Abstract
The migration of dendritic cells (DCs) to secondary lymphoid organs plays a crucial role in the initiation of adaptive immune responses. Although lipopolysaccharide enhances chemokine receptor 7 (CCR7) expression on DCs, the second signal for the migration of DCs toward the chemokine CCL19 remains unknown. In this study, we show that sphingosine kinase inhibitor (SKI) inhibits the migration of DCs toward CCL19 through the down-regulation of CCR7. Inhibition of p38 mitogen-activated protein kinase (MAPK) activation by SKI may be responsible for the SKI-mediated effects on the regulation of chemokine receptor expression. Impairment of DC migration by the inhibition of p38 MAPK and down-regulation of CCR7 expression may contribute to the protective effects of SKI in DC-related disorders. These results suggest that sphingosine kinase-mediated signalling plays a role in the innate and adaptive immune responses by altering DC migration.
Keywords: chemokine receptor, dendritic cell, migration, sphingosine kinase
Introduction
Bone marrow-derived dendritic cells (DCs), as antigen-presenting cells, have a unique ability to enhance T-cell and B-cell responses as well as immune tolerance.1,2 Upon maturation, DCs in peripheral tissues migrate into the afferent lymphatics, move to the T-cell area of draining lymph nodes, and then initiate adaptive immune responses.2–4 Migration of DCs into tissues depends on a cascade of discrete events, including chemokine production and regulation of its receptors.5,6 Chemokine receptors (CKRs) are important for the regulation of DC localization and homing.7,8 Certain CKRs, including CCR1, CCR2, CCR5, CCR6, CXCR1 and CXCR2, are highly expressed in immature monocyte-derived DC. CCR1 and CCR5 are down-regulated and CCR7 is newly expressed during maturation by interleukin-1β (IL-1β), tumour necrosis factor (TNF) or lipopolysaccharide (LPS). CCR7+ DCs enter targeted lymph nodes through the afferent lymphatics or the blood vessels by sensing CCL19/MIP-3β and CCL21/SLC.4,9
Sphingosine-1-phosphate (S1P) has been shown to be associated with signals for calcium mobilization, cytoskeletal reorganization, migration and proliferation in immune cells.10–13 The level of S1P is tightly regulated by the balance between synthesis through sphingosine kinase (Sphk), irreversible cleavage by S1P lyase, and reversible dephosphorylation to sphingosine by S1P phosphatases. Until now, the biological activity of S1P in the immune system has been well understood. Recently, several groups have shown that extracellular S1P modulates chemotaxis14,15 and the maturation toward the T helper type 2 (Th2) immune response14 in DCs and inhibits T-cell proliferation.10 However, the cellular targets of Sphk in DCs are not yet known, leaving open the question of its global function in the migration of DCs. Despite the high level of concern regarding the role of sphingolipid-derived signalling, very few inhibitors of the enzymes of this pathway have yet been established. In particular, the field suffers from a lack of potent and selective inhibitors of Sphk. To date, pharmacological studies have used sphingosine analogues, especially N,N-dimethylsphingosine (DMS). However, these lipids are well-known to inhibit several protein kinases.16–19 Very recently, a few natural product inhibitors (compounds I–V) of Sphk were isolated. It has been reported that compound II is the most selective Sphk inhibitor among the natural product inhibitors of Sphk and therefore may be the most attractive candidate for additional medicinal chemistry efforts.20
In this study, we used compound II as a sphingosine kinase inhibitor (SKI) to investigate the effect of a non-cytotoxic concentration of SKI on the migration in bone marrow DCs. Our findings demonstrated that SKI and SB203580 inhibit LPS-induced migration of DCs toward CCL19 by regulating the levels of CCR1 and CCR7 expression. In addition, SKI decreases LPS-induced p38 mitogen-activated protein kinase (MAPK) activation. Taken together, these data suggest that SKI may affect the migration of DCs through the regulation of CKR expression mediated by p38 MAPK.
Materials and methods
Mice
Male 8- to 12-week-old C57BL/6 (H-2Kb and I-Ab) and BALB/c (H-3Kd and I-Ad) mice were purchased from the Korean Institute of Chemistry Technology (Daejeon, Korea). The mice were housed in a specific pathogen-free environment within our animal facility for at least 1 week before use.
Reagents and antibodies
Materials were obtained from the following sources. Recombinant mouse granulocyte–macrophage colony-stimulating factor (rmGM-CSF) and rmIL-4 were purchased from R & D Systems (Minneapolis, MN). Various inhibitors, such as SP600125, SB203580, U0126 and SKI, were obtained from Calbiochem (San Diego, CA); phycoerythrin (PE)-conjugated CCR1 and CCR7, anti-phospho-ERK, anti-extracellular signal-regulated kinase (ERK), anti-phospho-p38, anti-p38, anti-phospho-JNK and anti-Jun N-terminal kinase (JNK) were purchased from Santa Cruz (Santa Cruz, CA). LPS (from Escherichia coli 055:B5) was obtained from Sigma (St Louis, MO); fluorescein isothiocyanate (FITC) or PE-conjugated monoclonal antibodies (mAbs) of CD11c (HL3), CD83 (Michel-19), CD86 (GL1), IAbβ-chain (AF-120.1), IL-12 p40/p70 (C15.6), IL-10 (JESS-16E3) or TNF-α (TN3-19) by flow cytometry, as well as isotype-matched control mAbs and biotinylated-anti-CD11c (N418) mAbs were purchased from eBioscience (San Diego, CA).
Generation and isolation of DCs
Dendritic cells were generated from murine bone marrow cells according to the procedure of Inaba et al.21 with minor modifications. In brief, bone marrow was flushed from the tibias and femurs of 6- to 8-week-old male C57BL/6 mice and depleted of red blood cells using Red Blood Cell Lysing buffer (Sigma). The cells were plated in six-well culture plates (1 × 106 cells/ml; 2 ml/well) in RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, 100 mg/ml streptomycin, 20 ng/ml rmGM-CSF and 10 ng/ml rmIL-4 at 37° in 5% CO2. On days 3 and 5 of culturing, floating cells were gently removed and fresh medium was added. On day 6 of the culture, non-adherent cells and loosely adherent proliferating DC aggregates were harvested for analysis or stimulation, or, in some experiments, were replated in 60-mm dishes (1 × 106 cells/ml; 5 ml/dish). On day 7, 80% or more of the non-adherent cells expressed CD11c. In certain experiments, to obtain highly purified populations for subsequent analysis, the DCs were labelled with bead-conjugated anti-CD11c mAb (Miltenyi Biotec, Bergisch Gladbach, Germany) followed by positive selection through paramagnetic columns (LS columns; Miltenyi Biotec) performed according to the instructions of the manufacturer. The purity of the selected cell fraction was > 90%.
Determination of DC viability
The SKI was added to cultures of isolated DCs in six-well plates (1 × 106 cells/ml; 2 ml/well). For the determination of cell viability, DCs were stimulated with LPS or left without any stimuli, and viability was analysed by double-staining with 5 μg/ml propidium iodide (PI) and annexin V followed by flow cytometry.
Flow cytometry
On day 7, DCs were harvested, washed with phosphate-buffered saline (PBS) and resuspended in fluorescence-activated cell sorter (FACS) washing buffer (2% FBS and 0·1% sodium azide in PBS). Cells were first blocked with 10% (v/v) normal goat serum for 15 min at 4° and stained with PE-conjugated mouse mAbs against CD83, CD86 and major histocompatibility complex class II with FITC-conjugated CD11c for 30 min at 4°, and then cells were analysed on a FACSCalibur cytometer (Becton Dickinson, Franklin Lakes, NJ). For intracellular cytokine staining, the cells were treated with brefeldin A (10 μg/ml; 4 hr), washed with 1% v/v FBS-PBS (staining buffer), stained with FITC- or PE-conjugated CD11c mAb, and fixed in 4% w/v paraformaldehyde (20 min at room temperature). Subsequently, the cells were washed twice in staining buffer, rendered permeable in 100 μl 0·1% saponin in 1% FBS-PBS, and incubated with FITC- or PE-conjugated anti-IL-12 p40/p70, anti-IL-10 or anti-TNF-α mAb (for 30 min at 4°). To calculate the percentage of positive cells, a proportion of 1% false-positive events was accepted in the negative control samples throughout.
Western blot
Dendritic cells were washed twice with Tris-buffered saline and lysed by the addition of ice-cold lysis buffer, containing 0·5% Triton X-100, 50 mm Tris–HCl (pH 8·0), 150 mm NaCl, 5 mm ethylenediaminetetraacetic acid, 5 mm NaF, 1 mm Na3VO4, 10 μg/ml aprotinin and pepstatin A, and 5 μg/ml leupeptin. Lysates were left on ice for 20 min and centrifuged for 30 min at 12 000 g in a microcentrifuge at 4° to remove nuclei. Proteins were separated on 12% sodium dodecyl sulphate–polyacrylamide gels and transferred to polyvinylidene membranes. Membranes were blocked with 5% non-fat dried milk in T-PBS (0·2% Tween-20 in PBS) and incubated with antibody against phospho-JNK, phospho-p38 MAPK or phosphor-ERK1/2 antibody (Santa Cruz) for overnight (O/N) to detect phosphorylation of JNK, p38 MAPK or ERK1/2 and ERK2 or p38 MAPK antibody to detect the loading amount. Membranes were subsequently washed and incubated for 1 hr with secondary antibody conjugated to horseradish peroxidase. Immunolabelling was detected using an enhanced chemiluminescence detection system (Millipore Corporation, Billerica, MA).
Boyden chamber chemotaxis assay
Dendritic cell migration was assayed by a modification of the Boyden chamber method22 performed in a microchemotaxis chamber (NeuroProbe, Gaithersburg, MD) using polycarbonate membrane (NeuroProbe) with a pore size of 5·0 μm. The membranes were coated with mouse type IV collagen (20 μg/ml in PBS) and placed between the chambers. First, the lower well of the chamber was filled with 27 μl RPMI-1640 supplemented with 0·1% bovine serum albumin (BSA) following the addition of CCL19 at the indicated concentration. The DCs were washed with RPMI-1640 containing 0·1% BSA resuspended at a concentration of 1 × 106 cells/ml in RPMI-1640 supplemented with 0·1% BSA. The cells were then placed in the upper well of the chamber (50 μl/well) and incubated at 37° in 95% air and 5% CO2 for 2 hr. At the end of the incubation, the filters were removed and all non-migrated cells on the upper side of the filter were scraped off with wet tissue paper. The migrated cells on the other side of the filter were fixed for 2 min with fixative solution from the HEMA 3 stain set and stained with solutions 1 and 2 of the HEMA 3 stain set, each for 2 min (Fisher Scientific, Kalamazoo, MI). The numbers of stained cells were quantified densitometrically using Image Gauge Version 2·54 (Fujifilm) for data analysis.
Real-time horizontal chemotaxis assay
Real-time horizontal chemotaxis assays using the EZ-TAXIScan chamber (Effector Cell Institute, Tokyo, Japan) were performed as previously described23. The EZ-TAXIScan chamber consists of an etched silicon substrate and a flat glass plate, both of which form two compartments with a 5-μm deep microchannel. The DCs (1 μl of 106 cells/ml) were put into one hole in the stainless steel holder with which the device is held together, and 1 μl of 0·5 μg/ml CCL19 was put into another contra-hole. The chamber was incubated for 1 hr at 37°. A charge-coupled device (CCD) camera was used to record the migration of DC toward the high concentration of CCL19 on the microchannel where the gradient of CCL19 was. To count the migrated cells in each channel, images of the cells in each channel were digitally recorded onto a computer hard disk with time-lapse interval of 30 seconds.
Confocal microscopy
Cells grown on glass coverslips coated with poly l-lysine were washed with PBS and incubated with FITC-conjugated anti-CD11c+ with PE-conjugated anti-CCR1 or anti-CCR7 antibody for 1 hr at 4°. The coverslips were then washed twice with PBS and fixed with PBS containing 4% paraformaldehyde for 15 min at room temperature. After three washes and a final rinse in PBS, the coverslips were inverted onto Aqua Poly Mount mounting medium (Polyscience, Inc., Warrington, PA). Fluorescence was assessed using a 63 × oil immersion objective in an inverted Zeiss LSM-510 Meta confocal laser scanning microscope.
Quantitative real-time polymerase chain reaction
The CCR7 polymerase chain reaction (PCR) primers used were as follows: forward 5′-GTGTGCTTCTGCCAAGATGA-3′, reverse 5′-CCACGAAGCAGATGACAGAA-3′. The CCR1 PCR primers used were as follows: forward 5′-AGGGCCCGAACTGTTACTTT-3′, reverse 5′- TTCCACTGCTTCAGGCTCTT-3′. Quantitative amounts of each gene were standardized against the GAPDH housekeeping gene. Real-time PCR was performed using a Bio-Rad MiniOpticon System (Bio-Rad Laboratories Ltd, Hercules, CA) with SYBR green fluorophore. Reactions were performed in a total volume of 20 μl, which included 10 μl 2× SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA), 1 μl of each primer at a 10 μm concentration and 1 μl of the previously reverse-transcribed cDNA template. The protocols used were as follows: denaturation (95° for 10 min), amplification repeated 40 times (95° for 30 seconds, 52° for 30 seconds, 72° for 30 seconds, and acquisition temperature for 15 seconds). For each sample, delta delta Threshold cycle (ddCT) (crossing point) values were calculated as the Ct of the target gene minus the Ct of the GAPDH gene. Gene expression was derived according to the equation 2–ddCt; changes in gene expression were expressed as relative to basal levels.
Statistical analysis
Experiments were repeated at least three times with consistent results. Unless otherwise stated, data are expressed as the mean ± SEM. Analysis of variance was used to compare experimental groups to control values while comparisons between multiple groups were made using Tukey's multiple comparison test. Statistical significance was determined as P < 0·05.
Results
SKI inhibits the expression of costimulatory molecules and IL-12 production but not IL-10 in LPS-stimulated DCs
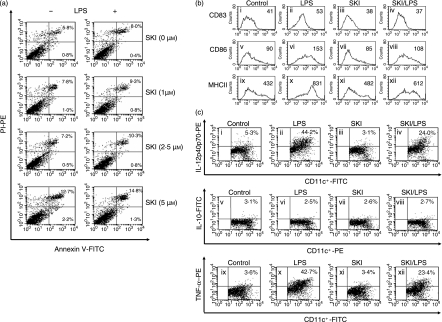
To study the survival effect of SKI in DCs, we observed the necrotic and apoptotic changes as revealed by annexin V and PI staining using flow cytometry. On day 6 of DC culture, SKI at a various concentrations (0, 1, 2·5 and 5 μm) was preincubated for 3 hr and then incubated with or without 200 ng/ml of LPS for a further 24 hr. Because concentrations of SKI > 5 μm were found to be somewhat cytotoxic to DCs, SKI was used at a concentration ≤ 2·5 μm. There were no marked differences observed until reaching a concentration of 2·5 μm in the percentage of dead cells according to the CD11c+ cells and annexin-V/PI staining (Fig. 1a). Next, to determine whether SKI would affect the development of DCs derived from bone marrow, we measured the costimulatory molecules (CD83, CD86 and major histocompatibility complex class II). On day 6 of DC culture, SKI (2·5 μm) was preincubated for 3 hr and then incubated with or without 200 ng/ml of LPS for a further 24 hr. As shown in Fig. 1(b), stimulation of cells with LPS for 24 hr from day 6 resulted in the up-regulation of costimulatory molecules (Fig. 1b, panels ii, vi and x). It was found that 2·5 μm SKI was sufficient to inhibit the expression of costimulatory molecules on CD11c+ cells in LPS-treated DCs (Fig. 1b, panels iv, viii and xii). These data suggest that SKI-treated DCs were at least partially resistant to phenotype maturation.
Figure 1.
SKI suppresses costimulatory molecules and IL-12 production during DC maturation. bone marrow-derived DCs were generated as described in the Material and methods. (a) DCs were treated with the indicated concentrations of SKI for 3 hr and further incubated with or without LPS (200 ng/ml) for 24 hr. Then, DCs were stained with annexin-V and PI. The percentage within each positive cell represents the incidence of annexin-V+PI+. (b) DCs were preincubated with SKI 2·5 μm for 3 hr and then further incubated with 200 ng/ml LPS for 24 hr. Co-stimulatory molecules were then analysed by flow cytometry. The cells were gated on CD11c+. The mean fluorescence intensity (MFI) values were shown for each panel. (c) DCs were preincubated with SKI 2·5 μm for 3 hr and then further incubated with 200 ng/ml LPS for 24 hr. The analysis of IL-12 p40/p70, IL-10 and TNF-α in CD11c+ DCs was measured by flow cytometry. The numbers indicate the percentages of CD11c+ cells expressing IL-12, IL-10 or TNF-α. Values are mean ± SEM obtained from at least three separate experiments. The results are from one representative experiment of three performed.
A major attribute of mature DCs is the synthesis and release of cytokines with important modulatory functions in inflammation and T-cell differentiation. DCs produce proinflammatory cytokines (such as IL-1β, IL-6, IL-12 and TNF-α). Especially, specific cytokine patterns, IL-12 and IL-10, are known to select Th1 and Th2 dominant states of T-cell-mediated immune responses, respectively.24 Matured DCs treated with LPS produced IL-12 to regulate the Th1 response.25 To assess whether SKI may modulate LPS-induced production of IL-12 or IL-10 in DCs, the respective productions of IL-12 and IL-10 were detected by flow cytometry. FITC-labelled anti-CD11c+ DCs with PE-labelled anti-IL-12 p40/p70 and PE-labelled anti-CD11c+ DCs with FITC-labelled anti-IL-10 mAbs indicated that LPS-treated DCs in the presence of SKI expressed a low level of IL-12 p40/p70 (Fig. 1c, panel iv) compared with those of LPS-treated DCs in the absence of SKI (Fig. 1c, panel ii), whereas IL-10 was not detected (Fig. 1c, panels vi and viii). These findings suggest that SKI attenuated the capability of DC to enhance high levels of IL-12 p40/p70 and TNF-α. In addition, SKI inhibited endogenously produced TNF-α by DCs stimulated with LPS (Fig. 1c, panel xii). The above results indicate that exposure to SKI impaired the capability of DCs to produced large amounts of IL-12 p70 and TNF-α. Thus, these phenomena suggest that SKI suppresses the maturation function of DCs stimulated with LPS.
SKI impairs LPS-induced DC migration toward CCL19
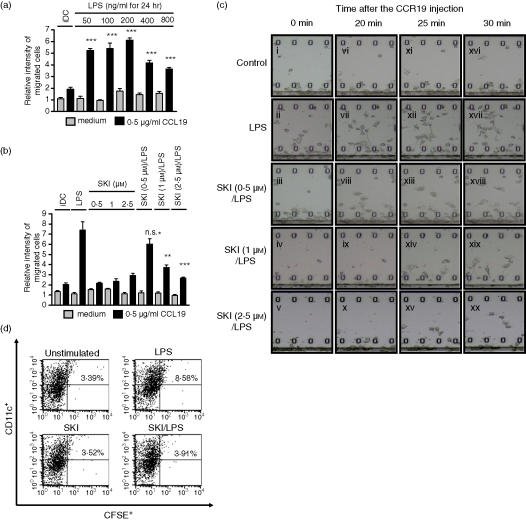
We tested the effects of SKI on DC migration by using a Boyden chamber and an EZ-TAXIScan chamber. We found that LPS stimulated the migration of DCs in a dose-dependent manner, with maximum migration at a concentration of 200 ng/ml LPS (Fig. 2a). While the migration of immature DCs was not affected by the application of SKI, migration of matured DCs was inhibited by SKI in a dose-dependent manner, which indicated that migration of matured DCs might be mediated by Sphk (Fig. 2b). To further confirm the inhibitory effect of SKI, we used the EZ-TAXIScan chamber, which is capable of detecting the real-time horizontal migration of cells. On day 6 of DC culture, SKI (0, 0·5, 1 and 2·5 μm) was preincubated for 3 hr and then incubated with 200 ng/ml LPS for a further 24 hr; 1 μl of DCs (1 × 106 cell/ml) was put into the one hole of the EZ-TAXIScan chamber which consists of an etched silicon substrate and a flat glass plate, both of which form two compartments with a 5-µm-deep microchannel in between. After the addition of 1 μl of 0·5 μg/ml CCL19, the chamber was incubated for 1 hr at 37° and the migration of DCs under the gradient of CCL19 ws recorded with a CCD camera (Fig. 2c). When DCs were incubated with LPS only, some of the DCs started to migrate toward a higher concentration of CCL19 within 10 min. These migrating cells spread lamellipodia toward the high concentration of CCL19 in 20, 25 and 30 min (Fig. 2c, panels vi, xii and xvii). In contrast, when DCs were incubated with LPS in the presence of 0·5, 1 and 2·5 μm of SKI, directional migration was inhibited in a dose-dependent manner.
Figure 2.
LPS-induced migration is impaired by SKI in DCs. (a) Effect of LPS on DC migration. On day 6, DCs were incubated with LPS at the indicated concentrations for 24 hr. On day 7, cells were harvested and an in vitro Boyden chamber chemotaxis assay was performed in response to medium or CCL19 (0·5 μg/ml). (b) Effect of SKI on the LPS-induced DC migration in vitro. On day 6, DCs were preincubated with SKI at the indicated concentrations for 3 hr, and were further incubated with or without LPS (200 ng/ml) for 24 hr. On day 7, cells were harvested and an in vitro Boyden chamber chemotaxis assay was performed in response to medium or CCL19 (0·5 μg/ml). (c) Time-lapse camera monitoring of chemotaxis. On day 6, DCs were preincubated with SKI (0, 0·5, 1 and 2·5 μm) at the indicated concentrations for 3 hr, and were further incubated with LPS (200 ng/ml) for 24 hr. On day 7, cells were harvested and a real-time horizontal chemotaxis assay was performed in response to medium or CCL19 (0·5 μg/ml). Migration of DCs under the gradation of CCL19 was recorded by a CCD camera. These images show portions of individual frames. (d) On day 6, DCs were preincubated with SKI (2·5 μm) for 3 hr, and were then further incubated with or without LPS (200 ng/ml) for 24 hr. A representative flow cytometry profile is shown for the groups of mice receiving unstimulated DC, LPS-DC, SKI-DC or SKI + LPS-DC. Numbers represent the percentage of CFSE+ and CD11c+ cells in the total LN DCs. The results shown are from one representative experiment of three performed (c) and (d). The asterisks in (a) indicate significant increases compared to that of the black bar of iDC at ***P < 0·001. For (b), asterisks indicate significant increases compared to the black bar of LPS-only treated DCs at **P < 0·01 and ***P < 0·001, P > 0·05 not significant (n.s.).
To further investigate the possibility that SKI treatment impaired the capacity of DCs to immigrate into secondary lymphoid organs, we examined in vivo experiments to test the migratory capacity of DCs in mice. To confirm this, unstimulated DCs, LPS-DCs, SKI-DCs and LPS + SKI-DCs were labelled with the fluorescent cell tracker carboxyfluorescein succinimidyl ester (CFSE) and injected intravenously into naive mice. The inguinal lymph node (LN) cells were harvested 48 hr later. The migratory capacities of DCs were evaluated by measuring CFSE-labelled DCs in inguinal LN by flow cytometry. Although the percentage of the drained DCs in inguinal LN that were double-positive with CFSE and CD11c was low, it was significantly enhanced in LPS-treated DCs. However, pretreatment of SKI significantly decreased the percentage of the drained DCs (Fig. 2d). These data demonstrated that SKI treatment impairs the migration of DCs in vitro and in vivo.
SKI regulates chemokine receptor expression
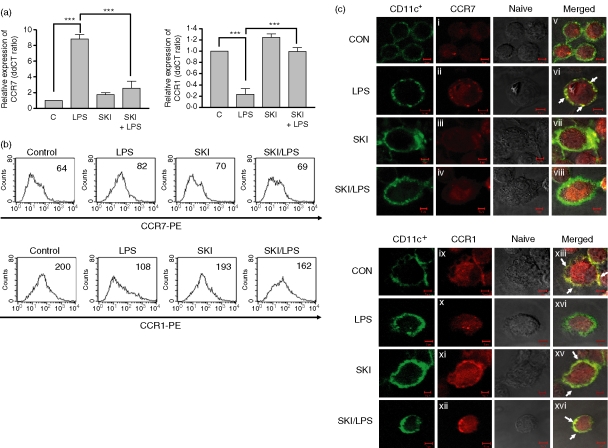
CCL19, which induces LPS-treated DC migration (Fig. 2), is a specific ligand for CCR7. We hypothesized that SKI treatment alters chemokine receptor (CKR) expression, which in turn impairs DC migration. To prove this hypothesis, we measured the expressions of CCR1 and CCR7 after LPS stimulation in the presence or absence of SKI using quantitative PCR to detect mRNA level (Fig. 3a) and flow cytometry to detect protein level (Fig. 3b). While the expression of CCR7 was significantly enhanced in LPS-treated DCs, SKI inhibited the up-regulation of CCR7 expression by LPS. Otherwise, CCR1 expression was down-regulated by LPS stimulation; pretreatment with SKI reversed the down-regulation of CCR1 expression (Fig. 3a,b).
Figure 3.
CKR expressions are regulated by SKI in DCs. (a) Effect of SKI on the expression of CCR1 and CCR7. On day 6, DCs were preincubated with SKI (2·5 μm) for 3 hr and were further incubated with or without LPS (200 ng/ml) for 24 hr. On day 7, cells were harvested and total RNA was extracted. Quantitative mRNA expression was measured as described in the Material and methods. (b) Effect of SKI on the expression of CCR1 and CCR7. DCs were preincubated with SKI for 3 hr and then further incubated with 200 ng/ml LPS for 24 hr. PE-conjugated CCR1 and CCR7 were then analysed by flow cytometry. The cells were gated on CD11c+. The mean fluorescence intensity (MFI) values are shown for each panel. (c) Effect of SKI on the expression of CCR1 and CCR7. On day 5, cells were harvested, and seeded in six-well culture dish contained glass coverslips, and left overnight. On day 6, cells were preincubated with SKI (2·5 μm) for 3 hr and were further incubated with or without LPS (200 ng/ml) for 24 hr. On day 7, coverslips were stained with FITC-conjugated CD11c+ plus PE-conjugated CCR1 or CCR7 for 1 hr. A confocal microscopy assay was performed to examine CKR expression. Double-positive co-localization signal appears yellow (arrows). The asterisks in (a) indicate ***P < 0·001. The results are from one representative experiment of three performed (b) and (c).
To further confirm these SKI-mediated effects on CKR expression, the expression of CCR1 and CCR7 was determined by confocal microscopy (Fig. 3c). After DCs were incubated with LPS for 24 hr in the presence and absence of SKI, cells were stained with PE-conjugated CKR antibody and a DC surface marker, FITC-conjugated CD11c antibody. The expression of CKR (red) colocalized with CD11c (green) appeared as yellow. CCR7 proteins were strongly enriched by LPS stimulation in the cell surface, as demonstrated by their colocalization with CD11c proteins at the cell surface (Fig. 3c, panels ii and vi), whereas pretreatment with SKI diminished the colocalization of CCR7 and CD11c (Fig. 3c, panels iv and viii). In contrast, the expression of CCR1 (red) colocalized with CD11c (green) already showed a yellow colour in untreated DCs (Fig. 3c, panels ix and xiii), while the colocalization of CCR1 and CD11c was decreased in LPS-treated DCs (Fig. 3c, panels x and xiv). The colocalization of CCR1 and CD11c was not decreased in LPS-treated DCs in the presence of SKI (Fig. 3c, panels xii and xvi).
p38 MAPK regulates migration of DCs via the control of CCR7 expression
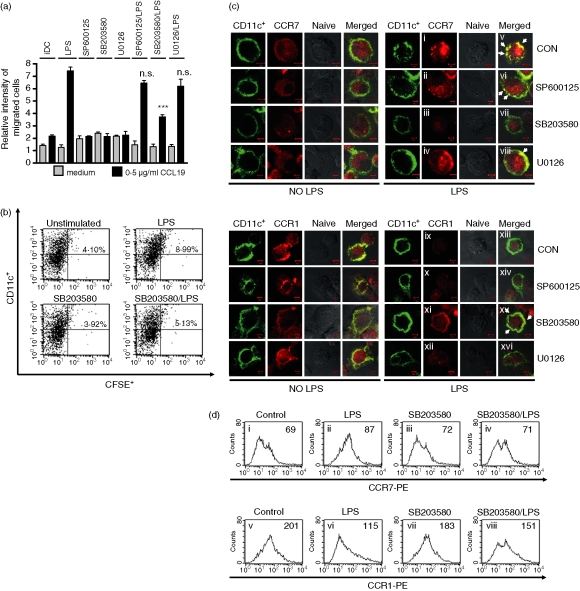
Previous reports have shown that MAPK activation is an important event in the maturation of DCs.26 We wondered whether MAPKs were involved in LPS-induced migration of DCs. LPS-induced cell migration was measured by a Boyden chamber assay in the presence or absence of SP600125 (JNK inhibitor), SB203580 (p38 MAPK inhibitor) and U0126 (ERK1/2 inhibitor). These inhibitors, except SB203580, did not affect the migration of immature DCs. SB203580 significantly inhibited the migration of mature DCs, which indicated that the LPS-induced migration of DCs was mediated by p38 MAPK. JNK and ERK1/2 inhibitors did not block the migration of DCs (Fig. 4a). To further investigate the possibility that SB203580 treatment impaired the capacity of DCs to immigrate into secondary lymphoid organs, we examined in vivo experiments to test the migratory capacity of DCs in mice. To confirm this, unstimulated DCs, LPS-DCs, SB203580-DCs and LPS + SB203580-DCs were labelled with the fluorescent cell tracker CFSE and injected intravenously into naive mice. The inguinal LN cells were harvested 48 hr later. The migratory capacities of DCs were evaluated by measuring CFSE-labelled DCs in inguinal LN by flow cytometry. As expected, the percentage of the drained DCs in inguinal LN which were double-positive with CFSE and CD11c were increased in LPS-treated DCs, while pretreatment of SB203580 significantly decreased the percentage of the drained DCs (Fig. 4b). The data demonstrated that p38 MAPK might affect the migration of DCs by LPS stimulation in vitro and in vivo. Next, we hypothesized whether SB203580 may modulate the expression of CKR in a similar manner to that of SKI. We measured the expressions of CCR1 and CCR7 after LPS stimulation in the presence or absence of SP600125, SB203580 and U0126 using confocal microscopy (Fig. 4c). As shown in Fig. 4(c), LPS-induced CCR7 expression was down-regulated (Fig. 4c, panels iii and vii), but, as expected, LPS-decreased CCR1 expression was up-regulated in SB203580-treated DCs (Fig. 4c, panels xi and xv). However, SP600125 and U0126 had no effect on CCR1 or CCR7 expression in LPS-treated DCs. To further confirm these p38 MAPK-mediated effects on CKR expression, we measured CKR expression after LPS stimulation in the presence and absence of SB203580 by flow cytometry (Fig. 4d). In the presence of SB203580, LPS-induced up-regulation of CCR7 expression was diminished (Fig. 4d, panel iv), while LPS-induced down-regulation of CCR1 expression was increased (Fig. 4d, panel viii).
Figure 4.
Analysis of MAPKs on migration and CKR expression in DCs. (a) Effect of MAPKs on the LPS-induced migration of DCs. On day 6, DCs were preincubated with SP600125 (5 μm), SB203580 (10 μm), or U0126 (5 μm) for 1 hr, and were then further incubated with or without LPS (200 ng/ml) for 24 hr. On day 7, cells were harvested and in vitro migration was assessed in response to medium or CCL19 (0·5 μg/ml). (b) On day 6, DCs were preincubated with SB203580 (10 μm) for 1 hr, and were then further incubated with or without LPS (200 ng/ml) for 24 hr. A representative flow cytometry profile is shown for the groups of mice receiving unstimulated DC, LPS-DC, SB203580-DC or SB203580 + LPS-DC. Numbers represent the percentage of CFSE+ and CD11c+ cells in the total LN DCs. (c) Effect of p38 MAPK on the expression of CCR1 and CCR7. On day 5, cells were harvested, seeded in six-well culture dish contained glass coverslips, and left overnight. On day 6, cells were preincubated with SB203580 (10 μm) for 1 hr, and were further incubated with or without LPS (200 ng/ml) for 24 hr. On day 7, coverslips were stained with FITC-conjugated CD11c+ plus PE-conjugated CCR1 or CCR7 for 1 hr. A confocal microscopy assay was performed to examine CKR expression. Double-positive co-localization signal appears yellow (arrows). (d) Effect of SB203580 on the expression of CCR1 and CCR7. DCs were preincubated with SB203580 (10 μm) for 1 hr and then further incubated with 200 ng/ml LPS for 24 hr. PE-conjugated CCR1 and CCR7 were then analysed by flow cytometry. The cells were gated on CD11c+. The mean fluorescence intensity (MFI) values were shown for each panel. The asterisks in (a) indicate significant increases compared to the black bar of only LPS-treated DCs at **P < 0·01 and ***P < 0·001, P > 0·05 not significant (n.s.). The results are from one representative experiments of the three performed (b), (c) and (d).
Sphk is an upstream molecule of p38 MAPK in LPS-induced DCs migration
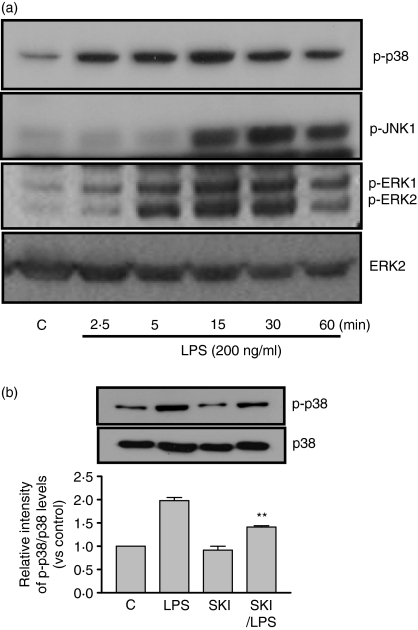
To investigate the relationship between Sphk and p38 MAPK in LPS-mediated signalling, we measured p38 MAPK phosphorylation in the presence or absence of SKI in DCs. Recently, it has been demonstrated that SKI acts a highly specific inhibitor of sphingosine kinase (50% inhibitory concentration = 0·5 μm for GST-human sphingosine kinase (hSK)) and does not affect the kinase activity of human ERK2, human PI3K, or PKCα even at concentrations at high as 60 μm.20 We found that LPS stimulates the phosphorylation of JNK, p38 MAPK and ERK1/2 in DCs in a time-dependent manner (Fig. 5a). We also established that the phosphorylation of p38 MAPK was inhibited in LPS-treated DCs in the presence of SKI, which means that the Sphk event is upstream of p38 MAPK in LPS-induced DCs migration (Fig. 5b).
Figure 5.
The effect of SKI on MAPK activation by LPS in DCs. (a) Effect of LPS in MAPK phosphorylation. On day 5, cells were incubated with serum-free media for 6 hr. DCs were incubated with LPS (200 ng/ml) for the indicated times. Cells were then harvested and total protein was extracted. Western blot assays were performed to examine phospho-p38 MAPK, phospho-JNK, phospho-ERK, and total p38 MAPK expression. (b) Effect of SKI on the phosphorylation of p38 MAPK. On day 5, cells were incubated with serum-free media for 6 hr. DCs were preincubated with SKI (2·5 μm) for 3 hr, and were further incubated with or without LPS (200 ng/ml) for 15 min. Cells were then harvested and total protein was extracted. Western blot assays were performed to assess phospho-p38 MAPK expression. The asterisks in (b) indicate significant increases compared to that of LPS treatment at **P < 0·01. The results are from one representative experiment of three performed (a).
Discussion
To the best of our knowledge, this is the first description of the effects of SKI on the migration of murine bone marrow DCs. Sphingosine kinase has generally been considered a cytosolic protein that is constitutively active and, thus, generates basal levels of S1P within the cells. Therefore, although S1P produced by Sphk can regulate various cellular processes including growth, survival, differentiation, cytoskeletal rearrangement, chemotaxis, angiogenesis and immunity27–29 the effects of Sphk on the migration of DCs have not yet been elucidated.
Dendritic cells are considered to play an important role in deciding the establishment of immunity and tolerance.30,31 Thus, we performed a series of functional assays to ascertain the phenotype and function of SKI in murine bone marrow DCs. To confirm that the observed effects of SKI could be attributed to DCs and not to contaminating cells persisting in the bone marrow-derived DC cultures, DCs were purified (> 90%) before analysis in each of the conducted assays. The level of costimulatory molecule stimulated by LPS in the presence of SKI was found to be significantly attenuated; thereby suggesting that SKI is a potent inhibitor of LPS-induced DC maturation. Recently collected evidence suggests that the production of cytokines by DCs is dependent on either the specific type of DCs, or on the stimuli received by DCs.32 IL-12, in particular, exerts multiple immunoregulatory functions, inducing the activation of the Th1 subset, which performs a critical role in the induction of inflammation.33,34 The results obtained in this report indicated that Sphk1 caused CD11c+ DC to generate IL-12 in the presence of LPS, and also confirmed that SKI exerts inhibitory effects on the expression of intracellular IL-12 p40/p70.
DCs developmentally regulate the expression of chemokine receptors; CCR7, the receptor for CCL19 and CCL21, is highly expressed in mature DCs, but not immature DCs.8,9 In contrast, CCR1 and CCR5 are down-regulated in immature DCs during maturation.35 Immature DCs express CCR1 and CCR5, which confer responsiveness to inflammatory chemokines, directing the migration of DCs toward inflammatory stimuli and peripheral tissues in general.35 Upon exposure to maturation-inducing stimuli, such as inflammatory cytokine and pathogen products, DCs down-regulate CCR1 and CCR5, up-regulate CCR7 expression; CCR7 expression in DCs eventually allows them to obtain the migratory properties from peripheral tissues to T-cell zones of the spleen and lymph nodes, where constitutively high levels of CCL19 or CCL21 expression exist. Chemokines and their receptors modulate the migration of DCs into the blood during inflammation.36 Migration of the DCs is an important function in T-cell-mediated immunity for selective elimination of infected cells.36 Whether the migration occurs in mature DCs15 or immature DCs14 remains a point of controversy. With this knowledge, we then attempted to explore the properties of Sphk in the migration of DCs with regard to the effect of SKI treatment on CCR1 and CCR7 expression in LPS-induced mature DCs. SKI-treated DCs demonstrated inhibited CCR7 up-regulation upon maturation (Fig. 3). However, SKI-treated DCs displayed normal expression of CCR1, which implies that DC infiltration into tissues may be unaffected (Fig. 3). Therefore, it could be inferred that migration of DCs into the T-cell areas of draining lymph nodes would be impaired, which was demonstrated by our in vivo migration assay (Fig. 2d). The inability of SKI-treated DCs to turn off CCR1 expression and their inability to turn on CCR7 expression upon maturation both contributed to impaired migration of SKI-treated DCs into secondary lymphoid organs.
The MAPKs are key mediators of signal transduction from the activated receptor to the nucleus. Growth factors stimulate the activation of a protein kinase cascade, which sequentially involves Ras, Raf, MAP kinase kinase (MEK), and MAPK.37 Recently, it has been demonstrated that Sphk plays an important role in the regulation of MAPKs. ERK1/2 activation by TNF-α and platelet-derived growth factor is at least partially dependent on Sphk.38,39 Also, S1P stimulates ERK1/2 MAPK in airway smooth muscle cells.40 Intracellular signalling cascades with MAPKs, the archetypal molecules for cell signalling, have long been described for functional and phenotypic changes of DCs stimulated by various agents (LPS, TNF-α and toxic stress).30,41,42 We also intended to demonstrate the possible role of Sphk in the migration of DCs via the alteration of cellular expression levels of ERK1/2, JNK, and p38 MAPK. As shown in Fig. 4(a), inhibition of p38 MAPK blocked DC migration, but this was not the case with the other molecules. Accordingly, the associated decrease of LPS-induced CCR7 expression to the basal level and the increase of CCR1 expression (Fig. 4b) by p38 MAPK inhibition as a result of the phosphorylation of p38 MAPK indicate a critical step in DC migration. Additionally, we found that p38 MAPK phosphorylation was significantly dependent on the effect of Sphk in mature DCs by LPS stimuli (Fig. 5b), in addition to the recently revealed involvement of JNK and Rho-dependent DC migration in response to the CCR7-mediated migration of mature DCs.43 Therefore, SKI might play a role in DC migration via the reciprocal control of CCR7 expression by augmenting the p38 MAPK signalling pathway. In fact, the effects of Sphk on DC biology are much more complex. In this study, we have just suggested that Sphk might play a potential role in the physiological responses of DCs to provide an essential defence against infections and interference with Sphk-dependent pathways might become a novel pharmaceutical target for preventing LPS-dependent DC trafficking and the associated spread of infection.
Acknowledgments
This work was supported by grants from the Korea Science and Engineering Foundation through National Research Laboratory Program Grant M106–0000000805J000000810.
Glossary
Abbreviations:
- BSA
bovine serum albumin
- CCD
charge-coupled device
- CFSE
carboxyfluorescein succinimidyl ester
- CKR
chemokine receptor
- DCs
dendritic cells
- ERK
extracellular signal-regulated kinase
- FACS
fluorescence-activated cell sorting
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- IL
interleukin
- JNK
Jun N-terminal kinase
- LN
lymph node
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- mAb
monoclonal antibody
- PBS
phosphate-buffered saline
- PE
phycoerythrin
- PI
propidium iodide
- rm
recombinant murine
- Sphk
sphingosine kinase
- SKI
sphingosine kinase inhibitor
- S1P
sphingosine-1-phosphate
- Th2
T helper type 2
- TNF
tumour necrosis factor
References
- 1.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–28. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 4.Cyster JG. Chemokines and the homing of dendritic cells to the T cell areas of lymphoid organs. J Exp Med. 1999;189:447–50. doi: 10.1084/jem.189.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allavena P, Sica A, Vecchi A, Locati M, Sozzani S, Mantovani A. The chemokine receptor switch paradigm and dendritic cell migration: its significance in tumor tissues. Immunol Rev. 2000;177:141–9. doi: 10.1034/j.1600-065x.2000.17714.x. [DOI] [PubMed] [Google Scholar]
- 6.Cavanagh LL, Von Andrian UH. Travellers in many guises: the origins and destinations of dendritic cells. Immunol Cell Biol. 2002;80:448–62. doi: 10.1046/j.1440-1711.2002.01119.x. [DOI] [PubMed] [Google Scholar]
- 7.Sozzani S, Allavena P, Vecchi A, Mantovani A. The role of chemokines in the regulation of dendritic cell trafficking. J Leukoc Biol. 1999;66:1–9. doi: 10.1002/jlb.66.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Sallusto F, Lanzavecchia A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol Rev. 2000;177:134–40. doi: 10.1034/j.1600-065x.2000.17717.x. [DOI] [PubMed] [Google Scholar]
- 9.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 10.Jin Y, Knudsen E, Wang L, Bryceson Y, Damaj B, Gessani S, Maghazachi AA. Sphingosine 1-phosphate is a novel inhibitor of T-cell proliferation. Blood. 2003;101:4909–15. doi: 10.1182/blood-2002-09-2962. [DOI] [PubMed] [Google Scholar]
- 11.Baumruker T, Prieschl EE. Sphingolipids and the regulation of the immune response. Semin Immunol. 2002;14:57–63. doi: 10.1006/smim.2001.0342. [DOI] [PubMed] [Google Scholar]
- 12.Spiegel S. Sphingosine 1-phosphate: a prototype of a new class of second messengers. J Leukoc Biol. 1999;65:341–4. doi: 10.1002/jlb.65.3.341. [DOI] [PubMed] [Google Scholar]
- 13.Ott VL, Cambier JC. Introduction: multifaceted roles of lipids and their catabolites in immune cell signaling. Semin Immunol. 2002;14:1–6. doi: 10.1006/smim.2001.0336. [DOI] [PubMed] [Google Scholar]
- 14.Idzko M, Panther E, Corinti S, et al. Sphingosine 1-phosphate induces chemotaxis of immature and modulates cytokine-release in mature human dendritic cells for emergence of Th2 immune responses. Faseb J. 2002;16:625–7. doi: 10.1096/fj.01-0625fje. [DOI] [PubMed] [Google Scholar]
- 15.Czeloth N, Bernhardt G, Hofmann F, Genth H, Forster R. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J Immunol. 2005;175:2960–7. doi: 10.4049/jimmunol.175.5.2960. [DOI] [PubMed] [Google Scholar]
- 16.Igarashi Y, Hakomori S, Toyokuni T, et al. Effect of chemically well-defined sphingosine and its N-methyl derivatives on protein kinase C and src kinase activities. Biochemistry. 1989;28:6796–800. doi: 10.1021/bi00443a002. [DOI] [PubMed] [Google Scholar]
- 17.Megidish T, White T, Takio K, Titani K, Igarashi Y, Hakomori S. The signal modulator protein 14-3-3 is a target of sphingosine- or N,N-dimethylsphingosine-dependent kinase in 3T3 (A31) cells. Biochem Biophys Res Commun. 1995;216:739–47. doi: 10.1006/bbrc.1995.2684. [DOI] [PubMed] [Google Scholar]
- 18.King CC, Zenke FT, Dawson PE, Dutil EM, Newton AC, Hemmings BA, Bokoch GM. Sphingosine is a novel activator of 3-phosphoinositide-dependent kinase 1. J Biol Chem. 2000;275:18108–13. doi: 10.1074/jbc.M909663199. [DOI] [PubMed] [Google Scholar]
- 19.McDonald OB, Hannun YA, Reynolds CH, Sahyoun N. Activation of casein kinase II by sphingosine. J Biol Chem. 1991;266:21773–6. [PubMed] [Google Scholar]
- 20.French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JL, Yun JK, Smith CD. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003;63:5962–9. [PubMed] [Google Scholar]
- 21.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wuyts A, Menten P, Van Osselaer N, Van Damme J. Assays for chemotaxis. Meth Mol Biol. 2004;249:153–66. doi: 10.1385/1-59259-667-3:153. [DOI] [PubMed] [Google Scholar]
- 23.Kanegasaki S, Nomura Y, Nitta N, et al. A novel optical assay system for the quantitative measurement of chemotaxis. J Immunol Meth. 2003;282:1–11. doi: 10.1016/j.jim.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 24.de Jong EC, Smits HH, Kapsenberg ML. Dendritic cell-mediated T cell polarization. Springer Semin Immunopathol. 2005;26:289–307. doi: 10.1007/s00281-004-0167-1. [DOI] [PubMed] [Google Scholar]
- 25.Langrish CL, McKenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 26.Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med. 1998;188:2175–80. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 28.Saba JD, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ Res. 2004;94:724–34. doi: 10.1161/01.RES.0000122383.60368.24. [DOI] [PubMed] [Google Scholar]
- 29.Anliker B, Chun J. Lysophospholipid G protein-coupled receptors. J Biol Chem. 2004;279:20555–8. doi: 10.1074/jbc.R400013200. [DOI] [PubMed] [Google Scholar]
- 30.Arrighi JF, Rebsamen M, Rousset F, Kindler V, Hauser C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J Immunol. 2001;166:3837–45. doi: 10.4049/jimmunol.166.6.3837. [DOI] [PubMed] [Google Scholar]
- 31.Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, Shlomchik MJ, Emerson SG. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–5. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 32.Kadowaki N, Antonenko S, Ho S, Rissoan MC, Soumelis V, Porcelli SA, Lanier LL, Liu YJ. Distinct cytokine profiles of neonatal natural killer T cells after expansion with subsets of dendritic cells. J Exp Med. 2001;193:1221–6. doi: 10.1084/jem.193.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment. Downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Triantaphyllopoulos KA, Williams RO, Tailor H, Chernajovsky Y. Amelioration of collagen-induced arthritis and suppression of interferon-gamma, interleukin-12, and tumor necrosis factor alpha production by interferon-beta gene therapy. Arthritis Rheum. 1999;42:90–9. doi: 10.1002/1529-0131(199901)42:1<90::AID-ANR12>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 35.Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay CR, Qin S, Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760–9. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 36.Yoneyama H, Matsuno K, Matsushimaa K. Migration of dendritic cells. Int J Hematol. 2005;81:204–7. doi: 10.1532/IJH97.04164. [DOI] [PubMed] [Google Scholar]
- 37.Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci USA. 1993;90:5889–92. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rani CS, Wang F, Fuior E, et al. Divergence in signal transduction pathways of platelet-derived growth factor (PDGF) and epidermal growth factor (EGF) receptors. Involvement of sphingosine 1-phosphate in PDGF but not EGF signaling. J Biol Chem. 1997;272:10777–83. doi: 10.1074/jbc.272.16.10777. [DOI] [PubMed] [Google Scholar]
- 39.Xia P, Gamble JR, Rye KA, et al. Tumor necrosis factor-alpha induces adhesion molecule expression through the sphingosine kinase pathway. Proc Natl Acad Sci USA. 1998;95:14196–201. doi: 10.1073/pnas.95.24.14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rakhit S, Conway AM, Tate R, Bower T, Pyne NJ, Pyne S. Sphingosine 1-phosphate stimulation of the p42/p44 mitogen-activated protein kinase pathway in airway smooth muscle. Role of endothelial differentiation gene 1, c-Src tyrosine kinase and phosphoinositide 3-kinase. Biochem J. 1999;338:643–9. [PMC free article] [PubMed] [Google Scholar]
- 41.Sato K, Nagayama H, Tadokoro K, Juji T, Takahashi TA. Extracellular signal-regulated kinase, stress-activated protein kinase/c-Jun N-terminal kinase, and p38mapk are involved in IL-10-mediated selective repression of TNF-alpha-induced activation and maturation of human peripheral blood monocyte-derived dendritic cells. J Immunol. 1999;162:3865–72. [PubMed] [Google Scholar]
- 42.Puig-Kroger A, Relloso M, Fernandez-Capetillo O, Zubiaga A, Silva A, Bernabeu C, Corbi AL. Extracellular signal-regulated protein kinase signaling pathway negatively regulates the phenotypic and functional maturation of monocyte-derived human dendritic cells. Blood. 2001;98:2175–82. doi: 10.1182/blood.v98.7.2175. [DOI] [PubMed] [Google Scholar]
- 43.Iijima N, Yanagawa Y, Clingan JM, Onoe K. CCR7-mediated c-Jun N-terminal kinase activation regulates cell migration in mature dendritic cells. Int Immunol. 2005;17:1201–12. doi: 10.1093/intimm/dxh297. [DOI] [PubMed] [Google Scholar]