Abstract
Through the production of stimulatory and suppressive cytokines, dendritic cells (DCs) regulate virus-specific immune responses that are crucial to virus eradication. To explore a possible role of DCs in the persistence of hepatitis C virus (HCV) infection, in this study we analysed peripheral blood DCs (PBDCs) in patients with chronic hepatitis C (CHC) compared with those in both healthy seronegative (HSN) controls and a group of subjects who had spontaneously resolved infection, defined as healthy HCV-seropositive (HSP), and we evaluated the relationships between PBDCs and HCV-specific CD4+ T-cell reactivity. The number of PBDCs, their immunophenotype and expression of regulatory cytokines were evaluated by flow cytometry on whole-blood samples. HCV-specific CD4+ T-cell activation, proliferation and cytokine production were evaluated in cultures of peripheral blood mononuclear cells (PBMCs) stimulated in vitro with HCV peptides. We found that PBDCs from CHC subjects were numerically reduced and showed lower interleukin-12 (IL-12) and higher IL-10 expression than those from HSN controls. PBDCs from HSP subjects were similar to those from HSN controls. HCV-specific CD4+ T-cell proliferation was less frequent and vigorous in CHC than in HSP patients and was directly related to the number of PBDCs and their IL-12 production but inversely related to their IL-10 production. Taken together, these results seem to suggest that cytokines of DC origin contribute to the regulation of HCV-specific immunity in CHC patients and indicate that PBDCs may represent a novel non-invasive tool for immune monitoring of these patients.
Keywords: hepatitis C virus, peripheral blood dendritic cells, interleukin-10, interleukin-12, T-cell proliferation
Introduction
Dendritic cells (DCs) are the most potent antigen-presenting cells in antiviral host defence and many viruses directly or indirectly affect DCs to subvert the immune response and establish infection in the host.1 Modifications of DCs during hepatitis C virus (HCV) infection are currently under intense investigation. Maturation and functional defects of monocyte-derived DCs, obtained by in vitro culture of peripheral blood monocytes (PBMCs) from patients with chronic HCV hepatitis (CHC), have been reported by some authors but not confirmed by others, possibly because of differences in culturing conditions [reviewed in ref. 2]. Because data obtained from monocyte-derived DCs may not be representative of in vivo functions,3 more recent studies have been performed by analysing directly ex vivo peripheral blood DCs (PBDCs). PBDCs can be divided into two subpopulations on the basis of their expression of the β-integrin CD11c. CD11c+ myeloid DCs (mDCs) express myeloid markers and, upon encounter with pathogens, secrete interleukin-12 (IL-12), which drives type 1 helper T-cell (Th1) responses associated with the generation of cellular immunity.4 Plasmacytoid DCs (pDCs) lack myeloid markers, express the IL-3 receptor α-chain CD123 on their surface and are the main producers of interferon-α (IFN-α), which also drives Th1 responses and is endowed with direct antiviral properties.5 Both subpopulations may play therefore an important role in controlling viral infections. Most authors agree that in CHC patients PBDCs are substantially reduced, with reduction involving both mDCs and pDCs.6–11 Moreover, PBDCs appear to be functionally impaired, with reduced production of IL-12 and IFN-α documented in various studies.7–9,12 However, to our knowledge, the production of IL-10 by PBDCs from CHC subjects has never been investigated. IL-10 of DC origin plays a central role in the regulation of T-cell responses, with predominant suppressive effects that counteract the action promoted by the above mentioned stimulatory cytokines.13 Increased production of IL-10 during HCV infection may therefore contribute to the high propensity of HCV to cause chronic infection, this last associated with weak HCV-specific T-cell responses.14,15 In the present study we analysed PBDCs from CHC patients, including their production of IL-10, and we correlated PBDC parameters with T-cell responses against HCV peptides. To explore the role of DCs in the persistence of infection, PBDCs from a cross-sectional cohort of CHC patients were compared not only with PBDCs from healthy seronegative (HSN) controls, but also with a group of subjects who spontaneously resolved HCV-infection (healthy HCV-seropositive; HSP). Finally, because monocytes are antigen-presenting cells and represent DC precursors both in vitro and in vivo, we extended our analyses to peripheral blood monocytes.
Materials and methods
Study population
A group of CHC patients and two control groups of HSN and HSP subjects were enrolled into the study. CHC were selected in our centre from among patients attending for regular follow-up, while the two control groups were enrolled among blood donors in Milan. Peripheral fresh heparinized blood samples were obtained from 42 HSN (male/female: 23/19; mean age 46 ± 15 years), 19 HSP (9/10; 50 ± 13) and 26 sex-matched and age-matched CHC (12/14; 54 ± 11) subjects. All donors tested negative for human immunodeficiency virus (HIV) and hepatitis B virus. All HSP individuals tested negative for HCV RNA and had normal indices of cytolysis. The clinical characteristics of CHC patients are summarized in Table 1. Histology data were analysed if a liver biopsy taken within 24 months of enrolment was available. Data were evaluated according to an international scoring system for chronic hepatitis.16,17 All CHC patients were treatment-naive at the time of blood collection. Ethics approval was obtained from the local Institutional Review Committee and a signed informed consent was obtained from all participants.
Table 1.
Characteristics of CHC patients participating in the study
| Patient ID | Age (years) | Sex | Genotype | Viral load (UI/ml) | ALT | AST | Histology (Knodell HAI score) |
|---|---|---|---|---|---|---|---|
| CH1 | 47 | M | 3a | NA | 120 | 50 | 9 + 4 = 13; cirrhosis |
| CH2 | 53 | F | 2a/2c | 530000 | 135 | 75 | 5 + 1 = 6 |
| CH3 | 47 | F | 2a | 690000 | 85 | 37 | 3 + 0 = 3 |
| CH4 | 61 | M | 1b | 610000 | 44 | 40 | 7 + 3 = 10 |
| CH5 | 64 | M | 2a/2c | 580000 | 166 | 90 | 6 + 1 = 7 |
| CH6 | 52 | F | 1b | 365000 | 68 | 48 | 3 + 1 = 4 |
| CH7 | 32 | F | 3a | NA | 81 | 60 | 7 + 1 = 8 |
| CH8 | 52 | F | 1b | 850000 | 93 | 62 | 7 + 3 = 10 |
| CH9 | 62 | F | 1a/1b | NA | 75 | 46 | 3 + 1 = 4 |
| CH10 | 37 | M | 3a | NA | 61 | 27 | 6 + 2 = 8 |
| CH11 | 65 | F | 1b | NA | 88 | 75 | 9 + 3 = 12 |
| CH12 | 60 | M | 1b | 510000 | 69 | 45 | 7 + 3 = 10 |
| CH13 | 59 | M | 1b | 61000 | 69 | 44 | NA |
| CH14 | 54 | M | 1b | 585500 | 70 | 29 | 4 + 1 = 5 |
| CH15 | 38 | M | 4 | 575000 | 66 | 38 | 3 + 0 = 3 |
| CH16 | 63 | F | 1a | 620000 | 87 | 46 | 9 + 1 = 10 |
| CH17 | 59 | F | 2a/2c | NA | 77 | 57 | NA |
| CH18 | 42 | M | 3a | 62800 | 50 | 30 | 9 + 1 = 10 |
| CH19 | 65 | F | 2a/2c | 22200 | 86 | 74 | NA |
| CH20 | 63 | M | 2a/2c | 253000 | 83 | 88 | NA |
| CH21 | 51 | F | 4 | NA | 28 | 34 | 5 + 4 = 9; cirrhosis |
| CH22 | 68 | F | 2a/2c | 162 | 86 | 73 | 8 + 4 = 12; cirrhosis |
| CH23 | 66 | F | 2a/2c | 450000 | 409 | 332 | 8 + 4 = 12; cirrhosis |
| CH24 | 63 | M | 2a/2c | 520000 | 34 | 23 | NA; clinical signs of cirrhosis |
| CH25 | 33 | M | 3a | 139000 | 318 | 160 | 9 + 4 = 13; cirrhosis |
| CH26 | 55 | F | 1b | 51200 | 84 | 93 | 9 + 4 = 13; cirrhosis |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; NA, not available.
Viral load defined by quantitative polymerase chain reaction (PCR) at time of blood draw (Amplicor; Roche Molecular Diagnostics, Pleasanton, CA).
Virological methods
Anti-HCV antibodies were assayed by a third generation enzyme-linked immunosorbent assay (HCV 3·0 ELISA, Ortho Clinical Diagnostic System, Raritan, NJ). Reactive samples were confirmed using a supplemental recombinant immunoblot assay (RIBA 3·0, Ortho Clinical Diagnostic Systems). HCV RNA was detected by an in-house reverse transcription–polymerase chain reaction assay with nested primers of the 5′ non-coding region of the HCV genome. HCV genotype was determined using the Innogenetic Line Probe assay (Inno Lipa HCVII, Immunogenetics, Ghent, Belgium).
Immunophenotypic analysis and counting of PBDCs
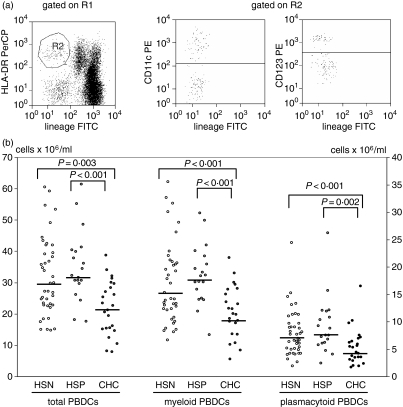
Whole peripheral blood samples were analysed by three-colour flow cytometry (FACScan; Becton Dickinson, San Jose, CA), as previously described.18–20 PBDCs were identified as mononuclear cells that lacked lineage cell markers (Lin–: CD3– CD14– CD16– CD19– CD20–; Caltag Laboratories, Burlingame, CA) but expressed human leucocyte antigen (HLA)-DR (Becton Dickinson); myeloid and plasmacytoid subpopulations were identified as DCs expressing CD11c (Caltag Laboratories) or CD123 (IL-3 receptor α-chain) (Pharmingen, San Diego, CA), respectively. A representative analysis is shown in Fig. 1(a). Estimates of the absolute numbers of PBDCs were calculated from the proportion of DCs recorded by flow cytometry in the mononuclear gate multiplied by absolute mononuclear cell count measured using a standard haemocytometer. The expression of CD80, CD83 and CD86 (Pharmingen) by Lin– HLA-DR+ cells was measured to assess the activation/maturation state of the PBDCs.
Figure 1.
PBDCs are decreased in patients with chronic HCV infection. (a) Gated on a mononuclear cell analysis region (R1), PBDCs were identified on the basis of their lack of labelling for cell lineage markers, but positive staining for HLA-DR (R2); gated on R2 events, myeloid DCs and plasmacytoid DCs were identified for their surface expression of CD11c or CD123, respectively. (b) The number of PBDCs (left axis) was significantly lower in CHC patients compared with HSN and HSP controls. Both myeloid and plasmacytoid PBDC subpopulations (right axis) were decreased in CHC patients. Each symbol represents a single sample. Median values represented by horizontal lines in each series. P-values determined using the Mann–Whitney U-test.
Cytoplasmic cytokine expression by PBDCs and monocytes
The expression of IL-12 and IL-10 by PBDCs and monocytes was determined by flow cytometry in whole-blood samples, as previously described.18–20 Lipopolysaccharide (LPS) was used as a stimulator because it is known to be the optimal stimulus for this experimental model.18,21,22 Whole-blood samples were incubated with or without 100 ng/ml LPS (Sigma, St Louis, MO) for 5 hr, in the presence of the protein transport inhibitor brefeldin-A (10 μg/ml; Sigma) added during the last 4 hr to allow intracellular accumulation of cytokines. Cells were then fixed, rendered permeable and stained with cytokine-directed monoclonal antibodies (Caltag Laboratories).
In vitro assays for CD4+T-cell reactivity
The reactivity of CD4+ T cells was assessed by flow cytometry as previously described.23 The PBMCs were cultured for 6 days without stimulation, or with phytohaemagglutinin (PHA) (10 μg/ml; Sigma), influenza virus vaccine (FLU) (A/Taiwan, A/Shangai, B/Victoria; 24 pg/ml; a kind gift from Prof. Clerici, University of Milan), or 10 μg/ml synthetic HCV peptides. One peptide from the core (C39–63), one from the envelope (E1238–262), and two from the non-structural region (NS31384–1401, and NS31406–1415) were used; these had been previously selected as the most immunogenic among 38 peptides chosen from sequences of HCV 1b strain on the basis of their ability to bind to the most common HLA molecules present in the Italian population.23 A non-antigenic HIV peptide (p23) was used as negative control. Cultures were performed in duplicate. 5-bromo-2′-deoxyuridine (BrdU, 20 μm; Sigma) was added during the last 6 hr and lymphocyte proliferation was assessed by flow cytometry as BrdU incorporation by CD4+ CD25+ lymphocytes. Supernatants were stored at −20° for further analyses. The degree of HCV-specific CD4+ cell proliferation was expressed as the proliferation index (PI), calculated from the fraction of CD25+ BrdU+ CD4+ cells found with antigen divided by that found without antigen. A significant PI in response to HCV peptides was defined as being >2·75, which was the cut-off value greater than the mean PI plus three times the standard deviation obtained in a group of 16 HSN controls. The degree of CD4+ T-cell incomplete activation was evaluated in the same samples and was expressed as the incomplete activation index (iAI), similarly calculated from the fraction of CD25+ BrdU– CD4+ cells.
Cytokine measurement in culture supernatants
The release of IFN-γ and IL-10 in the supernatants was measured by specific sandwich ELISA, using commercially available pairs of monoclonal antibodies (Endogen, Woburn, MA), according to the manufacturer's instructions.
Statistical analysis
All statistical analyses assumed a two-sided significance level of 0·05. The Mann–Whitney U-test and the Fisher exact test were used for comparisons between patients and controls; the Wilcoxon signed-rank test was used to analyse the effects of in vitro stimulation. The Spearman rank test was used to describe correlations. Overall P-values for secondary comparisons used a Kruskal–Wallis test (a non-parametric analysis of variance) and, given an overall P < 0·05, pairwise comparisons to the control group were conducted using Dunn's multiple comparison adjustment.24 Data analyses were performed with Openstat3 software.
Results
Enumeration of PBDCs and monocytes
The median frequency of total PBDCs in the mononuclear cell population was significantly lower in CHC (0·80%) compared with HSN (1·52%, P < 0·001) and HSP (1·25%, P < 0·001) subjects. As shown in Fig. 1(b), the absolute number of PBDCs was also significantly lower in CHC than in HSN and HSP subjects. The decrease in PBDCs involved both PBDC subsets: the median frequency of mDCs in CHC patients was 0·44%, compared with 0·72% (P < 0·001) in HSN controls, and 0·69% (P < 0·001) in HSP subjects; the median frequency of pDCs was 0·20% in patients with CHC, compared with 0·32% (P < 0·001) in HSN and 0·27% (P = 0·002) in HSP subjects. The absolute numbers of PBDC subsets are reported in Fig. 1(b). Within the group of CHC patients, the frequency of mDCs was significantly lower in subjects infected with HCV genotype-2 than in those infected with the other genotypes (Kruskal–Wallis test followed by Dunn's multiple comparison adjustment, P < 0·05). The frequency and absolute number of PBDCs and their subsets were not correlated with HCV viral load, nor with hepatic disease activity, measured as transaminase levels or Knodell index. The number of monocytes was similar in the three groups (HSN: 0·57 × 103/μl; HSP: 0·60 × 103/μl; CHC: 0·59 × 103/μl).
Immunophenotype of PBDCs
PBDCs from CHC patients appeared less activated than PBDCs from healthy controls, as the percentage of CD80+ PBDCs was lower in CHC (median 2·77%) than in HSN (4·55%, P = 0·03) and HSP (3·53%, P = ns) subjects, although the frequency of CD86+ PBDCs did not significantly differ between patients and controls (median percentage HSN, HSP and CHC: 71·43, 69·36 and 64·35, respectively). Similar results were obtained when data were expressed as mean fluorescence intensity (not shown). PBDCs from CHC were also less mature, as assessed by a lower percentage of CD83+ PBDCs in these subjects (0·66%) than in HSN (1·44%; P = 0·035) and HSP (1·20%; P = 0·021) subjects. The activation and maturation states of PBDCs did not correlate, in the CHC group, with either HCV viral load or hepatic disease activity.
Cytokine production by PBDCs and monocytes
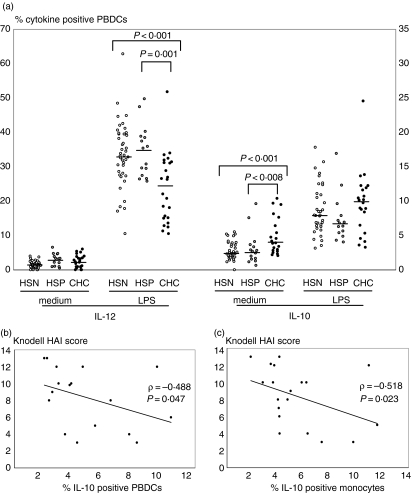
PBDCs from patients with CHC differed from PBDCs from healthy controls in their expression of the regulatory cytokines IL-12 and IL-10. Incubation of whole-blood samples with LPS induced in all the groups a significant increase in the percentage of PBDCs expressing either IL-12 or IL-10 (HSN, HSP and CHC: P < 0·001). Yet, as shown in Fig. 2(a), the percentage of IL-12+ PBDCs upon LPS stimulation was significantly lower in the CHC group than in the HSN and HSP groups. Moreover, the percentage of PBDCs constitutively expressing IL-10 was significantly higher in the CHC group than in the HSN and HSP groups. Similar results were obtained when data were expressed as mean fluorescence intensity (not shown). Within CHC patients, the frequency of IL-10+ PBDCs inversely correlated with the Knodell index (Fig. 2b). No significant correlation was found between PBDC expression of cytokines and viral load or specific HCV genotypes.
Figure 2.
PBDCs from patients with chronic HCV-infection have altered expression of cytokines. (a) The percentage of PBDCs expressing LPS-stimulated IL-12 was significantly lower in CHC patients compared with HSN and HSP controls. The percentage of PBDCs constitutively expressing IL-10 was significantly higher in CHC than in HSN and HSP. After incubating whole blood samples for 5 hr in the presence or absence of LPS with brefeldin A (BFA) added during the last 4 hr, PBDCs were identified on the basis of their lack of labelling for cell lineage markers, but positive staining for HLA-DR. Intracellular accumulation of cytokines within PBDCs was evaluated with mAbs directed against either IL-12 or IL-10, after DC permeabilization. Each symbol represents a single sample. Median values represented by horizontal lines in each series. P-values determined using the Mann–Whitney U-test. Within the group of CHC patients, the frequency of PBDCs (b) and monocytes (c) constitutively expressing IL-10 was inversely correlated with the Knodell HAI score. Each symbol represents a single sample. P-values determined using the Spearman rank test.
The production of regulatory cytokines by monocytes was similarly partially impaired. Incubation of blood samples with LPS induced a significant increase in the percentage of monocytes expressing either IL-12 or IL-10 (HSN, HSP and CHC: P < 0·001). The percentage of IL-12+ monocytes upon LPS stimulation was significantly lower in CHC patients (19·67%) than in the HSN (26·97%; P = 0·05) and HSP (24·11%; P = ns) groups. The percentage of IL-10+ monocytes in unstimulated or LPS-stimulated conditions did not differ between patients and controls; nevertheless, similarly to PBDCs, in CHC patients the frequency of IL-10+ monocytes was inversely correlated with Knodell index (Fig. 2c).
HCV-specific CD4+ T-cell responses
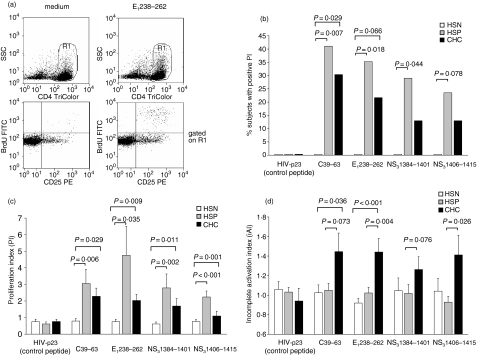
Proliferative responses to PHA and FLU did not differ between the CHC, HSN and HSP groups (median PI to PHA: 125·3, 116·3, 110·8; FLU: 10·4, 11·6, 12·7, respectively), thus confirming the notion that CHC patients are immune-competent.25 The analysis of the HCV-specific CD4+ T-cell responses revealed that lymphocytes from HSP and CHC patients were reactive to viral peptides, while lymphocytes from HSN controls did not show any positive proliferative response to any HCV peptide used. Lymphocytes from CHC patients had positive proliferative responses to HCV peptides at a lower frequency than those from HSP patients, with positive responses (i.e. with PI greater than the positive cut-off value of 2·75) to at least one peptide observed in 52·2% of CHC compared with 70·6% of HSP patients, and positive responses to at least two peptides in 21·7% compared with 47·1% (P = ns). Figure 3(a) shows the frequency of subjects with positive proliferation to individual HCV-peptides in the three groups. The proportion of responders among HSP patients was significantly higher than among HSN controls upon stimulation with any peptide. The proportion of responders among CHC was lower than among HSP (P = ns). As shown in Fig. 3(b), similar results were observed when the intensity of the proliferative responses, expressed as PI, was evaluated. PI in response to all individual peptides were significantly higher in HSP than HSN, and lower in CHC than HSP. The reduction of proliferative responses in CHC reached statistical significance when the overall responses to individual peptides were considered (PI: 2·12 versus 3·06; P = 0·007). To investigate whether the impaired HCV-specific proliferation of CHC lymphocytes could be related to an incomplete activation, we measured the CD4+ T cells that did express CD25 after stimulation but did not incorporate BrdU. Indeed, the iAI was higher in CHC patients than in HSP patients upon stimulation with all the individual HCV peptides (Fig. 3c).
Figure 3.
HCV-specific CD4+ T-cell proliferation is vigorous in subjects with spontaneous control of HCV-infection, but impaired in patients with chronic infection. (a) Representative flow cytometric analysis of CD4+ T-cell proliferation upon stimulation of PBMCs with (right column) or without (left column) an HCV peptide. After culture of PBMCs for 6 days with BrdU incorporation allowed during the last 6 hr, cells were double stained with TriColor-cojugated anti-CD4 and PE-conjugated anti-CD25 mAbs. Cells were then fixed, permeabilized, subjected to partial DNA denaturation, and stained with FITC-conjugated anti-BrdU. Flow cytometric analysis was performed within 24 hr. Gated on CD4+ cells (R1, upper row), cell proliferation was assessed as incorporation of BrdU by CD25+ cells (lower row). The degree of HCV-specific CD4+ T-cell proliferation was expressed as the proliferation index (PI), calculated from the fraction of CD25+ BrdU+ CD4+ cells found with antigen divided by that found without antigen. (b) Percentage of subjects, in the groups of HSN (white bars), HSP (grey bars) and CHC (black bars), with a significant PI to the indicated HCV peptides. A significant PI was defined as being >2·75, which was the cut-off value calculated from the group of 16 healthy HCV-seronegative controls. P-values were calculated using the Fisher exact test. (c) Intensity of CD4+ T-cell proliferation to the indicated HCV-peptides in the three groups. Mean ±SEM from 16 HSN (white bars), 17 HSP (grey bars), and 23 CHC (black bars). P-values were calculated using the Mann–Whitney U-test. (d) The intensity of CD4+ T-cell incomplete activation was calculated in the same subjects as in (b), from the proportion of CD25+ BrdU– cells.
Cytokine production by PBMCs
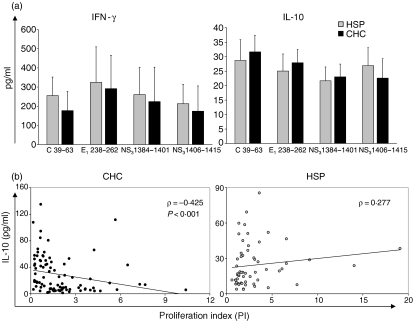
The production of IFN-γ and IL-10 upon stimulation of PBMCs with individual HCV peptides did not differ significantly between CHC and HSP subjects (Fig. 4a). In CHC, but not in HSP, subjects the levels of IL-10 were inversely correlated with PI (Fig. 4b).
Figure 4.
The production of IL-10 by PBMCs stimulated with HCV peptides inversely correlates with HCV-specific CD4+ T-cell proliferation in patients with chronic HCV-infection. (a) The in vitro production of IFN-γ and IL-10 upon stimulation of PBMCs with individual HCV-peptides is similar in HSP and CHC subjects. Mean ±SEM from 15 HSP (grey bars) compared with 22 CHC (black bars). P-values calculated using the Mann–Whitney U-test. (b) and (c) Within the group of CHC patients, but not in the group of HSP subjects, the levels of IL-10 were inversely correlated with the intensity of the proliferative responses. Each symbol represents a single sample. P-values were calculated using the Spearman rank test.
Correlations between PBDCs and CD4+ T-cell reactivity
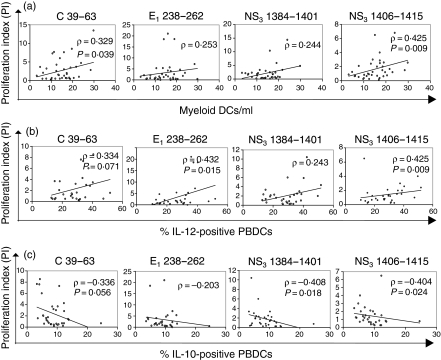
As shown in Fig. 5(a), in the comprehensive group of HCV-exposed individuals composed of CHC and HSP patients, the CD4+ T-cell proliferative responses to HCV peptides were directly correlated with the number of mDCs. The same proliferative responses were correlated directly with the percentage of IL-12+ PBDCs (Fig. 5b) and inversely with the percentage of IL-10+ PBDCs (Fig. 5c). These correlations were more evident when the production of cytokines upon LPS stimulation was considered. No other significant correlations were found between CD4+ T-cell reactivity or cytokine production and parameters measured on PBDCs and monocytes.
Figure 5.
HCV-specific proliferation of CD4+ T cells correlates with PBDC number and functions. In the comprehensive group of HCV-exposed individuals composed of CHC and HSP subjects, the intensity of CD4+ T-cell proliferation in response to individual HCV-peptides was directly correlated with the number of myeloid PBDCs (a) and with the percentage of PBDCs producing IL-12 upon LPS stimulation (b), and inversely with the percentage of PBDCs producing IL-10 (c). Each symbol represents a single sample. P-values were determined using the Spearman rank test.
Discussion
Reduction of PBDCs in CHC patients has recently been reported by several groups, with some conflicting results on whether either mDCs or pDCs or both subsets are affected.6–9,11,26,27 In this study we observed a significant reduction of both mDCs and pDCs that was comparable with the other reports. The reason for this reduction is still unclear. It has been proposed that liver injury per se may lead to a paucity of PBDCs either by affecting DC mobilization from the bone marrow, or by increasing DC migration to the liver.8 Another possibility is that, as demonstrated for other viral infections,28–33 HCV itself is responsible for DC reduction, by mechanisms that may include injury of infected DCs or DC precursors, recruitment of infected cells to the lymph nodes, or interference with the differentiation process of DCs from their precursors. Our results seem to suggest that direct and indirect, viral and non-viral effects may all contribute to the reduction of PBDCs in CHC. In fact, the lack of correlation between PBDC count and viral load in our patients, yet reported in other studies,6,8,9,27 may suggest the contribution of liver injury and indirect viral mechanisms. On the other hand, the more pronounced mDC reduction in patients infected with HCV genotype 2 may reflect a different cell tropism of the different viral clusters, suggesting a role for direct viral effects. What is evident from our results is that, whatever the mechanisms involved, active HCV infection is needed to affect the number of DCs, because HSP subjects, characterized by the spontaneous resolution of their HCV infection with persistently undetectable viraemia, have PBDC numbers similar to HSN controls.
By including the HSP group we could also demonstrate that in HCV-exposed subjects the number of mDCs, which are the main promoters of T-cell immunity,4 directly correlate with the proliferative responses of HCV-specific CD4+ T lymphocytes. This is an important new finding that may allow better clarification of the significance of DC reduction in these patients. In a similar manner to previous observations in a different setting composed of CHC women and their children,23 here we could confirm that CD4+ T-cell-proliferative responses to HCV peptides are less frequent and vigorous in CHC than in HSP, possibly because of an incomplete cell activation. As in that study, here we could also demonstrate that the production of IL-10 by PBMCs stimulated with HCV peptides was inversely correlated with CD4+ T-cell proliferation in CHC patients but not in HSP patients. The role for IL-10 in impaired T-cell reactivity during chronic HCV infection, which we had previously demonstrated with inhibition experiments,23 seems therefore to be supported in this clinical setting.
Moreover, the centrality of IL-10 in the modulation of immune responses during HCV infection seems to be strengthened by the inverse correlation found in this study between the production of IL-10 by PBDCs, expressed as percentage of cytokine-secreting cells, and HCV-specific CD4+ T-cell proliferation. This observation is worthy of note, because IL-10 of DC origin is known to direct the differentiation of anergic T cells from naive precursors and is therefore expected to contribute to the impaired T-cell reactivity that is associated with the persistence of HCV infection.13,34,35 We also observed that PBDCs from CHC have an impaired ability to produce IL-12, the main regulatory cytokine that is able to promote the cell-mediated immune responses that are crucial for the detection and elimination of virus-infected cells.36 This finding is in accordance with previous studies that reported reduced IL-12 production in different experimental models.7,9,12 What we add is that IL-12 production by PBDCs is preserved in HSP patients and that it directly correlates with HCV-specific CD4+ T-cell proliferation. Notably, monocytes showed a pattern of cytokine production similar to that of PBDCs. But, unlike cytokines of DC origin, IL-12 and IL-10 produced by monocytes did not correlate with T-cell functions. Therefore, taken together these findings seem to support the growing conviction that DCs play a central role in the immune dysregulation leading to chronic HCV persistence.
While T-cell functions correlated uniquely with DC parameters, the Knodell histological activity index (HAI) score inversely correlated with the production of IL-10 by either DCs or monocytes. This observation may highlight the dual role of IL-10 in CHC patients. In fact, by skewing tolerogenic T-cell responses, IL-10 of DC origin can hamper the Th1 responses, which on the one hand are fundamental to the eradication of HCV infection, but on the other hand are responsible for liver injury caused by an immune Th1 response against HCV-infected hepatocytes.37,38 Moreover, IL-10 of whatever origin can potently inhibit the production of almost all pro-inflammatory cytokines and chemokines.39 Therefore, the production of IL-10 in CHC patients, while it may be detrimental to the control of viral infection, may be favourable to the prevention of overwhelming liver inflammation and injury.
In conclusion, the present study clearly indicates that during chronic HCV infection PBDCs show numerical defects and functional alterations that are not present following spontaneous resolution of HCV infection and that correlate with HCV-specific CD4+ T-cell impairment. Although further studies are needed to clarify whether the number and function of peripheral DCs reflect those of liver DCs, the present study seems to indicate that cytokines of DC origin may contribute to the regulation of HCV-specific immunity in CHC patients, and provides evidence that PBDCs may represent a novel non-invasive tool for immune monitoring of these patients.
Acknowledgments
This work was supported in part by grant 2003069391–001 from the Ministero dell'Istruzione, dell'Università e della Ricerca. It was also supported in part by a grant from Fondazione Cariplo (Milan, Italy).
Abbreviations
- BrdU
5-bromo-2′-deoxyuridine
- CHC
chronic hepatitis C
- DC
dendritic cell
- ELISA
enzyme-linked immunosorbent assay
- FLU
influenza virus vaccine
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- HSN
healthy seronegative
- HSP
healthy HCV-seropositive
- iAI
incomplete activation index
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- mDC
myeloid dendritic cell
- PBDC
peripheral blood dendritic cell
- PBMC
peripheral blood mononuclear cell
- pDC
plasmacytoid dendritic cell
- PHA
phytohaemagglutinin
- PI
proliferation index
- Th1
type 1 helper T cell
References
- 1.Rinaldo CR, Piazza P. Virus infection of dendritic cells: portal for host invasion and host defense. Trends Microbiol. 2004;12:337–45. doi: 10.1016/j.tim.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Pachiadakis I, Pollara G, Chain BM, Naoumov NV. Is hepatitis C virus infection of dendritic cells a mechanism facilitating viral persistence? Lancet Infect Dis. 2005;5:296–304. doi: 10.1016/S1473-3099(05)70114-6. [DOI] [PubMed] [Google Scholar]
- 3.O'Neill HC, Wilson HL. Limitations with in vitro production of dendritic cells using cytokines. J Leukoc Biol. 2004;75:600–3. doi: 10.1189/jlb.0903446. [DOI] [PubMed] [Google Scholar]
- 4.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 5.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nature Immunol. 2004;12:1219–26. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 6.Kunitani H, Shimizu Y, Murata H, Higuchi K, Watanabe A. Phenotypic analysis of circulating and intrahepatic dendritic cell subsets in patients with chronic liver diseases. J Hepatol. 2002;36:734–41. doi: 10.1016/s0168-8278(02)00062-4. [DOI] [PubMed] [Google Scholar]
- 7.Anthony DD, Yonkers NL, Post AB, Asaad R, Heinzel FP, Lederman MM, Lehmann PV, Valdez H. Selective impairment in dendritic-cell associated function distinguish hepatitis C virus and HIV infection. J Immunol. 2004;172:4907–16. doi: 10.4049/jimmunol.172.8.4907. [DOI] [PubMed] [Google Scholar]
- 8.Wertheimer AM, Bakke A, Rosen HR. Direct enumeration and functional assessment of circulating dendritic cells in patients with liver disease. Hepatol. 2004;40:335–45. doi: 10.1002/hep.20306. [DOI] [PubMed] [Google Scholar]
- 9.Kanto T, Inoue M, Miyatake H, et al. Reduced numbers and impaired ability of myeloid and plasmacytoid dendritic cells to polarize T helper cells in chronic hepatitis C virus infection. J Infect Dis. 2004;190:1919–26. doi: 10.1086/425425. [DOI] [PubMed] [Google Scholar]
- 10.Piccioli D, Tavarini S, Nuti S, et al. Comparable functions of plasmacytoid and monocyte-derived dendritic cells in chronic hepatitis C patients and healthy donors. J Hepatol. 2005;42:61–7. doi: 10.1016/j.jhep.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Longman RS, Talal AH, Jacobson IM, Rice CM, Albert ML. Normal functional capacity in circulating myeloid and plasmacytoid dendritic cells in patients with chronic hepatitis C. J Infect Dis. 2005;192:497–503. doi: 10.1086/431523. [DOI] [PubMed] [Google Scholar]
- 12.Tsubouchi E, Akbar SMF, Murakami H, Horiike N, Onji M. Isolation and functional analysis of circulating dendritic cells from hepatitis C virus (HCV) RNA-positive and HCV RNA-negative patients with chronic hepatitis C. role of antiviral therapy. Clin Exp Immunol. 2004;137:417–23. doi: 10.1111/j.1365-2249.2004.02544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levings MK, Bacchetta R, Schulz U, Roncarolo MG. The role of IL-10 and TGF-beta in the differentiation and effector function of T regulatory cells. Int Arch Allergy Immunol. 2002;129:263–76. doi: 10.1159/000067596. [DOI] [PubMed] [Google Scholar]
- 14.Lechner F, Gruener NH, Urbani S, et al. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur J Immunol. 2000;30:2479–87. doi: 10.1002/1521-4141(200009)30:9<2479::AID-IMMU2479>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 15.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 16.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–20. [PubMed] [Google Scholar]
- 17.Knodell RG, Ishak KJ, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–5. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 18.Della Bella S, Gennaro M, Vaccari M, et al. Altered maturation of peripheral blood dendritic cells in patients with breast cancer. Br J Cancer. 2003;89:1463–72. doi: 10.1038/sj.bjc.6601243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Della Bella S, Nicola S, Brambilla L, et al. Quantitative and functional defects of dendritic cells in classic Kaposi's sarcoma. Clin Immunol. 2006;119:317–29. doi: 10.1016/j.clim.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Della Bella S, Bierti L, Presicce P, Arienti R, Valenti M, Saresella M, Vergani C, Villa ML. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin Immunol. 2006 doi: 10.1016/j.clim.2006.09.012. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 21.Almeida J, Bueno C, Alguero MC, et al. Extensive characterization of the immunophenotype and pattern of cytokine production by distinct subpopulations of normal human peripheral blood MHC+/lineage– cells. Clin Exp Immunol. 1999;118:392–401. doi: 10.1046/j.1365-2249.1999.01078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bueno C, Almeida J, Alguero MC, et al. Flow cytometric analysis of cytokine production by normal human peripheral blood dendritic cells and monocytes: comparative analysis of different stimuli, secretion-blocking agents and incubation periods. Cytometry. 2001;46:33–40. [PubMed] [Google Scholar]
- 23.Della Bella S, Riva A, Tanzi E, et al. Hepatitis C virus-specific reactivity of CD4+-lymphocytes in children born from HCV-infected women. J Hepatol. 2005;43:394–402. doi: 10.1016/j.jhep.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 24.Barron MA, Blyveis N, Palmer BE, MaWhinney S, Wilson CC. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals. J Infect Dis. 2003;187:26–37. doi: 10.1086/345957. [DOI] [PubMed] [Google Scholar]
- 25.Longman RS, Talal AH, Jacobson IM, Albert ML, Rice CM. Presence of functional dendritic cells in patients chronically infected with hepatitis C virus. Blood. 2004;103:1026–9. doi: 10.1182/blood-2003-04-1339. [DOI] [PubMed] [Google Scholar]
- 26.Murakami H, Akbar SMF, Matsui H, Horiike N, Onji M. Decreased interferon-α production and impaired T helper 1 polarization by dendritic cells from patients with chronic hepatitis C. Clin Exp Immunol. 2004;137:559–65. doi: 10.1111/j.1365-2249.2004.02550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szabo G, Dolganiuc A. Subversion of plasmacytoid and myeloid dendritic cell functions in chronic HCV infection. Immunobiol. 2005;210:237–47. doi: 10.1016/j.imbio.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 28.Fugier-Vivier I, Servet-Delprat C, Rivailler P, Rissoan MC, Liu YJ, Rabourdin-Combe C. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J Exp Med. 1997;186:813–23. doi: 10.1084/jem.186.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donaghy H, Pozniak A, Gazzard B, Qazi N, Gilmour J, Gotch F, Patterson S. Loss of blood CD11c+ myeloid and CD11c–- plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 2001;98:2574–6. doi: 10.1182/blood.v98.8.2574. [DOI] [PubMed] [Google Scholar]
- 30.Bartz H, Turkel O, Hoffjan S, Rothoeft T, Gonschorek A, Schauer U. Respiratory syncytial virus decreases the capacity of myeloid dendritic cells to induce interferon-γ in naive T cells. Immunol. 2003;109:49–57. doi: 10.1046/j.1365-2567.2003.01629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beckebaum S, Cicinnati VR, Zhang X, Ferencik S, Frilling A, Grosse-Wilde H, Broelsch CE, Gerken G. Hepatitis B virus-induced defect of monocyte-derived dendritic cells leads to impaired T helper type 1 response in vitro: mechanisms for viral immune escape. Immunol. 2003;109:487–95. doi: 10.1046/j.1365-2567.2003.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jimenez-Flores R, Mendez-Cruz R, Ojeda-Ortiz J, et al. High-risk human papilloma virus infection decreases the frequency of dendritic Langerhans' cells in the human female genital tract. Immunol. 2006;117:220–8. doi: 10.1111/j.1365-2567.2005.02282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larsson M, Beignon AS, Bhardwaj N. DC-virus interplay: a double edged sword. Semin Immunol. 2004;16:147–61. doi: 10.1016/j.smim.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–93. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 35.Steinbrink K, Graulich E, Kubsch S, Knop J, Enk AH. CD4+ and CD8+ anergic T cells induced by interleukin-10-treated human dendritic cells display antigen-specific suppressor activity. Blood. 2002;99:2468–76. doi: 10.1182/blood.v99.7.2468. [DOI] [PubMed] [Google Scholar]
- 36.Watford WT, Moriguchi M, Morinobu A, O'Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14:361–8. doi: 10.1016/s1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 37.McCaughan GW, Zekry A. Effects of immunosuppression and organ transplantation on the natural history and immunopathogenesis of hepatitis C virus infection. Transpl Infect Dis. 2000;2:166–85. doi: 10.1034/j.1399-3062.2000.020403.x. [DOI] [PubMed] [Google Scholar]
- 38.Doherty DG, O'Farrelly C. Dendritic cells: regulators of hepatic immunity or tolerance? J Hepatol. 2001;34:156–60. doi: 10.1016/s0168-8278(00)00020-9. [DOI] [PubMed] [Google Scholar]
- 39.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]