Abstract
We have previously shown that iodotyrosyl formation within certain innocuous thyroglobulin (Tg) peptides confers on them immunopathogenic properties. In this report, we generated a panel of T-cell hybridoma clones specific for the immunogenic 16 mer Tg peptide p179 (amino acids 179–94) or its iodinated analogue (I-p179), with a view to examining the effects of a single iodine atom at the Y192 amino acid residue on T-cell recognition. We found that the peptide p179 was subdominant, and its binding to both Ak and Ek molecules was not significantly influenced by iodine. T-cell receptor (TCR) engagement was unaffected by the bulky iodine atom in two clones that responded to both analogues but it was sterically hindered in two other clones that recognized only p179. One clone was reactive only to I-p179, suggesting that the iodine atom is an integral part of its TCR ligand. Truncation analysis localized the determinant seen by all clones within the 11 mer peptide p184 (amino acids 184–194), suggesting that the cross-reactive clones were not activated by a minimal epitope lacking Y192 and that the negative influence of iodine was not the result of a flanking residue effect. These results demonstrate, at the clonal level, variable influences of a single iodine atom on the recognition of a single Tg peptide. Iodination of tyrosyl-containing, immunopathogenic Tg peptides may have unpredictable effects at the polyclonal level, depending on the extent of iodination at the particular site, and the relative number or effector function of autoreactive T-cell clones that are switched on or off by the neoantigenic determinant.
Keywords: autoimmunity, iodination, T-cell epitope, thyroglobulin, thyroiditis
Introduction
Excessive iodine intake has been associated with the incidence of autoimmune thyroiditis in clinical and experimental studies.1–7 The mechanisms underlying this association remain unknown but they may be, at least in part, related to the immunogenicity of thyroglobulin (Tg), the only molecule that incorporates iodine in vivo to facilitate the synthesis of the thyroid hormones tri-iodothyronine and thyroxine.8 Tg represents up to 75% of the total protein in the thyroid gland9 and it is the major thyroid autoantigen causing experimental autoimmune thyroiditis (EAT), a T-cell-mediated disease10–12 that is considered to be a model for Hashimoto's thyroiditis in humans.13,14
Several studies have supported the view that the iodine content in Tg, which varies according to dietary intake, can influence its immunogenicity.15–17 For example, iodine-deficient Tg fails to induce EAT in mice15 and BB/W rats17 and conversely, highly iodinated Tg is more immunogenic than normal Tg and elicits severe EAT.16,18 Removal of iodine, and presumably hormone, from the human Tg (hTg) fragment 2513–2713 was also reported to convert an immunogenic epitope that is localized within this region into a tolerogenic one.19 Iodination has been shown to enhance Tg immunogenicity in two ways: (1) by formation of neoantigenic determinants encompassing either hormonogenic or iodotyrosyl-containing sites,20–22 and (2) by alteration of Tg processing, resulting in generation of non-iodinated but pathogenic cryptic epitopes.18,23 We have previously reported that iodotyrosyl formation can render certain innocuous Tg peptides (p304, p117 and p1931) immunopathogenic.21 Additional results, however, have suggested that iodotyrosyl formation can have variable effects on the immune recognition of other Tg peptides, i.e. it can increase, decrease or not alter their established immunogenicity.21 In this study, we have used as a model antigen the mouse Tg (mTg) peptide p179 [amino acids (aa) 179–194] for two interesting reasons: (1) an overlapping peptide analogue (aa 181–195) has been reported to result from the natural processing of hTg in HLA-DR3-transgenic mice,24 and (2) the iodinated analogue I-p179, carrying an iodotyrosyl at position Y192, elicits stronger proliferative T-cell responses than p179, but remains, unexpectedly, mildly pathogenic as p179 (Li et al. submitted for publication). Therefore, we aimed to examine if p179 could be similarly generated following the in vivo processing of mTg and, in addition, investigate at the clonal T-cell level, the effects of a single iodine atom on T-cell recognition of p179 that may account for the immunopathogenic behaviour of the iodinated analogue.
Materials and methods
Animals, antigens and antibodies
Female CBA/J (H-2k) mice purchased from Jackson Laboratories (Bar Harbor, ME) were used in experiments at 6–8 weeks of age. The following mTg peptides were synthesized at 80–90% purity: p179, I-p179, I-p304 (aa 304–318) (Dalton Chemical Laboratories Inc., Toronto, Canada),21 p2496 (aa 2496–2504) (Alberta Peptide Institute, Edmonton, Canada)25 and p1826 (aa 1826–1835) (Sigma-Genosys, The Woodlands, TX).26 All truncated peptides (> 90% purity) were synthesized at Biosynthesis, Inc. (Lewisville, TX). All peptides were blocked with an N-terminal acetyl group and a C-terminal amide group. F-moc-3-iodo-tyrosine was used for the synthesis of all iodinated peptide analogues. The mTg was purified from frozen thyroids of outbred ICR mice (Bioproducts for Science, Indianapolis, IN) by passing thyroid homogenates through a Sepharose CL-4B column, as previously described.27 Tg concentrations are expressed as the molarity of the monomeric form. Monoclonal antibodies (immunoglobulin G2a) specific for Ak (10-3.6.2),28 Ek (14-4-4s)29 and influenza A nucleoprotein (H16-L10-4R5)30 were purified by protein G–Sepharose 4 Fast Flow affinity chromatography (Amersham Biosciences, Uppsala, Sweden) from culture supernatants of cell lines (American Type Culture Collection, Manassas, VA).
Proliferation assay of antigen-specific lymph node cells
Mice were immunized subcutaneously with 100 nmol Tg peptide or 100 μg mTg in complete Freund's adjuvant (CFA; with Mycobacterium butyricum; Difco Laboratories, Detroit, MI). Nine days later, the inguinal, brachial, and axillary lymph node cells (LNC) were cultured (5 × 105 cells/200 μl/well) in Dulbecco's modified Eagle's minimal essential medium supplemented with 10% heat-inactivated fetal bovine serum (PAA Laboratories, Etobicoke, Ontario), 20 mm HEPES buffer, 2 mm l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin (all from Gibco) and 5 × 10−5m 2-mercaptoethanol (Sigma Chemical Co., St Louis, MO) (complete medium) for 4 days with or without antigen. Eighteen hours before harvesting, 1 μCi methyl[3H]thymidine (PerkinElmer, Boston, MA) was added to each well. Cell harvesting and radioactivity counting were performed as previously described.26 The stimulation index (S.I.) is defined as [counts per minute (c.p.m.) in the presence of antigen]/(c.p.m. in the absence of antigen).
Generation of peptide-specific T-cell hybridoma clones
Interleukin-2 (IL-2) -secreting T-cell hybridomas were generated following a modified method described by Perkins et al.31. Briefly, CBA/J mice were challenged subcutaneously with 100 nmol p179 or I-p179 in 100 μl of phosphate-buffered saline/CFA emulsion and 9 days later, draining LNC were collected and 4 × 106 cells/ml were cultured in the presence of 20 μm of the respective peptides. After 3 days, the stimulated cells were fused by polyethylene glycol at a 1 : 2 ratio with the BW5147α–β– variant,32 a gift from P. Marrack (Howard Hughes Medical Institute, Denver, CO). Hybridomas appeared after 7–10 days of culture, and were successively passaged through hypoxanthine–aminopterin–thymidine-containing medium and hypoxanthine–thymidine-containing medium, and then into complete medium. Peptide-specific hybridomas were cloned by limiting dilution at 0·3 cell/well in medium with 20% fetal bovine serum and 1% syngeneic red blood cells as feeder cells.
Activation assay of T-cell clones
Hybridoma clones (105 cells/well) were cultured with LK35.2 cells (H-2Ak/d, H-2Ek/d)33 (105 cells/well) in the presence of relevant antigen. After 24 hr of incubation, 100 μl supernatant was transferred from each well into new plates and stored at − 70° for at least 2 hr. The release of IL-2 into the culture supernatant was assessed by measuring the proliferation of the IL-2-dependent cytotoxic T cells (CTLL-2), as described previously.34 T-cell clones 10C1 (Ak-restricted, I-p304-specific)21 and 8F9 (Ek-restricted, p2496-specific)25 were used in the competitive inhibition assays.
Reverse transcription–polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from 5 × 106 cells using TRIZOL reagent (Invitrogen, Carlsbad, CA) following the manufacturer's instructions. First-strand cDNA synthesis was performed using a commercial kit (Amersham Bioscience, Buckinghamshire, UK) in a 33-μl reaction solution containing 5 μg total RNA. The RT-PCR reaction mixture contained: 1 × PCR buffer, 1·5 mm MgCl2 (both from Invitrogen), 0·2 mm of each dNTP (Gibco-BRL), 2 μl cDNA template, 20 pmol of each primer, 2·5 U Taq polymerase (Invitrogen) and RNase-free water (Sigma) to 100 μl final volume. Forward primers for Vβ families, a reverse primer for Cβ35 as well as primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH)36 were synthesized by the GSD Center for Biomaterials (Toronto, Canada). Amplification was performed in 35 cycles (1 min denaturation at 95°, 1 min annealing at 55° and 1 min extension at 72°) on a Perkin Elmer DNA thermocycler (Cetus, Norwalk, CT) preceded by a 5-min denaturation step at 94° and followed by a 10-min extension step at 72°. The PCR products were visualized by electrophoresis on 1·5% agarose gels containing 0·5 μg/ml ethidium bromide.
Results
p179 contains subdominant epitope(s)
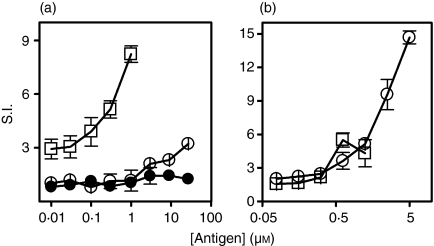
We first tested whether the Tg peptides p179 and I-p179 contained dominant T-cell epitope(s). CBA/J mice were immunized with 100 μg mTg emulsified in CFA and 9 days later, draining LNCs were cultured in the presence of mTg or free peptides. Tg-primed LNCs responded strongly against mTg (S.I. = 2·9–8·2 in the 0·01–1 μm range), weakly to higher concentrations of p179 (S.I. = 3·2 ± 0·3 at 27 μm), but failed to respond to equimolar amounts of I-p179 (Fig. 1a). Conversely, in vivo priming with 100 nmol p179 elicited strong recall responses to the priming peptide (S.I. = 3·6–14·7 in the 0·6–5 μm range) and a weak but significant response (S.I. = 4·3–5·5) to equimolar amounts of mTg in vitro (Fig. 1b). A proliferative LNC response was not detected against the control Tg peptide p2496 in any of the assays (data not shown). These data support the view that p179 encompasses subdominant T-cell determinant(s).
Figure 1.
p179 contains a subdominant epitope. CBA/J mice (three mice per group) were challenged with either Tg (a) or p179 (b). Antigen-specific proliferation of LNCs, in the presence of Tg (□), p179 (○) or I-p179 (•) was examined 9 days later. Data show the mean ± SD of S.I. values of triplicate wells and are representative of two or three experiments. Background c.p.m. varied from 3499 to 5653.
Iodination of p179 does not influence major histocompatibility complex (MHC) binding
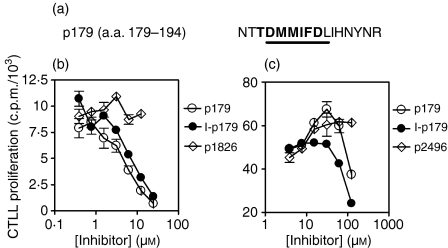
The Tg peptide p179 was initially identified as a site of putative pathogenic epitope(s) because it contains an Ak-binding motif.37 Subsequently, it was found that p179 also has an Ek-binding motif (Fig. 2a) and that in bulk cultures the majority of T cells recognize p179 as well as I-p179 in the context of Ek (Li et al. submitted for publication). To determine whether iodination of p179 can influence its binding to MHC, we used two hybridoma clones: the Ek-restricted 8F9 clone, specific for the Tg peptide p2496,25 and the Ak-restricted 10C1 clone, specific for the Tg peptide I-p304,21 to examine their activation by their cognate ligands in the presence of p179 or its iodinated analogue in a competitive inhibition assay. This pair of peptides inhibited 8F9 activation equally well, suggesting equivalent binding affinities to Ek (Fig. 2b). Similarly, both analogues strongly inhibited 10C1 activation but this effect was noticeable at high competitor peptide concentrations (125 μm), with the I-p179 showing a slightly higher, although not significantly different, inhibition (Fig. 2c). The inhibitory effect was specific because it was not detected at equimolar concentrations of control peptides (Fig. 2b,c). These results indicate that the addition of an iodine atom to the Y192 residue does not significantly alter the MHC-binding properties of p179.
Figure 2.
Iodine modification of p179 does not significantly alter its MHC-binding properties. (a) Amino acid sequence of p179 indicating the Ak-binding (underlined) and Ek-binding (bold) motifs. (b) Activation of the Ek-restricted clone 8F9 by its p2496 ligand (25 nm), in the presence of the inhibitor peptides p179 and I-p179. The Ak-restricted Tg peptide p1826 was used as a negative control. (c) Activation of the Ak-restricted clone 10C1 by its I-p304 ligand (0·15 µm), in the presence of increasing concentrations of the inhibitor peptides p179 and I-p179. The Ek-restricted Tg peptide p2496 was used as a negative control. IL-2 secretion by the activated T-cell hybridomas was assessed by CTLL proliferation.
Variable effects of iodine on T-cell recognition at the clonal level
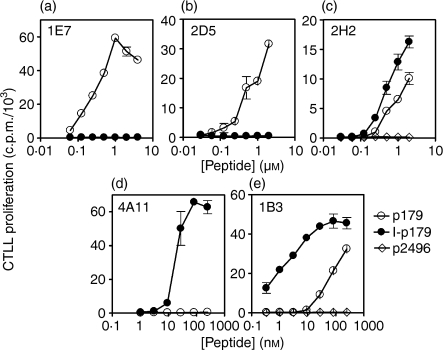
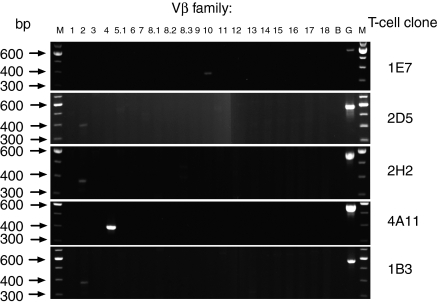
To examine the possible effects of the iodinated Y192 on T-cell recognition, we generated a panel of T-cell hybridoma clones: 1E7, 2D5 and 2H2 clones were obtained from p179-primed LNC, whereas 4A11 and 1B3 clones were generated from I-p179-primed LNC. Within the first set, it was found that 1E7 and 2D5 were activated only by p179 and that the incorporation of one iodine atom to Y192 abrogated the engagement of their receptor (Fig. 3a,b). In contrast, clone 2H2 was activated by both p179 and I-p179, suggesting that the presence of iodine had a neutral effect on its T-cell receptor (TCR) engagement (Fig. 3c). Within the second set, clone 4A11 was activated only by the I-p179 analogue within the 1–125 nm range, demonstrating that the iodine atom was critical for TCR recognition (Fig. 3d). On the other hand, clone 1B3 showed a preference for the iodinated analogue but remained reactive to p179, indicating that the iodine atom was not an integral part of its ligand (Fig. 3e). The monoclonal antibody-mediated blocking assays confirmed that all the above clones were Ek-restricted with the exception of 2D5, which is Ak-restricted (data not shown). Furthermore, RT-PCR analysis showed that 1E7 utilized Vβ10, 4A11 utilized Vβ4, while 2D5, 1B3 and 2H2 utilized Vβ2 gene families. These results supported the clonal nature of these hybridomas and further suggested that there may be no strict segregation of Vβ family use according to the fine TCR specificity (Fig. 4).
Figure 3.
Variable influences of a single iodine atom on TCR recognition. Hybridoma clones 1E7 (a), 2D5 (b) and 2H2 (c) were generated from p179-primed LNCs and clones 4A11 (d) and 1B3 (e) were generated from I-p179-primed LNCs. The LK35.2 cell line was used as APC. Data represent mean ± SD of c.p.m. for triplicate wells.
Figure 4.
TCR Vβ family utilization examined by RT-PCR, using Vβ-specific primers. M, markers; B, blank control; G, GAPDH.
Examination of fine specificity of hybridoma clones by truncation analysis
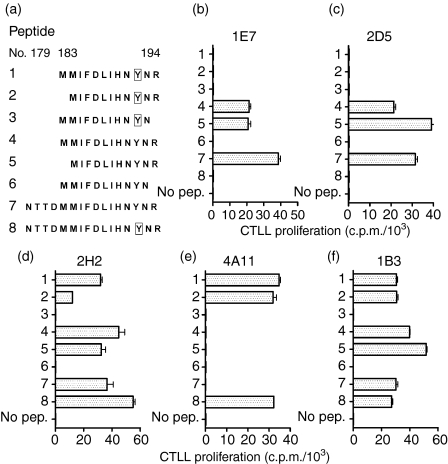
To investigate whether the fine specificity of the above clones was linked to recognition of distinct determinants within the aa 179–194 sequence, we performed truncation analysis using the peptide panel shown in Fig. 5(a). The clones 1E7 and 2D5, which reacted only against the p179 analogue (pep.7), were found to respond to the 11 mer Tg peptide p184 (pep.5, aa 184–194) but remained unresponsive to the iodinated analogue I-p184 (pep.2) (Fig. 5b,c). In contrast, the clone 2H2 responded to both 11 mer analogues (pep.2 and pep.5) (Fig. 5d). Conversely, the clone 4A11 recognized only I-p184 (pep.2), not p184 (pep.5) (Fig. 5e), whereas clone 1B3 responded to both p184 and I-p184 analogues (Fig. 5f). No clone was activated by either pep.3 or pep.6, suggesting that R194 is a critical residue. These results established: (1) that the R194 residue is the C-terminal residue of the minimal epitope recognized by all clones, and (2) that the cross-reactive clones 2H2 and 1B3 recognize an epitope that contains the Y192, i.e. they can tolerate the presence of the bulky iodine atom in this position.
Figure 5.
(a) Peptide panel used for truncation analysis. Peptides 7 and 8 represent the full amino acid sequence of p179 and I-p179, respectively. Boxes denote iodotyrosyls. (b) to (f) Activation of 1E7, 2D5, 2H2, 4A11 and 1B3 clones in the presence of 10 µm of indicated peptides. The LK35.2 cell line was used as APC. Data depict mean ± SD c.p.m. of triplicate wells.
Discussion
Tg is the only self antigen that stores iodide and organifies it in the form of iodotyrosyl or iodothyronine residues. This post-translational modification varies according to the environmental supply of iodine and has immunological consequences because of formation of neoantigenic determinants. For example, thyroid-infiltrating T cells have been characterized to be elicited by Tg peptides containing hormonogenic sites20,38 or iodotyrosyls.21 Iodine can also have variable influences on Tg peptides of known immunogenicity because it can increase, decrease or not alter their antigenic and immunogenic behaviour.21 In this context, we have described in a parallel study that iodine enhances the immunogenicity of the p179 peptide with no apparent effects on its pathogenicity (Li et al. submitted for publication). These observations and the previous finding that processing of hTg in HLA-DR3 transgenic mice generates the overlapping peptide 181–195,24 prompted us to examine how iodine influences recognition of p179 at the clonal T-cell level.
Our results demonstrate that processing of mTg in vivo or in vitro seems to weakly prime p179-specific T cells, confirming its subdominant nature. However, mTg-primed LNC do not react against I-p179, suggesting that mTg does not harbour the iodinated analogue under steady-state conditions. So far, subdominant Tg T-cell epitopes have been localized within seven Tg peptides: (179–194), (418–432), (1518–1532) (2079–2093), (2340–2359), (2549–2560) and (2695–2713).24,39–41 Subdominance has been defined by the capacity of peptide-primed T cells to occasionally respond to intact Tg in vivo or in vitro but at molar concentrations much higher than those required for activation by free peptide. Identification of a dominant Tg epitope that can activate mTg-primed LNC at concentrations equimolar to those of intact Tg remains elusive, and it is uncertain to what extent a 330 000 molecular weight Tg monomer can be quantitatively processed in vitro to yield any given peptide with an approximately 150-fold smaller molecular mass.39,41
The I-p179 analogue binds to Ak or Ek molecules almost as well as p179, yet the presence of one iodine atom at Y192 can exert extremely variable influences at the clonal T-cell level. Clones 1E7 and 2D5 must represent subsets of T cells whose fine specificity is negatively impacted by iodine, presumably through a steric hindrance effect. Conversely, the absence of iodine abrogates completely the reactivity of clone 4A11, which probably represents T-cell subsets that are monospecific for the neoantigenic determinant. These cells will be elicited following animal challenge with I-p179, and they may even occur at a frequency higher than p179-specific T cells to account for the higher immunogenicity of I-p179. However, they are not expected to contribute to EAT if I-p179 is not constitutively expressed in normal Tg. Their putative effector function would be demonstrable only when increased ingestion of iodine might promote iodination of the Y192 residue, but experimental evidence for this hypothesis is currently lacking. Lastly, the cross-reactive receptors on clones 2H2 and 1B3 may be expressed on effector T cells in EAT elicited by either analogue, and may account, to a large extent, for the mild thyroiditogenicity of p-179 and I-p179.
The truncation analysis data mapped the minimal epitope recognized by all clones within the 11 mer p184 (184–194) peptide. The R194 residue comprises the C-terminal aa residue of this epitope because its removal abrogates the reactivity of all clones. Although the N-terminal end of the minimal epitope has not been formally assigned, this sequence differs only at two aa positions (I185–V185 and N191–S191) from the homologous thyroiditogenic sequence in hTg.24 The data also demonstrate that the cross-reactivity of clones 2H2 and 1B3 cannot be explained by recognition of an identical minimal epitope lacking Y192. Instead, these findings argue for the presence, within the normal receptor repertoire, of TCR capable of tolerating the bulky iodine atom regardless of whether the initiating stimulus is p179 or I-p179.
Under steady-state conditions, only five out of 132 tyrosyls in a dimeric hTg molecule with 0·5% iodine content adopt, on average, the form of mono-iodotyrosyl residues.42 As mentioned above, the present studies with mouse Tg do not support the view that the Y192 residue is one of them, in agreement with biochemical investigations reporting on the localization of mono-iodotyrosyls in Tg subjected to very low levels of iodination.43 Earlier in vitro studies have indicated that only 12–15 Tyr residues can be available for non-enzymatic iodination in native Tg44 but it remains unknown whether thyroperoxidase-mediated iodination of Tg in vivo, over long periods of enhanced dietary iodine intake, may yield different results. Interestingly, the peptide p179 is localized within a non-repetitive sequence that disrupts the cysteine-rich motifs of tandem repeats on the N-terminal side of Tg, namely within a 40-residue insert of motif 1·3. It has been suggested that these intervening, non-repetitive sequences may represent solvent-exposed regions of Tg that may be inherently sensitive to proteolysis.45 In addition, the asparagine N179 is a confirmed glycosylation site that contains galactose but no fucose, representing either a hybrid oligosaccharide structure or a complex oligosaccharide moiety.46
In conclusion, the current data support the view that iodination of Tg may have pleiotropic effects on Tg-reactive T-cell clones. Any given iodinated neoantigenic determinant may turn on some autoreactive T cells but simultaneously switch off others specific for the non-iodinated analogue. Thyroiditogenicity may be determined by the combined net effect of this modification at the polyclonal level. Overall, highly iodinated Tg may become more immunopathogenic than normal Tg because of: (1) the creation of iodinated neoantigenic peptides that preferentially activate monospecific or cross-reactive thyroiditogenic T cells; and/or (2) generation of non-iodinated but cryptic pathogenic peptides generated by altered processing of Tg in antigen-presenting cells.18 The potential role of each category of determinant in the development of clinical disease remains to be elucidated.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research.
Abbreviations
- aa
amino acid
- CFA
Freund's complete adjuvant
- EAT
experimental autoimmune thyroiditis
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IL-2
interleukin-2
- LNC
lymph node cells
- MHC
major histocompatibility complex
- RT-PCR
reverse transcription–polymerase chain reaction
- S.I.
stimulation index
- Tg
thyroglobulin
- hTg
human thyroglobulin
- mTg
mouse thyroglobulin
- p179
mTg peptide (179–194)
- I-p179
iodinated analogue of p179
- I-p304
iodinated analogue of mTg peptide (304–318)
- p1826
mTg peptide (1826–1835)
- p2496
mTg peptide (2496–2504)
References
- 1.Braverman LE, Ingbar SH, Vagenakis AG, Adams L, Maloof F. Enhanced susceptibility to iodide myxedema in patients with Hashimoto's disease. J Clin Endocrinol Metab. 1971;32:515–21. doi: 10.1210/jcem-32-4-515. [DOI] [PubMed] [Google Scholar]
- 2.Boyages SC, Bloot AM, Maberly GF, et al. Thyroid autoimmunity in endemic goitre caused by excessive iodine intake. Clin Endocrinol (Oxf) 1989;31:453–65. doi: 10.1111/j.1365-2265.1989.tb01269.x. [DOI] [PubMed] [Google Scholar]
- 3.Robuschi G, Safran M, Braverman LE, Gnudi A, Roti E. Hypothyroidism in the elderly. Endocr Rev. 1987;8:142–53. doi: 10.1210/edrv-8-2-142. [DOI] [PubMed] [Google Scholar]
- 4.Tajiri J, Higashi K, Morita M, Umeda T, Sato T. Studies of hypothyroidism in patients with high iodine intake. J Clin Endocrinol Metab. 1986;63:412–17. doi: 10.1210/jcem-63-2-412. [DOI] [PubMed] [Google Scholar]
- 5.Kahaly GJ, Dienes HP, Beyer J, Hommel G. Iodide induces thyroid autoimmunity in patients with endemic goitre: a randomised, double-blind, placebo-controlled trial. Eur J Endocrinol. 1998;139:290–7. doi: 10.1530/eje.0.1390290. [DOI] [PubMed] [Google Scholar]
- 6.Bagchi N, Brown TR, Urdanivia E, Sundick RS. Induction of autoimmune thyroiditis in chickens by dietary iodine. Science. 1985;230:325–7. doi: 10.1126/science.4048936. [DOI] [PubMed] [Google Scholar]
- 7.Mooij P, de Wit HJ, Drexhage HA. An excess of dietary iodine accelerates the development of a thyroid-associated lymphoid tissue in autoimmune prone BB rats. Clin Immunol Immunopathol. 1993;69:189–98. doi: 10.1006/clin.1993.1169. [DOI] [PubMed] [Google Scholar]
- 8.Taurog A. The biosynthesis of thyroxine. Mayo Clin Proc. 1964;39:569–85. [PubMed] [Google Scholar]
- 9.Malthiery Y, Marriq C, Berge-Lefranc JL, et al. Thyroglobulin structure and function: recent advances. Biochimie. 1989;71:195–209. doi: 10.1016/0300-9084(89)90057-6. [DOI] [PubMed] [Google Scholar]
- 10.Braley-Mullen H, Johnson M, Sharp GC, Kyriakos M. Induction of experimental autoimmune thyroiditis in mice with in vitro activated splenic T cells. Cell Immunol. 1985;93:132–43. doi: 10.1016/0008-8749(85)90394-6. [DOI] [PubMed] [Google Scholar]
- 11.Flynn JC, Conaway DH, Cobbold S, Waldmann H, Kong YC. Depletion of L3T4+ and Lyt-2+ cells by rat monoclonal antibodies alters the development of adoptively transferred experimental autoimmune thyroiditis. Cell Immunol. 1989;122:377–90. doi: 10.1016/0008-8749(89)90085-3. [DOI] [PubMed] [Google Scholar]
- 12.Conaway DH, Giraldo AA, David CS, Kong YC. In situ kinetic analysis of thyroid lymphocyte infiltrate in mice developing experimental autoimmune thyroiditis. Clin Immunol Immunopathol. 1989;53:346–53. doi: 10.1016/0090-1229(89)90063-9. [DOI] [PubMed] [Google Scholar]
- 13.Rose NR, Witebsky E. Studies on organ specificity. V. Changes in the thyroid glands of rabbits following active immunization with rabbit thyroid extracts. J Immunol. 1956;76:417–27. [PubMed] [Google Scholar]
- 14.Rose NR, Twarog FJ, Crowle AJ. Murine thyroiditis. importance of adjuvant and mouse strain for the induction of thyroid lesions. J Immunol. 1971;106:698–704. [PubMed] [Google Scholar]
- 15.Champion BR, Rayner DC, Byfield PG, Page KR, Chan CT, Roitt IM. Critical role of iodination for T cell recognition of thyroglobulin in experimental murine thyroid autoimmunity. J Immunol. 1987;139:3665–70. [PubMed] [Google Scholar]
- 16.Sundick RS, Herdegen DM, Brown TR, Bagchi N. The incorporation of dietary iodine into thyroglobulin increases its immunogenicity. Endocrinology. 1987;120:2078–84. doi: 10.1210/endo-120-5-2078. [DOI] [PubMed] [Google Scholar]
- 17.Ebner SA, Lueprasitsakul W, Alex S, Fang SL, Appel MC, Braverman LE. Iodine content of rat thyroglobulin affects its antigenicity in inducing lymphocytic thyroiditis in the BB/Wor rat. Autoimmunity. 1992;13:209–14. doi: 10.3109/08916939209004826. [DOI] [PubMed] [Google Scholar]
- 18.Dai YD, Rao VP, Carayanniotis G. Enhanced iodination of thyroglobulin facilitates processing and presentation of a cryptic pathogenic peptide. J Immunol. 2002;168:5907–11. doi: 10.4049/jimmunol.168.11.5907. [DOI] [PubMed] [Google Scholar]
- 19.Gardine CA, Gentile F, Pellegrini C, et al. Multiple fragments of human TG are capable of inducing oral tolerance to whole human TG. J Endocrinol Invest. 2003;26:294–300. doi: 10.1007/BF03345175. [DOI] [PubMed] [Google Scholar]
- 20.Kong YC, McCormick DJ, Wan Q, et al. Primary hormonogenic sites as conserved autoepitopes on thyroglobulin in murine autoimmune thyroiditis. Secondary role of iodination. J Immunol. 1995;155:5847–54. [PubMed] [Google Scholar]
- 21.Li HS, Carayanniotis G. Iodination of tyrosyls in thyroglobulin generates neoantigenic determinants that cause thyroiditis. J Immunol. 2006;176:4479–83. doi: 10.4049/jimmunol.176.7.4479. [DOI] [PubMed] [Google Scholar]
- 22.Gentile F, Conte M, Formisano S. Thyroglobulin as an autoantigen: what can we learn about immunopathogenicity from the correlation of antigenic properties with protein structure? Immunology. 2004;112:13–25. doi: 10.1111/j.1365-2567.2004.01861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carayanniotis G. The cryptic self in thyroid autoimmunity. the paradigm of thyroglobulin. Autoimmunity. 2003;36:423–8. doi: 10.1080/08916930310001602975. [DOI] [PubMed] [Google Scholar]
- 24.Flynn JC, McCormick DJ, Brusic V, et al. Pathogenic human thyroglobulin peptides in HLA-DR3 transgenic mouse model of autoimmune thyroiditis. Cell Immunol. 2004;229:79–85. doi: 10.1016/j.cellimm.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Rao VP, Balasa B, Carayanniotis G. Mapping of thyroglobulin epitopes: presentation of a 9mer pathogenic peptide by different mouse MHC class II isotypes. Immunogenetics. 1994;40:352–9. doi: 10.1007/BF01246676. [DOI] [PubMed] [Google Scholar]
- 26.Verginis P, Stanford MM, Carayanniotis G. Delineation of five thyroglobulin T cell epitopes with pathogenic potential in experimental autoimmune thyroiditis. J Immunol. 2002;169:5332–7. doi: 10.4049/jimmunol.169.9.5332. [DOI] [PubMed] [Google Scholar]
- 27.Chronopoulou E, Carayanniotis G. Identification of a thyroiditogenic sequence within the thyroglobulin molecule. J Immunol. 1992;149:1039–44. [PubMed] [Google Scholar]
- 28.Oi VT, Jones PP, Goding JW, Herzenberg LA, Herzenberg LA. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–20. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- 29.Ozato K, Mayer N, Sachs DH. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980;124:533–40. [PubMed] [Google Scholar]
- 30.Yewdell JW, Frank E, Gerhard W. Expression of influenza A virus internal antigens on the surface of infected P815 cells. J Immunol. 1981;126:1814–19. [PubMed] [Google Scholar]
- 31.Perkins DL, Berriz G, Kamradt T, Smith JA, Gefter ML. Immunodominance: intramolecular competition between T cell epitopes. J Immunol. 1991;146:2137–44. [PubMed] [Google Scholar]
- 32.White J, Blackman M, Bill J, et al. Two better cell lines for making hybridomas expressing specific T cell receptors. J Immunol. 1989;143:1822–5. [PubMed] [Google Scholar]
- 33.Kappler J, White J, Wegmann D, Mustain E, Marrack P. Antigen presentation by Ia+ B cell hybridomas to H-2-restricted T cell hybridomas. Proc Natl Acad Sci U S A. 1982;79:3604–7. doi: 10.1073/pnas.79.11.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gillis S, Smith KA. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977;268:154–6. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- 35.Casanova JL, Romero P, Widmann C, Kourilsky P, Maryanski JL. T cell receptor genes in a series of class I major histocompatibility complex-restricted cytotoxic T lymphocyte clones specific for a Plasmodium berghei nonapeptide: implications for T cell allelic exclusion and antigen-specific repertoire. J Exp Med. 1991;174:1371–83. doi: 10.1084/jem.174.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ju ST, Panka DJ, Cui H, et al. Fas (CD95) /FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–8. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 37.Altuvia Y, Berzofsky JA, Rosenfeld R, Margalit H. Sequence features that correlate with MHC restriction. Mol Immunol. 1994;31:1–19. doi: 10.1016/0161-5890(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 38.Champion BR, Page KR, Parish N, et al. Identification of a thyroxine-containing self-epitope of thyroglobulin which triggers thyroid autoreactive T cells. J Exp Med. 1991;174:363–70. doi: 10.1084/jem.174.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carayanniotis G, Chronopoulou E, Rao VP. Distinct genetic pattern of mouse susceptibility to thyroiditis induced by a novel thyroglobulin peptide. Immunogenetics. 1994;39:21–8. doi: 10.1007/BF00171793. [DOI] [PubMed] [Google Scholar]
- 40.Karras E, Yang H, Lymberi P, Christadoss P. Human thyroglobulin peptide p2340 induces autoimmune thyroiditis in HLA-DR3 transgenic mice. J Autoimmun. 2005;24:291–6. doi: 10.1016/j.jaut.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Hutchings PR, Cooke A, Dawe K, et al. A thyroxine-containing peptide can induce murine experimental autoimmune thyroiditis. J Exp Med. 1992;175:869–72. doi: 10.1084/jem.175.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunn JT, Dunn AD. Thyroglobulin. chemistry, biosynthesis, and proteolysis. In: Lewis E, Braverman R, Utiger D, editors. Werner and Ingbar's the Thyroid: a Fundamental and Clinical Text. Philadelphia: Lippincott, Williams & Wilkins; 2000. [Google Scholar]
- 43.Lamas L, Anderson PC, Fox JW, Dunn JT. Consensus sequences for early iodination and hormonogenesis in human thyroglobulin. J Biol Chem. 1989;264:13541–5. [PubMed] [Google Scholar]
- 44.Edelhoch H, Lippoladt RE. The properties of thyroglobulin. IX. The molecular properties of iodinated thyroglobulin. J Biol Chem. 1962;237:2788–94. [PubMed] [Google Scholar]
- 45.Gentile F, Salvatore G. Preferential sites of proteolytic cleavage of bovine, human and rat thyroglobulin. The use of limited proteolysis to detect solvent-exposed regions of the primary structure. Eur J Biochem. 1993;218:603–21. doi: 10.1111/j.1432-1033.1993.tb18414.x. [DOI] [PubMed] [Google Scholar]
- 46.Yang SX, Pollock HG, Rawitch AB. Glycosylation in human thyroglobulin: location of the N-linked oligosaccharide units and comparison with bovine thyroglobulin. Arch Biochem Biophys. 1996;327:61–70. doi: 10.1006/abbi.1996.0093. [DOI] [PubMed] [Google Scholar]