Abstract
Pemphigus vulgaris (PV) is considered to be an autoimmune disease affecting skin and mucous membranes. Traditionally, PV autoantibodies are thought to recognize antigens located in the intercellular substance (ICS) of keratinocytes; antigens represented mainly by the desmosomal cadherin desmoglein 3 (Dsg3). Accordingly, titres of anti-ICS and anti-Dsg3 immunoglobulin G (IgG) are considered to be major laboratory criteria when making a diagnosis of PV. In this paper, we demonstrated for the first time that PV IgG bind antigen(s) expressed on the surface of peripheral blood mononuclear cells (PBMC), as revealed by immunofluorescence studies. This novel autoantigen is immunoprecipitated by PV IgG as a 130 000 molecular weight protein. However, Western blot analysis of the immunocomplexes failed to show reactivity with anti-Dsg3 monoclonal and polyclonal antibodies. Taken together, our data provide strong evidence that PV autoimmunity targets a 130 000 antigen other than Dsg3 on PBMC. This shifting from epidermis to blood cells may open new perspectives for a better understanding of pemphigus autoimmunity and more rational approaches to its treatment.
Keywords: autoantigen, autoimmunity, desmoglein 3, pemphigus, peripheral blood mononuclear cells
Introduction
Pemphigus vulgaris (PV) is an autoimmune blistering disease that primarily affects stratified epithelia.1,2 Almost all patients with PV have pathogenic circulating immunoglobulin G (IgG) autoantibodies, which bind to normal components of keratinocyte cell membrane belonging to the cadherin supergene family. Immunoblotting and immunoprecipitation studies demonstrated the autoantigen of PV as the 130 000–140 000 molecular weight (MW) desmoglein 3 (Dsg3)3 type I transmembrane glycoprotein that mediates adhesion by engaging calcium-dependent interactions with apposed cells.4 Some putative non-desmoglein PV antigens include desmocollins,5 plakoglobin,6 desmoplakin7 and acetylcholine receptors,8,9 all of which are involved in regulating cell–cell adhesion of keratinocytes. The binding of the autoantibodies to their targets generates a plethora of biological effects as a result, on the one hand, of their direct interference with the adhesive function of desmogleins and, on the other, of more complex events involving intracellular pathways that modify cell phosphorylation status, protease activity or calcium metabolism, leading to loss of cell–cell adhesion (reviewed in ref. 10).
Recently, the discovery of a circulating 30 000 MW fragment of desmoglein 3 (sDsg3) has moved the attention from tissue-restricted epithelial Dsg3 to that found in serum.11 Indeed, recognition of immunogenic epitopes of PV autoantigen may be crucial for the initiation and perpetuation of specific T-cell and B-cell responses, as well as for the induction of tolerance. In line with this concept, the availability of the self antigen represents a key point for immune function regulation. We have supposed that sDsg3 can stem from cleavage of keratinocyte Dsg3; however, the presence of full-length Dsg3 on blood cells has never been investigated.
In this study, we tried to assess whether PV antigens exist that are expressed by cells other than keratinocytes. We found that PV IgG recognizes a 130 000 MW autoantigen on the surface of peripheral blood mononuclear cells (PBMC) but, surprisingly, it is not Dsg3.
Materials and methods
Antibodies and reagents
The 5H10 mouse monoclonal antibody recognizing the N-terminal residues 49–60 within the extracellular domain of Dsg3,11 anti-Dsg3 H-145 rabbit polyclonal antibodies raised against the cytoplasmic domain of Dsg3, H-290 rabbit antibodies against C-terminal residues 760–1046 of Dsg1 and horseradish peroxidase-conjugated anti-rabbit and anti-mouse antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The H44211M mouse monoclonal antibody against the extracellular domain of Dsg112 was obtained from Biodesign International (Saco, MA). The 5G11 monoclonal antibody against the extracellular domain of Dsg3 was from Zymed Laboratories (Invitrogen immunodetection, San Francisco, CA) and fluorescein isothiocyanate (FITC)-conjugated anti-human, anti-mouse and anti-rabbit IgG antibodies were from DAKO (Dako Denmark A/S). Polyvinyldifluoride (PVDF) filters and RPMI-1640 were purchased from Invitrogen (Carlsbad, CA); the enhanced chemiluminescent immunodetection system and Hyperfilms were from Amersham (Buckinghamshire, UK) and the protease inhibitors and all cell culture reagents, with the exception of RPMI-1640, were supplied by Sigma (St Louis, MO).
Patients and sera
Sera of patients with active PV (n = 4, named PV1–PV4), bullous pemphigoid (BP, n = 2) and healthy volunteers without any skin disease (n = 3, controls) were used in the experiments. The diagnoses of PV and BP were made based on criteria reported elsewhere.13,14 The presence of autoantibodies was determined by indirect immunofluorescence using monkey oesophagus as substrate [values above 1 : 40 of circulating anti-intercellular substance (ICS) antibodies were considered positive]. All sera were heated to 56° for 30 min to inactivate complement.
The serum IgG fractions were isolated following standard procedures.8
Unless otherwise stated, the figures in this paper represent data obtained using PV1 and control 1 sera. Results were confirmed in independent experiments with sera from PV2 to PV4.
Cell cultures and treatments
HaCaT cells, a non-tumorigenic human keratinocyte cell line which exhibits normal differentiation and is capable of forming epidermal tissue when transplanted in vivo,15 were maintained in Dulbecco's modified Eagle's minimal essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (50 U/ml), streptomycin (50 μg/ml) and fungizone (2·5 μg/ml) in a humidified atmosphere with 5% CO2.
Peripheral blood was collected from two healthy donors into heparinized tubes, transferred to polypropylene tubes and diluted with RPMI-1640 medium. PBMC were isolated using Ficoll–HyPaque density gradient centrifugation. Aliquots of 1 × 106 freshly isolated PBMC were washed with ice-cold phosphate-buffered saline (PBS), pelleted at 800 g and stored at − 80° until further analysis. PBMC were cultured in vitro in RPMI-1640 supplemented with non-essential amino acids and 10% FBS.
Protein extraction and Western blot analysis
Pooled cells were rinsed with complete PBS supplemented with protease inhibitors [phenylmethylsulphonylfluoride (PMSF) at 1 mm, 10 μg/ml leupeptin and 5 μg/ml aprotinin] and pellets (800 g for 10 min) were resuspended in Triton buffer (50 mm Tris–HCl, pH 7·5, 150 mm NaCl, 5 mm ethylenediaminetetraacetic acid, 1% Triton X-100, 1 mm dithiothreitol, 1 mm PMSF). Equal amounts of protein (60 μg per lane) were mixed with 4 × Laemmli sample buffer and loaded onto an 8% sodium dodecyl sulphate (SDS)–polyacrylamide gel after heating for 5 min at 95°. Western blot analysis was carried out according to standard procedures.16 Briefly, proteins were transferred overnight onto PVDF filters at 20 V and then stained with Ponceau red. Blocked membranes were incubated for 1 hr with the appropriate antibody (1 : 1000) and then with species-specific horseradish peroxidase-conjugated IgG (1 : 10000) as secondary antibody. Bound antibodies were detected using an enhanced chemiluminescent immunodetection system.
Immunoprecipitation
Pelleted cells were suspended in immunoprecipitation buffer (50 mm Tris–HCl, pH 7·5, 150 mm NaCl, 0·5% Nonidet P-40, 1 mm dithiothreitol, 1 mm PMSF) and centrifuged for 30 min at 16 000 g. Supernatants containing equal amounts of protein (300 μg) were precleared with 15 μl protein A–Sepharose and then incubated for 1 hr with PV serum, after which 15 μl protein A–Sepharose was added for 2 hr. After centrifugation at 2300 g for 10 min, beads containing antigen–antibody complexes were washed as described elsewhere to increase the efficiency of immunoprecipitation17 and Western blotting was performed as detailed above.
Gel purification of 130 000 MW bands
For purification of both the keratinocyte and PBMC 130 000 bands, the protein samples immunoprecipitated from cell lysates were loaded onto an 8% preparative polyacrylamide gel and separated at 100 V for 2 hr; a 5 mm-wide band, corresponding to the 133 000 MW prestained marker, was excised and dehydrated in acetonitrile; subsequently, gel containing the 130 000 MW protein(s) was incubated for 2 hr at 37° in bicarbonate elution buffer (50 mm ammonium bicarbonate, 0·1% SDS) after which a solution of isopropanol–formic acid was added to a final concentration of 45/5% (v/v) for 30 min at room temperature. The eluted proteins were lyophilized, and the SDS was removed by washings with cold 80% acetone. Finally, for control studies, PV IgG was incubated with 1 ml of gel-pure 130 000 MW protein(s) for 1 hr and then diluted in appropriate antibody solution and used for immunoblotting or immunofluorescence.
Immunofluorescence microscopy
Keratinocytes were grown to confluence on glass coverslips in DMEM plus 10% FBS. Cultured PBMC were collected in 2-ml tubes and pelleted at 800 g. Immunofluorescence microscopy was performed as reported previously.18 Briefly, after washing with PBS, cells were fixed and permeabilized in paraformaldehyde solution (3% paraformaldehyde in PBS containing 0·1% Triton X-100) for 20 min at room temperature.19 Samples were then washed three times in PBS containing 2% bovine serum albumin (BSA) to block non-specific sites, incubated with primary antibody (1 : 10) or sera for 1 hr at room temperature, washed in BSA/PBS, and exposed to species-specific antibodies (1 : 100) conjugated to FITC. Specimens were examined with a Zeiss Axiophot microscope (Calr Zeiss Inc., Thornwood, NY) at × 400 magnification and fluorescence images were acquired with an Evolution VF fast digital camera (MediaCybernetics, Marlow, UK).
Results
Recognition of keratinocyte antigen(s) by PV sera
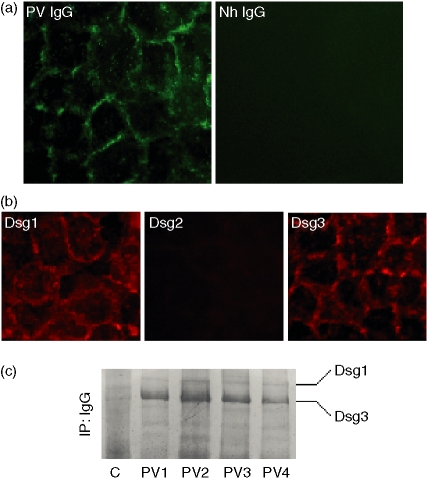
Normal and PV sera were used for this study. Immunofluorescence on HaCaT keratinocytes showed that PV IgG, but not normal human (Nh) IgG, described a fishnet-like pattern (Fig. 1a), indicating that in our experimental cell system PV IgG targeted antigen(s) located in the intercellular substance (ICS) of keratinocytes. At the time of the experiment, HaCaT expressed Dsg1, Dsg3 and virtually undetectable levels of Dsg2 (Fig. 1b). This expression pattern enabled us to further identify the immunoreactivity of PV IgG by immunoprecipitation of HaCaT extracts. Western blot analysis of the immunocomplexes revealed that IgG from active PV (n = 4) recognized Dsg3 and Dsg1 while Nh IgG did not (Fig. 1c).
Figure 1.
(a) PV IgG, but not Nh IgG, bound antigen(s) within the intercellular substance (ICS), describing the typical fishnet-like pattern (b). As revealed by immunofluorescence, cells expressed Dsg1 and Dsg3, whereas Dsg2 was virtually undetectable. Figures are representative of three independent experiments. (c) PV IgG (n = 4), but not control IgG, immunoprecipitated the 130 000 MW Dsg3 (n = 4) and were slightly positive to the 160 000 MW Dsg1. The immunoreactivity of sera was first probed with anti-Dsg3 and anti-Dsg1 IgG separately, in three independent experiments. Then the filter shown in the figure was stripped and incubated with both antibodies to visualize the autoantigenic pattern in the same blot.
PBMC do not express desmogleins 1 and 3
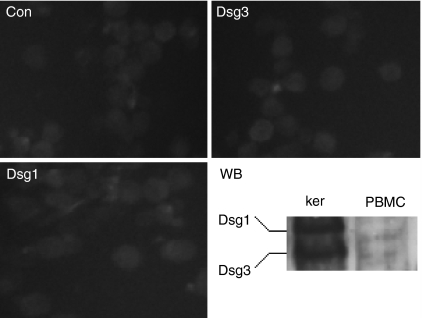
To investigate whether PV antigens were expressed by PBMC, we carried out immunofluorescence studies with monoclonal antibodies against Dsg1 and Dsg3. Consistent with the assumption that blood cells do not assemble desmosomes, these desmosomal proteins were found to be undetectable on PBMC (Fig. 2). Slight green fluorescence was found both in negative controls (Con) and in PBMC probed for Dsg1 and Dsg3. This was probably reflected the unspecific binding of FITC-conjugated secondary antibody on the cell surface. Western blotting on cell lysates confirmed that PBMC expressed neither Dsg1 nor Dsg3 (Fig. 2, WB).
Figure 2.
Immunofluorescence studies and Western blotting (WB) showed that PBMC lack Dsg1 and Dsg3. Anti-Dsg1 and anti-Dsg3 were first probed separately, then the results were summarized in the same blot by incubating PVDF filters with both antibodies. Keratinocyte lysates (ker) served as a positive control. Con, negative control PBMC obtained by using only FITC-conjugated anti-human antibodies. The figures are representative of three independent experiments.
PV IgG immunoprecipitates a PBMC 130 000 MW protein other than Dsg3
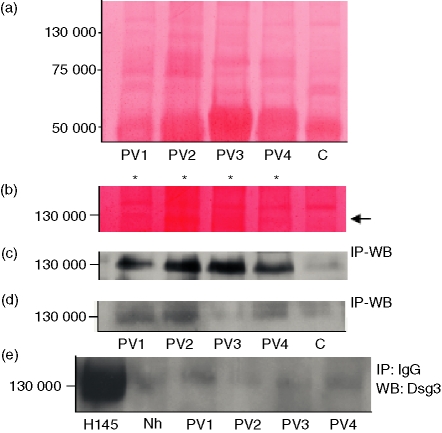
Since PV IgG binds antigens located in the epidermis, the immunoreactivity of PV sera is classically assessed by using keratinocyte extracts as the antigen source. To test whether antigen(s) exist in blood cells that are recognized by PV sera, IgG of patients with PV were used to immunoprecipitate protein extracts from PBMC. Surprisingly, Ponceau staining of PVDF filters showed that PV IgG, but not normal IgG, immunoprecipitated a 130 000 MW protein (Fig. 3a,b). By using combined immunoprecipitation–Western blotting with PV IgG as primary antibody, the 130 000 MW band was confirmed to be selectively recognized by IgG of patients with PV (Fig. 3c). Furthermore, preincubation of PV IgG with the 130 000 MW protein(s) purified from keratinocyte extracts strongly reduced the signal (Fig. 3d), suggesting that the novel PV antigen was expressed on both keratinocytes and PBMC. To definitively exclude that this 130 000 band corresponded to Dsg3, immunoprecipitates were assayed for Dsg3 reactivity. Western blot of the immunocomplexes using H-145 polyclonal IgG as primary antibody revealed that the 130 000 MW antigen was not Dsg3 (Fig. 3e). This finding was further ascertained by probing filters with the 5H10 and 5G11 monoclonal antibodies raised against the extracellular domain of human Dsg3 (not shown).
Figure 3.
(a) Ponceau staining of PVDF membranes blotted with immunocomplexes shows a slight 130 000 band immunoprecipitated by PV (n = 4) but not control sera. (b) By increasing the contrast of the surface of interest (adobe Photoshop), the 130 000 band (arrow) clearly appeared in lanes 1–4 (PV sera, asterisks) but not in lane 5 (control). (c) Combined immunoprecipitation (IP)-Western blotting (WB) with PV IgG demonstrated that IgG from PV sera recognized a 130 000 MW protein. (d) Pre-incubation of PV IgG (primary antibody) with gel-pure 130 000 MW protein(s) from keratinocyte extracts strongly reduced the ability of PV IgG to bind the 130 000 PBMC antigen. (e) Immunoprecipitation of keratinocyte extracts with H-145 anti-Dsg3 (positive control), Nh or PV IgG followed by Western blotting against Dsg3 revealed that immunocomplexes other than positive controls did not contain Dsg3. Results were confirmed in at least two experiments carried out independently.
Taken together, these findings demonstrate that PV IgG recognizes an antigen synthesized by PBMC other than Dsg3 (PV130) with a molecular weight of 130 000.
The novel 130 000 antigen is expressed on PBMC surface
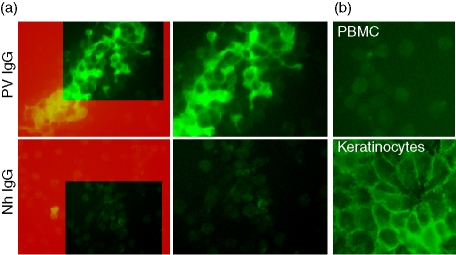
Immunofluorescence microscopy on permeabilized PBMC was performed to assess the cellular localization of PV130. PV, BP and normal sera were incubated with PBMC and then bound antibodies were left to react with FITC-conjugated anti-human IgG. Immunofluorescence analysis revealed that PV IgG, but not Nh IgG, recognized antigen(s) located on the PBMC surface (Fig. 4a). Indeed, FITC fluorescence appeared to be localized all around the cell membrane, describing a fishnet-like pattern on aggregated PBMC similar to that observed in the epidermis. Moreover, when IgG from another immune-mediated bullous disease such as BP was probed as a further control, the immunostaining on PBMC was found to be negative (not shown), confirming that the above fluorescence features were PV-specific.
Figure 4.
(a) PBMC were fixed and permeabilized in paraformaldehyde solution and then incubated with PV or normal IgG. Binding of the antibodies on PBMC surface was revealed by FITC-conjugated anti-human IgG. Only PV IgG stained all around cell membrane of PBMC. The right-hand panels represent magnifications of the highlighted boxes in the left panels. (b) Pre-incubation of PV serum with purified keratinocyte 130 000 protein(s) abolished the staining of PV IgG on PBMC surface (left panel), whereas preincubation with gel-pure 130 000 protein(s) from PBMC extracts apparently did not impaire the ability of PV IgG to recognize keratinocyte antigens. The figure is representative of at least two independent experiments.
Interestingly, preincubation of PV IgG with gel-pure 130 000 MW protein(s) of keratinocyte origin abolished the PBMC fluorescence (Fig. 4b, PBMC), indicating that the novel autoantigen was expressed in both keratinocytes and PBMC. It is possible that PV IgG exhibits cross-reaction between Dsg3 and PV130. In contrast, preincubation with PBMC extracts did not grossly affect the reactivity of PV IgG against keratinocyte surface antigens (Fig. 4b, keratinocytes).
Overall, these data definitively demonstrated that PV patients can develop autoimmunity against blood cell antigen(s).
Discussion
In the present study, we demonstrated for the first time that PBMC can be targeted by PV autoimmunity. Indeed, our results clearly showed that PV IgG recognized a 130 000 MW autoantigen other than Dsg3 located on the surface of PBMC. This novel autoantigen, PV130, seemed to be expressed also by keratinocytes, because preabsorption with HaCaT extracts strongly impaired the reactivity of PV IgG against the PBMC surface.
Under certain aspects, pemphigus represents an enigma for investigators. Although a series of papers shed light on the pathophysiological mechanisms of epithelial acantholysis,19–21 the current explanation for pemphigus autoimmunity appears not to be completely satisfactory.22,23 Until the antigenic targets and their functions are not fully elucidated, we will be unable to clarify the complex phenomena that drive the disease. Observations reported in the present paper open new roads towards a better understanding of PV autoimmunity by shifting the search from the epidermis to the blood cells. For example, the regulation of the autoimmune response by a PV antigen on PBMC could explain the inconsistency, not seldom observed, between immunological findings and clinical phenomenology.
Previous reports described autoantigens other than Dsg1 and Dsg3 in PV.24 Indeed, the epidermis of Dsg3-null mice was found to describe a fishnet-like pattern when incubated with Dsg1-depleted PV sera in an immunofluorescence analysis. The novelty of our findings is that the 130 000 antigen recognized by PV IgG is located on PBMC. Hence, PV autoimmunity targets non-epithelial cells, which neither assemble desmosomes nor express desmogleins. Further studies will elucidate the nature of this novel 130 000 MW antigen, allowing clarification of whether PV130 plays a pathogenic role in the pathophysiology of PV. However, to generalize our observations, a larger number of patients is needed.
In summary, here we provide the first evidence that PV IgG recognizes antigen(s) located outside the epidermis. The novel PV autoantigen, PV130, is found on both the PBMC surface and keratinocytes but, although displaying a molecular mass of 130 000, it is not Dsg3. This finding represents a potential breakthrough in the knowledge of PV autoimmunity and might permit the amelioration of its therapeutic approach.
Acknowledgments
This study was supported by research funds from Regional Center on Craniofacial Malformations-MRI, Regione Campania.
References
- 1.Bystryn JC, Rudolph JL. Pemphigus Lancet. 2005;366:61–73. doi: 10.1016/S0140-6736(05)66829-8. [DOI] [PubMed] [Google Scholar]
- 2.Wolff K, Schreiner E. Ultrastructural localization of pemphigus autoantibodies within the epidermis. Nature. 1971;229:59–61. doi: 10.1038/229059a0. [DOI] [PubMed] [Google Scholar]
- 3.Amagai M, Klaus-Kovtun V, Stanley JR. Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell. 1991;67:869–77. doi: 10.1016/0092-8674(91)90360-b. [DOI] [PubMed] [Google Scholar]
- 4.Chitaev NA, Troyanovsky SM. Direct Ca2+-dependent heterophilic interaction between desmosomal cadherins, desmoglein and desmocollin, contributes to cell–cell adhesion. J Cell Biol. 1997;138:193–201. doi: 10.1083/jcb.138.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dmochowski M, Hashimoto T, Garrod DR, Nishikawa T. Desmocollins I and II are recognized by certain sera from patients with various types of pemphigus particularly Brazilian pemphigus foliaceus. J Invest Dermatol. 1993;100:380–4. doi: 10.1111/1523-1747.ep12471934. [DOI] [PubMed] [Google Scholar]
- 6.Korman NJ, Eyre RW, Klaus-Kovtun V, Stanley JR. Demonstration of an adhering-junction molecule (plakoglobin) in the autoantigens of pemphigus foliaceus and pemphigus vulgaris. N Engl J Med. 1989;321:631–5. doi: 10.1056/NEJM198909073211002. [DOI] [PubMed] [Google Scholar]
- 7.Mimouni D, Foedinger D, Kouba DJ, et al. Mucosal dominant pemphigus vulgaris with anti-desmoplakin autoantibodies. J Am Acad Dermatol. 2004;51:62–7. doi: 10.1016/j.jaad.2003.11.051. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen VA, Ndoye A, Grando SA. Pemphigus vulgaris antibody identifies pemphaxin, a novel keratinocyte annexin-like molecule binding acetylcholine. J Biol Chem. 2000;275:29466–76. doi: 10.1074/jbc.M003174200. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen VT, Ndoye A, Grando SA. Novel human alpha9 acetylcholine receptor regulating keratinocyte adhesion is targeted by pemphigus vulgaris autoimmunity. Am J Pathol. 2000;157:1377–91. doi: 10.1016/s0002-9440(10)64651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanza A, Cirillo N, Femiano F, Gombos F. How does acantholysis occur in pemphigus vulgaris: a critical review. J Cutan Pathol. 2006;33:401–12. doi: 10.1111/j.0303-6987.2006.00523.x. [DOI] [PubMed] [Google Scholar]
- 11.Lanza A, Femiano F, De Rosa A, Cammarota M, Lanza M, Cirillo N. The N-terminal fraction of desmoglein 3 encompassing its immunodominant domain is present in human serum: implications for pemphigus vulgaris autoimmunity. Int J Immunopathol Pharmacol. 2006;19:399–407. doi: 10.1177/039463200601900216. [DOI] [PubMed] [Google Scholar]
- 12.Lanza A, Cirillo N. Caspase-dependent cleavage of desmoglein 1 depends on the apoptotic stimulus. Br J Dermatol. 2007;156:400–2. doi: 10.1111/j.1365-2133.2006.07654.x. [DOI] [PubMed] [Google Scholar]
- 13.Cirillo N, Femiano F, Gombos F, Lanza A. Serum from pemphigus vulgaris reduces desmoglein 3 half-life and perturbs its de novo assembly to desmosomal sites in cultured keratinocytes. FEBS Lett. 2006;580:3276–81. doi: 10.1016/j.febslet.2006.04.089. [DOI] [PubMed] [Google Scholar]
- 14.Frezzolini A, Cianchini G, Ruffelli M, Cadoni S, Puddu P, De Pita O. Interleukin-16 expression and release in bullous pemphigoid. Clin Exp Immunol. 2004;137:595–600. doi: 10.1111/j.1365-2249.2004.02570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–71. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cirillo N, Gombos F, Lanza A. Changes in desmoglein 1 expression and subcellular localization in cultured keratinocytes subjected to anti-desmoglein 1 pemphigus autoimmunity. J Cell Physiol. 2007;210:411–16. doi: 10.1002/jcp.20856. [DOI] [PubMed] [Google Scholar]
- 17.Pasdar M, Nelson WJ. Regulation of desmosome assembly in epithelial cells. Kinetics of synthesis, transport, and stabilization of desmoglein I, a major protein of the membrane core domain. J Cell Biol. 1989;109:163–77. doi: 10.1083/jcb.109.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cirillo N, Femiano F, Gombos F, Lanza A. Metalloproteinase 9 is the outer executioner of desmoglein 3 in apoptotic keratinocytes. Oral Dis. 2006 doi: 10.1111/j.1601-0825.2006.01287.x. DOI 10.1111/j.1601-2006.0825.01287.x. [DOI] [PubMed] [Google Scholar]
- 19.Sato T, Irie S, Kitada S, Reed JC. FAP-1: a protein tyrosine phosphatase that associates with Fas. Science. 1995;268:411–15. doi: 10.1126/science.7536343. [DOI] [PubMed] [Google Scholar]
- 20.Mahoney MG, Wang Z, Rothenberger K, Koch PJ, Amagai M, Stanley JR. Explanations for the clinical and microscopic localization of lesions in pemphigus foliaceus and vulgaris. J Clin Invest. 1999;103:461–8. doi: 10.1172/JCI5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caldelari R, de Bruin A, Baumann D, Suter MM, Bierkamp C, Balmer V, Muller E. A central role for the armadillo protein plakoglobin in the autoimmune disease pemphigus vulgaris. J Cell Biol. 2001;153:823–34. doi: 10.1083/jcb.153.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waschke J, Bruggeman P, Baumgartner W, Zillikens D, Drenckhahn D. Pemphigus foliaceus IgG causes dissociation of desmoglein 1-containing junctions without blocking desmoglein 1 transinteraction. J Clin Invest. 2005;115:3157–65. doi: 10.1172/JCI23475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cirillo N, Lanza A. Pemphigus acantholysis and steric hindrance: an unsolved equation. J Clin Invest. 2006 http://www.jci.org/cgi/eletters/115/11/3157. [Google Scholar]
- 24.Nguyen VT, Ndoye A, Shultz LD, Pittelkow MR, Grando SA. Antibodies against keratinocyte antigens other than desmogleins 1 and 3 can induce pemphigus vulgaris-like lesions. J Clin Invest. 2000;106:1467–79. doi: 10.1172/JCI10305. [DOI] [PMC free article] [PubMed] [Google Scholar]