometry (HP 6890/5973 GCMS with HP-225 column; Agilent Technologies, Bracknell, UK). Plasma glucose enrichments, taken approximately every 4 hr during infusion, were analysed similarly (mass/charge ratio (m/z) 328 and 330). Results were expressed as the fraction of labelled cells present on each day (F), being the ratio of the enrichment of label in DNA (E) to the mean glucose enrichment over 24 hr ×0·65.25 The proliferation rate (p) was taken as the peak value for F, but this assumes no cell death between proliferation and appearance at day 10 and is therefore a minimum estimate of the true proliferation rate. A disappearance rate constant for labelled cells (d*) was calculated from the slope of the log-plot against time of labelling from the peak value to the last sampling point, assuming exponential decay. This is also a minimum estimate as it assumes no ongoing appearance of labelled cells in blood after day 10. Proliferation and disappearance were expressed as doubling time (T2) and half-life (T½), where T2 = ln2/p and T½ = ln2/d*. Data were expressed as mean ± 1 standard deviation (SD). Comparisons between groups by two-tailed Student's t-tests of log-transformed data were made for age by comparing young and elderly healthy subjects, for HTLV-I infection by comparing HTLV-I subjects with both young and elderly controls, and for EBV infection (AIM) by comparison with the young control group.
Results
Normal NK cell kinetics
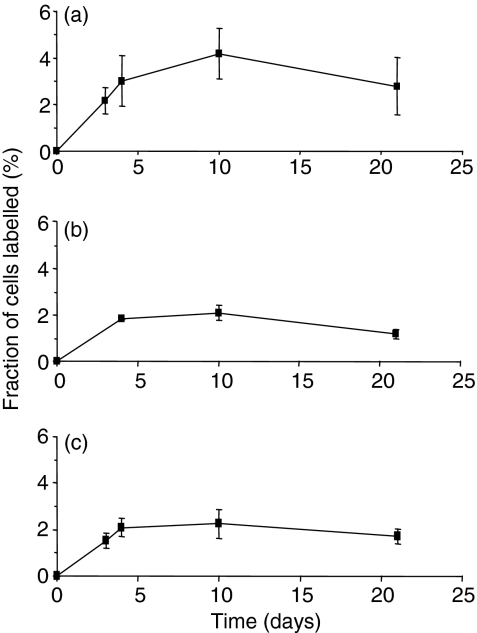
Analysis of deuterium enrichment in DNA of NK cells following labelling in healthy young subjects yielded enrichment–time curves as shown in Fig. 1(a). Three characteristics of these labelling curves were noted. Firstly, peak labelling was delayed from the end of labelling to day 10 in four subjects and to day 21 in one, suggesting a significant postmitotic maturation phase of about 10 days in bone marrow before emergence into peripheral blood (although confidence in estimating the exact duration of this lag time is limited by the number of sampling points). This delay was longer than that seen with T or B lymphocytes using the same protocol, where peak labelling was seen at day 3 or 4 postinfusion.25,27 Interestingly, a significant proportion of labelled cells was found in samples taken at day 3 (mean 2·2%), suggesting either that some cells have very short lag times between mitosis and release or that there is significant division of recirculating mature NK cells in the periphery. Indeed, if one made the extreme assumption that all labelled cells detected at day 3 represent cells arising from division of the mature peripheral NK cell pool, one would conclude that about 50% of newly formed circulating NK cells derive from division in the periphery rather than from bone marrow precursors.
Figure 1.
Labelling of natural killer (NK) cells following infusion of 6,6-2H2-glucose in (a) healthy young subjects, (b) healthy elderly subjects and (c) young subjects with acute Epstein–Barr virus (EBV) infection. Values represent the mean ± standard deviation for labelled cells at time-points after commencement of infusion for 24 hr. Subjects infected with human T-cell lymphotropic virus type I (HTLV-I) are not shown as blood samples were not taken after day 10.
Secondly, mean peak labelling of 4·3 ± 2·4% was observed in this group of subjects, consistent with a mean minimum proliferation rate within this population of at least 4·3%/day (Table 1). (This estimate of proliferation represents a minimum because it assumes no cell death between division and sampling at day 10.) This rate is equivalent to a doubling time (T2) of no more than 16 days. As in T- and B-cell studies, there was significant inter-individual variation (range 1·9–7·7%/days; Table 1). The overall production rate of NK cells was estimated to range between 6 and 25 (mean 15) × 106 cells/day per litre of whole blood (Table 1).
Table 1.
Natural killer (NK) cell kinetics from deuterated glucose labelling
| Group | Subject identifier | NK cell number (× 106 cells/l) | Peak fraction labelled cellsa (%) | Doubling time (T2) (days) | Production rateb (× 106 cells/l/day) | Disappearance rate (d*)c (%/day) | Half-life (T1/2) (days) |
|---|---|---|---|---|---|---|---|
| Young, healthy | C05 | 422 | 5·91 | 12 | 24·9 | 11·1 | 6 |
| C06 | 272 | 7·68 | 9 | 20·9 | 8·9 | 8 | |
| C07 | 599 | 1·88 | 37 | 11·3 | 1·3 | 53 | |
| C08 | 462 | 2·56 | 27 | 11·8 | 4·5 | 16 | |
| C10 | 180 | 3·60 | 19 | 6·5 | 8·8 | 8 | |
| Mean | 387 | 4·33 | 16d | 15·1 | 6·9 | 10d | |
| SD | 164 | 2·42 | 7·6 | 4·0 | |||
| Elderly, healthy | E01 | 298 | 2·08 | 33 | 6·2 | 9·3 | 7 |
| E02 | 181 | 3·45 | 20 | 6·2 | 4·8 | 14 | |
| E03 | 198 | 2·87 | 24 | 5·7 | 6·1 | 11 | |
| E04 | 706 | 0·85 | 81 | 6·0 | 2·5 | 27 | |
| E05 | 588 | 1·22 | 57 | 7·2 | 5·3 | 13 | |
| E06 | 509 | 3·04 | 23 | 15·5 | 8·2 | 9 | |
| E07 | n/a | 3·35 | 21 | n/a | 5·8 | 12 | |
| E08 | 163 | 2·80 | 25 | 4·6 | 7·1 | 10 | |
| Mean | 377 | 2·46 | 28d | 7·3* | 6·1 | 11d | |
| SD | 221 | 0·98 | 3·7 | 2·1 | |||
| HTLV-1-infected | L06 | 148 | 6·22 | 11 | 9·2 | n/a | n/a |
| L08 | 192 | 2·42 | 29 | 4·6 | n/a | n/a | |
| L09 | 220 | 4·03 | 17 | 8·9 | n/a | n/a | |
| L10 | 200 | 2·09 | 33 | 4·2 | n/a | n/a | |
| L11 | 496 | 1·35 | 51 | 6·7 | n/a | n/a | |
| Mean | 251 | 3·22 | 22d | 6·7* | |||
| SD | 139 | 1·94 | 2·3 | ||||
| Young, with AIMe | M01 | 1256 | 3·04 | 23 | 38·1 | n/a | n/a |
| M03 | 1234 | 1·74 | 40 | 21·5 | 1·9 | 37 | |
| M04 | 364 | 4·27 | 16 | 15·5 | 7·1 | 10 | |
| M05 | 2598 | 1·84 | 38 | 47·9 | n/a | n/a | |
| M06 | 619 | 3·09 | 22 | 19·1 | 7·9 | 9 | |
| Mean | 1214* | 2·79 | 25d | 28·4 | 5·6 | 12d | |
| SD | 865 | 1·04 | 13·9 | 3·3 |
The peak fraction of labelled cells gives a minimum estimate of the proliferation rate, p, in percentage/day.
The production rate is expressed per litre of blood.
The disappearance rate estimates represent a minimum value as the model does not account for continued appearance of labelled cells after peak labelling.
Values represent doubling-time/half-life equivalents of the mean p or d (ln2/p or ln2/d), rather than the mean of doubling-time/half-lives which have a skewed distribution.
For acute infectious mononucleosis (AIM) subjects, the NK cell number shown is on the day of labelling.
P < 0·05 versus young healthy group by two-tailed Student's t-test.
Thirdly, labelled cells disappeared from the circulation between days 10 and 21 in four of the subjects. The mean disappearance rate constant for labelled cells (d*) in these subjects was 6·4%/day, although this is a minimum estimate, because it assumes no further appearance of labelled cells after day 10. The equivalent half-life, about 12 days, is similar to that found in murine studies.16
NK cell kinetics in elderly humans
When elderly subjects were studied, NK cell counts in blood were found to be similar to those of young controls (Table 1). Similar patterns of enrichment were obtained (Fig. 1b) to those found in young subjects, but peak enrichment and estimated proliferation rates tended to be lower (2·5%/day versus 4·3%/day; Table 1) and the mean total production rate was significantly reduced compared with the young controls (7·3 versus 15·1 × 106 cells/l/day; P =0·03; Table 1). These data suggest that NK cell production from bone marrow may be impaired by ageing.
The effect of HTLV-I infection on NK cell dynamics
Infection with the human retrovirus HTLV-I did not appear to affect NK cell numbers, which were similar to those in the young and elderly healthy subjects. Peak labelling (mean 3·2%/day) tended to be reduced and production rates were significantly lower than in young controls (Table 1; P = 0·03) but similar to those in the healthy elderly subjects. As HTLV-I subjects were nearer in age to the elderly than to the young healthy group, this reduction may be age-related. Follow-up blood samples were not taken after day 10 in this group and thus no disappearance rates were estimated; an underestimate of p caused by peak enrichment after day 10 cannot be excluded but peak enrichments beyond day 10 were not seen in the other groups.
The effect of acute viral infection on NK cell dynamics
In five young individuals with clinically diagnosed AIM, mean NK cell numbers in blood were increased by a factor of about three compared with values for uninfected young subjects (Table 1). However, enrichment curves (Fig. 1c) and labelling and disappearance rates (Table 1) in AIM subjects were very similar to those for the healthy young controls, except that, in three of the five AIM subjects, peak labelling was seen earlier (day 3 or 4 rather than day 10 postinfusion), suggesting either shortening of the lag time between division and release from marrow or increased division of peripheral, as opposed to marrow-derived, NK cells. Although this lack of increased enrichment in NK cells in AIM is surprising, when increased cell numbers were taken into account, mean production rates tended to be higher than in healthy young controls, although not reaching statistical significance in this small group (P = 0·08). There was good correlation of the total NK production rate with the clinical severity score26 and with the total blood lymphocyte count (r = 0·81 and r = 0·76, respectively), although with only five subjects these relationships were not statistically significant.
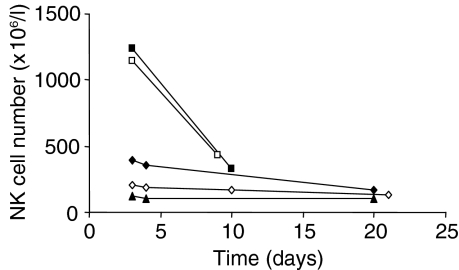
The surprising lack of enrichment in NK cells in AIM may be related to the timing of the study. Serial NK cell counts following the day on which the label was given (Fig. 2) showed that, at the time of investigation, the circulating NK cell pool was contracting in all subjects, rapidly in two individuals.
Figure 2.
Time–course of natural killer (NK) cell numbers in acute infectious mononucleosis. Time is from the beginning of in vivo labelling. Curves represent individual subjects: M01 (filled square), M03 (filled diamond), M04 (triangle), M05 (open square) and M06 (open diamond).
Discussion
This study has demonstrated the dynamic nature of NK cell homeostasis in humans. In healthy young individuals, substantial daily turnover, with a mean of 4%/day in the five subjects studied here, equivalent to a doubling time/half-life of the order of 1–2 weeks, is fuelled by the total production of about 70 × 106 NK cells/day, taking blood volume into account. This high rate of NK cell turnover is analogous to that seen in mice, where adoptive transfer experiments suggest a half-life for mature NK cells of 7–10 days.1
True NK cell turnover may be greater than the estimates given in this preliminary study, as estimates of p do not account for cell death before measurement of labelled cells in the circulation and estimates of d* only relate to labelled cells (not to the whole population)25 and do not account for ongoing late release of labelled cells. These factors will contribute to uncertainty in the minimum values given to p and d* and thus to inter-individual variation. This limitation would be mitigated by more frequent sampling following labelling; additional time-point estimates of enrichment would allow more complete modelling of the data, as has been applied to T-cell kinetics,25 and thus more accurate estimation of kinetic parameters. Expression of life span as doubling time/half-life tends to exaggerate the apparent variance because of the reciprocal relationship with turnover. Clinically, there were no apparent differences in health status among the ‘healthy’ young controls to explain such variation, although subclinical infection cannot be excluded. We therefore conclude that most of the inter-individual variation seen in this study is probably physiological, as seen with T cells.25 Indeed, in this small number of subjects there was a good correlation between proliferation rates for NK cells and for CD4+ CD45R0+ T cells (r = 0·89, n = 5, P = 0·045), but not for other T-cell subtypes.
Both ageing and viral infection have an impact on NK cell kinetics. In the elderly subjects, NK cell numbers were well preserved, although in this small number of subjects we did not see an increase in NK cell numbers as described in some previous studies.17,19–21 However, we did find evidence for a reduction in total NK cell production rates of about 50%, despite the small numbers of subjects. This combination of reduced production rate with preserved cell numbers would be most consistent with an increased proportion of long-lived NK cells in the elderly subjects and may be related to an increased proportion of CD56dim cells, as previously reported in elderly subjects.23 Although such an increased proportion of long-lived cells might be expected to result in an increase in the estimated disappearance rate, d*, no change was seen in the elderly subjects (Table 1). However, the value given to d* represents the disappearance rate of dividing cells only,25 and thus for it to remain unchanged does not exclude an increase in the proportion of non-dividing (and thus unlabelled and ‘invisible’) long-lived cells in the NK population. This is the first demonstration of impaired NK production in elderly people, and these age-related changes in NK kinetics merit further investigation.
The effect of ageing on NK kinetics probably also explains the lower NK production rates in subjects with chronic HTLV-I infection when compared with young controls. The absence of a proliferative response in this condition contrasts with the accelerated turnover rates of CD8 cells seen in the same group (B. Asquith et al. 2005, unpublished results), consistent with independent regulation of T-cell and NK cell proliferation, and may be related to impaired IL-2 responsiveness, as seen in NK cells from elderly subjects.23
The lack of increased NK cell labelling in acute EBV infection is surprising and contrasts with the dramatic proliferative responses seen in mice with MCMV infection1 and with the massive CD4 and CD8 lymphocyte proliferative response occurring at the same time in the same individuals in whom proliferation rates for CD8+ CD45R0+ T cells were 100 times normal.26 Despite this lack of labelling, there did appear to be expansion of the circulating NK cell pool, consistent with, although less marked than, the findings of a previous study in AIM in which NK cell numbers at diagnosis were increased 6-fold24 and the total production rate of NK cells did appear to be related to clinical severity.
How then do we explain the apparent lack of proliferation of NK cells in AIM? The most likely explanation is that NK cell expansion had indeed occurred in our subjects but was resolving by the time our investigations were performed. Despite recruiting and investigating subjects as early as logistically possible, median time to investigation was 12 days (range 4–35 days) from onset of symptoms and 5 days (range 0–28 days) from the time of maximal symptoms. NK cell numbers in blood were falling at the time of the study (Fig. 2), suggesting rapid contraction of the circulating NK cell pool, analogous to the down-regulatory ‘phase-III’ of the murine response to MCMV (from about day 6 postinfection).1 Activation of the adaptive immune response, which was very evident in our AIM subjects, down-regulates NK cell proliferation and there is evidence that IL-21 mediates this effect.31 If this is the case, much earlier studies would be required to demonstrate the magnitude of the NK proliferative response to EBV, which may not be feasible in human clinical investigation; indeed, the phase of NK expansion may even precede symptoms and clinical presentation, which are largely related to the excessive lymphocytic response induced by EBV.
An alternative, but not mutually exclusive, explanation for the paucity of NK proliferative response in acute EBV would be that the proliferative response for NK cells is genuinely much smaller than that for T cells. Whereas T-cell responses are clonally restricted, NK responses are not, activation occurring via multiple germline-encoded receptors with considerable overlap;1,32 thus a large number of NK cells may have responded to EBV infection, within the context of an already dynamic NK cell pool, without extensive clonal expansion.
The proliferation rates measured in these studies represent an average value for the whole NK cell population defined by a CD3– CD16+ phenotype. However, NK cells are not homogenous and include two important subpopulations which may have distinct kinetics. Firstly, some NK cells are CD56+ CD83+ CCR7+ CD25+ and have helper activity.33 Such cells are likely to home preferentially to the lymph node as they express high levels of CCR7 and this may affect their dwell time in the circulation; further studies with additional antibody staining and sorting would elucidate whether this affects their dynamics. Secondly, the CD56bright subpopulation differs from the rest of the NK cell pool in IL-2 responsiveness and interferon (IFN)-γ production.14 These cells may have been underrepresented in our sorts, as CD56bright cells tend to be CD16dim. We estimated the magnitude of this effect on our data by dual staining with CD16 and CD56 in a further five (healthy) subjects. We found that about half (range 33–71%) of the CD56bright population, constituting a mean of 4% (range 0·9–8%) of total NK cells in blood, would have been excluded from our analysis as CD16–. This may be particularly relevant in AIM subjects who have an increased proportion of CD56bright cells,24 although omission of this population from the analysed cells is unlikely to explain the paucity of labelled NK cells in AIM. Further studies using CD56 expression as a sort criterion would demonstrate whether this population has a disproportionately high proliferation rate.
This study represents the first report of in vivo kinetics of NK cells in humans. Although subject numbers were limited, we have demonstrated the highly kinetic nature of the NK cell population, with turnover rates of the same order of magnitude as in murine data. The early appearance of some labelled cells suggests that division of mature peripheral NK cells may contribute to NK cell homeostasis in vivo. Immunosenescence appears to reduce bone marrow production of new NK cells, although circulating numbers are well maintained. Chronic infection with HTLV-I does not measurably perturb NK dynamics, whilst in acute EBV infection NK cell proliferation occurs early in the infection, with rapid restoration of proliferation rates to normal once the adaptive response has been initiated. These preliminary findings need confirmation in larger groups of subjects and could be extended to include further NK subsets; however, the data presented represent the first in vivo human kinetic data and have important implications for approaches that might seek to utilize or perturb NK cells within the context of a therapeutic strategy.
Acknowledgments
We are grateful to the research subjects who willingly took part in this study, to the staff of the St George's Vaccine Institute for help in executing the clinical studies, and to Professor Charles Bangham, Imperial College, London for advice on experimental design and preparation of the manuscript. YZ was supported by the Wellcome Trust and HG by the Medical Research Council (UK); BA acknowledges support from the Leverhulme trust.
References
- 1.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Ann Rev Immunol. 2004;22:405–29. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 2.Orange JS, Ballas ZK. Natural killer cells in human health and disease. Clin Immunol. 2006;118:1–10. doi: 10.1016/j.clim.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 3.French AR, Yokoyama WM. Natural killer cells and viral infections. Curr Opin Immunol. 2003;15:45–51. doi: 10.1016/s095279150200002x. [DOI] [PubMed] [Google Scholar]
- 4.Yokoyama WM. Natural killer cells. In: Paul WE, editor. Fundamental Immunology. New York: Lippincott-Raven; 1999. pp. 575–603. [Google Scholar]
- 5.Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002;4:1545–58. doi: 10.1016/s1286-4579(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 6.Parolini S, Bottino C, Falco M, et al. X-linked lymphoproliferative disease: 2B4 molecules displaying inhibitory rather than activating function are responsible for the inability of natural killer cells to kill Epstein-Barr virus-infected cells. J Exp Med. 2000;192:337–46. doi: 10.1084/jem.192.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bukowski JF, Woda BA, Habu S, Okumura K, Welsh RM. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J Immunol. 1983;131:1531–8. [PubMed] [Google Scholar]
- 8.Galy A, Travis M, Cen D, Chen B. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 1995;3:459–73. doi: 10.1016/1074-7613(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 9.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–72. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 10.Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, Yokoyama WM. Specific and nonspecific NK cell activation during virus infection. Nat Immunol. 2001;2:951–6. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 11.Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. J Exp Med. 2003;197:967–76. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jamieson AM, Isnard P, Dorfman JR, Coles MC, Raulet DH. Turnover and proliferation of NK cells in steady state and lymphopenic conditions. J Immunol. 2004;172:864–70. doi: 10.4049/jimmunol.172.2.864. [DOI] [PubMed] [Google Scholar]
- 13.Dokun AO, Chu DT, Yang L, Bendelac AS, Yokoyama WM. Analysis of in situ NK cell responses during viral infection. J Immunol. 2001;167:5286–93. doi: 10.4049/jimmunol.167.9.5286. [DOI] [PubMed] [Google Scholar]
- 14.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–7. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 15.Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, Aguila HL, Caligiuri MA. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–8. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 16.Wang JW, Howson JM, Ghansah T, et al. Influence of SHIP on the NK repertoire and allogeneic bone marrow transplantation. Science. 2002;295:2094–7. doi: 10.1126/science.1068438. [DOI] [PubMed] [Google Scholar]
- 17.Mocchegiani E, Malavolta M. NK and NKT cell functions in immunosenescence. Aging Cell. 2004;3:177–84. doi: 10.1111/j.1474-9728.2004.00107.x. [DOI] [PubMed] [Google Scholar]
- 18.Ogata K, Yokose N, Tamura H, An E, Nakamura K, Dan K, Nomura T. Natural killer cells in the late decades of human life. Clin Immunol Immunopathol. 1997;84:269–75. doi: 10.1006/clin.1997.4401. [DOI] [PubMed] [Google Scholar]
- 19.Facchini A, Mariani E, Mariani AR, Papa S, Vitale M, Manzoli FA. Increased number of circulating Leu 11+ (CD 16) large granular lymphocytes and decreased NK activity during human ageing. Clin Exp Immunol. 1987;68:340–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Krishnaraj R, Blandford G. Age-associated alterations in human natural killer cells. 2. Increased frequency of selective NK subsets. Cell Immunol. 1988;114:137–48. doi: 10.1016/0008-8749(88)90261-4. [DOI] [PubMed] [Google Scholar]
- 21.Ligthart GJ, Schuit HR, Hijmans W. Natural killer cell function is not diminished in the healthy aged and is proportional to the number of NK cells in the peripheral blood. Immunology. 1989;68:396–402. [PMC free article] [PubMed] [Google Scholar]
- 22.Mariani E, Meneghetti A, Formentini I, Neri S, Cattini L, Ravaglia G, Forti P, Facchini A. Different rates of telomere shortening and telomerase activity reduction in CD8 T and CD16 NK lymphocytes with ageing. Exp Gerontol. 2003;38:653–9. doi: 10.1016/s0531-5565(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 23.Borrego F, Alonso MC, Galiani MD, Carracedo J, Ramirez R, Ostos B, Pena J, Solana R. NK phenotypic markers and IL2 response in NK cells from elderly people. Exp Gerontol. 1999;34:253–65. doi: 10.1016/s0531-5565(98)00076-x. [DOI] [PubMed] [Google Scholar]
- 24.Williams H, McAulay K, Macsween KF, Gallacher NJ, Higgins CD, Harrison N, Swerdlow AJ, Crawford DH. The immune response to primary EBV infection: a role for natural killer cells. Br J Haematol. 2005;129:266–74. doi: 10.1111/j.1365-2141.2005.05452.x. [DOI] [PubMed] [Google Scholar]
- 25.Macallan DC, Asquith B, Irvine A, et al. Measurement and modeling of human T cell kinetics. Eur J Immunol. 2003;33:2316–26. doi: 10.1002/eji.200323763. [DOI] [PubMed] [Google Scholar]
- 26.Macallan DC, Wallace DL, Irvine A, et al. Rapid turnover of T cells in acute infectious mononucleosis. Eur J Immunol. 2003;33:2655–65. doi: 10.1002/eji.200324295. [DOI] [PubMed] [Google Scholar]
- 27.Macallan DC, Wallace DL, Zhang Y, et al. B cell kinetics in humans: rapid turnover of peripheral blood memory cells. Blood. 2005;105:3633–40. doi: 10.1182/blood-2004-09-3740. [DOI] [PubMed] [Google Scholar]
- 28.Mohri H, Perelson AS, Tung K, et al. Increased turnover of T lymphocytes in HIV-1 infection and its reduction by antiretroviral therapy. J Exp Med. 2001;194:1277–87. doi: 10.1084/jem.194.9.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace DL, Zhang Y, Ghattas H, et al. Direct measurement of T cell subset kinetics in vivo in elderly men and women. J Immunol. 2004;173:1787–94. doi: 10.4049/jimmunol.173.3.1787. [DOI] [PubMed] [Google Scholar]
- 30.Macallan DC, Wallace D, Zhang Y, et al. Rapid turnover of effector-memory CD4(+) T cells in healthy humans. J Exp Med. 2004;200:255–60. doi: 10.1084/jem.20040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasaian MT, Whitters MJ, Carter LL, et al. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity. 2002;16:559–69. doi: 10.1016/s1074-7613(02)00295-9. [DOI] [PubMed] [Google Scholar]
- 32.Smith HR, Chuang HH, Wang LL, Salcedo M, Heusel JW, Yokoyama WM. Nonstochastic coexpression of activation receptors on murine natural killer cells. J Exp Med. 2000;191:1341–54. doi: 10.1084/jem.191.8.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mailliard RB, Alber SM, Shen H, Watkins SC, Kirkwood JM, Herberman RB, Kalinski P. IL-18-induced CD83+CCR7+ NK helper cells. J Exp Med. 2005;202:941–53. doi: 10.1084/jem.20050128. [DOI] [PMC free article] [PubMed] [Google Scholar]