Abstract
Throughout the body, the distribution and differentiation of T-cell subsets varies in a way that optimizes host responses. The role of activation-induced cell death (AICD) in altering the distribution of T-lymphocyte subsets at an immune or inflammatory sites has been unexplored. The objective of this study was to assess whether pleural macrophages modulate AICD of specific pleural T-lymphocyte subsets. We found that pleural T-lymphocytes spontaneously undergo apoptosis, which is associated to increased expression of both FAS and FAS ligand, to decreased expression of Bcl 2 and to caspase 8 and 3 activation. While pleural T lymphocytes were partly protected from apoptosis, autologous peripheral blood T lymphocytes increased their apoptosis when cultured with exudative pleural fluids. Pleural CD45RO+ T cells, in comparison to pleural CD45RA+ T cells, were more susceptible to apoptosis, but were preferentially protected by exudative pleural fluids. Pleural prostaglandin E 2 (PGE2) was implicated in protecting T-lymphocytes from apoptosis because exudative pleural T lymphocytes highly express PGE2 receptors, and because exudative pleural fluid contained high concentrations of PGE2. Activated pleural macrophages released PGE2 and reduced the spontaneous apoptosis of pleural T lymphocytes and depletion of PGE2 from pleural fluids decreased this protective effect. This study demonstrates that PGE2, released in the pleural fluids following pleural macrophage activation, prolongs the survival of specific T-cell subsets, resulting in differentiation of the T-cell repertoire within the inflamed pleural space.
Keywords: pleura, apoptosis, T lymphocytes
Introduction
Chronic pleural inflammation can have devastating consequences as it promotes formation of an inelastic membrane around the lung leading to lung entrapment as well as to fibrothorax and consequently to impaired respiratory function. Consequently, the immune response in tissue compartments such as the pleural space is regulated to optimize host defence with a minimum of damage. The aetiological agents or malignant cells penetrate into the pleural space resulting in local activation of both structural cells such as mesothelial cells1 and immunocompetent resident cells such as pleural macrophages.2
Pleural macrophages represent the major cellular component of the pleural lavage of normal subjects.3 Activated pleural macrophages actively contribute to the regulation of inflammation and cell-mediated immunity by promoting the accumulation2 and the activation of T lymphocytes in the pleural space4 as well as possibly regulating the T-lymphocyte response.
Multiple regulatory mechanisms contribute to immune homeostasis. These include functional inactivation of cells and killing of no-longer needed or potentially dangerous immune cells.5 Activation-induced cell death (AICD), a form of apoptosis characterized by specific cell morphological features, is one of the important mechanisms involved in the limitation of inflammatory and immune responses by deleting activated lymphocytes.6 Apoptotic lymphocytes are removed from the site of inflammation by macrophages that ingest the lymphocytes without releasing inflammatory agents, thus contributing to the resolution of the inflammatory process.7 The accumulation of T lymphocytes at the site of inflammation in other compartments8–10 has been demonstrated to be related to a dysregulation of T cell apoptosis. Appropriate signals may prevent cell apoptosis in specific cell subsets, thus allowing their accumulation in specific compartments.11 Recently, the contribution of CD62L and chemokine receptors such as CCR7 to the homing of memory T cells has been defined12 but it is not known to what extent selective apoptosis might also contribute to that process. Although it has been previously demonstrated that in pleural fluids there is an increased numbers of CD45R0+ T cells11 it is not known whether apoptosis contributes to the altered distribution of CD45R0 positive T-cells.
Furthermore, although the role of lymphocyte apoptosis in the resolution of inflammation has been demonstrated in a variety of diseases,8–10 it is unknown whether, in the pleural space, this phenomenon is regulated by pleural macrophages during pleural inflammation.
The aims of the present study were to determine whether: (1) pro- or anti-apoptotic factors are present in exudative pleural fluids; (2) pleural macrophages control T lymphocyte apoptosis in the pleural space via the release of a specific soluble factor; or (3) apoptosis was in part responsible for the increased numbers of CD45RO+ T cells in the pleural compartment.
Materials and methods
Pleural fluid collection
Pleural fluid was collected by thoracentesis from hospitalized patients with congestive heart failure (CHF) (n = 9, age range 48–78 years), tuberculosis (TB) (n = 7, age range 38–64), cancer (n = 24, age range 45–73 years). All subjects gave informed written consent and the study was approved by the institutional review board for human studies. The effusions were first classified as transudates or exudates by meeting at least one of the criteria described by Light.13 Standard clinical, laboratory, and radiological investigations were used to establish the diagnosis for each patient, as previously described.14 No patients were undergoing anti-inflammatory or steroid therapies. The fluids were drawn into polypropylene bags containing heparin (10–20 IU/ml) and were subsequently centrifuged at 400 g for 10 min. The identification of cancer cells was performed by the Department of Pathology of our Hospital (Ospedale V. Cervello, Palermo, Italy, or Foothills Medical Centre, Calgary, Alberta), following the standard diagnostic procedures, using a panel of monoclonal antibodies (mAbs), including anti-human calretinin (Dako, Glostrup, Denmark), as recommended by the current consensus.15
Cell-free fluids were immediately frozen at −70° until they were analysed for total protein and prostaglandin E2 (PGE2) concentrations.
Isolation of T lymphocytes
Lymphocyte-enriched fractions were obtained from pleural fluids and from peripheral blood by Ficoll-Hypaque centrifugation followed by fractionation on a one-step Percoll (Pharmacia, Uppsala, Sweden) gradient. The lymphocyte-enriched fraction was passed through a nylon wool column to further purify the T-cell population (1 hr, 37°, 5% CO2) as previously described.2 The recovered T-cell fraction was >95% CD3-positive as assessed by cytofluorimetric analysis. Pleural T lymphocytes were cultured at 37° and 5% CO2 for 24, 72, and 144 hr in complete medium (RPMI-1640 + 10% fetal calf serum + 25 mm HEPES + 2 mm glutamine + 100 ng/ml penicillin + 100 ng/ml streptomycin) (Gibco, Paisley, UK) and, in some experiments, with pleural fluid supernatants, with autologous pleural macrophages, or with PGE2-depleted pleural fluid supernatants.
Determination of T lymphocyte apoptosis
Pleural T lymphocyte apoptosis was evaluated by staining with annexin V–fluorescein isothiocyanate (FITC) and propidium iodide using a commercial kit (Bender MedSystem, Vienna, Austria) following the manufacturer's directions.6 Cells were analysed using a FACStar Plus (Becton Dickinson, Mountain View, CA) analyser equipped with an Argon ion Laser (Innova 70 Coherent) and Consort 32 computer support. We evaluated as apoptotic cells, the cells that were annexin V positive and propidium iodide negative.
Expression of Bcl 2, Fas and FasL
The expression of Bcl2, Fas and Fas ligand (FasL) was evaluated at the same experimental conditions described above. To evaluate intracellular expression of Bcl 2, cells were permeabilized using a commercial fix-perm cell permeabilization kit (Caltag Laboratories, Burlingame, CA). Cells were then washed, resuspended in phosphate-buffered saline (PBS) at the concentration of 5 × 106/ml and 0·1 ml aliquots were incubated in the dark (30 min, 4°) with a FITC-conjugated mouse anti-Bcl2 clone 124 (Dakopatts, Glostrup, Denmark), with RPE-conjugated mouse anti-human CD95 clone DX2 (Dakopatts) and with mouse anti-human FITC- conjugated FasL clone H11 (Alexis Corporation, Lausen, Switzerland). RPE-conjugated (Dakopatts) and FITC-conjugated mouse anti-human immunoglobulin G1 (IgG1, Dakopatts) were used as negative controls. Results of Fas and FasL are expressed as percentage of positive cells.
Determination of the expression of caspases
The expression of caspases was evaluated by Western blot analysis as previously described.6 Fifty µg of total protein underwent sodium dodecyl sulphate–ployacrylamide gel electrophoresis on 4–12% gradient gels (Novex, San Diego, CA) and were blotted onto nitrocellulose membranes. These were blocked with PBS containing 3% bovine serum albumin (BSA), 0·1% Tween-20 and then probed with a mouse antibody specific to caspase 3 and 8 (PharMingen, San Diego, CA) at 4° overnight. After serial washes with PBS containing 0·1% Tween-20, membranes were incubated with peroxidase-conjugated anti-mouse antibody (Dako). Labelled proteins were revealed with an enhanced chemiluminescence system (NEN, Boston, MA) followed by autoradiography. Negative controls were performed in the absence of primary antibody or an isotype control antibody. Beta-actin (Sigma, St Louis, MO) was used as control. To confirm caspase 3 activation, we performed a caspase activity assay using a commercially available kit (PharMingen).
Cell death in CD45RO+ and CD45RA+ positive T cells
CD3+ T cells were labelled with anti-CD45RO (phycoerythrin, PE) and with CD45RA (FITC) or with isotype control antibodies, all purchased from eBioscience (San Diego, CA). The percentage of CD3/CD45RO+ or CD3/CD45RA+ cells undergoing cell death was assayed by uptake of 7-amino-actinomycin D (7-AAD) (Guava Technologies, Hayward, CA). Data was acquired using a Guava EasyCyte® 96-well flow cytometer and the Guava Express Plus Software (Guava Technologies). Data analysis was performed using FlowJo© 6.0 (Treestar, Inc, OR).
Measurement of PGE2
PGE2 concentrations were evaluated in cell-free pleural fluids and in macrophage cell culture supernatants using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Amersham Bioscences, Amersham, UK) following the manufacturer's directions. Results are expressed as pg/ml.
Expression of prostaglandin receptors (EP)
The expression of EP2 was evaluated by Western blot as described above using specific antibody (Cayman Chemical Ann Arbor, MI).
Release of PGE2 by pleural macrophages (PleM)
Macrophages were isolated from exudative and transudative pleural effusions using a previously described methodology developed in our laboratory omitting the adherence step.14 The isolated cell population was composed of >90% of macrophages, as confirmed by expression of CD68. Pleural macrophages were resuspended in RPMI-1640 (+ 1% fetal calf serum + 25 mm HEPES + 2 mm glutamine + 100 ng/ml penicillin + 100 ng/ml streptomycin) (Gibco) at 106 cells/ml and incubated (4, 18 hr; 37°; 5% CO2) with lipopolysaccharide (LPS) 1 µg/ml. In some experiments a specific inhibitor of COX-2 (SC58236) (10 µm) (COX-2 INI) (Searle Corporation, St Louis, MO) was also included. The tubes were then centrifuged (600 g, 10 min) and culture supernatants were collected and stored at −70° until used in subsequent experiments to test PGE2 concentrations.
Depletion of PGE 2
To deplete pleural fluids of PGE2, pleural fluids were mixed gently for 2 hr with a commercially available PGE2 affinity sorbent (a mouse anti-PGE2 IgG covalently bound to Sepharose 4B) with a 100% specificity (Cayman Chemical, Ann Arbor, MI), as previously described.16,17 Subsequently, the samples were centrifuged at 1500 g and filtered on 0·2-mm filters (Lida Manufacturing Corp, Kenosha, WI), with the aim of separating the PGE2 affinity sorbent and therefore eliminating PGE2 from supernatants. The efficiency of the specific extraction of PGE2 by the PGE2 affinity sorbent was evaluated in three separate pleural fluid samples that were spiked with 1 ng of PGE2 standard (Sigma-Aldrich, Milan, Italy). Final concentrations of PGE2 in samples incubated in the presence and in the absence of PGE2 affinity sorbent were assessed by specific commercial ELISA kit as described above. The concentrations of PGE2 following affinity sorbent were below the lower sensitivity limit of ELISA kit. To further assess the specificity of the PGE2 affinity sorbent, we measured leukotriene B4 (LTB4), before and after PGE2 depletion in three different exudates. The concentrations of LTB4 before and after PGE2 depletion were similar (before = 1579 ± 176 pg/ml; after = 1619 ± 176 pg/ml) thus confirming the specificity of the system for PGE2.
Statistics
Data are expressed as mean counts ± standard deviation. The Mann–Whitney U-test was used for comparison between patient groups, while comparison between different experimental conditions was evaluated by analysis of variance (anova). The Spearman test was used for correlations. Linear regression was done using anova and linear integration was done using MINITAB 12 Student Edition Copyright 1999 (Minitab Inc., State College, PA). P < 0·05 was accepted as statistically significant.
Results
Exudative pleural T lymphocytes cultured in vitro undergo spontaneous apoptosis
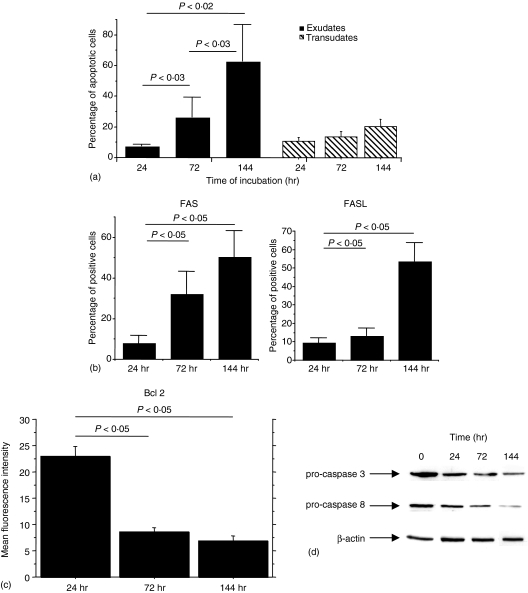
Activation of cell apoptosis following T-lymphocyte activation is a regulatory mechanism limiting T-lymphocyte expansion and activation. The first step of this study was to assess whether pleural T lymphocytes isolated from exudative and transudative effusions underwent spontaneous apoptosis when removed from the pleural compartment and placed in tissue culture. T lymphocytes underwent spontaneous apoptosis in a time dependent fashion (Fig. 1a). Interestingly, pleural T lymphocytes isolated from exudative pleural fluids (TB and cancer) underwent apoptosis to a greater extent when compared to T lymphocytes isolated from transudative pleural effusions (CHF) (Fig. 1a). In addition, the activation of apoptosis was associated with increased expression of Fas and Fas ligand (Fig. 1b), with a decreased expression of Bcl 2 (Fig. 1c) and with the activation of caspases 8 and 3 as detected by the decreased expression of the procaspases (Fig. 1d). A caspase 3 activity assay showed an increase in caspase activity during the cultures (data not shown). No significant modifications in the expression of all the markers studied were observed for cells isolated from transudates (data not shown).
Figure 1.
Spontaneous apoptosis of pleural T lymphocytes. (a) Pleural T lymphocytes isolated from exudative (n = 7) and transudative (n = 5) pleural fluids (see Materials and methods for details) were cultured in medium for 24, 72 and 144 hr. T-cell apoptosis was evaluated by annexin V binding and expressed as percentage of annexin V positive cells (mean ± SD). (b) Fas and FasL expression by pleural T lymphocytes isolated from exudative (n = 7) pleural fluids. (c) Bcl2 expression by pleural T lymphocytes from exudative (n = 5) pleural fluids. (d) Western blot analysis of pro-caspase 8 and 3 and β-actin expression by pleural T lymphocytes isolated from exudative pleural fluids (representative experiment). Lane 1 = pleural T lymphocytes cultured for 0 hr; lane 2 = pleural T lymphocytes cultured for 24 hr; lane 3 = pleural T lymphocytes cultured for 72 hr; lane 4 = pleural T lymphocytes cultured for 144 hr.
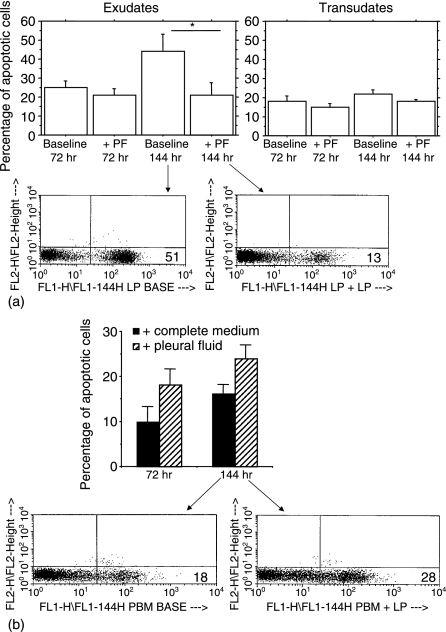
Exudative pleural fluids protect pleural T lymphocytes from spontaneous apoptosis
We next explored whether the spontaneous apoptosis of exudative pleural T lymphocytes was caused by a deprivation of some soluble factors present in the pleural fluids. We evaluated the apoptosis of T lymphocytes isolated from exudative and transudative pleural fluids and from autologous peripheral blood in the presence and in the absence of autologous pleural fluids. Interestingly, when pleural T lymphocytes isolated from exudates were cultured in the presence of autologous pleural fluids, apoptosis was significantly decreased in comparison to T lymphocytes cultured in medium (Fig. 2a). In contrast, autologous peripheral blood T lymphocytes increased their apoptosis when cultured in the presence of pleural fluid supernatants. (Fig. 2b). This suggests that factors in exudative pleural fluid protect pleural fluid T cells from apoptosis, but promote apoptosis in autologous blood T cells. However, when pleural T lymphocytes isolated from transudates were cultured in the presence of autologous pleural fluids, no significant decrease in cell apoptosis was observed (Fig. 2a).
Figure 2.
Exudative pleural fluids protect pleural T lymphocytes from apoptosis. T lymphocytes isolated from exudative (n = 5) and transudative (n = 2) pleural fluids (a) and from autologous peripheral blood (see Materials and methods for details) (b) were cultured with autologous pleural fluid or with medium for 72 and for 144 hr. T-cell apoptosis was evaluated by annexin V binding and expressed as percentage of annexin V positive cells (mean ± SD). *P < 0·05 compared to the corresponding group cultured with medium.
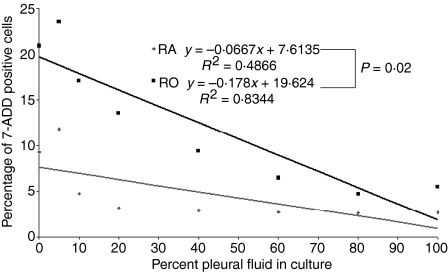
Exudative pleural fluids preferentially protect pleural CD45RO+ T cells
It has previously been determined that there is a preponderance of CD45RO+, and a paucity of CD45RA+ T cells in the human pleural space.11 To determine whether the protective effect of pleural fluid was specific for certain pleural T-cell subsets, the incidence of death among CD45RA+ T cells was compared to CD45RO+ T cells when cultured with or without pleural fluid. In two independent experiments, the percentage of CD45RO+ T cells undergoing cell death was higher than the percentage of CD45RA+ T cells undergoing cell death (19% and 16% versus 9·3% and 9·3%). However, the degree of protection for CD45RO+ T cells was greater than the protection afforded to CD45RA+ T cells. Addition of pleural fluid decreased the percentage of CD45RO+ apoptotic T cells by 13·5 and 9·9%, while it only decreased the percentage of CD45RA+ T cells by 6·3 and 2·3%. (the first of these two experiments is shown in Fig. 3). When comparing the effect of increasing concentrations of pleural fluid on the percentage of dead cells by a linear integration analysis, there was a significant difference between CD45RO+ and CD45RA+ T cells (P = 0·02) (Fig. 3). This suggests that pleural fluid had a greater effect on the survival of CD45 RO+ T cells when compared to the survival of CD45RA+ T cells.
Figure 3.
Exudative pleural fluids preferentially maintain the survival of CD45RO+ T cells. Pleural lymphocytes isolated from exudative pleural fluids were cultured with different concentrations of pleural fluid for 72 hr. Cells were considered dead if they stained positive for 7-AAD that were either CD3+ CD45RA+ or CD3+ RO. A linear regression analysis was performed using anova to determine the relationship between the percent 7-AAD+ cells and the percentage of pleural fluid in culture. Linear integration analysis was performed to determine if there was a statistical difference between the relationships for the CD45RO+ and CD45RA+ cells. The experiment was repeated with similar results.
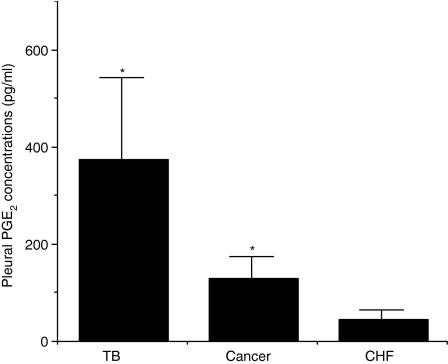
PGE2 is present in exudative pleural effusions
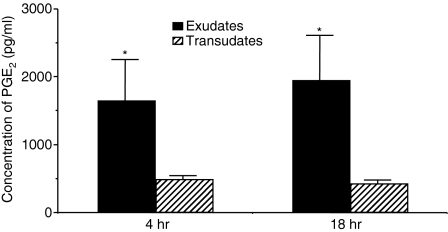
Because PGE2 has been demonstrated to prevent the apoptosis of activated T lymphocytes18 we determined whether PGE2 was present in pleural fluids. Interestingly, TB and cancer exudative pleural fluids contained higher concentrations of PGE2 in comparison to transudative (CHF) pleural fluids (Fig. 4) suggesting that the production of PGE2 within the pleura is associated to pleural inflammation. In addition, PGE2 concentrations positively correlated with the total numbers of pleural T lymphocytes (Spearman correlation: ρ = 0·64; P < 0·0001).
Figure 4.
PGE2 is present in exudative pleural fluids. Pleural fluids were obtained from CHF patients (n = 8); TB patients (n = 7); and cancer patients (n = 24). PGE2 concentrations were measured as described in ‘ materials and methods’ and are expressed as pg/ml (mean ± SD). *TB versus CHF P < 0·03; *Cancer versus CHF P < 0·03; TB versus Cancer = ns.
Exudative pleural T lymphocytes express EP2 receptors
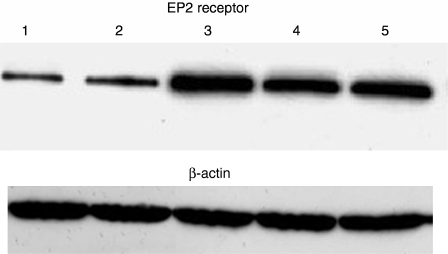
We assessed whether pleural T lymphocytes from exudates express the PGE2-specific EP2 receptors differently from pleural T lymphocytes from transudates. Pleural T lymphocytes isolated from exudative pleural fluids expressed EP2 receptors at higher level in comparison to pleural T lymphocytes isolated from transudates (Fig. 5).
Figure 5.
Pleural T lymphocytes highly express EP2 receptors. Representative Western blot analysis of EP2 receptor by pleural T lymphocytes from pleural transudates and from pleural exudates. Lanes 1 and 2 = pleural T lymphocytes from transudates; lanes 3, 4, 5 = pleural T lymphocytes from exudates.
Pleural macrophages protect autologous pleural T lymphocytes from apoptosis
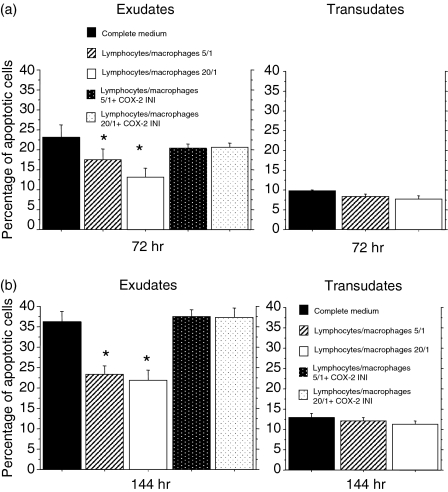
Experiments were performed to assess whether pleural macrophages contributed to the protection of pleural T lymphocytes from apoptosis. We first evaluated whether pleural macrophages isolated from exudates actively contribute to the release of PGE2. Pleural macrophages isolated from exudates and stimulated with LPS (1 µg/ml) released higher concentrations of PGE2 at 4 and 18 hr when compared to pleural macrophages isolated from transudates (Fig. 6). Furthermore, using cells isolated from exudates, activated pleural macrophages that were separated from T lymphocytes to avoid direct macrophage-lymphocyte contacts protected T lymphocytes from spontaneous apoptosis. This phenomenon, present after 72 hr of incubation, was more evident after 144 hr of incubation. No significant differences were observed at the two different lymphocyte/macrophage tested ratios. The addition of a specific COX-2 inhibitor restored T lymphocyte apoptosis to baseline. No differences were observed for cells isolated from transudates (Fig. 7).
Figure 6.
Activated pleural macrophages highly release PGE2. Pleural macrophages recovered from transudative effusions (CHF) (n = 5) and from exudative effusions (n = 5) were used to assess the release of PGE2 (see Materials and methods for details). *P < 0·05 (exudates versus transudates).
Figure 7.
Activated pleural macrophages protect autologous pleural T lymphocytes from apoptosis. Pleural T lymphocytes and autologous pleural macrophages (lymphocyte/macrophage ratios 5/1 and 20/1) isolated from exudative and from transudative pleural fluids (see Materials and methods for details) were cultured in transwells to avoid direct macrophage-lymphocyte contacts for 72 hr (a) and for 144 hr (b). T-cell apoptosis was evaluated by annexin V binding and expressed as percentage of annexin V positive cells (mean ± SD). *P < 0·05 compared to the corresponding group cultured in complete medium without autologous pleural macrophages.
Pleural PGE2 contributes to the protection of pleural T lymphocytes
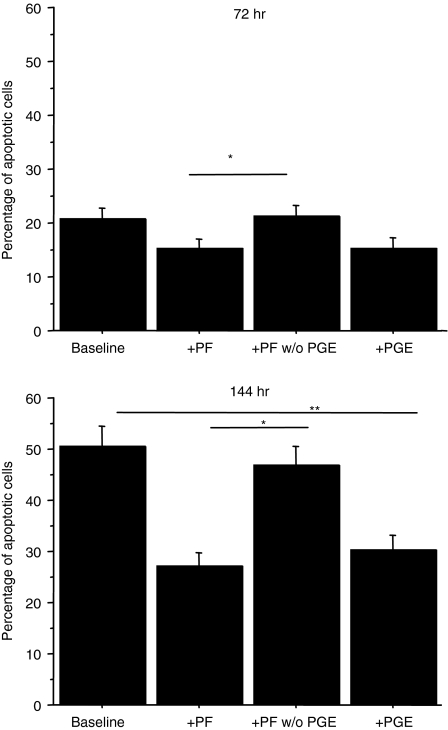
The findings that PGE2 was present in pleural fluids and that its concentrations positively correlated with the number of pleural T lymphocytes prompted us to assess whether PGE2 present in exudative pleural fluids contributed to the prevention of T lymphocyte apoptosis exerted by pleural fluids. Depletion of PGE2 from exudative pleural fluids significantly increased the apoptosis of autologous activated pleural T lymphocytes (Fig. 8) suggesting that PGE2 is the factor that protects T lymphocytes from apoptosis.
Figure 8.
Pleural PGE2 protects pleural T lymphocytes from apoptosis. Pleural T lymphocytes isolated from exudative pleural fluids (n = 5) (see Materials and methods for details) were cultured with synthetic PGE2, with pleural fluids and with PGE2 depleted pleural fluids for 72 hr (a) and for 144 hr (b). T-cell apoptosis was evaluated by annexin V binding and expressed as percentage of annexin V positive cells (mean ± SD). *P < 0·05.
Discussion
The results of the present study demonstrate that PGE2 is increased in the inflamed pleural space and that this correlates with the number of T lymphocytes in the pleural space. Exudative pleural T lymphocytes are capable of responding to PGE2 because they highly expressing the EP2 receptor. Pleural fluid, which contains PGE2 selectively protects CD45RO+ T lymphocytes from becoming apoptotic. Among the cells present in the inflamed pleural space, activated pleural macrophages release PGE2 and PGE2 was shown to be the factor that protects T cells from spontaneous apoptosis.
Naïve T lymphocytes are induced to proliferate after immune stimulation by antigens. At the end of an immune response, the majority of the expanded population is removed by apoptosis by two major routes: AICD and cytokine deprivation.5 Mechanisms that prevent the apoptosis of a proportion of activated T lymphocytes may lead to the persistence of a memory T-cell population. Dysregulation of this physiological process appears to be involved in the pathogenesis of several human diseases. For example, lack of apoptosis plays a causative role in malignancies (haematological) and autoimmune diseases, whereas excess apoptosis has been associated with infectious processes including human immunodeficiency virus infection, malaria, and trypanosomiasis.19 AICD is activated by the restimulation of activated T lymphocytes and requires ligation of Fas by Fas L. Upon activation T cells potently induce FasL expression on the surface of cells already expressing Fas, thus causing autocrine or paracrine cell death.20 This phenomenon may occur principally in foci of hyperactivity where large numbers of T lymphocytes and considerable concentrations of antigens are present.20 The pleural space is a closed compartment where both large numbers of T lymphocytes and considerable concentrations of antigens may be present. Consistent with these concepts, we focused our attention on the mechanisms governing the apoptosis of pleural T lymphocytes in an inflammatory milieu. It represented an unique opportunity to evaluate this phenomenon on naturally, in vivo-activated T lymphocytes and offered the possibility to better understand the mechanisms conditioning the fate of activated T lymphocytes at the site of inflammation. We demonstrated that activated pleural T lymphocytes, when cultured in vitro with medium, increased the expression of both Fas and FasL decreased the expression of Bcl2 and underwent spontaneous apoptosis. Our data extend and integrate previous results provided by Hirsch et al.19 showing that apoptosis of pleural fluid mononuclear cells (T cells, mononuclear phagocytes) isolated from pleural fluids of TB patients and cultured in complete medium was augmented in comparison with autologous peripheral blood mononuclear cells. We demonstrated that T lymphocytes isolated from exudates caused by cancer and tuberculosis undergo spontaneous apoptosis to a greater extent than autologous peripheral T lymphocytes or T lymphocytes isolated from transudative pleural effusions. These findings may be indicative of the general immune activation of lymphocytes present in the inflamed pleural space and suggest the presence of mechanisms not exclusively related to specific pathological contexts such as TB or malignancies. Our approach is novel as we also evaluated the apoptosis of pleural T lymphocytes partially reproducing the pleural inflammatory milieu by culturing the cells with pleural fluid. However, the available previous studies evaluated, in vitro, cell apoptosis of T lymphocytes cultured in medium.
Several pieces of evidence suggest that the pleural microenvironment (cells and mediators) tightly participates in the regulation of immune responses within the pleural space.21 Pleural macrophages play a crucial role in regulating T-cell functions in the pleural compartment.2,22,23 Our recent reports demonstrate that pleural macrophages also contribute to the regulation of immune responses by secreting chemokines2 by increasing the expression of adhesion molecules14 or directly interacting with lymphocytes.4 Here we demonstrate that a soluble factor present in exudative pleural fluids and a soluble factor released from activated pleural macrophages are able to protect T lymphocytes from apoptosis. This protective effect was mainly exerted on the CD45RO+ T cells rather than on CD45RA+ T cells, suggesting that this mechanism may contribute to the selection of specific T-cell subsets within the inflamed pleural compartment.
The distribution of T-lymphocyte subsets in various organ compartments is very different than the distribution of subsets in the peripheral blood. This altered distribution and compartmentalization is essential for optimal host defense. In part, the altered distribution of CD62L and CCR7 cells bind to O-glycans of GlyCAM-1, CD34 and MAdCAM-1, and CCL21, respectively, and are selectively recruited to different organ compartments.24 In addition to selective recruitment, it is possible for apoptosis to alter the distribution of naïve and memory T-cell subsets.25 It has previously been noted that T-cell growth factors such as interleukin (IL)-7 and IL-15 prevent apoptosis of memory T cells.25 Moreover, IL-6 and CXCL12 have also been implicated in rescue from apoptosis.26 It has been found that the majority of T lymphocytes in the pleural compartment are CD45RO,11 a surface molecule that has been correlated with the memory phenotype; however, the factors involved in the preservation of this phenotype have not been identified. The current studies extend the observations of Atreya et al. in the human intestine26 and demonstrate that factors in the diseased pleural space selectively regulate apoptosis resulting in the maintenance of the CD45RO+ memory T cells.
PGE2, the primary cyclo-oxygenase breakdown product, is primarily released by mononuclear phagocytes.27 In this study we show that higher concentrations of PGE2 were associated with greater numbers of T cells in the pleural apace, that synthetic PGE2 decreased apoptosis and that depletion of PGE2 from exudative pleural fluids significantly increases the apoptosis of autologous activated pleural T lymphocytes. Moreover, activated pleural macrophages protect T lymphocytes from spontaneous apoptosis and this phenomenon, while present at 72 hr of incubation, is more evident after 144 hr of incubation. Taken together, these findings indicate that PGE2 released following a prolonged stimulation of pleural macrophages prevents apoptosis and might play a role in augmenting and perpetuating T-cell responses in the pleural space.
PGE2 has been associated with the prolonged survival of immune effector cells and the persistence of inflammatory responses.28 PGE2 inhibits AICD by down-regulating FasL expression and caspase 8 activation.18,28 Considering the short in vivo half-life of PGE224 the pleural compartment is likely characterized by a continuous production of PGE2 to maintain PGE2 at biologically relevant levels. The biological activity of PGE2 is mediated by G protein-coupled receptors by conventionally designated EP receptors.29,30 The EP receptors can be divided into four classes and couple with distinct signalling pathways. The existence of this family of EP receptors coupled with different signalling pathways provides a molecular basis for the different biological activities of PGE2. The interaction with EP2 and with EP4 promotes an increase in the intracellular levels of cAMP which in turn, triggering PKA-I-mediated signals, increases the survival of T cells and enhances memory T cell commitment.31 On the other hand, cAMP-dependent protein kinase (PKA) signalling, due to PGE2, suppresses T lymphocyte proliferation30 and affects immune effector functions by decreasing T helper 1 (Th1) cytokines resulting in a shift in the Th1/Th2 balance in favour of a Th2 response.32 The effects of PGE2 on T lymphocyte apoptosis are dependent on the activation state of the cell. In this regard it has been demonstrated that PGE2 induces apoptosis in resting T lymphocytes.33 Consistently, with this finding, we demonstrate here that only exudative pleural T lymphocytes sense the protective effect of PGE2 present in pleural fluids as EP2 receptors are highly expressed on exudative pleural T lymphocytes rather than on transudative pleural T lymphocytes. It is conceivable that, in the pleural space during inflammation, the release of soluble mediators increases the production of PGE2 by pleural macrophages, and the expression of EP2 receptors by T lymphocytes. In this regard, it has been previously demonstrated that during inflammation, intestinal epithelial cells newly and significantly expressed EP2 receptors.34
In conclusion, this study demonstrates that PGE2 released into the pleural fluid, following pleural macrophage activation, prolongs the survival and alters the differentiation of specific T lymphocytes subsets within the inflamed pleural space.
Acknowledgments
This work was supported by the Italian National Research Council and by the Alberta Lung Association.
C.H.M. is a Senior Scholar of the Alberta Heritage Foundation for Medical Research.
Abbreviations
- PGE2
prostaglandin E2
- EP
prostaglandin E receptors
- mAb
monoclonal antibody
- CM
complete medium
- CHF
congestive heart failure
References
- 1.Kroegel C, Antony VB. Immunobiology of pleural inflammation: potential implications for pathogenesis, diagnosis and therapy. Eur Respir J. 1997;10:2411–8. doi: 10.1183/09031936.97.10102411. [DOI] [PubMed] [Google Scholar]
- 2.Pace E, Gjomarkaj M, Melis M, Profita M, Spatafora M, Vignola M, Bonsignore G, Mody CH. Interleukin-8 induces lymphocyte chemotaxis within the pleural space that is regulated by pleural macrophages. Am J Respir Crit Care Med. 1999;159:1592–9. doi: 10.1164/ajrccm.159.5.9806001. [DOI] [PubMed] [Google Scholar]
- 3.Noppen M, De Waele M, Li R, Gucht KV, D'Haese J, Gerlo E, Vincken W. Volume and cellular content of normal pleural fluid in humans examined by pleural lavage. Am J Respir Crit Care Med. 2000;162:1023–6. doi: 10.1164/ajrccm.162.3.9910050. [DOI] [PubMed] [Google Scholar]
- 4.Gjomarkaj M, Melis M, Pace E, Spatafora M, Toews GB. Mononuclear cells in exudative malignant pleural effusions: characterization of pleural phagocytic cells. Chest. 1994;106:1042–9. doi: 10.1378/chest.106.4.1042. [DOI] [PubMed] [Google Scholar]
- 5.Strasser A, Pellegrini M. T-lymphocyte death during shutdown of an immune response. Trends Immunol. 2004;25:610–5. doi: 10.1016/j.it.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Pace E, Gagliardo R, Melis M, et al. Synergistic effects of fluticasone propionate and salmeterol on in-vitro T-cell activation and apoptosis in asthma. J Allergy Clin Immunol. 2004;114:1216–23. doi: 10.1016/j.jaci.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 7.Fan X, Krahling S, Smith D, Williamson P, Schlegel RA. Macrophage surface expression of annexins I and II in the phagocytosis of apoptotic lymphocytes. Mol Biol Cell. 2004;15:2863–72. doi: 10.1091/mbc.E03-09-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manganelli P, Fietta P. Apoptosis and Sjögren syndrome. Semin Arthritis Rheum. 2003;33:49–65. doi: 10.1053/sarh.2003.50019. [DOI] [PubMed] [Google Scholar]
- 9.Itoh J, de La Motte C, Strong SA, Levine AD, Fiocchi C. Decreased Bax expression by mucosal T cells favours resistance to apoptosis in Crohn's disease. Gut. 2001;49:35–41. doi: 10.1136/gut.49.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vignola AM, Chanez P, Chiappara G, et al. Evaluation of apoptosis of eosinophils, macrophages, and T lymphocytes in mucosal biopsy specimens of patients with asthma and chronic bronchitis. J Allergy Clin Immunol. 1999;103:563–73. doi: 10.1016/s0091-6749(99)70225-3. [DOI] [PubMed] [Google Scholar]
- 11.Prado-Garcia H, Aguilar-Cazares D, Flores-Vergara H, Mandoki JJ, Lopez-Gonzalez JS. Effector, memory and naive CD8+ T cells in peripheral blood and pleural effusion from lung adenocarcinoma patients. Lung Cancer. 2005;47:361–71. doi: 10.1016/j.lungcan.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 12.Rivino L, Messi M, Jarrossay D, Lanzavecchia A, Sallusto F, Geginat J. Chemokine receptor expression identifies Pre-T helper (Th) 1, Pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J Exp Med. 2004;200:725–35. doi: 10.1084/jem.20040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Light RW. Pleural diseases. Dis Mon. 1992;38:261–331. doi: 10.1016/0011-5029(92)90007-c. [DOI] [PubMed] [Google Scholar]
- 14.Melis M, Pace E, Siena L, et al. Biologically active intercellular adhesion molecule-1 is shed as dimers by a regulated mechanism in the inflamed pleural space. Am J Respir Crit Care Med. 2003;167:1131–8. doi: 10.1164/rccm.200207-654OC. [DOI] [PubMed] [Google Scholar]
- 15.Comin CE, Novelli L, Boddi V, Paglierani M, Dini S. Calretinin, thrombomodulin, CEA, and CD15: a useful combination of immunohistochemical markers for differentiating pleural epithelial mesothelioma from peripheral pulmonary adenocarcinoma. Hum Pathol. 2001;32:529–36. doi: 10.1053/hupa.2001.24329. [DOI] [PubMed] [Google Scholar]
- 16.Profita M, Sala A, Bonanno A, et al. Increased prostaglandin E2 concentrations and cyclooxygenase-2 expression in asthmatic subjects with sputum eosinophilia. J Allergy Clin Immunol. 2003;112:709–16. doi: 10.1016/s0091-6749(03)01889-x. [DOI] [PubMed] [Google Scholar]
- 17.Pace E, Siena L, Ferraro M, et al. Role of prostaglandin E2 in the invasiveness, growth and protection of cancer cells in malignant pleuritis. Eur J Cancer. 2006;42:2382–9. doi: 10.1016/j.ejca.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Yarovinsky TO, Hunninghake GW. Lung fibroblasts inhibit activation-induced death of T cells through PGE (2)-dependent mechanisms. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1248–56. doi: 10.1152/ajplung.2001.281.5.L1248. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch CS, Toossi Z, Johnson JL, et al. Augmentation of apoptosis and interferon-gamma production at sites of active Mycobacterium tuberculosis infection in human tuberculosis. J Infect Dis. 2001;183:779–88. doi: 10.1086/318817. [DOI] [PubMed] [Google Scholar]
- 20.Akbar AN, Salmon M. Cellular environments and apoptosis: tissue microenvironments control activated T-cell death. Immunol Today. 1997;18:72–6. doi: 10.1016/s0167-5699(97)01003-7. [DOI] [PubMed] [Google Scholar]
- 21.Antony VB. Immunological mechanisms in pleural disease. Eur Respir J. 2003;21:539–44. doi: 10.1183/09031936.03.00403902. [DOI] [PubMed] [Google Scholar]
- 22.Pace E, Profita M, Melis M, et al. LTB4 is present in exudative pleural effusions and actively contributes to neutrophil recruitment in the inflamed pleural space. Clin Exp Immunol. 2004;135:519–27. doi: 10.1111/j.1365-2249.2003.02387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8 C T cells. J Exp Med. 2001;194:953–66. doi: 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grayson JM, Harrington LE, Lanier JG, Wherry EJ, Ahmed R. Differential sensitivity of naive and memory CD8+ T cells to apoptosis in vivo. J Immunol. 2002 October 1;169:3760–70. doi: 10.4049/jimmunol.169.7.3760. [DOI] [PubMed] [Google Scholar]
- 25.Schluns KS, Williams K, Ma A, Zheng XX, Lefrançois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168:4827–31. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 26.Atreya R, Mudter J, Finotto S, et al. Blockade of interleukin 6 trans signaling suppresses T cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–8. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 27.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–50. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 28.Porter BO, Malek TR. Prostaglandin E2 inhibits T cell activation-induced apoptosis and Fas-mediated cellular cytotoxicity by blockade of Fas-ligand induction. Eur J Immunol. 1999;29:2360–5. doi: 10.1002/(SICI)1521-4141(199907)29:07<2360::AID-IMMU2360>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 29.Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors. multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103:147–66. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Nataraj C, Thomas DW, Tilley SL, Nguyen MT, Mannon R, Koller BH, Coffman TM. Receptors for prostaglandin E (2) that regulate cellular immune responses in the mouse. J Clin Invest. 2001;108:1229–35. doi: 10.1172/JCI13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goetzl EJ, An S, Zeng L. Specific suppression by prostaglandin E2 of activation-induced apoptosis of human CD4+ CD8+ T lymphoblasts. J Immunol. 2001;154:1041–7. [PubMed] [Google Scholar]
- 32.Miles EA, Aston L, Calder PC. In vitro effects of eicosanoids derived from different 20-carbon fatty acids on T helper type 1 and T helper type 2 cytokine production in human whole-blood cultures. Clin Exp Allergy. 2003;33:624–32. doi: 10.1046/j.1365-2222.2003.01637.x. [DOI] [PubMed] [Google Scholar]
- 33.Pica F, Franzese O, D'Onofrio C, Bonmassar E, Favalli C, Garaci E. Prostaglandin E2 induces apoptosis in resting immature and mature human lymphocytes: a c-Myc-dependent and Bcl-2-independent associated pathway. J Pharmacol Exp Ther. 1996;277:1793–800. [PubMed] [Google Scholar]
- 34.Takafuji V, Cosme R, Lublin D, Lynch K, Roche JK. Prostanoid receptors in intestinal epithelium: selective expression, function, and change with inflammation. Prostaglandins Leukot Essent Fatty Acids. 2000;63:223–35. doi: 10.1054/plef.2000.0144. [DOI] [PubMed] [Google Scholar]