Abstract
Human CD32B (FcγRIIB), the low-affinity inhibitory Fcγ receptor (FcγR), is highly homologous in its extracellular domain to CD32A (FcγRIIA), an activating FcγR. Available monoclonal antibodies (mAb) against the extracellular region of CD32B recognize both receptors. Through immunization of mice transgenic for human CD32A, we generated a set of antibodies specific for the extracellular region of CD32B with no cross-reactivity with CD32A, as determined by enzyme-linked immunosorbent assay and surface plasmon resonance with recombinant CD32A and CD32B, and by fluorescence-activated cell sorting analysis of CD32 transfectants. A high-affinity mAb, 2B6, was used to explore the expression of CD32B by human peripheral blood leucocytes. While all B lymphocytes expressed CD32B, only a fraction of monocytes and almost no polymorphonuclear cells stained with 2B6. Likewise, natural killer cells, which express CD32C, a third CD32 variant, did not react with 2B6. Immune complexes co-engage the inhibitory receptor with activating Fcγ receptors, a mechanism that limits cell responses. 2B6 competed for immune complex binding to CD32B as a monomeric Fab, suggesting that it directly recognizes the Fc-binding region of the receptor. Furthermore, when co-ligated with an activating receptor, 2B6 triggered CD32B-mediated inhibitory signalling, resulting in diminished release of inflammatory mediators by FcεRI in an in vitro allergy model or decreased proliferation of human B cells induced by B-cell receptor stimulation. These antibodies form the basis for the development of investigational tools and therapeutics with multiple potential applications, ranging from adjuvants in FcγR-mediated responses to the treatment of allergy and autoimmunity.
Keywords: Fcγ receptors, CD32B (Fcγ RIIB), CD32A (Fcγ RIIA), monoclonal antibodies, B lymphocytes
Introduction
Immunoglobulin G Fc receptors (FcγRs) provide a crucial link between the humoral and cellular immune responses. Binding of antigen–antibody complexes to certain FcγRs contributes to the reaction of the host against pathogens by delivering activation signals that lead to endocytosis, phagocytosis and the release of inflammatory mediators. Inhibitory signals, concomitantly triggered during immune stimulation, coincide to prevent deleterious consequences of unchecked cell activation. The low-affinity Fcγ-receptor IIB (FcγRIIB, CD32B) delivers such an inhibitory signal and has emerged as one of the critical regulatory receptors in immune homeostasis.1
CD32B counterbalances cell activation by a whole spectrum of activating receptors, including the high-affinity FcγR, CD64 (FcγRI), the low-affinity FcγRs, CD16A (FcγRIIIA) and CD32A (FcγRIIA),2,3 and the high-affinity receptor for immunoglobulin E (IgE), FcεRI.4,5 CD32B is coexpressed with activating Fc receptors on monocytes, macrophages, basophils and mast cells. CD32B is also expressed by B lymphocytes, the sole FcγR expressed by these cells, where it functions by countering B-cell receptor (BCR)-induced activation,6 evidence that the inhibitory properties of CD32B are not limited to FcγRs. Mice deficient in CD32B show enhanced antibody responses, including increased IgG- and IgE-induced anaphylactic reactions,7–9 hypersensitivity to collagen-induced arthritis,10 the spontaneous development of lupus-like symptoms in C57BL/6 mice11,12 and an improved outcome to antibody-based immunotherapy in tumour models.13 Furthermore, the in vivo cell-depleting activity of monoclonal antibodies of different IgG subclasses correlates with their differential binding to the murine activating FcγRs compared to CD32B.14 CD32B appears also to mediate the anti-inflammatory effect of high-dose intravenous immunoglobulins in some murine models of autoimmune disease, including nephrotoxic nephritis15 and KRN × NOD immune complex (IC)-induced arthritis.16,17 In immune thrombocytopenic purpura, the therapeutic effects of intravenous immunoglobulins may be mediated, at least in part, by an effect on CD32B expression18 in addition to an effect on activating FcγR present on dendritic cells.19 Taken together, these data demonstrate that CD32B contributes to controlling both the threshold and the extent of immune activation by maintaining immune tolerance and preventing the emergence of autoimmune disease.1,20
In view of its critical homeostatic role, CD32B is a promising target for therapeutic purposes. The high degree of sequence similarity between human CD32B and CD32A within their extracellular regions, however, has been a formidable obstacle in the generation of antibodies specific for the inhibitory receptor. In contrast to CD32B, CD32A leads to cell activation via the immune tyrosine-based activation motifs present in its cytoplasmic domain. Of the anti-human CD32 monoclonal antibodies (mAbs) previously described,21–23 one group reacts equally well with both myeloid and lymphoid cells, indicative of shared reactivity with both CD32A and CD32B. A second group is strongly reactive with monocytes, macrophages and neutrophils but only weakly with lymphocytes or B-cell lines, an indication of the ability of these mAbs to recognize exclusively or prevalently CD32A. We have succeeded in generating a panel of mAbs exhibiting specific recognition of human CD32B with no cross-reactivity with CD32A and used them to characterize CD32B expression by peripheral blood human leucocytes. A subset of these antibodies was functionally characterized for their ability to prevent IC binding to CD32B or to induce an inhibitory signal.
Materials and methods
Antibodies
Commercially available anti-CD32 antibodies were obtained from the following vendors: FLI8.26 from Research Diagnostics; Flanders, NJ, and KB61 from Accurate Chemical; Westbury, NY. The anti-CD32A antibody, IV.3 (IgG2b, k), was derived from the corresponding hybridoma [American Type Culture Collection (ATCC), Manassas, VA] and purified from conditioned media. Mouse IgG1 and IgG2b isotype control mAbs were purchased from BD Bioscience-Pharmingen (San Diego, CA) and the F(ab′)2 fragment of an Fc-fragment-specific, goat anti-mouse (GAM) IgG, was obtained from Jackson Laboratories (West Grove, PA).
Human samples and cell lines
Human whole blood was collected in heparin from healthy donors (BRT Laboratory, Baltimore, MD and Research Blood Component, L.L.C., Brighton, MA). Human B lymphoblastoid cell lines, Daudi and Raji, and the human monocytic cell line, U-937, were maintained in RPMI-1640 supplemented with 10% fetal calf serum, 2 mm l-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin and 1 mm sodium pyruvate. Human embryonic kidney cell line 293-HEK, Chinese hamster ovary line, CHO-K1, and rat basophilic RBL-2H3 cell line (ATCC) were maintained in Dulbecco's modified Eagle's minimal essential medium (DMEM; Invitrogen, Carlsbad, CA) supplemented as for RPMI-1640. The 293-HEK (293H, Invitrogen), CHO-K1 and RBL-2H3 cells were transfected using Lipofectamine 2000 (Invitrogen), as described by the manufacturer. Transfectants expressing the recombinant receptors were cultured in DMEM containing 10% fetal calf serum and G418 (BioSource, Camarillo, CA).
Genotyping
The CD32A-H/H131, CD32A-R/R131 and CD32A-H/R131 genotype of each donor was determined by DNA sequence analysis using a sequence-specific forward primer (5′-TGTAAAACGACGGCCAGA-TGGAAAATCCCAGAAATTC-3′) and reverse primer (5′-CAGGAAACAGCTATG-ACCCTTGGACAGTGATGGTCACAG-3′). Sequencing reactions were run and analysed on an automated sequencer (ABI3100, Applied BioSystems, Foster City, CA).
Fc-receptor cDNAs encoding the human CD32A-R131 and CD32B1 have been described elsewhere.24 The full-length coding region of the CD32B1 gene was isolated as an EcoRI fragment and cloned into the polylinker of the pCIneo expression vector (Promega, Madison, WI). Plasmids expressing the soluble forms of either CD32A-R131 or CD32B1 were constructed by ligation of an EcoRI–NotI fragment encoding the receptor's extracellular domain (180 amino acids) into pcDNA3 (Invitrogen). Soluble recombinant CD32A-R131 or CD32B1 present in the conditioned media of 293H transfectants were purified by affinity chromatography on human IgG, resulting in a product homogeneity of ≥ 95%. Concentration was determined by measurement of optical density at 280 nm (OD280) and homogeneity was determined by sodium dodecyl sulphate–polyacrylamide gel electrophoresis.
The CD32B1-Fc (sFcRIIb-G2) gene was constructed by amplifying the extracellular region of human CD32B1, including the signal sequence, from a cDNA clone followed by joining it to the hinge-CH2-CH3 domains of human IgG2 by overlapping polymerase chain reaction (PCR). The resulting fragment was cloned into pCIneo as a NheI–EcoRI fragment. The single glycosylation site in the IgG2 Fc was eliminated by site-directed mutagenesis of Asn297 to Gln using the Quick Change kit (Stratagene, La Jolla, CA). The resulting expression plasmid was then transfected into 293H cells and a stable expression cell line was obtained. Cultures of this cell line were expanded and the conditioned medium was used for purification. Purification was accomplished as described above.
Generation of mAbs and antibody engineering
Monoclonal antibodies against human CD32B were generated by immunizing mice transgenic for human FCGR2A25 (kindly provided by Dr Jeffrey V. Ravetch, Rockefeller University, New York) with the soluble extracellular domain of recombinant human CD32B1. A & G Pharmaceutical (Baltimore, MD) performed immunizations and fusions. Briefly, three female mice (4–7 weeks old) were immunized multiple times and tail-bleeds were screened by enzyme-linked immunosorbent assay (ELISA) against the antigen. Harvested splenocytes were fused to SP2/0 mouse myeloma cells and supernatants were harvested 14 days post-fusion and screened for antibody production. Antibody-positive fusions were analysed for binding using an antigen-specific ELISA assay (see below). A Clonotyping System/AP kit was used for immunoglobulin isotyping assay according to the manufacturer's instructions (Southern Biotechnology, Birmingham, AL).
The anti-fluorescein chimeric mAb, 4-4-20 (ch4-4-20), was constructed by fusing the murine 4-4-20 VH region to a signal peptide and a human Cγ1 constant region by overlapping PCR. To facilitate cloning, a SacI site was introduced into the VH-Cγ1 junction sequence. To construct the ch4-4-20 light-chain gene, the murine 4-4-20 VL segment was fused to both a signal sequence and a human CK constant region using overlapping PCR amplification. Heavy-chain and light-chain plasmids were cotransfected into 293H cells for antibody expression.
ELISA
For direct binding ELISA, MaxiSorp Immunoplates (Nalge Nunc International, Rochester, NY) were coated with 50 ng/well of CD32A-R131 or CD32B1 soluble receptors. Plates were washed with phosphate-buffered saline (PBS) containing 0·1% Tween-20 (PBS-T), blocked for 1 hr with PBS containing 1% bovine serum albumin (BSA), then 10-fold dilutions of the purified antibodies were incubated for 1 hr at room temperature. Bound antibodies were detected with GAM horseradish peroxidase-conjugated secondary antibody at a 1 : 10 000 dilution (Jackson Immunoresearch, West Grove, PA.). The colorimetric reaction was developed with 3,3′,5,5′-tetramethylbenzidine (TMB) peroxidase substrate and absorbance was measured at 650 nm using a Versamax microplate reader (Molecular Devices, Sunnyvale, CA).
Flow cytometric analysis
Indirect immunofluorescence analysis was performed similarly for all cells. Briefly, cells were incubated with primary mAb for 1 hr at 4° in PBS containing 1% BSA, fraction V (PBS-BSA; Sigma-Aldrich, St Louis, MO) and 0·1% sodium azide (SA) followed by a cyanine 5 (Cy5)-conjugated F(ab′)2 GAM (Jackson Immunoresearch) using the concentrations indicated in the figure legends.
Peripheral blood leucocytes from healthy donors were stained with fluorescein isothiocyanate-(FITC)-conjugated KB61 (KB61-FITC; Accurate Chemical, Westbury, NY), 2B6-FITC (2 μg/ml) or 2B6 (0·2 μg/ml) plus Cy5-F(ab′)2 GAM. CD20-FITC or phycoerythrin (PE)-conjugated CD20, CD3-FITC, CD56-FITC or CD56-PE, CD14-FITC and CD16-FITC (BD Bioscience-Pharmingen, San Diego, CA) mAbs were used to define the lymphocyte subpopulations. In all assays, 5000 cultured cells or 10 000 peripheral blood leucocytes were analysed on a FACScalibur (Becton-Dickinson, San Diego, CA). To measure IC binding, CD32B1-transduced CHO-K1 cells were incubated with 5 μg/ml mAb in PBS-BSA, followed by one wash with PBS-BSA and incubation with 9 μg/ml heat-aggregated human IgG in PBS-BSA. After a second wash, cells were incubated with FITC-conjugated F(ab′)2 goat anti-human (GAH) IgG (Jackson Immunoresearch) and analysed by flow cytometry.
Surface plasmon resonance
Antibody binding to CD32A-R131 or CD32B1 was analysed by surface plasmon resonance using a BIAcore 3000 biosensor (Biacore AB, Uppsala, Sweden) as previously described.26
Competition ELISA
Analysis of IgG-FcR binding was performed by using IC formed with ch4-4-20 and FITC-conjugated S-protein (Novagen, San Diego, CA). MaxiSorp Immunoplates were coated with S-protein-FITC (50 μl/well), blocked with PBS containing 0·1% Tween-20 and 0·5% BSA (PBS/T-BSA) for 30 min at room temperature, and followed by addition of 50 μl (1 μg/ml) ch4-4-20 in PBS/T-BSA to each well to form ICs. CD32B mAb, Fab fragments of CD32B mAb, or isotype controls were titrated in PBS/T-BSA at the concentrations indicated, mixed with an equal volume of 0·5 μg/ml CD32B1-Fc in PBS/T-BSA, and transferred to the IC-containing 96-well plate for incubation. Plates were washed and incubated with a biotin-conjugated, Fc-specific mouse anti-human IgG2 mAb (Calbiochem, San Diego, CA) followed by horseradish peroxidase-conjugated streptavidin (Amersham Pharmacia Biotech, UK). Plates were developed and assayed as previously described for the ELISA.
β-hexosaminidase release assay
Transfected RBL-2H3 cells were resuspended in fresh medium containing 0·01 μg/ml murine anti-dinitrophenyl (DNP) IgE mAb (Serotec Inc., Raleigh, NC) and dispensed in a 96-well plate at a concentration of 2 × 104 cells/well for overnight incubation at 37° in the presence of 5% CO2. Cells were washed twice with PBS and re-suspended with pre-warmed release buffer (HEPES 10 mm, NaCl 137 mm, KCl 2·7 mm, sodium phosphate monobasic 0·4 mm, glucose 5·6 mm, calcium chloride 1·8 mm, magnesium sulphate 1·3 mm and 0·04% BSA, pH 7·4). Washed cells were treated at 37° for 1 hr with either 3 μg/ml anti-CD32B mAb or a murine IgG1 isotype control followed by serial dilutions (0·03 μg/ml to 30 μg/ml) F(ab′)2 fragment of GAM (Jackson Immunoresearch). The reaction was stopped after 30 min by placing the cells on ice. To measure the released enzyme, 50 μl supernatant was removed from each well, while total enzyme content was determined from osmotically lysed cells. Supernatants and cell lysates were incubated with p-nitrophenyl-N-acetyl-β-d-glucosaminide (5 mm) for 90 min, the reaction was stopped with glycine (0·1 m, pH 10·4), and the absorbance at 405 nm was measured after 3 min. The percentage of β-hexosaminidase released was calculated as follows: (supernatant)/(supernatant +cell lysates).
B-cell purification and proliferation assay
Peripheral blood mononuclear cells were separated by a Ficoll/Paque Plus (Amersham Pharmacia Biotech, UK) gradient method using blood from healthy donors. B lymphocytes were isolated using a Dynal B Cell Negative Isolation Kit (Dynal Biotechnology Inc., NY) following the manufacturer's instructions. The purity of the isolated B cells (CD20+) was greater than 95% as estimated by fluorescence-activated cell sorting (FACS) analysis. For the proliferation assay, purified B cells were seeded in complete RPMI-1640 medium in flat-bottomed 96-well microtitre plates at a cell density of 1 × 105 cells per well in a final volume of 200 μl and incubated for 48 hr in the presence or absence of antibodies at 37° in 5% CO2. Then, 1 μCi/well [3H]thymidine (Perkin Elmer, Wellesley, MA) was added and the incubation was continued for an additional 16–18 hr before harvesting. Incorporation of [3H]thymidine was measured by liquid scintillation counting.
Results
Generation of murine mAbs directed against the extracellular domain of human CD32B
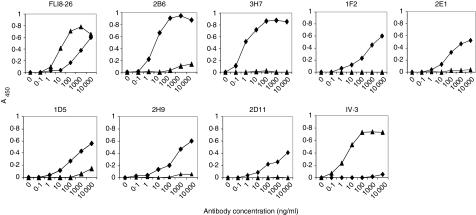
The extracellular regions of CD32A and CD32B differ in only a few amino acids (Fig. 1). While several differences are scattered throughout the whole molecule, some cluster between amino acids 127 and 135. This region is particularly relevant because allelic variations in the fcgr2a gene generate molecules that differ in the amino acid corresponding to position 131.24 While CD32B displays an invariant arginine (R) in this position, two polymorphic variants displaying either histidine (H) or arginine in position 131 have been described for CD32A. To improve the probability of generating mAbs capable of discriminating CD32B from its closest CD32A variant, mice transgenic for human CD32A-R131,25 and hence tolerant to this receptor, were immunized with the extracellular 180-amino acid domain of human CD32B. Splenocytes from three mice were isolated, fused with SP2/0 mouse myeloma cells, and the resulting hybridomas were tested for their differential ability to bind the inhibitory and activating receptors in direct-binding ELISA using soluble monomeric forms of either CD32B or CD32A-R131. Several clones reacted with CD32B at high titres with no or marginal reactivity with CD32A-R131(Fig. 2). In contrast, a pan-CD32 antibody, FLI8.26, recognized either form of the receptor, while a CD32A-specific antibody, IV.3, reacted with CD32A-R131 exclusively, confirming the specificity of the ELISA. Isotype analysis showed that six of the anti-CD32B clones (designated 2D11, 1D5, 1F2, 2E1, 3H7 and 2B6) were murine IgG1(κ), while a single clone, 2H9, was a murine IgG2a(κ).
Figure 1.
Amino acid alignment of the extracellular domains of human CD32A and CD32B. Amino acid differences between the two receptors are marked with an asterisk (*). Residues 127–135 (underlined) correspond to the IgG Fc binding site. Residue 131, which is polymorphic in CD32A (H/R), is labelled (^). The entire 180-amino acid sequence of CD32B extracellular domain was used for immunization.
Figure 2.
Anti-CD32B mAb binding to human CD32A and CD32B ELISA. Plates coated with either soluble CD32A-R131 (▴) or CD32B (♦) were used to analyse binding of the different antibodies. A pan-CD32 mAb, FLI8.26, and a CD32A-specific mAb, IV.3, were used as controls.
Antigen binding of the antibodies was further analysed by surface plasmon resonance. The antibodies of interest or isotype-matched controls were captured on the surface with an immobilized F(ab′)2 fragment of an Fc-specific GAM and soluble monomeric forms of either human CD32A-R131 or CD32B were injected at a constant flow rate to monitor the interaction between the receptors and the captured antibodies in real time. All mAbs showed binding to CD32B in the absence of detectable interaction with CD32A (Table 1 and data not shown). Two commercial anti-huCD32 antibodies, KB61 and FLI8.26, bound both receptors. Clones 2B6 and 3H7 showed the highest affinity for CD32B (Table 1), consistent with the ELISA data. Although 2B6 and 3H7 have similar affinity constants (KD), they differ in their kinetic parameters, with clone 2B6 demonstrating faster association (ka) and dissociation rates (kd) compared to 3H7.
Table 1.
Kinetic constants of monoclonal antibodies (mAbs) directed against the extracellular domains of CD32B1
| CD32B | CD32A-R131 | |||||
|---|---|---|---|---|---|---|
| mAb | ka (× 10−5/m/s) | kd (× 104/s) | KD (nm) | ka (× 10−5/m/s) | kd (× 104/s) | KD (nm) |
| 2B6 | 26·1 ± 12·4 | 6·9 ± 0·9 | 0·3 ± 0·1 | ND | ND | ND |
| 3H7 | 3·8 ± 0·4 | 2·2 ± 0·3 | 0·6 ± 0·1 | ND | ND | ND |
| 1F2 | 2·7 (2·4–3·0) | 335 (300–370) | 123 (122–124) | ND | ND | ND |
| FLI8·26 | 12·9 (9·5–16·3) | 178 (150–206) | 14·3 (12·6–16·0) | 10·5 (5·4–15·6) | 31 (22–40) | 3·3 (2·5–4·2) |
| KB61 | 13·9 (13·2–14·7) | 82 (80–84) | 5·9 (6·4–5·4) | 11·5 (11·1–12·0) | 220 (212–228) | 19 (19·0–19·1) |
Surface plasmon resonance was used to determine the kinetic constants for CD32 binding of 2B6, 3H7 and 1F2. The pan-CD32 mAbs, FLI8.26 and KB61, were used for comparison. Binding experiments were performed using receptor concentrations of 0, 3·13, 6·25, 12·5, 25, 50 and 100 nm. For 2B6 and 3H7, data shown are averages ± standard deviation of three or more independent determinations. All other evaluations were obtained from two independent experiments; shown are the average values and, between parentheses, individual data. ND = no detectable binding.
Discrimination of cell-surface-expressed CD32B from CD32A
To document further the ability of the antibodies to discriminate CD32B from CD32A, human embryonic kidney (293-HEK) were transfected with expression vectors encoding human CD32A-R131 or CD32B. Clones expressing similar receptor levels were selected by FACS analysis with the pan-anti-CD32 antibody, FLI.826. While none of the antibodies recognized the CD32A-R131 transfectants, all mAbs stained the inhibitory receptor expressed in 293-HEK cells (Fig. 3). All antibodies also reacted with the human Burkitt's lymphoma-derived lymphoblastoid lines, Daudi and Raji, cells known to express high levels of CD32B (Table 2). A subset of antibodies weakly stained the monocytic cell line, U-937,27 which is known to express low levels of both CD32B and CD32A (Table 2). These data show that the antibody panel specifically recognized CD32B with no apparent cross-reactivity with CD32A.
Figure 3.
Binding of anti-CD32B mAbs to human CD32A and CD32B cell transfectants. 293H cells stably transfected with either full-length CD32A-R131 or CD32B were incubated with 5 μg/ml 1F2, 2D11, 3H7, 2E1, 1D5, 2D11, or FLI8.26 or 1 μg/ml 2B6 in the presence of 10% human serum. Binding was detected by F(ab′)2 fragments of Cy5-GAM and FACS analysis. Plots show relative level of staining as MFI (x-axis) versus the number of events recorded (y-axis). Staining by the anti-CD32 mAb (filled histograms) is overlaid with that of the corresponding isotype controls (solid line).
Table 2.
FACS analysis of CD32-positive human cell lines1
| mAb | Raji | Daudi | U−937 |
|---|---|---|---|
| 2B6 | + + + | + + + | + |
| 3H7 | + + | + + | + |
| 1F2 | + + | + + | 0 |
| 2E1 | + + | + + | + |
| 1D5 | + + | + + | + |
| 2D11 | + + | + + | 0 |
| 2H9 | + + | + + | 0 |
| FLI8·26 | + + + | + + + | + |
Cells were incubated with saturating concentrations of the indicated monoclonal antibody (mAb) followed by detection with F(ab′)2 fragments of Cy5-GAM. Background fluorescence was detected by using the respective mouse isotype controls. 0, undetectable; + to + + + indicate increasing mean fluorescence intensity.
CD32B expression by human peripheral blood leucocytes
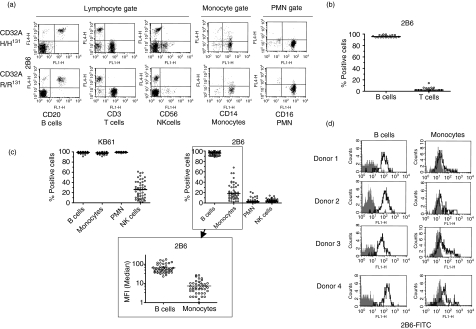
Having established that the antibody panel reacted specifically with CD32B, we selected 2B6, the clone with the highest affinity, to investigate the expression of the inhibitory receptor by peripheral blood human leucocytes. Initial analysis was performed by dual-colour indirect immunofluorescence analysis on peripheral blood leucocytes from 19 normal donors. Figure 4(a) shows FACS plots from two representative donors homozygous for either alloform of CD32A, while Fig. 4(b) shows a summary of 2B6 staining of B and T cells from all 19 donors. The data confirmed CD32B expression on all circulating CD20+ B lymphocytes and a variable fraction of CD14+ monocytes, while all other leucocytes were consistently negative.
Figure 4.
CD32 expression by peripheral blood leucocytes from healthy donors. (a) Representative 2B6 staining of peripheral blood leucocytes (PBL) from CD32A-H/H131 and CD32A-R/R131 homozygous normal donors. Immunofluorescence analysis of PBL was performed using CD20-FITC, CD3-FITC, CD56-FITC, CD14-FITC and CD16-FITC mAbs to define specific cell populations. Detection of 2B6 was performed by indirect immunofluorescence using Cy5-conjugated GAM F(ab′)2. (b) B lymphocytes (CD20-positive cells) and T lymphocytes (CD3-positive cells) from 19 healthy donors were stained with murine 2B6 by indirect immunofluorescence as described in (a). The scatter plot shows the percentages of CD32B-positive cells of each donor defined as the population above the threshold established by an isotype-matched control antibody. The black line indicates the median value. (c) CD32 and CD32B-specific staining of PBL from 48 healthy donors by direct immunofluorescence using KB61-FITC (top left panel) or 2B6-FITC (top right panel). In this analysis, PMN cells and monocytes were identified using forward and side scatter. B lymphocytes and NK cells were identified by staining with CD20-PE or CD56-PE, respectively. Scatter plots show the percentages of CD32 positive cells in each individual, defined as the population above the threshold established by isotype-matched control antibodies. The bottom panel shows a comparison of B-lymphocyte and monocyte MFI values for 2B6 staining. The MFI value shown is the value of the experimental sample after subtraction of the value of the isotype-matched control mAb in the same gate. In all panels, the black line indicates the median value. (d) FACS plots of four representative donors from the 48 donors summarized in (c). FACS histograms illustrate staining using mIgG1-FITC (filled) or 2B6-FITC (bold line).
To investigate further CD32B expression by peripheral blood leucocytes, 48 additional subjects were analysed by direct immunofluorescence with FITC-conjugated 2B6 and the FITC-conjugated pan-CD32 mAb, KB61. All B cells, monocytes and polymorphonuclear (PMN) cells reacted with KB61 (Fig. 4c, top left panel). While all B lymphocytes reacted with 2B6 as well, monocytes showed variable 2B6 staining, ranging from near 0 to ∼ 60% of the population, with a median value of ∼ 20% (Fig. 4c, top right panel). Furthermore, even when positive for CD32B, receptor density on the monocytes was low, as judged by comparing their mean-channel fluorescence intensity (MFI) to that of B lymphocytes (Fig. 4c, bottom panel and Fig. 4d, which show FACS histograms from four subjects representative of the monocyte reactivity spectrum). These data confirmed that all circulating monocytes expressed CD32A, but only a fraction, variable from subject to subject, appeared to express CD32B based on 2B6 reactivity.
Natural killer (NK) cells from approximately 50% of normal individuals have been shown to react to various degrees with KB61.28,29 This property has been attributed to the expression of CD32C, a molecule resulting from a crossover between fcgr2a and fcgr2b, whose extracellular domain is expected to be identical to that of CD32B.28,29 Staining of NK cells with KB61 from the 48 normal donors analysed confirmed these previous findings,28,29 with a fraction of the subjects being positive for the antibody (Fig. 4c, top left panel). None of the donors, however, showed staining of NK cells with 2B6 (Fig. 4c, top right panel) or 3H7 (data not shown), suggesting that these antibodies recognize different molecules or different forms of the receptor. Similarly, no donor showed 2B6-positive PMN cells, while strong staining of PMN cells was observed with KB61 in all subjects.
The high level of expression of CD32A by monocytes and PMN cells makes them ideal populations to analyse the possible contribution of the CD32A alloforms to 2B6 binding. Owing to its invariant arginine in position 131, CD32B shows increased homology to CD32A-R131 than CD32A-H131. Since residue 131 is located in the binding domain of either receptor (a region targeted by 2B6, as examined below), it is possible that the amino acid in position 131 of CD32A will have an impact on the cross-reactivity of 2B6 with this receptor. Furthermore, because 131 polymorphism also affects CD32A binding to the IgG Fc, it is also theoretically possible that Fc-CD32A engagement will contribute to 2B6 binding via its Fc domain rather than the paratope. Genotyping data were obtained for 46 of the 48 healthy donors analysed above in Fig. 4(c). When the data were compiled based on the homozygous and heterozygous genotype categories, both KB61 and 2B6 staining of B cells, monocytes, and PMN cells were unaffected by the CD32A polymorphism (Table 3). Therefore, 2B6 binding was independent of CD32A expression.
Table 3.
The reactivity of B cells, monocytes and polymorphonuclear (PMN) cells with KB61 or 2B6 is independent of the alloform of CD32A1
| B cells | Monocytes | PMN cells | |
|---|---|---|---|
| CD32A-R/R131 (n = 18) | |||
| KB61 | 99 ± 1 | 98 ± 2 | 99 ± 1 |
| 2B6 | 95 ± 4 | 21 ± 18 | 4 ± 4 |
| CD32A-H/H131 (n = 8) | |||
| KB61 | 97 ± 3 | 97 ± 1 | 99 ± 1 |
| 2B6 | 94 ± 4 | 26 ± 14 | 4 ± 4 |
| CD32A-H/R131 (n = 20) | |||
| KB61 | 99 ± 1 | 97 ± 2 | 99 ± 1 |
| 2B6 | 95 ± 3 | 21 ± 15 | 4 ± 5 |
Forty-six of the 48 healthy donors shown in Fig. 4(c) were genotyped for 131 polymorphism of CD32A as described in the Materials and Methods. B lymphocytes were defined by costaining with anti-CD20-PE while monocytes and PMN cells were identified by using FSC versus SSC plots. Cells were stained with either KB61-FITC or 2B6-FITC and threshold levels were determined by staining cells with an isotype-matched, FITC-labelled control mAb (mIgG1-FITC). The percentage of CD32-positive cells (± SD) in each gate is indicated.
The anti-CD32B clone, 2B6, blocks immune complex binding to CD32B
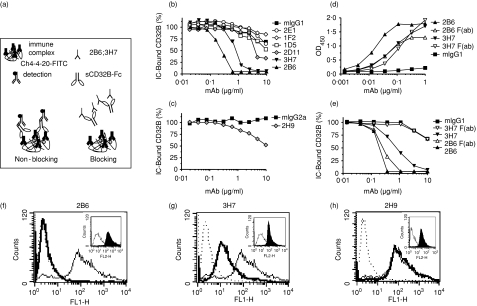
Since the IC interaction with CD32B delivers a negative signal, antibodies capable of selectively blocking this receptor may have useful investigational properties and potential clinical applications. A competition ELISA with immobilized IC and a soluble dimeric CD32B IgG2 fusion protein (sCD32B-Fc) was used to characterize the ability of the antibodies to block IC binding to human CD32B. The Fc domain of IgG2 was selected as a fusion partner to eliminate the potential formation of CD32B-Fc concatamers, because this Fc does not efficiently bind human FcγR. The Fc domain was further engineered with a mutation in Asn297 (N297Q), a glycosylation site, to reduce residual FcγR binding further. The IC were constructed by using plate-immobilized S-protein-FITC complexed with ch4-4-20, a chimeric mAb directed against fluorescein. A saturating concentration of sCD32B-Fc was incubated with the preformed immobilized IC in the presence of competing concentrations of anti-CD32B mAb or isotype-matched controls. Bound sCD32B-Fc was detected with a biotin-conjugated mouse anti-human IgG2 mAb, as illustrated in Fig. 5(a).
Figure 5.
Inhibition of immune complex binding by anti-CD32B antibodies. (a) Diagram illustrating the experimental set-up of the competition ELISA. (b,c) CD32B-specific mAb were tested for their ability to block IC binding to sCD32B-Fc in a competition ELISA. Each plot compares the binding to the corresponding isotype-matched control mAb. (d) Binding of 2B6, 3H7 or their corresponding Fab fragments to sCD32B-Fc in a direct binding ELISA. Bound antibodies were detected with a κ-chain-specific, horseradish peroxidase-conjugated GAM. (e) IC binding competition by 2B6, 3H7, or their corresponding Fab fragments. (f–h) CD32B-transduced CHO-K1 cells were preincubated with 2B6 (f), 3H7 (g) or 2H9 (h) followed by aggregated human IgG and the F(ab′)2 fragment of an FITC-conjugated GAH IgG. Murine isotype + GAH-FITC (dotted line), murine isotype + aggregated human IgG + GAH-FITC (thin line), and anti-CD32B mAb + aggregated human IgG + GAH-FITC (bold line). The level of binding of each of the mAbs to the CHO-CD32B cell line was determined by FACS analysis (insets).
Of the anti-CD32B antibodies, only 2B6 and 3H7 effectively competed for IC binding to the sCD32B-Fc fusion protein (Fig. 5b) when used as whole molecule. The only IgG2a mAb, 2H9, showed a modest effect compared to its isotype-matched control antibody, but only at the highest concentration tested (Fig. 5c). All remaining mAbs were indistinguishable from the murine isotype control (Fig. 5b).
To exclude Fc–CD32B interactions as a contributing mechanism to 2B6 or 3H7 competition, monomeric Fab fragments of the two antibodies were generated. Either Fab fragment bound sCD32B-Fc with similar potency in a direct binding ELISA (Fig. 5d), albeit 2B6 Fab binding was reduced with respect to that of the corresponding whole molecule. In IC competition, however, only the Fab fragment of 2B6 blocked IC binding (Fig. 5e), indicating that the mAb itself recognized a region within the Fc binding site of CD32B. The Fab fragment of 3H7 was unable to compete for IC binding, suggesting that 3H7 recognizes an epitope different from that recognized by 2B6 and distinct from the Fc-binding site of CD32B (Fig. 5e). Therefore, the results observed with the whole 3H7 mAb were probably the result of its Fc competing for the receptor.
The ability of 2B6 to block the IC interaction with the inhibitory receptor expressed on the cell surface was investigated in CHO-KI cells transduced with CD32B and compared to that of 2H9 and 3H7. The two mAbs and their isotype controls were incubated with the CHO-CD32B cell line, followed by aggregated human IgG and F(ab′)2 fragment of an FITC-conjugated GAH IgG to detect the bound IC by flow cytometry. As predicted by the ELISA data, 2B6 fully competed for IC binding to the receptor, because cells treated with the antibody show no detectable binding of aggregated human IgG (Fig. 5f). The 3H7 partially competed for IC binding (Fig. 5g), while 2H9 did not interfere with the binding of the aggregated IgG to the CD32B expressed on the cell surface (Fig. 5h).
Inhibitory signalling by anti-CD32B mAbs
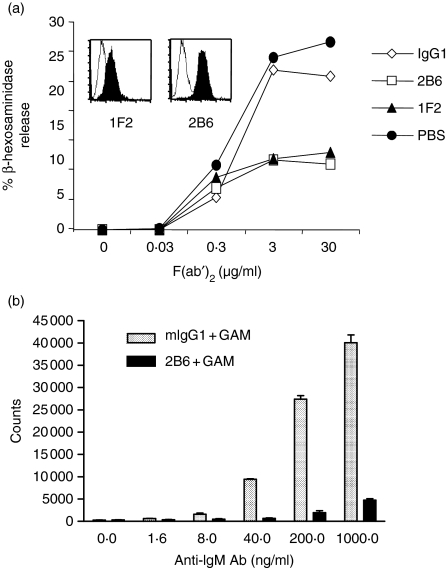
Two models were developed to test the ability of anti-CD32B antibodies to deliver an inhibitory signal: one based on the RBL-2H3 rat basophilic leukaemia line, a system extensively used to study the signalling mechanism underlying IgE-mediated cell activation in allergy,30–32 and a second system that explored the ability of CD32B to inhibit antigen receptor-induced activation of human B cells. Since none of the antibodies reacted with rodent CD32B (data not shown), RBL-2H3 cells were stably transfected with the human CD32B (RBL-2H3-hCD32B cells). No release of β-hexosaminidase, a marker of degranulation,33 was detected in cells treated with the anti-CD32B antibodies, alone or in the presence of a secondary antibody (data not shown). When RBL-2H3-hCD32B cells were sensitized with a mouse IgE mAb and challenged with increasing concentrations of a F(ab′)2 fragment of a polyclonal GAM that recognizes the heavy and light chains of the bound IgE, a dose-dependent release of β-hexosaminidase was observed (Fig. 6a). Co-ligation of the rat FcεRI with human CD32B was obtained by preincubating cells with either 2B6 or 1F2, resulting in a significant decrease in β-hexosaminidase release when compared to sensitized cells preincubated with an irrelevant isotype-matched control antibody.
Figure 6.
Anti-CD32B-induced inhibition of both FcεRI activation and B-cell proliferation. (a) β-hexosaminidase release was measured in RBL-2H3-hCD32B cells. Cells were sensitized with mouse IgE, followed by incubation with 2B6, 1F2 or a murine IgG1 isotype control, and stimulated with GAM F(ab′)2. Binding of 2B6 and 1F2 to RBL-2H3-hCD32B by FACS analysis is shown in the inset. Results are representative of three independent experiments. (b) Purified B cells were activated using increasing concentrations of anti-human IgM mAb (x-axis) and 50 μg/ml of F(ab′)2 fragment of GAM IgG Fc specific in the presence of 5 μg/ml of either mouse IgG1 (speckled bars) or 2B6 mAb (black bars). The proliferation was assessed on day 3 by [3H]thymidine incorporation (y-axis), the reactions were performed in triplicate and standard deviations were calculated. Results are representative of three independent experiments.
Co-aggregation of CD32B with the BCR has been shown to inhibit B-cell activation.2,34,35 To further explore the inhibitory properties of CD32B, B cells were negatively selected from human blood and activated through treatment with increasing concentrations of a mouse anti-human IgM as a polyclonal activator together with a fixed concentration (5 μg/ml) of 2B6 or an equivalent amount of mouse IgG1 isotype control (Fig. 6b). Coligation of the BCR with control mouse IgG1 or 2B6 was obtained by the addition of a F(ab′)2 fragment of an Fc-specific GAM as a secondary reagent. Cell proliferation, measured as [3H]thymidine incorporation, increased with increasing concentrations of the polyclonal anti-IgM activator in the presence of the irrelevant control antibody (Fig. 6b, mIgG1), indicating that the secondary cross-linking reagents were not limiting. In the presence of 2B6, coligation of BCR and CD32B via the secondary antibody led to a profound reduction in B-cell proliferation at all concentrations of anti-human IgM (Fig. 6b, 2B6). These data further confirm that mAb-mediated CD32B coligation with activating receptors can deliver an inhibitory signal that is capable of blocking antigen-receptor-induced cell activation.
Discussion
Recent knowledge about the regulatory function of CD32B has produced great interest in understanding its role in disease, an effort that has been significantly limited by the lack of antibodies capable of selectively recognizing the extracellular domain of the human inhibitory receptor. We describe here the generation of a panel of mAbs specific for human CD32B with unique properties and no demonstrable cross-recognition of CD32A. These mAbs provide essential investigative reagents and form the basis for the development of clinical tools for the treatment of human diseases.
None of the anti-CD32 mAbs previously described has been able to distinguish CD32B from CD32A. This also holds true for II8D2, a mAb initially described to be specific for the inhibitory receptor based on its reported ability to selectively recognize this isoform in Western blots.23,36 While we have been unable to obtain this antibody for direct comparison with our panel, II8D2 has been more recently shown to react with both CD32A and CD32B using indirect immunofluorescence staining and immunoprecipitation assays.21 It was further shown that II8D2 reacts poorly in FACS analysis,21 a feature that was ascribed to limited accessibility of the epitope recognized by this antibody in intact cells or non-denatured proteins.
The CD32B selectivity of the antibody panel herein described was demonstrated with a combination of ELISA, BIAcore analysis, and cell-based FACS assays. Two high-affinity mAb, 2B6 and 3H7, were identified and 2B6 was selected for extensive characterization of CD32B expression and function. FACS analysis of leucocytes from normal donors showed the expected expression of CD32B by all peripheral blood B lymphocytes. Surprisingly, no peripheral blood PMN cells (mostly neutrophils) were observed, while only a fraction of the monocyte population showed significant 2B6 staining. In addition, 2B6 showed no reactivity with human platelets, whereas platelets as well as PMN cells and monocytes completely stained with IV.3, a CD32A-specific mAb (data not shown; staining of PMN cells and monocytes with KB61, a pan-CD32 mAb, are shown in Fig. 4c). Furthermore, 2B6 reactivity with CD32B, whose sequence displays an invariant arginine in position 131, was unaffected by concomitant expression of any of the CD32A alloforms. In particular, CD32A-R/R131 subjects did not show 2B6 reactivity with the monocytes that was different from that of CD32A-H/H131 or heterozygous subjects. This property was also confirmed in a limited series of subjects (19 donors) with the other mAb of the CD32B panel (data not shown).
The absence of 2B6 staining of PMN cells and the low levels and frequency of monocyte reactivity observed in our study appear to contradict reports of expression of the inhibitory receptor by these leucocytes.27,36,37 Similarly, the lack of recognition of CD32C, the CD32 isoform expressed by NK cells in ∼ 45% of normal subjects,28,29 was unexpected, because the unequal crossover between fcgr2a and fcgr2b that generates CD32C contains the extracellular region of CD32B. Since the optimal mAb concentration for FACS analysis was determined from saturation studies with peripheral blood B lymphocytes and B-cell lines, it is unlikely that low levels of expression by myeloid peripheral blood leucocytes went undetected because of insufficient reagent. Furthermore, both we and others38 noted good 2B6 staining of in vitro monocyte-derived macrophages and dendritic cells (unpublished observations), consistent with previous reports of cytokine-induced CD32B transcription.27,36,37,39 Therefore, 2B6 can detect CD32B expressed by differentiated mononuclear phagocytes.
While the exact reason for the discrepancy between our data and previously published information remains unclear, there are several possible explanations. Past studies have frequently relied on comparisons of CD32A and CD32B transcription levels,36,37 which do not directly correlate with protein expression, because translational efficiency of each receptor may be profoundly different. A complete dissociation between transcription and translation, however, is unlikely, because it has been recently shown that the activating-to-inhibitory FcγR mRNA ratio in PMN cells correlates with responsiveness to phagocytic stimuli.37 Therefore, some levels of protein must be expressed, consistent with the identification of CD32B in PMN cells and monocytes by immunoblotting or immunoprecipitation with sera directed against intracellular epitopes unique to this receptor.36 It is possible, however, that translated FcγR may fail to reach the cell surface, as shown for a splice variant of FcγRI (CD64).40 Whether this is true for the CD32B2 splice variant prevalently expressed by PMN cells and peripheral blood monocytes remains to be determined.
With respect to NK cells, it should be noted that 2B6 is not the only anti-CD32 mAb that does not recognize CD32C: in contrast to KB61, another CD32 mAb, AT10, reacts poorly with CD32C,28,29 which led to speculation that this mAb might have been sensitive to a CD32C-specific amino acid change at position 161.29 An alternative explanation for the differential reactivity of AT10 and that of our antibodies is that CD32B expressed by monocytes and PMN cells as well as CD32C may be post-translationally modified, e.g. glycosylated, in a manner different from that of B lymphocytes, thus masking the epitope recognized by these mAb. Further studies will be aimed at dissecting these different possibilities.
The 2B6 Fab blocked IC binding to CD32B, while 3H7 did so only as a whole antibody, but failed in its monomeric Fab form. None of the other anti-CD32B mAbs interfered with IC binding. It is likely that the high affinity of 3H7 for its epitope and its slow off-rate allows its Fc portion to compete effectively for the CD32B binding site. Blockade of CD32B has multiple investigational and pharmacological application, such as the enhancement of dendritic cell functions38,41 or the improvement of the antitumour responses of therapeutic antibodies whose effector mechanism may involve antibody-dependent cytotoxicity, as suggested by the enhanced activity of immunotherapy observed in tumour-bearing CD32B knock-out mice.26
Another application of antibodies targeting CD32B is the exploitation of the receptor's negative signal. This function was demonstrated for BCR-induced proliferation of human B cells and antigen receptor-induced activation of a rat basophilic leukaemia line commonly used as an in vitro allergy model. Co-aggregation via 2B6 may have the potential to modulate the function of all coexpressed immunoreceptor tyrosine-based activation motif (ITAM)-coupled activating immunoreceptors2 and control proliferation of malignant B cells. Interestingly, 2B6 and 1F2 were equally capable of inhibiting IgE-induced activation of RBL-2H3 cells, irrespective of their receptor blocking capability. In fact, non-blocking mAbs may be preferable for agonistic inhibitory applications, such as the control of allergic responses or autoimmune diseases, to minimize interference with the physiological triggering of CD32B by circulating IC.
In conclusion, we have generated a panel of CD32B-specific mAbs whose binding characteristics and functional properties make them suitable for applications ranging from the study and treatment of allergy and autoimmune diseases to the potential use as adjuvant in immunotherapy and vaccination.
Acknowledgments
The authors would like to thank Kalpana Shah and Tosan Tutse-Towne for their excellent technical assistance, Dr. Christopher T. Rankin for helpful discussions and Dr. Jeffrey L. Nordstrom for critical review of the manuscript.
Abbreviations
- BCR
B-cell receptor
- BSA
bovine serum albumin
- Cy5
cyanine 5
- ELISA
enzyme-linked immunosorbent assay
- FACS
fluorescence-activated cell sorting
- FcγR
IgG Fc receptor
- FcγRIIB
low-affinity Fcγ-receptor IIB
- FcεRI
high-affinity receptor for IgE
- FITC
fluorescein isothiocyanate
- GAH
goat anti-human
- GAM
goat anti-mouse IgG
- IC
immune complex
- IgG
immunoglobulin G
- ITAM
immunoreceptor tyrosine-based activation motif
- mAb
monoclonal antibody
- MFI
mean-channel fluorescence intensity
- NK
natural killer cells
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PE
phycoerythrin
- PMN
polymorphonuclear cells
- SA
sodium azide
References
- 1.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–90. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 2.Daeron M, Latour S, Malbec O, et al. The same tyrosine-based inhibition motif, in the intracytoplasmic domain of Fc gamma RIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity. 1995;3:635–46. doi: 10.1016/1074-7613(95)90134-5. [DOI] [PubMed] [Google Scholar]
- 3.Hunter S, Indik ZK, Kim MK, Cauley MD, Park JG, Schreiber AD. Inhibition of Fcgamma receptor-mediated phagocytosis by a nonphagocytic Fcgamma receptor. Blood. 1998;91:1762–8. [PubMed] [Google Scholar]
- 4.Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–34. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 5.Zhu D, Kepley CL, Zhang M, Zhang K, Saxon A. A novel human immunoglobulin Fc gamma Fc epsilon bifunctional fusion protein inhibits Fc epsilon RI-mediated degranulation. Nat Med. 2002;8:518–21. doi: 10.1038/nm0502-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coggeshall KM. Inhibitory signaling by B cell Fc gamma RIIb. Curr Opin Immunol. 1998;10:306–12. doi: 10.1016/s0952-7915(98)80169-6. [DOI] [PubMed] [Google Scholar]
- 7.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–9. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 8.Ujike A, Ishikawa Y, Ono M, et al. Modulation of immunoglobulin (Ig) E-mediated systemic anaphylaxis by low-affinity Fc receptors for IgG. J Exp Med. 1999;189:1573–9. doi: 10.1084/jem.189.10.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clatworthy MR, Smith KG. FcgammaRIIb balances efficient pathogen clearance and the cytokine-mediated consequences of sepsis. J Exp Med. 2004;199:717–23. doi: 10.1084/jem.20032197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuasa T, Kubo S, Yoshino T, et al. Deletion of fcgamma receptor IIB renders H-2(b) mice susceptible to collagen-induced arthritis. J Exp Med. 1999;189:187–94. doi: 10.1084/jem.189.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma) RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–85. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 12.Bolland S, Yim YS, Tus K, Wakeland EK, Ravetch JV. Genetic modifiers of systemic lupus erythematosus in FcgammaRIIB(–/–) mice. J Exp Med. 2002;195:1167–74. doi: 10.1084/jem.20020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 14.Hamaguchi Y, Xiu Y, Komura K, Nimmerjahn F, Tedder TF. Antibody isotype-specific engagement of Fcgamma receptors regulates B lymphocyte depletion during CD20 immunotherapy. J Exp Med. 2006;203:743–53. doi: 10.1084/jem.20052283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaneko Y, Nimmerjahn F, Madaio MP, Ravetch JV. Pathology and protection in nephrotoxic nephritis is determined by selective engagement of specific Fc receptors. J Exp Med. 2006;203:789–97. doi: 10.1084/jem.20051900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruhns P, Samuelsson A, Pollard JW, Ravetch JV. Colony-stimulating factor-1-dependent macrophages are responsible for IVIG protection in antibody-induced autoimmune disease. Immunity. 2003;18:573–81. doi: 10.1016/s1074-7613(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 17.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–3. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 18.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–6. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 19.Siragam V, Crow AR, Brinc D, Song S, Freedman J, Lazarus AH. Intravenous immunoglobulin ameliorates ITP via activating Fc gamma receptors on dendritic cells. Nat Med. 2006;12:688–92. doi: 10.1038/nm1416. [DOI] [PubMed] [Google Scholar]
- 20.Clynes R, Dumitru C, Ravetch JV. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science. 1998;279:1052–4. doi: 10.1126/science.279.5353.1052. [DOI] [PubMed] [Google Scholar]
- 21.Budde P, Weinrich V, Sondermann P, et al. Leukocyte typing V: white cell differentiation antigens. Oxford: Oxford University Press; 1995. Specificity of CD32 mAb for FcgammaRIIA, FcgammaRIIb1, and FcgammaRIIb2 expressed in transfected mouse B cells and BHK-21 cells; pp. 828–32. [Google Scholar]
- 22.Ierino FL, Hulett MD, McKenzie IF, Hogarth PM. Mapping epitopes of human Fc gamma RII (CDw32) with monoclonal antibodies and recombinant receptors. J Immunol. 1993;150:1794–803. [PubMed] [Google Scholar]
- 23.Weinrich V, Sondermann P, Bewarder N, Wissel K, Frey J. Epitope mapping of new monoclonal antibodies recognizing distinct human FcRII (CD32) isoforms. Hybridoma. 1996;15:109–16. doi: 10.1089/hyb.1996.15.109. [DOI] [PubMed] [Google Scholar]
- 24.Brooks DG, Qiu WQ, Luster AD, Ravetch JV. Structure and expression of human IgG FcRII (CD32). Functional heterogeneity is encoded by the alternatively spliced products of multiple genes. J Exp Med. 1989;170:1369–85. doi: 10.1084/jem.170.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKenzie SE, Taylor SM, Malladi P, et al. The role of the human Fc receptor Fc gamma RIIA in the immune clearance of platelets: a transgenic mouse model. J Immunol. 1999;162:4311–18. [PubMed] [Google Scholar]
- 26.Rankin CT, Veri MC, Gorlatov S, et al. CD32B, the human inhibitory Fc-gamma receptor IIB, as a target for monoclonal antibody therapy of B-cell lymphoma. Blood. 2006;108:2384–91. doi: 10.1182/blood-2006-05-020602. [DOI] [PubMed] [Google Scholar]
- 27.Tridandapani S, Siefker K, Teillaud JL, Carter JE, Wewers MD, Anderson CL. Regulated expression and inhibitory function of Fcgamma RIIb in human monocytic cells. J Biol Chem. 2002;277:5082–9. doi: 10.1074/jbc.M110277200. [DOI] [PubMed] [Google Scholar]
- 28.Ernst LK, Metes D, Herberman RB, Morel PA. Allelic polymorphisms in the FcgammaRIIC gene can influence its function on normal human natural killer cells. J Mol Med. 2002;80:248–57. doi: 10.1007/s00109-001-0294-2. [DOI] [PubMed] [Google Scholar]
- 29.Metes D, Ernst LK, Chambers WH, Sulica A, Herberman RB, Morel PA. Expression of functional CD32 molecules on human NK cells is determined by an allelic polymorphism of the FcgammaRIIC gene. Blood. 1998;91:2369–80. [PubMed] [Google Scholar]
- 30.Ott VL, Tamir I, Niki M, Pandolfi PP, Cambier JC. Downstream of kinase, p62 (dok), is a mediator of Fc gamma IIB inhibition of Fc epsilon RI signaling. J Immunol. 2002;168:4430–9. doi: 10.4049/jimmunol.168.9.4430. [DOI] [PubMed] [Google Scholar]
- 31.Daeron M, Malbec O, Latour S, Arock M, Fridman WH. Regulation of high-affinity IgE receptor-mediated mast cell activation by murine low-affinity IgG receptors. J Clin Invest. 1995;95:577–85. doi: 10.1172/JCI117701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malbec O, Fong DC, Turner M, et al. Fc epsilon receptor I-associated lyn-dependent phosphorylation of Fc gamma receptor IIB during negative regulation of mast cell activation. J Immunol. 1998;160:1647–58. [PubMed] [Google Scholar]
- 33.Aketani S, Teshima R, Umezawa Y, Sawada J. Correlation between cytosolic calcium concentration and degranulation in RBL-2H3 cells in the presence of various concentrations of antigen-specific IgEs. Immunol Lett. 2001;75:185–9. doi: 10.1016/s0165-2478(00)00311-4. [DOI] [PubMed] [Google Scholar]
- 34.Brown KS, Blair D, Reid SD, Nicholson EK, Harnett MM. FcgammaRIIb-mediated negative regulation of BCR signalling is associated with the recruitment of the MAPkinase-phosphatase, Pac-1, and the 3′-inositol phosphatase, PTEN. Cell Signal. 2004;16:71–80. doi: 10.1016/s0898-6568(03)00113-x. [DOI] [PubMed] [Google Scholar]
- 35.Carter NA, Harnett MM. Dissection of the signalling mechanisms underlying FcgammaRIIB-mediated apoptosis of mature B-cells. Biochem Soc Trans. 2004;32:973–5. doi: 10.1042/BST0320973. [DOI] [PubMed] [Google Scholar]
- 36.Pricop L, Redecha P, Teillaud JL, et al. Differential modulation of stimulatory and inhibitory Fc gamma receptors on human monocytes by Th1 and Th2 cytokines. J Immunol. 2000;166:531–7. doi: 10.4049/jimmunol.166.1.531. [DOI] [PubMed] [Google Scholar]
- 37.van Mirre E, Breunis WB, Geissler J, et al. Neutrophil responsiveness to IgG, as determined by fixed ratios of mRNA levels for activating and inhibitory FcgammaRII (CD32), is stable over time and unaffected by cytokines. Blood. 2006;108:584–90. doi: 10.1182/blood-2005-12-4997. [DOI] [PubMed] [Google Scholar]
- 38.Boruchov AM, Heller G, Veri MC, Bonvini E, Ravetch JV, Young JW. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J Clin Invest. 2005;115:2914–23. doi: 10.1172/JCI24772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Masuda E, Blank MC, et al. Cytokine-mediated regulation of activating and inhibitory Fc gamma receptors in human monocytes. J Leukoc Biol. 2005;77:767–76. doi: 10.1189/jlb.0904532. [DOI] [PubMed] [Google Scholar]
- 40.van Vugt MJ, Reefman E, Zeelenberg I, Boonen G, Leusen JH, van de Winkel JG. The alternatively spliced CD64 transcript FcgammaRIb2 does not specify a surface-expressed isoform. Eur J Immunol. 1999;29:143–9. doi: 10.1002/(SICI)1521-4141(199901)29:01<143::AID-IMMU143>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 41.Dhodapkar KM, Kaufman JL, Ehlers M, et al. Selective blockade of inhibitory Fcgamma receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells. Proc Natl Acad Sci USA. 2005;102:2910–15. doi: 10.1073/pnas.0500014102. [DOI] [PMC free article] [PubMed] [Google Scholar]