Abstract
Numerous chemokine receptors are increased in number on T cells in inflamed tissues. Our objective was to examine CXCR6 expression on lymphocytes during immune and inflammatory reactions and its potential for mediating T-cell recruitment. The cDNA for rat CXCR6 was cloned and monoclonal antibodies (mAbs) to CXCR6 were developed. CXCR6 was present on 4–6% of CD4 and CD8 T cells in blood, normal lymph nodes (LNs) and the spleen, primarily on memory T cells. In vitro antigen re-stimulation of LN T cells from animals with autoimmune arthritis and experimental autoimmune encephalomyelitis (EAE) increased the proportion of CXCR6+ T cells to 35–50% and anti-T-cell receptor (TCR) activation to 60–80%. In vivo, after antigen challenge of LNs there was only a small increase in CXCR6+ T cells on the lymphoblasts in the LNs, and a much higher percentage of T cells were CXCR6+ in virus-induced peritoneal exudates (∼47%) and in allergen-induced lung inflammation (33%). Chemotaxis of CXCR6-expressing inflammatory T cells to CXCL16 was poor, but that to CXCL10 was robust. We conclude that few T cells in normal and antigen-challenged LNs are CXCR6+, whereas a high proportion of in vitro activated T cells and T cells from inflammatory sites are CXCR6+, but these cells migrate poorly to CXCL16. This suggests that CXCR6 may contribute to T-cell positioning and activation, rather than recruitment. CXCR6 is also expressed on T cells not only in T helper type 1 (Th1) inflammation (arthritis and EAE) but also, as shown here, in Th2 inflammation, where it is increased after allergen challenge.
Keywords: chemokines, cell trafficking, inflammation, asthma
Introduction
Chemokines induce a plethora of biological effects including cell activation, cell adhesion, and leucocyte extravasation.1 Several chemokine receptors (CKRs), CCR2, CCR4, CCR5, CXCR3 and others, are involved in the infiltration of lymphocytes into inflamed tissues and the pathogenesis of diseases, such as rheumatoid arthritis, multiple sclerosis, asthma and hepatitis.1–3 CXCR6, originally identified as a fusion cofactor for human immunodeficiency virus (HIV)-1 and simian immunodeficiency virus (SIV) and designated Bonzo, STRL33 and TYMSTR,4–7 is a member of the seven-transmembrane domain G-protein coupled CKR family.8 In humans, CXCR6 is preferentially expressed by subsets of memory/effector T cells,9,10 natural killer (NK) cells and NK T cells,11 and is also found on plasma cells.12
The only known ligand for CXCR6 is CXCL16, which is expressed in a membrane-bound form on antigen-presenting cells, such as dendritic cells, B cells, mononuclear phagocytes, and endothelial, epithelial and smooth muscle cells.13–16 CXCL16 can be shed from macrophages by the metalloproteinase ADAM10 in vitro17 and has also been described as a scavenger receptor for phosphatidylserine and oxidized lipoprotein on macrophages.18 However, this bifunctional role is not well understood.
Although the precise biological function of CXCR6/CXCL16 is unknown, their unique character and cellular distribution suggest an important role in T-cell trafficking and cell–cell contact during inflammation. Some reports have postulated that CXCR6 might mediate T helper type 1 (Th1) diseases, because high levels of CXCR6 were observed on Th1-polarized T cells and on T cells in inflamed tissues of Th1-mediated diseases, such as rheumatoid arthritis, hepatitis C and sarcoidosis.10,19–22 However, CXCR6+ T cells might contribute to Th2-mediated disease such as asthma, in which Th2 T cells are believed to initiate and perpetuate the inflammatory response. Characteristics of this chronic lung disease are airway hyperresponsiveness, mucus metaplasia, and thickened airway walls infiltrated by a plethora of cell types, i.e. mast cells, macrophages, neutrophils, eosinophils and lymphocytes (reviewed in Cohn et al.23). CXCL16 is also expressed in the lung.13,21
The objective of our study was to generate the first monoclonal antibodies (mAbs) to rodent CXCR6 in order to determine the expression of CXCR6 on various lymphocyte subpopulations, its change in expression during an immune response, and its potential role in inflammatory disease. Finally, CXCR6 expression on infiltrating T cells in the allergen-induced inflamed lung was compared with expression of CXCR3, which is associated with Th1 cells24,25 and found in lungs of asthmatic subjects.26
Materials and methods
Animals
Inbred male Lewis rats, weighing 200–250 g, from Charles River (St Constant, Quebec, Canada) were used in all experiments except for the allergen-induced lung inflammation experiments, for which Brown Norway (BN) rats (150–200 g; Harlan Sprague-Dawley Inc., Indianapolis, IN) were used.
Cloning of CXCR6 and generation of stable CHO-CXCR6 transfectants
Total RNA was isolated from in vivo activated rat lymph node (LN) T cells and reverse-transcribed. Based on the mouse CXCR6 sequence, the coding region was amplified by polymerase chain reaction (PCR) using primers containing restriction sites: 5′ primer: 5′-ATA AGA ATG CGG CCG CGG ATG ATG GGC ATC AAG AGT CAG C-3′; 3′ primer: 5′-TGG GGT ACC CTA CTA CAA TTG GAA CAT ACT GGT G-3′. The restriction-digested PCR product was ligated into pFLAG-CMV3 (Sigma, St Louis, MO) and stably transfected into CHO-K1 cells.
Generation of anti-CXCR6 mAbs
Armenian hamsters were immunized intraperitoneally (i.p.) at least four times with 2–3 × 107 CXCR6-expressing CHO cells. Splenocytes were fused with P3U1 myeloma cells and hybridomas were screened by enzyme-linked immunosorbent assay (ELISA) on untransfected and CXCR6-transfected CHO cells. Positive hybridomas were cloned and their specificity determined by immunofluorescence staining and ELISA on CHO transfectants stably expressing various rat CKRs.
Isolation of leucocytes from various tissues
T cells were isolated from the blood, spleen and LNs as previously described.27,28 Briefly, blood T cells were obtained by Percoll (Amersham Inc., Oakville, ON) gradient centrifugation of heparinized blood and passing of the mononuclear cells through a nylon wool column. Spleen T cells were prepared from a suspension of splenocytes after red cells had been lysed and passed through a nylon wool column. For antigen-activated T lymphoblasts, animals were immunized with 107 plaque-forming units (PFU) of Western Reserve (WR) strain vaccinia virus in the footpads. T lymphoblasts were isolated using a continuous Percoll gradient from the draining LNs 4 days later. For exudate T cells, animals were injected with 5 × 107 PFU of vaccinia virus i.p. and 5 days later the peritoneal cavity was lavaged with phosphate-buffered saline (PBS). Macrophages were depleted by adhesion for 1 hr, and the non-adherent T cells were passed through a nylon wool column to obtain peritoneal exudate T cells (PE-T).
Sensitization and allergen challenge of Brown Norway (BN) rats
Brown Norway rats were immunized with 1 mg of ovalbumin (OVA; grade V, Sigma) in Alum (Imject®Alum; Pierce, Rockford, IL) subcutaneously (s.c.) in the back of the neck. Bordetella pertussis vaccine containing 1010 heat-killed bacilli was injected i.p. as an adjuvant. After 16 days, animals were challenged with aerosolized OVA or bovine serum albumin (BSA) in saline [0·5% weight/volume (w/v)] for 1 hr using an Ultrasonic Nebulizer 670 (Monaghan Co., Littleton, CO). On day 18 after sensitization, lungs were perfused with PBS and bronchoalveolar lavage (BAL) was performed using 0·1% ethylenediaminetetraacetic acid (EDTA) in saline.29 Lung T cells were isolated by mincing the lungs and passing them through an 80 mesh screen and through a nylon wool column. Leucocytes were counted on a haemocytometer using 2% crystal violet, and eosinophils were counted using 0·05% phloxine B in 50% propylene glycol.
In vitro antigen activation of T cells
Lewis rats were immunized with 16 mg of guinea pig spinal cord in an emulsion of 200 µg Mycobacterium butyricum and mineral oil into one hind footpad to induce experimental autoimmune encephalomyelitis (EAE). Eleven days later, lymphocytes were isolated from the draining LNs and cultured for 4 days, with 12 µg/ml guinea pig myelin basic protein (MBP; Sigma).30
Adjuvant arthritis was induced in Lewis rats by s.c. immunization with 1 mg of M. butyricum in mineral oil at the base of the tail. Lymph node T cells were isolated from the draining LNs and re-stimulated in vitro with 12·5 µg/ml M. butyricum for 4 days.31
To induce polyclonal T-cell activation, spleen T cells were incubated with 2 µg/ml immobilized anti-T-cell receptor (TCR) mAb (R7·3) and 200 U/ml interleukin (IL)-2 in RPMI plus 10% fetal calf serum (FCS) for 2 days followed by 200 U/ml IL-2 + 5 ng/ml IL-12 for another 2 days, and then cultured overnight without cytokines and stained.
Flow cytometry
Cells were stained at 4° with the following mouse mAbs to rat antigens: W3/25 (anti-CD4), MRC OX-8 (anti-CD8), MRC OX-22 (anti-CD45RC), MRC OX-39 (anti-CD25), R7·3 (anti-β TCR) and NKR-P1a (anti-CD161a) from Serotec (Toronto, Ont., Canada). Mouse mAbs TA-2 (anti-α4), TA-5·1 (anti-CD62L) and TA-6 (anti-β7) were previously generated in our laboratory.27,32,33 Chemokine receptors were stained with hamster anti-CXCR6 and anti-CXCR3 mAbs.34 All primary mAbs were detected with appropriate conjugated polyclonal secondary Abs. The hamster anti-mouse CD3 mAb 145·2C11 immunoglobulin G (IgG) (ATCC, Manassas, VA) was used as a hamster negative control mAb. For measurement of ligand-induced CXCR6 down-regulation, PE-T cells were incubated with chemokines at 37° for 30 min followed by immunofluorescence staining. Cells were acquired using a FACSCalibur. Representative graphs are shown in combination with mean percentages calculated from at least three independent experiments.
Measurement of T-cell chemotaxis
Chemotaxis of PE-T cells was studied using 24-well Transwell chambers (Costar Corning, Corning, NY) as previously.35 Briefly, 2 × 105 51Cr-labelled T cells were added to the upper chamber of 5-µm pore size Transwells precoated with gelatin and fibronectin. Chemokines were added to the lower chamber, and after incubation for 1 hr at 37°, the migrated T cells in the lower chamber were collected for gamma counting. In some assays, unlabelled PE-T cells were allowed to chemotax for 2 hr, microbeads were added to the bottom well as an internal standard, and the migrated cells were collected for analysis by flow cytometry. Numbers of migrated cells are expressed as a percentage of the cell subset in the starting population.36 Recombinant CXCL16 and CXCL10 were from PeproTech (Rocky Hill, NJ).
Statistical analysis
Data are expressed as the mean ± standard error of the mean (SEM) of multiple assays. Analysis of variance (ANOVA) and Student's unpaired t-test were used for statistical analysis and P < 0·05 was considered significant.
Results
Cloning of rat CXCR6 and generation of CXCR6-specific mAbs
The rat cDNA was cloned from the spleen using reverse transcription–polyermase chain reaction (RT-PCR). The open reading frame (ORF) of rat CXCR6 (rCXCR6) located on chromosome 8 has been submitted to GenBank with the accession number DQ132631. The ORF is 1053 nucleotides and encodes a polypeptide of 351 amino acids. Per cent identities among rat, murine and human CXCR6 were: rat versus human, 74%; rat versus murine, 92% based on the nucleotide sequence; rat versus human, 71%; and rat versus murine, 87% based on the predicted protein sequence. The protein appears to be structurally identical to the human and murine analogues.
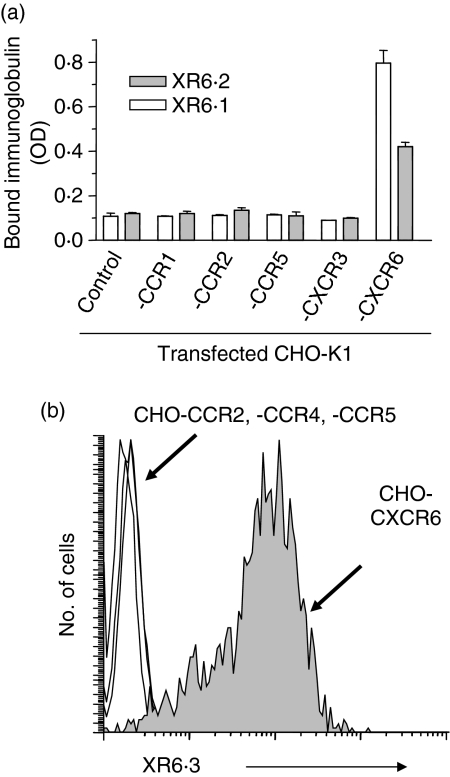
Monoclonal antibodies to rat CXCR6 were generated by immunizing hamsters with CXCR6-transfected CHO-K1 cells. Three mAbs, XR6·1, XR6·2 and XR6·3, reacting specifically with CXCR6 were identified. As shown in Fig. 1, only CHO cells that expressed CXCR6, but not other CKRs (CCR1, CCR2, CCR4, CCR5 or CXCR3), bound the mAbs, as determined by ELISA and immunofluorescence staining. Interestingly, the binding of all three mAbs, which were against different epitopes of CXCR6, was temperature sensitive and greatly reduced below 10° (data not shown), presumably as a result of conformational changes in the receptor at lower temperatures.
Figure 1.
Specificity of anti-CXCR6 monoclonal antibodies (mAbs). The binding of the XR6·1 and XR6·2 mAbs to CHO-K1 cells stably transfected with the indicated chemokine receptors or to untransfected cells was tested in a cell-based enzyme-linked immunosorbent assay (ELISA) in which bound Abs were detected with horseradish peroxidase-conjugated goat anti-hamster antibody and the optical density (OD) determined (a). Each bar indicates the mean ± standard deviation (n = 4) of one representative assay of three. (b) Immunofluorescence staining of CHO transfectants expressing the indicated chemokine receptors (CKRs) using mAb XR6·3. One representative staining of five is shown. Similar results were obtained with mAbs XR6·1 and XR6·2.
CXCR6 on T cells from normal lymphoid tissues and antigen-challenged LNs
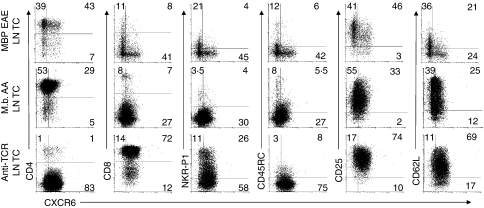
CXCR6 was present on a small subpopulation of blood, spleen and LN T cells (Fig. 2). Approximately 3–4% of CD4+ and 3–6% of CD8+ T cells, 7–10% of NK T cells and 17–22% of NK cells (Table 1), but not other leucocytes (data not shown), expressed CXCR6. Eighty to ninety per cent of the CXCR6+ T cells in these tissues were CD45RC– memory T cells. The mean intensity of staining on CXCR6+ was relatively similar among all the cells.
Figure 2.
CXCR6 expression on lymphocytes from normal lymphoid tissues and in vivo antigen-activated lymph nodes (LNs). CXCR6 expression was determined by immunofluorescence staining of T-cell receptor (TCR)-αβ-gated lymphocytes from blood mononuclear cells, splenocytes, normal LNs, and lymphoblasts from antigen-challenged LNs. Representative dot plots of anti-CXCR6 and isotype control mAb staining in each tissue for six to eight experiments are shown. The numbers indicate the mean percentage of cells in each quadrant obtained from all the experiments.
Table 1.
Expression of CXCR6 on normal rat blood, lymph node (LN) and spleen cells, and on in vivo antigen-challenged LN lymphoblasts
| Blood lymphocytes | LN lymphocytes | Spleen lymphocytes | LN lymphoblasts | |||||
|---|---|---|---|---|---|---|---|---|
| % positive | MFI | % positive | MFI | % positive | MFI | % positive | MFI | |
| CD4+ | 3·8 ± 0·7 | 11·2 ± 2·6 | 2·8 ± 0·7 | 14·3 ± 3·0 | 3·8 ± 0·6 | 12·0 ± 0·9 | 6·7 ± 1·3* | 9·1 ± 0·6 |
| CD8+ | 4·4 ± 1·0 | 7·2 ± 0·7 | 3·1 ± 1·0 | 9·9 ± 1·4 | 6·3 ± 1·4 | 10·2 ± 0·7 | 10·0 ± 3·1* | 8·2 ± 0·5 |
| CD45RC+ | 0·9 ± 0·3 | 8·9 ± 2·1 | 0·9 ± 0·2 | 11·8 ± 1·4 | 1·6 ± 0·5 | 10·9 ± 0·5 | 1·2 ± 0·6 | 15·3 ± 2·8 |
| CD45RC– | 8·2 ± 1·8 | 9·7 ± 0·8 | 5·1 ± 1·2 | 17·3 ± 1·4 | 8·0 ± 1·9 | 12·8 ± 0·9 | 9·5 ± 0·7** | 12·0 ± 0·9 |
| CD25+ | 12·3 ± 2·6 | 9·8 ± 0·7 | 12·3 ± 2·0 | 20·2 ± 3·4 | 15·2 ± 1·8 | 12·7 ± 1·1 | 21·3 ± 4·3* | 10·4 ± 0·6 |
| NK T cells | 7·3 ± 1·9 | 8·8 ± 1·1 | 10·2 ± 3·2 | 12·7 ± 3·5 | 9·8 ± 3·1 | 11·1 ± 0·7 | 14·1 ± 3·2 | 9·9 ± 0·7 |
| NK cells | 17·1 ± 3·5 | 15·3 ± 3·1 | 22·3 ± 4·3 | 14·8 ± 4·2 | 20·3 ± 5·3 | 11·5 ± 0·6 | 31·6 ± 5·0 | 10·1 ± 0·7 |
| CD62L+ | 2·2 ± 0·8 | 7·7 ± 1·0 | 1·9 ± 0·6 | 13·8 ± 1·7 | 1·7 ± 0·3 | 11·4 ± 0·7 | 8·5 ± 2·6* | 9·2 ± 0·7 |
| CD62L– | 6·5 ± 1·8 | 9·2 ± 0·9 | 4·0 ± 1·3 | 16·7 ± 2·9 | 4·9 ± 1·1 | 11·8 ± 0·9 | 11·3 ± 3·4* | 10·0 ± 0·5 |
Rat blood, LN and spleen cells were isolated and stained as outlined in the Materials and methods section. LN lymphoblasts were obtained from draining LNs of animals immunized 4 days previously in the footpads with vaccinia virus. Each entry shows the mean ± standard error of the mean (n = 6–8).
P < 0·05
P < 0·01, compared to normal LN T cells.
MFI, mean fluorescence intensity.
After s.c. immunization with vaccinia virus, the proportion of CXCR6+ activated T cells isolated from the regional LNs was about twice that of normal resting LN T cells, i.e. 7% of CD4+, 10% of CD8+, and 14% of NK T cells were CXCR6+.
Up-regulation of CXCR6 upon antigen-induced T-cell activation
CXCR6 on human lymphocytes is up-regulated upon activation and cytokine stimulation.37,38 Therefore, CXCR6 expression was determined on T cells activated in vitro by re-stimulation with antigen (MBP or M. butyricum) or with anti-TCR mAb plus IL-2 and IL-12 (Fig. 3 and Table 2). There was a marked increase in CXCR6+ T cells among the T-cell subsets examined. CXCR6 was expressed on 35–52% of LN T cells isolated from animals with EAE or M. butyricum-induced adjuvant arthritis and re-stimulated with antigen in vitro. On MBP-stimulated lymphocytes, more CD4+ (52%) and CD8+ (43%) T cells than NK T cells (16%) were CXCR6+ (P < 0·05). M. butyricum-activated T cells had a higher proportion of CXCR6+ NK T cells (55%), and similar proportions of CD4+ and CD8+ cells. Anti-TCR plus IL-2 activation produced mainly CD8+ CD45RC– T cells, and resulted in the highest percentage of CXCR6+ cells, with 61% of CD4+, 84% of CD8+ and 70% of NK T cells expressing CXCR6. The median fluorescence intensities of all of these T-cell subsets were equivalent (Table 2).
Figure 3.
CXCR6 expression on antigen- and anti-T-cell receptor (TCR)-activated lymphocytes. Expression of CXCR6 on T cells from lymph node (LN) lymphocytes from sensitized animals with experimental autoimmune encephalomyelitis (EAE) stimulated in vitro with myelin basic protein (MBP), animals with adjuvant arthritis (AA) stimulated with Mycobacterium butyricum (M.b), and normal animals stimulated by TCR cross-linking was determined. Representative TCR-αβ-gated events are shown in combination with the mean percentage in each quadrant obtained from four (anti-TCR) and eight (antigen) activation experiments.
Table 2.
CXCR6 expression on in vitro anti-T-cell receptor (TCR)-activated and antigen-activated lymph node (LN) cells and on in vivo antigen-induced exudate cells
| MBP-activated LN cells | M. butyricum-activated LN cells | Anti-TCR activated T cells | Peritoneal exudate cells | |||||
|---|---|---|---|---|---|---|---|---|
| % positive | MFI | % positive | MFI | % positive | MFI | % positive | MFI | |
| CD4+ | 52·1 ± 7·9*** | 18·6 ± 2·9 | 35·2 ± 7·1** | 10·5 ± 1·3 | 60·7 ± 11·9* | 13·0 ± 3·2 | 52·3 ± 2·5*** | 15·8 ± 1·0 |
| CD8+ | 43·2 ± 11·4* | 18·2 ± 2·0 | 46·6 ± 10·5** | 15·0 ± 3·1 | 83·5 ± 4·7*** | 12·0 ± 2·0 | 42·5 ± 3·0*** | 17·5 ± 1·3 |
| CD45RC+ | 34·4 ± 8·6*** | 24·0 ± 4·1 | 43·0 ± 9·1*** | 15·2 ± 2·4 | 74·2 ± 2·4** | 12·2 ± 0·2 | 31·5 ± 4·6*** | 14·1 ± 1·0 |
| CD45RC– | 50·6 ± 7·9*** | 18·7 ± 3·1 | 31·7 ± 6·1** | 9·2 ± 0·7 | 84·4 ± 1·8** | 13·4 ± 0·3 | 63·5 ± 2·8*** | 18·5 ± 1·7 |
| CD25+ | 52·8 ± 9·1** | 18·7 ± 3·4 | 48·4 ± 10·9* | 9·3 ± 0·6 | 81·6 ± 1·2*** | 12·9 ± 0·5 | 56·6 ± 4·3*** | 18·0 ± 1·3 |
| NK T cells | 15·7 ± 2·5 | 13·5 ± 3·0 | 55·0 ± 11·6**1 | 9·7 ± 0·7 | 69·7 ± 7·7** | 11·8 ± 1·8 | 37·4 ± 3·2** | 16·0 ± 1·2 |
| NK cells | ND | ND | ND | ND | ND | ND | 76·3 ± 13·7* | 15·2 ± 2·7 |
| CD62L+ | 36·7 ± 6·8** | 26·2 ± 9·2 | 39·2 ± 9·3** | 13·1 ± 2·0 | 85·7 ± 1·8*** | 13·9 ± 0·5 | 49·8 ± 3·9*** | 16·9 ± 1·0 |
| CD62L– | 56·6 ± 8·6*** | 27·5 ± 9·2 | 33·6 ± 7·2** | 12·9 ± 1·8 | 84·2 ± 1·8*** | 13·4 ± 1·4 | 74·4 ± 3·5*** | 21·2 ± 1·2 |
Activated T cells were generated by anti-TCR activation of spleen T cells, and MBP or M. butyricum antigen-activated LN cells from animals with experimental autoimmune encephalomyelitis or adjuvant arthritis, respectively, as outlined in the Materials and methods section. Peritoneal exudate T cells were obtained 5 days after injection of vaccinia virus intraperitoneally. Each entry shows the mean ± standard error of the mean (n = 5–9).
P < 0·05
P < 0·01
P < 0·001 compared with in vivo antigen-challenged LN T lymphoblasts shown inTable 1.
P < 0·01 compared with NK T cells from MBP-activated T cells.
M. butyricum, Mycobacterium butyricum; MBP, myelin basic protein; MFI, mean fluorescence intensity; ND, not determined; NK, natural killer.
CD45RC, CD25 and CD62L (l-selectin) expression was also analysed (Fig. 3). Whereas most of the CXCR6+ cells coexpressed CD25 and lacked CD45RC, the relationship to l-selectin was more heterogeneous: 50% of MBP-activated T cells lacked l-selectin, including the majority of the CXCR6+ subset; anti-TCR-activated T cells were mainly positive for l-selectin, including CXCR6+ cells; and M butyricum-activated cells were more variable but CXCR6 was present on both CD62L+ and CD62L– subsets.
CXCR6 expression and function on T cells from the inflamed peritoneum
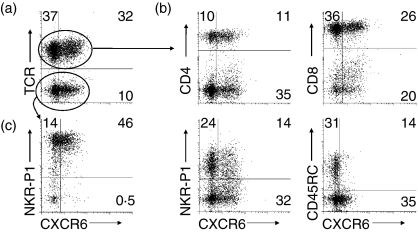
Vaccinia virus injected i.p. induced extensive leucocyte infiltration into the peritoneal cavity consisting of macrophages, NK cells and T cells, of which 21 ± 3% were CD4+, 62 ± 2% were CD8+, and 38 ± 3% were NK T cells. CXCR6 was expressed on 37–52% of the CD4+, CD8+ and NK T cells, and on 76% of NK cells at this inflammatory site (Fig. 4 and Table 2). T cells in the exudates are enriched in cells, which preferentially migrate to inflammation. Exudate T cells showed strong CXCR6 expression.
Figure 4.
CXCR6 expression on lymphocytes from the inflamed peritoneum. (a) CXCR6 expression was determined on lymphocytes from vaccinia-induced peritoneal inflammatory exudates. (b) Expression was determined on various T-cell receptor (TCR)-αβ+ subpopulations and (c) on TCR-αβ– natural killer (NK) cells. Representative graphs are shown in combination with the mean percentage in each quadrant obtained from nine experiments.
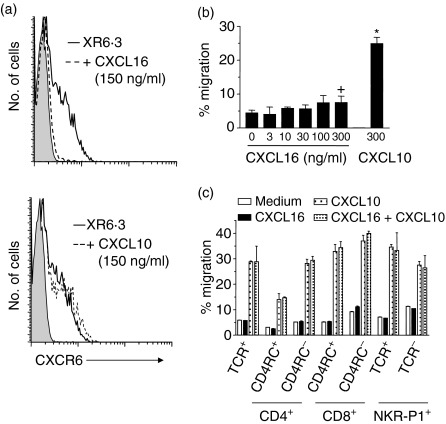
The functional activity of CXCR6 on PE T cells was examined using CXCL16-induced receptor internalization. Incubation with CXCL16 decreased CXCR6 cell surface expression from 47 to 7% on PE T cells (Fig. 5a), while incubation with CXCL10 had no effect on CXCR6, but down-regulated CXCR3 expression (data not shown). Despite their strong CXCR6 expression, chemotaxis of PE T cells to CXCL16 was remarkably poor (Fig. 5b). Only a small increase in PE T cell migration, from 4·4 to 7·5% of input cells, was observed at the highest concentration tested. Chemotaxis to the CXCR3 ligand, CXCL10, was much stronger, with 24·9% of cells migrating to CXCL10. To further analyse the subsets of PE T cells migrating to these chemokines, migrated cells were stained after chemotaxis. Only CD4+ CD45RC– and CD8+ CD45RC– T cells migrated very poorly to CXCL16 (Fig. 5c). In contrast, 30–40% of T cells migrated to CXCL10, mainly NK cells, NK T cells, CD4+ CD45RC– (memory) T cells and CD8+ CD45RChigh (effector) and CD45RC− (memory) T cells. The combination of the two ligands CXCL16 and CXCL10 showed neither a synergism in inducing chemotaxis of PE T cells nor an inhibition by CXCL16 of CXCL10-mediated chemotaxis. In conclusion, inflammatory PE T cells chemotaxed poorly to CXCL16, especially in comparison to CXCL10, despite their strong CXCR6 expression.
Figure 5.
Down-regulation of CXCR6 and chemotaxis of PE T cells. (a) PE T cells were preincubated with soluble CXCL16 (150 ng/ml) or CXCL10 (150 ng/ml) and stained with XR6·3 or control antibody (shadowed graph). (b) Inflammatory exudate T cells were radiolabelled and chemotaxis to CXCL16 and CXCL10 at the indicated concentrations was determined. (c) Lymphocytes were allowed to migrate to 300 ng/ml of the indicated chemokines, and the input and migrated cells were then subjected to immunofluorescence staining. Migration is expressed as a percentage of the input T cells. Each bar shows the mean ± standard deviation of three experiments. +P < 0·05; *P < 0·001 versus medium control. Random migration with the chemokine in the upper and lower chambers was the same as that in the absence of chemokine.
CXCR6 expression on T cells from the lung with allergen-induced inflammation
CXCR6 is reported to be expressed preferentially on Th1 T cells.10,21,39 Therefore, CXCR6+ T cells might represent a negative regulator in Th2-mediated disease. To assess this hypothesis, CXCR6 expression was determined on T cells from the lungs after allergen-induced inflammation, a classical Th2 disease. Compared with untreated animals, allergen challenge in sensitized animals led to an enormous increase in all leucocyte populations in the lung parenchyma and bronchial alveolar lavage (BAL). The number of leucocytes recovered from the lungs increased by 6-fold, including eosinophils (10-fold), neutrophils (5-fold) and T lymphocytes (> 10-fold).
As shown in Fig. 6(and Table 3), CXCR6 was expressed on 37% of CD4+ lung T cells, on 32% of CD8+ cells, on 31% of NK T cells, and on 34% of NK cells. Few T cells from normal or control challenged lungs expressed CXCR6 (3–7%; data not shown). Compared with the lymphocytes found within the lung, those isolated from BAL fluids expressed less CXCR6. CXCR6 was present on 18% of CD4+ cells, 19% of CD8+ cells, 9·8% of NK T cells and 7·6% of NK cells (data not shown). CXCR6 expression in other tissues such as blood, spleen and LNs showed no difference from that of non-challenged animals, i.e. 1–3% on average (data not shown).
Figure 6.
CXCR6 and CXCR3 expression on infiltrating lymphocytes in allergen-induced lung inflammation. CXCR6 (a) and CXCR3 (b) expression was determined on lymphocytes isolated from ovalbumin-challenged lungs. Representative graphs are shown in combination with the mean percentage in each quadrant obtained from six (CXCR6) and five (CXCR3) experiments.
Table 3.
CXCR6 and CXCR3 expression on lung lymphocytes from allergen-challenged animals
| CXCR6 | CXCR3 | |||
|---|---|---|---|---|
| % positive | MFI | % positive | MFI | |
| CD4+ | 36·9 ± 6·3* | 13·0 ± 2·3 | 10·2 ± 1·8 | 10·0 ± 0·2 |
| CD8+ | 31·7 ± 2·5** | 10·3 ± 1·3 | 11·9 ± 1·5 | 9·2 ± 0·1 |
| NK T cells | 31·2 ± 11·2 | 16·5 ± 3·7 | 40·0 ± 7·5 | 9·9 ± 0·4 |
| NK cells | 33·8 ± 3·7** | 16·4 ± 3·0 | 3·5 ± 1·3 | 7·8 ± 0·3 |
Lung cells were obtained from antigen-challenged Brown Norway rats as indicated in the Materials and methods section and stained by immunofluorescence for CXCR6 and CXCR3 expression. Each entry shows the mean ± standard error of the mean (n = 5–6).
P < 0·01
P < 0·001, compared with CXCR3 expression on lung cells.
Adhesion molecules such as α4 integrins and l-selectin have been shown to play an important role in allergic lung inflammation. Blocking α4 integrins decreased the early and late airway response,40,41 and blocking l-selectin inhibited allergen-induced airway hyper-responsiveness.42 The expression of α4 and β7integrins and CD62L on CXCR6+ T cells from the inflamed lungs was determined. Ninety-five per cent of CD4+ and 83% of CD8+ CXCR6+ T cells expressed α4, whereas β7 integrins were found on 36–66% of CD4+ and 20–40% of CD8+ CXCR6+ T cells. More than 90% of the T cells within the lung were CD62L– (data not shown).
CXCR3 expression on lung T cells was also determined for comparison to expression of CXCR6 and as a correlate of Th1 cells. CXCR3 was expressed on 10% of CD4+ T cells, 12% of CD8+ T cells, 40% of NK T cells and 3·5% of NK cells in the lung after allergen challenge (Fig. 6). Thus, the proportion of CD4+, CD8+ and NK cells expressing CXCR3 was significantly less than those expressing CXCR6.
Discussion
Our study describes (1) the first cloning of the cDNA for rat CXCR6; (2) the first mAbs to CXCR6 in rats, the expression of which in mice has until recently only been evaluated by using green fluorescent protein (GFP) reporter constructs or tagged ligand;9,13 (3) for the first time in detail, CXCR6 expression on subsets of rodent lymphocytes from various tissues and the effect of antigen challenge on CXCR6 expression in vivo; (4) the striking difference in CXCR6 expression on in vivo activated T cells compared with CXCR3 and on T cells activated in vitro by recall antigen or by TCR cross-linking, and (5) the greatly increased expression of CXCR6 on T cells in not only a viral, Th1 reaction, but for the first time also in an allergic Th2 inflammatory reaction. Our results also suggest that, although CXCR6 on T cells from an inflammatory site is functional, it is only weakly chemotactic and may be more important in activation or retention of T cells at the site.
CXCR6 was expressed on 3–6% of CD4+ and CD8+ cells in normal lymphoid tissues and blood, mainly on memory T cells. This is similar to the expression observed in humans,9 but substantially lower than that reported with the GFP reporter in mice, where CD8 T cells were uniformly GFP positive. Resting human NK T cells express a low level of CXCR6, in contrast to mice, where NK T cells based on a GFP reporter were mainly CXCR6+.43 In the rat, < 10% of NK T cells in the blood expressed CXCR6, but many NK T cells in the inflammatory sites were CXCR6+ (Figs 3 and 4; Table 2). These differences may be related to the species or to the different methods of detecting receptor expression.
After antigen challenge in vivo, the proportion of CD4+ and CD8+ LN T lymphoblasts expressing CXCR6 was 3-fold higher than that of normal LN T cells. This is much less than the 5-fold increase in CXCR3 on these activated T cells, especially on CD4 cells, which increase CXCR3 > 10-fold (Fig. 2 in ref. 34) on these cells.34 As activated T cells from antigen-challenged LNs migrate to sites of inflammation, the low proportion of CXCR6+-activated T cells that are CXCR6+ suggests that CXCR6 on the antigen-challenged LN T lymphoblasts is unlikely to mediate the migration of these cells to inflamed tissues. The migration of these activated T cells has been shown to be dependent on CXCR3.34,44
Lymphocytes from inflammatory peritoneal exudates induced with vaccinia virus, a predominantly Th1 response in the Th1-biased Lewis strain, chemotaxed very poorly to CXCL16, despite strong chemotaxis to CXCL10 and rapid receptor internalization by CXCL16. This also suggests that CXCR6 is unlikely to be required for migration of these T cells to this inflammatory site. This weak chemotactic response agrees with previous findings on resting T cells in mice,43 but also extends these to in vivo activated T cells from LNs (data not shown) and T cells from an inflammatory site, which are enriched in CXCR6+ lymphocytes (Fig. 5).
Recent studies have suggested that CXCR6 may have a special role in the migration of activated CD8 T cells into the liver in a graft-versus-host disease (GVHD) model of hepatic inflammation,45 and CXCR6+ T cells were specifically found associated with CXCL16-producing cholangiocytes in bile ducts of patients with liver disease.19 In addition, CXCR6 can mediate cell–cell interactions between T cells and antigen-presenting dendritic cells and stromal cells within lymphoid tissues,37,46 suggesting a primary role for CXCR6 in cell activation rather than migration.37 Therefore, we favour a model in which the ‘early’ up-regulated CKRs, such as CXCR3 (on Th1 cells), CCR4 (on Th2 cells) and others, promote migration of activated T cells to sites of inflammation, and at the site other CKRs, such as CXCR6, facilitate transmigration, positioning and further activation of the T cells within the tissue.
The highest in vivo expression of CXCR6 was in the inflamed peritoneal cavity in response to vaccinia virus, which induces a strong Th1 response. This is in agreement with reports that CXCR6 is associated with Th1 and T cytotoxic type 1 (Tc1) subsets in typical Th1 autoimmune diseases, such as rheumatoid arthritis, hepatitis, sarcoidosis and allograft rejection.10,19,47 However, Th2 cells can express CXCR6 in vitro, although to a lesser extent than Th1 cells.14,38 Our studies have shown that CXCR6 is also present in a Th2-mediated inflammatory disease, namely on lung-infiltrating T cells from allergen-challenged Th2-biased animals (Fig. 6). In asthmatics, in one study CCR4 and CCR7 expression was found to be low on T cells in BAL, while CXCR3 was expressed at a high level,26 and in another study after allergen challenge CCR4 and CCR7 were found to increase and CXCR3 to decline.48 CXCR6 recently was also found to be present on T cells in the BAL of asthmatics.22 In our studies using allergen-challenged animals, CXCR6 was present on about 30% of CD4+, CD8+ and NK T cells and NK cells, while CXCR3, a strong Th1-associated CKR, was present in the lung at much lower frequencies on CD4+ and CD8+ T cells and NK cells, but at higher frequencies on NK T cells. Thus, after allergen challenge, CXCR3 appears to decline in both human asthma and in the allergen-challenged lung in the Brown Norway rat, and this is associated with an increase in CXCR6 in this model and potentially in asthma.48 Furthermore, CXCR6 is expressed on lymphocytes infiltrating this Th2 disease, although CXCR6 has typically been associated with Th1 cells and is low on Th2-polarized cell lines.10,38 This suggests that in vivo, at least in some types of Th2 disease, CXCR6 may be an important inflammatory mediator. The expression of CXCR6 on lung T cells, the weak chemotactic effect of CXCR6, especially compared with CXCR3, and the presence of CXCL16 in the lung suggest that CXCR6 may have a particularly important role in stimulating or maintaining T-cell activation in this tissue, similar to the distinctive role it appears to have in inflammation of the liver, a tissue where CXCL16 is also normally found. The findings in this animal model of asthma also suggest that blockade of CXCR6 may be useful in the control of lung inflammation even after lymphocyte recruitment has occurred, by inhibiting a downstream process for continued inflammation.
Acknowledgments
The authors wish to thank the Canadian Institutes of Health Research for their support through grants MOP-42379 and MOP-57709 for these studies. We also would like to thank Dr Qingli Cheng, Ms Elizabeth Sexton, Ms Sarah Roberts, Karen Conrad and Mrs Maria Vaci for their expert technical assistance.
Glossary
Abbreviations:
- BAL
bronchial alveolar lavage
- BN
Brown Norway
- CKR
chemokine receptor
- EAE
experimental autoimmune encephalomyelitis
- LN
lymph node
- MBP
myelin basic protein
- NK
natural killer
- OVA
ovalbumin
- PE-T
peritoneal T-cells
References
- 1.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 2.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–8. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 3.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–R28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 4.Alkhatib G, Liao F, Berger EA, Farber JM, Peden KW. A new SIV co-receptor, STRL33. Nature. 1997;388:238. doi: 10.1038/40789. [DOI] [PubMed] [Google Scholar]
- 5.Deng HK, Unutmaz D, Kewa I, Ramani VN, Littman DR. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 6.Liao F, Alkhatib G, Peden KW, Sharma G, Berger EA, Farber JM. STRL33, a novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–23. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loetscher M, Amara A, Oberlin E, et al. TYMSTR, a putative chemokine receptor selectively expressed in activated T cells, exhibits HIV-1 coreceptor function. Curr Biol. 1997;7:652–60. doi: 10.1016/s0960-9822(06)00292-2. [DOI] [PubMed] [Google Scholar]
- 8.Murphy PM. International Union of Pharmacology. XXX. Update on chemokine receptor nomenclature. Pharmacol Rev. 2002;54:227–9. doi: 10.1124/pr.54.2.227. [DOI] [PubMed] [Google Scholar]
- 9.Unutmaz D, Xiang W, Sunshine MJ, Campbell J, Butcher E, Littman DR. The primate lentiviral receptor Bonzo/STRL33 is coordinately regulated with CCR5 and its expression pattern is conserved between human and mouse. J Immunol. 2000;165:3284–92. doi: 10.4049/jimmunol.165.6.3284. [DOI] [PubMed] [Google Scholar]
- 10.Kim CH, Kunkel EJ, Boisvert J, Johnston B, Campbell JJ, Genovese MC, Greenberg HB, Butcher EC. Bonzo/CXCR6 expression defines type 1-polarized T-cell subsets with extralymphoid tissue homing potential. J Clin Invest. 2001;107:595–601. doi: 10.1172/JCI11902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim CH, Johnston B, Butcher EC. Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among V alpha 24(+) V beta 11(+) NKT cell subsets with distinct cytokine-producing capacity. Blood. 2002;100:11–6. doi: 10.1182/blood-2001-12-0196. [DOI] [PubMed] [Google Scholar]
- 12.Nakayama T, Hieshima K, Izawa D, Tatsumi Y, Kanamaru A, Yoshie O. Cutting edge: profile of chemokine receptor expression on human plasma cells accounts for their efficient recruitment to target tissues. J Immunol. 2003;170:1136–40. doi: 10.4049/jimmunol.170.3.1136. [DOI] [PubMed] [Google Scholar]
- 13.Matloubian M, David A, Engel S, Ryan JE, Cyster JG. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat Immunol. 2000;1:298–304. doi: 10.1038/79738. [DOI] [PubMed] [Google Scholar]
- 14.Wilbanks A, Zondlo SC, Murphy K, et al. Expression cloning of the STRL33/BONZO/TYMSTRligand reveals elements of CC, CXC, and CX3C chemokines. J Immunol. 2001;166:5145–54. doi: 10.4049/jimmunol.166.8.5145. [DOI] [PubMed] [Google Scholar]
- 15.Hofnagel O, Luechtenborg B, Plenz G, Robenek H. Expression of the novel scavenger receptor SR-PSOX in cultured aortic smooth muscle cells and umbilical endothelial cells. Arterioscler Thromb Vasc Biol. 2002;22:710–1. doi: 10.1161/01.atv.0000012402.85056.45. [DOI] [PubMed] [Google Scholar]
- 16.Tabata S, Kadowaki N, Kitawaki T, Shimaoka T, Yonehara S, Yoshie O, Uchiyama T. Distribution and kinetics of SR-PSOX/CXCL16 and CXCR6 expression on human dendritic cell subsets and CD4+ T cells. J Leukoc Biol. 2005;77:777–86. doi: 10.1189/jlb.1204733. [DOI] [PubMed] [Google Scholar]
- 17.Gough PJ, Garton KJ, Wille PT, Rychlewski M, Dempsey PJ, Raines EW. A disintegrin and metalloproteinase 10-mediated cleavage and shedding regulates the cell surface expression of CXC chemokine ligand 16. J Immunol. 2004;172:3678–85. doi: 10.4049/jimmunol.172.6.3678. [DOI] [PubMed] [Google Scholar]
- 18.Minami M, Kume N, Shimaoka T, et al. Expression of SR-PSOX, a novel cell-surface scavenger receptor for phosphatidylserine and oxidized LDL in human atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2001;21:1796–800. doi: 10.1161/hq1001.096652. [DOI] [PubMed] [Google Scholar]
- 19.Heydtmann M, Lalor PF, Eksteen JA, Hubscher SG, Briskin M, Adams DH. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. J Immunol. 2005;174:1055–62. doi: 10.4049/jimmunol.174.2.1055. [DOI] [PubMed] [Google Scholar]
- 20.Boisvert J, Kunkel EJ, Campbell JJ, Keeffe EB, Butcher EC, Greenberg HB. Liver-infiltrating lymphocytes in end-stage hepatitis C virus: subsets, activation status, and chemokine receptor phenotypes. J Hepatol. 2003;38:67–75. doi: 10.1016/s0168-8278(02)00328-8. [DOI] [PubMed] [Google Scholar]
- 21.Agostini C, Cabrelle A, Calabrese F, et al. Role for CXCR6 and its ligand CXCL16 in the pathogenesis of T-Cell alveolitis in sarcoidosis. Am J Respir Crit Care Med. 2005;172:1290–8. doi: 10.1164/rccm.200501-142OC. [DOI] [PubMed] [Google Scholar]
- 22.Morgan AJ, Guillen C, Symon FA, et al. Expression of CXCR6 and its ligand CXCL16 in the lung in health and disease. Clin Exp Allergy. 2005;35:1572–80. doi: 10.1111/j.1365-2222.2005.02383.x. [DOI] [PubMed] [Google Scholar]
- 23.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 24.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–34. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim CH, Rott L, Kunkel EJ, Genovese MC, Andrew DP, Wu L, Butcher EC. Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest. 2001;108:1331–9. doi: 10.1172/JCI13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell JJ, Brightling CE, Symon FA, et al. Expression of chemokine receptors by lung T cells from normal and asthmatic subjects. J Immunol. 2001;166:2842–8. doi: 10.4049/jimmunol.166.4.2842. [DOI] [PubMed] [Google Scholar]
- 27.Issekutz TB. Inhibition of in vivo lymphocyte migration to inflammation and homing to lymphoid tissues by the TA-2 monoclonal antibody. A likely role for VLA-4 in vivo. J Immunol. 1991;147:4178–84. [PubMed] [Google Scholar]
- 28.Issekutz TB. Inhibition of lymphocyte endothelial adhesion and in vivo lymphocyte migration to cutaneous inflammation by TA-3, a new monoclonal antibody to rat LFA-1. J Immunol. 1992;149:3394–402. [PubMed] [Google Scholar]
- 29.Schneider T, Issekutz TB, Issekutz AC. The role of alpha4 (CD49d) and beta2 (CD18) integrins in eosinophil and neutrophil migration to allergic lung inflammation in the Brown Norway rat. Am J Respir Cell Mol Biol. 1999;20:448–57. doi: 10.1165/ajrcmb.20.3.3207. [DOI] [PubMed] [Google Scholar]
- 30.Paterson PY. Transfer of allergic encephalomyelitis in rats by means of lymph node cells. J Exp Med. 1960;111:119–36. doi: 10.1084/jem.111.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Issekutz AC, Nakazato S, Issekutz TB. Differential roles of VLA-4 (CD49d/CD29) and LFA-1 (CD11a/CD18) integrins and E- and P-selectin during developing and established active or adoptively transferred adjuvant arthritis in the rat. Immunol Cell Biol. 2003;81:397–408. doi: 10.1046/j.1440-1711.2003.01187.x. [DOI] [PubMed] [Google Scholar]
- 32.Issekutz TB, Wykretowicz A. Effect of a new monoclonal antibody, TA-2, that inhibits lymphocyte adherence to cytokine stimulated endothelium in the rat. J Immunol. 1991;147:109–16. [PubMed] [Google Scholar]
- 33.Palecanda A, Marshall JS, Li X, Briskin MJ, Issekutz TB. Selective antibody blockade of lymphocyte migration to mucosal sites and mast cell adhesion. J Leukoc Biol. 1999;65:649–57. doi: 10.1002/jlb.65.5.649. [DOI] [PubMed] [Google Scholar]
- 34.Mohan K, Cordeiro E, Vaci M, McMaster C, Issekutz TB. CXCR3 is required for migration to dermal inflammation by normal and in vivo activated T cells. differential requirements by CD4 and CD8 memory subsets. Eur J Immunol. 2005;35:1702–11. doi: 10.1002/eji.200425885. [DOI] [PubMed] [Google Scholar]
- 35.Mohan K, Ding Z, Hanly J, Issekutz TB. IFN-gamma-inducible T cell alpha chemoattractant is a potent stimulator of normal human blood T lymphocyte transendothelial migration. differential regulation by IFN-gamma and TNF-alpha. J Immunol. 2002;168:6420–8. doi: 10.4049/jimmunol.168.12.6420. [DOI] [PubMed] [Google Scholar]
- 36.Campbell JJ, Bowman EP, Murphy K, et al. 6-C-kine (SLC), a lymphocyte adhesion-triggering chemokine expressed by high endothelium, is an agonist for the MIP-3beta receptor CCR7. J Cell Biol. 1998;141:1053–9. doi: 10.1083/jcb.141.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim CH, Nagata K, Butcher EC. Dendritic cells support sequential reprogramming of chemoattractant receptor profiles during naive to effector T cell differentiation. J Immunol. 2003;171:152–8. doi: 10.4049/jimmunol.171.1.152. [DOI] [PubMed] [Google Scholar]
- 38.Langenkamp A, Nagata K, Murphy K, Wu L, Lanzavecchia A, Sallusto F. Kinetics and expression patterns of chemokine receptors in human CD4+ T lymphocytes primed by myeloid or plasmacytoid dendritic cells. Eur J Immunol. 2003;33:474–82. doi: 10.1002/immu.200310023. [DOI] [PubMed] [Google Scholar]
- 39.Calabresi PA, Yun SH, Allie R, Whartenby KA. Chemokine receptor expression on MBP-reactive T cells. CXCR6 is a marker of IFNgamma-producing effector cells. J Neuroimmunol. 2002;127:96–105. doi: 10.1016/s0165-5728(02)00106-6. [DOI] [PubMed] [Google Scholar]
- 40.Rabb HA, Olivenstein R, Issekutz TB, Renzi PM, Martin JG. The role of the leukocyte adhesion molecules VLA-4, LFA-1, and Mac-1 in allergic airway responses in the rat. Am J Respir Crit Care Med. 1994;149:1186–91. doi: 10.1164/ajrccm.149.5.8173758. [DOI] [PubMed] [Google Scholar]
- 41.Ramos-Barbon D, Suzuki M, Taha R, Molet S, Issekutz TB, Hamid Q, Martin JG. Effect of alpha4-integrin blockade on CD4+ cell-driven late airway responses in the rat. Am J Respir Crit Care Med. 2001;163:101–8. doi: 10.1164/ajrccm.163.1.2001093. [DOI] [PubMed] [Google Scholar]
- 42.Abraham WM, Ahmed A, Sabater JR, et al. Selectin blockade prevents antigen-induced late bronchial responses and airway hyperresponsiveness in allergic sheep. Am J Respir Crit Care Med. 1999;159:1205–14. doi: 10.1164/ajrccm.159.4.9806002. [DOI] [PubMed] [Google Scholar]
- 43.Johnston B, Kim CH, Soler D, Emoto M, Butcher EC. Differential chemokine responses and homing patterns of murine TCR alpha beta NKT cell subsets. J Immunol. 2003;171:2960–9. doi: 10.4049/jimmunol.171.6.2960. [DOI] [PubMed] [Google Scholar]
- 44.Hancock WW, Lu B, Gao W, et al. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192:1515–20. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato T, Thorlacius H, Johnston B, Staton TL, Xiang W, Littman DR, Butcher EC. Role for CXCR6 in recruitment of activated CD8+ lymphocytes to inflamed liver. J Immunol. 2005;174:277–83. doi: 10.4049/jimmunol.174.1.277. [DOI] [PubMed] [Google Scholar]
- 46.Hara T, Katakai T, Lee JH, Nambu Y, Nakajima-Nagata N, Gonda H, Sugai M, Shimizu A. A transmembrane chemokine, CXC chemokine ligand 16, expressed by lymph node fibroblastic reticular cells has the potential to regulate T cell migration and adhesion. Int Immunol. 2006;18:301–11. doi: 10.1093/intimm/dxh369. [DOI] [PubMed] [Google Scholar]
- 47.van der Voort R, van Lieshout AW, Toonen LW, Sloetjes AW, van den Berg WB, Figdor CG, Radstake TR, Adema GJ. Elevated CXCL16 expression by synovial macrophages recruits memory T cells into rheumatoid joints. Arthritis Rheum. 2005;52:1381–91. doi: 10.1002/art.21004. [DOI] [PubMed] [Google Scholar]
- 48.Kallinich T, Schmidt S, Hamelmann E, Fischer A, Qin S, Luttmann W, Virchow JC, Kroczek RA. Chemokine-receptor expression on T cells in lung compartments of challenged asthmatic patients. Clin Exp Allergy. 2005;35:26–33. doi: 10.1111/j.1365-2222.2004.02132.x. [DOI] [PubMed] [Google Scholar]