Abstract
Granulocyte–macrophage colony-stimulating factor (GM-CSF) has long been found to have growth-promoting effects on multipotent haematopoietic lineages, specifically granulocytes and macrophages. GM-CSF combined with interleukin-4 (IL-4) drives monocytes to become myeloid dendritic cells (mDCs) in vitro. We report that culturing human monocytes with GM-CSF alone generates myeloid cells (GM-Mono) that have lower expression of CD14 than monocytes and that fail to express DC-SIGN. GM-Monos, however, express CD83 and the transcription factor PU.1, although at a lower level than the conventional mDCs generated in the presence of GM-CSF and IL-4. On stimulation with tumour necrosis factor-α, interferon-γ and anti-CD40 monoclonal antibody, the GM-Monos predominantly produced IL-10 but were less efficient in IL-12 production. In a primary allogeneic mixed lymphocyte reaction, GM-Monos induced hyporesponsiveness and IL-10-biased cytokine production in CD4+ T cells. In fresh mixed lymphocyte reaction, GM-Monos inhibited conventional mDC-induced allogeneic CD4+ T-cell proliferation. GM-Mono-induced inhibition of allogeneic CD4+ T-cell proliferation was partially attributed to IL-10. Interestingly, GM-Monos neither induced hyporesponsiveness in allogeneic CD8+ T cells nor inhibited conventional mDC-induced allogeneic CD8+ T-cell proliferation. Taken together, we characterize monocyte-derived CD14low CD83+ cells generated by GM-CSF that can induce tolerance or stimulation of T cells depending on T-cell subsets.
Keywords: granulocyte–macrophage colony-stimulating factor, myeloid cells, tolerance
Introduction
Granulocyte–macrophage colony-stimulating factor (GM-CSF), a 23 000 molecular weight (MW) heavily glycosylated cytokine, is known to have growth-promoting activities on haematopoietic cell lineages. This cytokine was identified in lung-tissue-conditioned medium from lipopolysaccharide-injected mice, as a component showing stimulatory activity on the proliferation of mouse bone marrow cells in vitro, generating colonies of both granulocytes and macrophages.1 GM-CSF is known to stimulate multipotent progenitors depending on its concentration.2 GM-CSF, in both humans and mice, was cloned from a complementary DNA library of activated T lymphocytes.3,4 Various subsets of T lymphocytes have been found to produce GM-CSF on activation, although its direct effect on the T lymphocytes is not yet clear. Most of the demonstrated effects of GM-CSF are believed to be mediated by the antigen-presenting cells.5
Therapeutic uses of GM-CSF, like granulocyte colony-stimulating factor (G-CSF), mainly involve treating neutropenia of different aetiologies.6 Specifically, for treating chemotherapy-induced neutropenia and for expansion of haematopoietic progenitors before autologous transplantation, GM-CSF and G-CSF have been in extensive use. Although they surely have growth-promoting activity on multilineage haematopoietic cells, they might also have some immunomodulatory activities brought about by differential effects on different immune cells.
Conventionally, immature human myeloid dendritic cells (mDCs) are generated by culturing human peripheral blood monocytes with GM-CSF and interleukin-4 (IL-4).7 Dendritic cells are the most potent antigen-presenting cells and have an important role in the control of the adaptive immune response.8–11 In humans, at least two types of DCs have been established: plasmacytoid DCs (pDCs) and mDCs, which differ both phenotypically and functionally. The pDCs have features of a lymphoid lineage, produce type I/II interferons and elicit a T helper type 2 (Th2) response.12,13 The mDCs, on the other hand, express myeloid antigens, produce IL-12 and induce a Th1 response.12 Beyond the established heterogeneity of DCs in vivo as a result of the presence of these mDCs and pDCs, there is also speculation about DCs with tolerogenic properties in humans. Several studies have reported a role for DCs in the induction of peripheral tolerance.14,15In vitro experiments demonstrated that human mDCs with an immature phenotype induce differentiation of anergic regulatory T cells.16,17 However, other studies in mice have shown that semi-mature rather than completely immature DCs can yield tolerant naive CD4+ T cells.18 Although yet to be established in humans, reports have already indicated the presence of such tolerogenic DCs in mice.19 In mice, tolerogenic DCs could be induced by treating bone marrow cells with IL-10. Mouse tolerogenic DCs derived in vitro display plasmacytoid morphology and secrete high levels of IL-10 after stimulation.19
G-CSF has been reported to modify ex vivo cytokine production by the leucocytes.20 It has also been shown to generate tolerogenic antigen-presenting cells producing IL-10 that could suppress graft-versus-host disease when cotransplanted in allogeneic recipient animals.21 Other studies have reported antigen-specific T-cell suppression by G-CSF-treated DCs.22 On the other hand, GM-CSF had been shown to differentiate monocytes preferentially to DC2 type DCs in the presence of increased intracellular calcium.23 In the present study, we report that GM-CSF transforms human peripheral blood monocytes to CD14low CD83+ DC-SIGN– tolerogenic myeloid cells that predominantly produce IL-10 and simultaneously induces tolerance in the CD4+ T cells but stimulation in CD8+ T cells against alloantigens. Our data suggest immunomodulatory activities for GM-CSF in addition to its growth-promoting activities.
Materials and methods
Reagents
Complete medium consisted of RPMI-1640, 1%l-glutamine, 1% penicillin/streptomycin, 1% essential amino acids and 2% heat-inactivated fetal calf serum (all from Life Technologies, New Delhi, India). Monoclonal antibodies (mAbs) used in cell surface analysis by flow cytometry including fluorescein isothiocyanate- (FITC) or phycoerythrin-conjugated mouse anti-human CD3, CD4, CD8, CD16, CD32, CD64, CD40, CD80, CD83, CD86, DC-SIGN, HLA-DR, isotype-matched control mAbs, purified antibodies to human CD3, CD28, CD40, the neutralizing anti-human IL-10 without sodium azide and the recombinant human cytokines IL-4, GM-CSF, tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) were procured from BD Biosciences (Mountain View, CA). Purified mAbs to the transcription factor PU.1, human toll-like receptor 2 (TLR-2) and TLR-4 were from Santa Cruz Biotechnology (Santa Cruz, CA). The mAbs to human CD14, the microbead-tagged antibodies and the magnetic separation columns were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany).
Cell culture
Human peripheral blood mononuclear cells (PBMCs) were isolated from freshly drawn heparinized blood from healthy volunteers by Ficoll–Hypaque density gradient centrifugation. Peripheral blood samples were collected with due approval from the Human Ethics Committee of the institute and all experiments with human blood were conducted under an approved institutional Human Ethics Committee protocol. Informed consent was provided according to the Declaration of Helsinki. Monocytes were purified by seeding PBMCs in bacteriological plastic dishes coated with human immunoglobulin G for a 2-hr adherence followed by removal of the non-adherent cells.24 The adherent cells were found to be 95% monocytes as assessed by CD14 staining by flow cytometry. The monocytes (0·2 × 106/ml) were cultured in complete medium alone, in complete medium containing GM-CSF (30 ng/ml) alone or in complete medium containing GM-CSF (30 ng/ml) plus IL-4 (10 ng/ml) in a total volume of 1 ml for 4 days to generate the immature DCs. For maturation, a 4-day priming culture was followed by a 2-day differentiation culture in which IFN-γ (100 ng/ml), TNF-α (20 ng/ml) and anti-CD40 mAb (5 μg/ml) were added.
Flow cytometry
Flow cytometry was performed to define the phenotypic characteristics of the cells cultured in the presence of the indicated cytokines and to quantify cytokines in the culture supernatants by Cytometric Bead Array™ Multiplex assays. Cell surface markers were analysed after staining with FITC- or phycoerythrin-labelled antibodies and isotype-matched control antibodies. For staining with anti-TLR antibodies, cells were first stained with purified primary antibodies followed by staining with FITC-labelled goat anti-mouse secondary antibody (multiple adsorbed). For assessing the intracellular level of the transcription factor PU.1, staining was performed after permeabilizing the cells with FACS™ Permeabilizing Solution (BD Biosciences).25 Analysis was performed using a BD LSR™ flow cytometer (Becton Dickinson, San Jose, CA). Data on immunophenotyping were analysed on Cell Quest™ software (Becton Dickinson). Cytometric Bead Array™ (CBA) Multiplex assays for cytokines were performed following the manufacturer's instructions using the Cba Analysis software (Becton Dickinson). Results on cytokines obtained by CBA assay were validated by commercial enzyme-linked immunosorbent assay kits (R & D Systems, Minneapolis, MN).
Phagocytosis
The cells harvested from the cultures were resuspended (at 5 × 105 cells/ml) in complete medium with 5 μl PerCp-latex beads (3 μm in diameter; BD Biosciences) and were mixed well. The cells were incubated with the beads at 37° overnight. After incubation the cells were washed five times with ice-cold phosphate-buffered saline and then fixed in 1% paraformaldehyde before flow cytometric analysis.
T-cell subset isolation
Non-adherent PBMC were first depleted of B cells, natural killer cells and contaminating monocytes by incubation with microbead-coupled anti-CD19, anti-CD56 and anti-CD14 mAbs followed by magnetic separation. CD4+ CD45RO– and CD8+ CD45RO– T-cell subsets were purified from depleted non-adherent PBMC using CD4 or CD8 Multisort kits. After isolating positively selected CD4+ or CD8+ T-cell subsets, the magnetic particles were enzymatically released and further incubated with CD45RO microbeads for depletion of CD45RO+ cells following the manufacturer's instructions (Miltenyi Biotec). The purity (as determined by flow cytometry) and viability of each population was more than 95%.
Allogeneic mixed leucocyte reaction
Graded numbers of cultured monocytes, the GM-CSF-driven myeloid cell subset (GM-Mono) or conventional mDCs (GM + IL4-DCs), after being stimulated with TNF-α, IFN-γ and anti-CD40 mAb, were added to 1 × 105 allogeneic T-cell subsets (CD4+ CD45RO– or CD8+ CD45RO–) in 96-well, flat-bottomed tissue culture plates in the presence or absence of anti-IL-10 mAb (10 μg/ml) or isotype-matched control mAb and incubated for 6 days. Proliferation was determined by the addition of 0·5 μCi [3H]thymidine (New England Nuclear, Boston, MA) per well during the last 16 hr of the culture period and subsequent measurement of the incorporated radioactivity in a liquid scintillation counter (Perkin Elmer Life Sciences, Boston, MA). Parallel allogeneic mixed leucocyte reactions (MLRs) were set up with 1 × 105 of the above-mentioned cells and 1 × 106 allogeneic T-cell subsets per well in 48-well flat-bottomed tissue culture plates. After 6 days of culture, T-cell subsets were recovered from primary MLR, re-purified by magnetic separation and then re-stimulated on anti-CD3-coated and anti-CD28-coated 96-well plates at 1 × 105 cells per well. Supernatants were harvested 24 hr later and analysed for cytokines. In selected experiments allogeneic MLR were set up with mature conventional mDCs as stimulators (1 × 104 cells) along with graded numbers of stimulated GM-Monos or the mature GM + IL4-DCs from the same donor and allogeneic T-cell subsets (1 × 105) as responders in the presence or absence of anti-IL-10 antibody or isotype-matched control mAb. T-cell proliferation was measured by [3H]thymidine uptake.
Statistical analysis
Data are expressed as mean ± standard error of the mean and were assessed using the Student's t-test.
Results
GM-CSF induces differentiation of CD14+ human peripheral blood monocytes to CD14low CD83+ DC-SIGN− cells
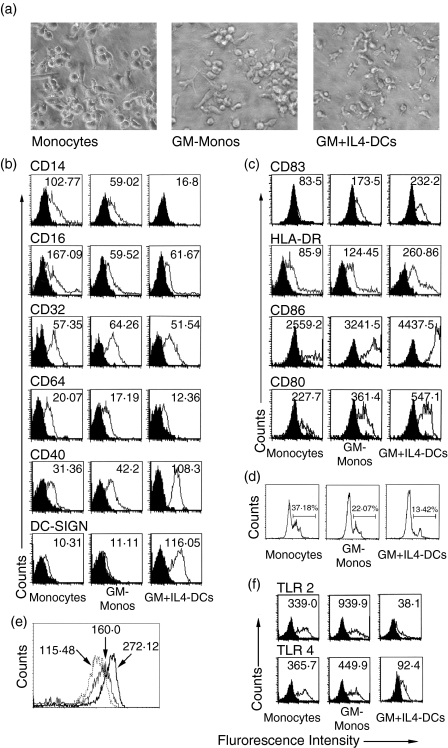
Conventionally, human mDCs are generated in vitro by culturing human peripheral blood monocytes in the presence of GM-CSF plus IL-4. Recently we reported that IL-4 alone, without the involvement of GM-CSF, transforms human peripheral blood monocytes to a CD1adim CD83+ DC subset.25 GM-CSF alone had also been shown to be sufficient to transform human monocytes to DCs in the presence of calcium ionophore23 while other studies have designated the GM-CSF-driven monocytes to be macrophages.26 To compare the phenotypic and functional properties of the GM-CSF-driven monocytes with that of conventional mDCs, human peripheral blood monocytes were cultured in medium alone, in medium containing GM-CSF alone or in medium containing GM-CSF plus IL-4 for 4 days followed by a 2-day maturation period in the presence of TNF-α, IFN-γ and anti-CD40 mAb. Morphologically, conventional mDCs (generated in the presence of GM-CSF plus IL-4; designated as GM + IL4-DC) had typical DC-like veiled structures. On the other hand, monocytes cultured with GM-CSF alone (designated as GM-Mono) had barely detectable DC-like veiled structures (Fig. 1a).
Figure 1.
Morphology, surface phenotype and phagocytic activity of human peripheral blood monocytes cultured in the presence of GM-CSF. (a) Morphological changes of human peripheral blood monocytes after culture for 4 days with media alone (monocytes), GM-CSF (30 ng/ml) alone (GM-Monos) or GM-CSF plus IL-4 (GM + IL4-DCs). Phase contrast micrographs, 400× using Olympus microscope and Olympus Camedia digital camera. (b) Flow cytometric determination of surface phenotypes of unstimulated cells after staining with isotype-matched control mAbs (filled histograms) or with the mAbs indicated (solid lines). Expression of the surface markers CD14, CD16, CD32, CD64, CD40 and DC-SIGN were assessed after 4 days of culture. (c) CD83, HLA-DR, CD86 and CD80 were analysed on the cells after stimulation with TNF-α, IFN-γ and anti-CD40 mAb. Values within histograms represent the specific mean fluorescence intensity (after subtracting the control values). (d) Comparative flow cytometric assessment of phagocytic activity of the cultured monocytes was performed by incubating the unstimulated cells with PerCp-labelled latex beads overnight at 37° and then analysing the washed cells by flow cytometry. The gated cells represent the fraction of cells showing phagocytosed beads. (e) The expression level of intracellular PU.1 was assessed by intracellular staining of the unstimulated cells with anti-PU.1 mAb and FITC-labelled secondary antibody. The respective mean fluorescence intensities are shown by arrowheads. The dotted line represents the cultured monocytes while the thin and thick lines represent the GM-Monos and the GM + IL4-DCs, respectively. (f) Flow cytometric evaluation of indicated toll-like receptors on the surface of unstimulated human peripheral blood monocytes after culture for 4 days in the presence or absence of indicated cytokines. Results are representative of four experiments.
The GM-CSF-driven monocytes were found to be low on the surface expression of CD14 compared to the cultured monocytes, while the GM + IL4-DCs did not show any expression of CD14 (Fig. 1b). The expression of the Fcγ receptors CD16, CD32 and CD64 were also assessed on these unstimulated cells. Both GM-Monos and GM + IL4-DCs had lower CD16 expression compared to the monocytes. However, expression of CD32 and CD64 were low and similar in cultured monocytes, GM-Monos and GM + IL4-DCs (Fig. 1b). The unstimulated GM-Monos were found to express CD40 but the level of expression was lesser than the conventional DCs, although higher than the monocytes (Fig. 1b). Of note, the unstimulated GM-Monos did not express the DC-specific marker DC-SIGN while this marker was readily expressed on unstimulated GM + IL4-DCs (Fig. 1b).
As the monocytes cultured in the presence of GM-CSF were CD14low and DC-SIGN–, we evaluated the expression of MHC class II, costimulatory molecules (CD80 and CD86) and another mature DC marker, CD83 on these cells after stimulation with TNF-α, IFN-γ and anti-CD40 mAb. The level of expression of these molecules in GM-Monos was higher than in monocytes but lower than in the conventional mDCs (Fig. 1c).
GM-Monos have reduced phagocytic efficacy compared to the monocytes
As the GM-Monos were CD83+, we evaluated the phagocytic efficacy of these cells. Myeloid DCs are known to rapidly lose their phagocytic activity in culture.28 After addition of PerCp-labelled latex beads to the cultures and incubation with the cells overnight, cells were assessed for their phagocytic activity by flow cytometry (Fig. 1d). While 37·18% of the monocytes were found to phagocytose the beads overnight, among the GM-Monos the fraction was 22·07%. The fraction of phagocytic cells among the conventional mDCs was even lower (13·42%).
GM-Monos have higher expression of PU.1 than the monocytes
We wanted to see whether the transcription factor PU.1, which is considered to be a marker for DC commitment for the myeloid cells,27 is expressed in the GM-Monos. The cells from all three groups after 4 days of culture were assessed for intracellular PU.1 levels by flow cytometry. The expression of PU.1 in GM-Monos was found to be midway between the expression found in cultured monocytes and GM + IL4-DCs (Fig. 1e), indicating that the GM-Monos attained a quasi-DC phenotype in terms of transcriptional control.
GM-Monos have higher expression of TLR-2 and TLR-4
It has been reported by several groups that the TLRs play a vital role in the DC phenotypes and fine tuning of the balance among the different TLRs expressed on the antigen-presenting cells is a very important determinant in the immunological decision making. Cell surface expression patterns of TLR-2 and TLR-4 were very different on cells cultured under different cytokine conditions (Fig. 1f). Expression of both TLR-2 and TLR-4 were higher on GM-Monos compared to the conventional mDCs (Fig. 1f).
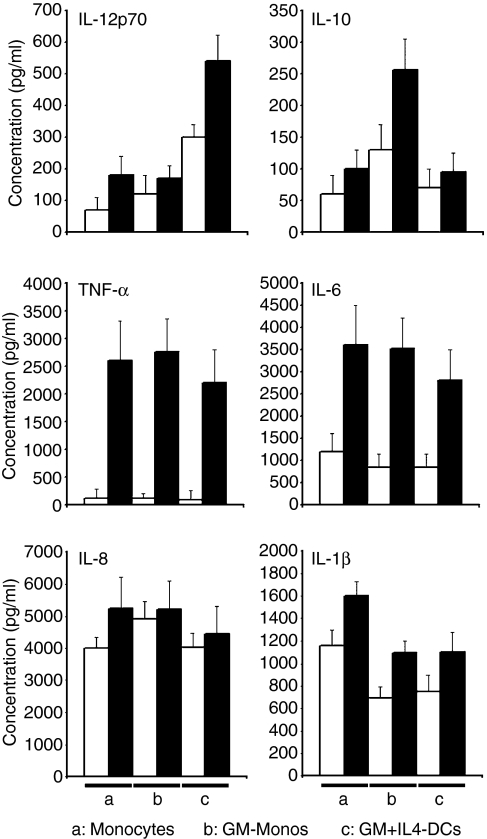
GM-Monos predominantly produce IL-10 after stimulation
The conventional mDCs predominantly produce IL-12 after stimulation with TNF-α, IFN-γ and anti-CD40 mAb. A large amount of IL-12 was also detectable in these DC cultures even before stimulation. These DCs produced detectable but small amounts of IL-10. In contrast, stimulated GM-Monos produced ∼ 2·5-fold more IL-10 than the conventional mDCs. GM-Monos showed less efficient IL-12 production (∼ 70% less compared to the conventional mDCs) (Fig. 2). No appreciable differences were noticed with respect to TNF-α, IL-6, IL-8 and IL-1β production by these cells.
Figure 2.
GM-Monos predominantly produce IL-10 after stimulation. Human monocytes were cultured as indicated and then either left unstimulated (white bars) or stimulated (black bars) with TNF-α (20 ng/ml), IFN-γ (100 ng/ml) and anti-CD40 mAb (5 μg/ml) for 1 day. Cytokines were quantified in the supernatants by Cytometric Bead Array™ Multiplex assays. Results are mean ± SEM of triplicate culture from a single experiment. Similar results were obtained from three other experiments.
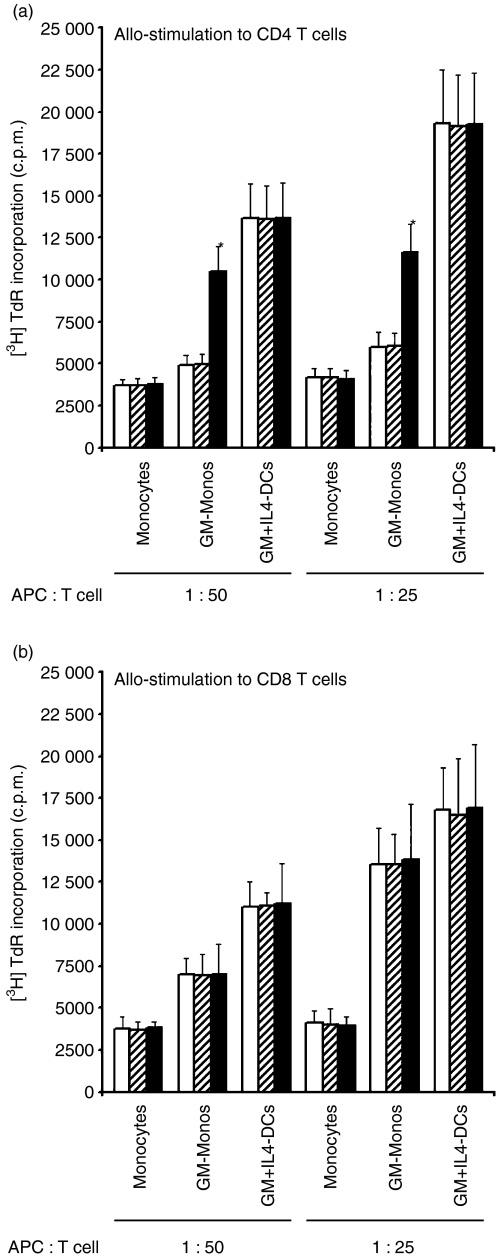
GM-Monos induce hyporesponsiveness of CD4+ T cells but stimulation of CD8+ T cells against alloantigens
To measure the effects of GM-Monos on responses to alloantigens, naive CD4+ and CD8+ T cells were cocultured with stimulated allogeneic cultured monocytes, GM-Monos and GM + IL4-DCs. In all five experiments, the [3H]thymidine uptake by CD4+ T cells in monocytes or GM-Mono cocultures was consistently less in all stimulator to responder ratios tested compared to GM + IL4-DC cocultures (Fig. 3a). Inhibition of allogeneic CD4+ T-cell proliferation in GM-Mono-stimulated culture was significantly reversed by the addition of neutralizing anti-IL-10 antibody while isotype-matched control mAb had no effects. However, cultured monocyte-induced hyporesponsiveness of allogeneic CD4+ T-cell proliferation was not reversed by the addition of neutralizing anti-IL-10 mAb. Addition of anti-IL-10 antibody or isotype-matched control mAb had no effects on allogeneic CD4+ T-cell proliferation in mature GM + IL4-DC-stimulated cultures (Fig. 3a). These data suggest that the tolerogenic effects on CD4+ T cells is induced by the GM-CSF treatment of monocytes and mediated at least in part by IL-10-driven mechanism.
Figure 3.
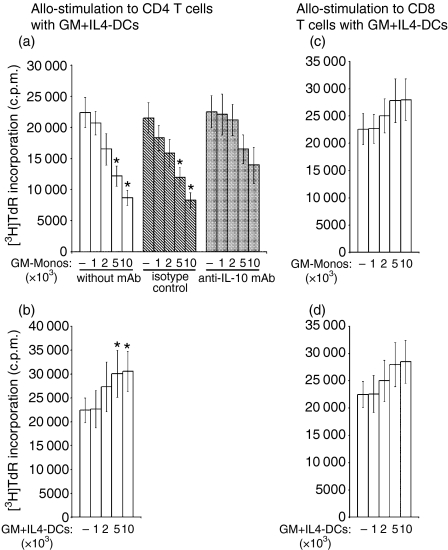
GM-Monos induce hyporesponsiveness to CD4+ T cells but stimulation to CD8+ T cells against alloantigens. (a) Allo-MLRs were initiated using the TNF-α, IFN-γ and anti-CD40 mAb stimulated monocyte cultures indicated as stimulators and purified allogeneic CD4+ T cells as responders. Experiments were set up with neutralizing anti-human IL-10 mAb (10 μg/ml) (black bars), isotype-matched control mAb (hatched bars) or without any antibody (white bars) at the onset of the assays. *P < 0·05 compared to cultures in the absence of anti-IL-10 mAb. (b) Allo-MLRs were initiated as described in the legend of Fig. 3(a) except that purified allogeneic CD8+ T cells were used as the responders. Results are mean ± SEM of triplicate cultures from a single experiment. Similar results were obtained from two other experiments.
Unlike CD4+ T cells, allogeneic CD8+ T-cell proliferation in GM-Mono-stimulated cultures was lower than that observed with mature GM + IL4-DC-stimulated cultures (Fig. 3b). However, the difference was only marginal at the higher antigen-presenting cell to T cell ratio of 1:25. Of note, anti-IL-10 antibody had no effects on the allogeneic CD8+ T-cell proliferation in any of these allo-MLRs (Fig. 3b).
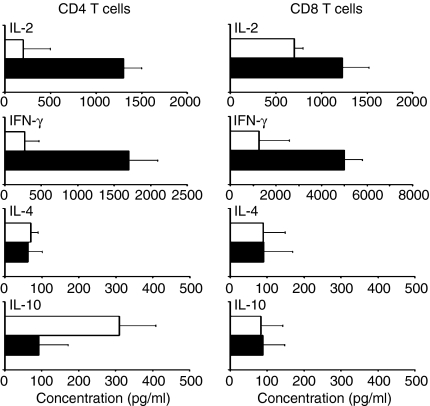
GM-Mono-stimulated allogeneic CD4+ and CD8+ T cells differ in IL-10 production
GM + IL4-DC-stimulated allogeneic CD4+ T cells produced mainly IFN-γ and IL-2 but little IL-10 after re-stimulation with anti-CD3 and anti-CD28 mAbs. In contrast, GM-Mono-stimulated allogeneic CD4+ T cells barely produced IFN-γ or IL-2 but their IL-10 production was enhanced (Fig. 4a). IL-10 was, however, barely detectable in GM-Mono-stimulated allogeneic CD8+ T cells. IL-2 and IFN-γ production by GM-Mono-stimulated allogeneic CD8+ T cells was also impaired (Fig. 4b).
Figure 4.
GM-Monos induce IL-10-biased cytokine production in allogeneic CD4+ T cells. Allogeneic CD4+ T cells (a) or CD8+ T cells (b) were cocultured with GM-Monos (white bars) or GM + IL4-DCs (black bars) after stimulation with ΤΝF-α, IFN-γ and anti-CD40 mAb (at a stimulator:responder ratio of 1:10) for 6 days. T cells were harvested, washed and restimulated on plates coated with anti-human CD3 and anti-human CD28 mAbs for 24 hr. Supernatants were collected and analysed by Cytometric Bead Array™ Multiplex assays. Results are mean ± SEM of triplicate culture from a single experiment out of four experiments with similar results.
GM-Monos can inhibit proliferation of allogeneic CD4+ T cells in a fresh MLR
We next evaluated the effects of GM-CSF-driven monocytes on fresh cocultures of allogeneic MLR. Graded numbers of semi-mature GM-Monos were added to a new MLR containing mature GM + IL4-DCs (104/well) from the same donor as stimulators and allogeneic CD4+ T cells (105/well) as responders. GM-Monos, in a dose-dependent manner, inhibited allogeneic CD4+ T-cell proliferation induced by the conventional mature GM + IL4-DCs (Fig. 5). This dampening of allostimulation was partially reversed by anti-IL-10 mAb. Autologous mature GM + IL4-DCs, under identical experimental conditions, had additive allostimulatory effects (Fig. 5) and remained unaffected by neutralizing anti-IL-10 mAb (data not shown). In contrast to the effect on allogeneic CD4+ T cells, the proliferation of mature GM + IL4-DC-induced allogeneic CD8+ T cells in fresh MLR was enhanced by the semi-mature GM-Monos in a dose-dependent manner (Fig. 5).
Figure 5.
GM-Monos inhibit CD4+ T cells but stimulate CD8+ T cells in a fresh allo-MLR induced by mature conventional mDCs (GM + IL4-DCs). GM-Monos and the conventional mDCs (GM + IL4-DCs) of the same donor were prepared as described in the Materials and methods. Fresh allo-MLRs were initiated with mature conventional mDCs and allogeneic CD4+ (a,b) or CD8+ (c,d) T cells (stimulator:responder ratio, 1:10). At the onset of the assays, graded numbers of stimulated GM-Monos (a,c) or GM + IL4-DCs (b,d) were added. Experiments with neutralizing anti-human IL-10 mAb (10 μg/ml) (grey bars), isotype-matched control mAb (hatched bars) were also set up in addition to cultures without any antibody (white bars). Results are mean ± SEM of triplicate cultures from a single representative experiment out of three experiments with similar results. *P < 0·05 compared to allo-MLR cultures containing mature GM + IL4-DCs and allogeneic CD4+ or CD8+ T cells only.
Discussion
In this study we demonstrate that GM-CSF, which is routinely used in combination with IL-4 to generate conventional mDCs (GM + IL4-DCs), if used alone can transform CD14+ human monocytes to a CD14low CD83+ DC-SIGN– cell subset. The GM-CSF-driven monocytes (GM-Monos) also showed moderate expression of HLA-DR, CD86 and CD80 after stimulation with TNF-α, IFN-γ and anti-CD40 mAb, thus attaining a quasi-DC phenotype, although they were DC-SIGN–. A previous report asserts that GM-CSF regulates the expression of MHC class II molecules through CIITA (class II transactivator) types I/III and enhances the expression of MHC II and other costimulatory molecules on monocytes.29
It has been shown earlier that mouse DCs generated by culturing bone marrow precursor cells in the presence of only low concentrations of GM-CSF are maturation-resistant and are capable of prolonging allograft survival in vivo.30 Our data indicate that human GM-CSF even at a concentration of 30 ng/ml, which is conventionally used along with IL-4 to generate human mDCs, when used alone can transform human monocytes to a semi-mature DC-like myeloid cell subtype. These GM-Monos differ from the conventional mDCs both phenotypically and functionally.
The transcription factor PU.1 is known to be required for proper differentiation of both lymphoid and myeloid lineage cells.31,32 Particularly for mDC development, PU.1 is considered indispensable.27 In the in vitro development of mDCs from monocytes, expression of PU.1 is known to be the marker for commitment towards DCs rather than macrophages. The level of expression of PU.1 in the GM-Monos was found to be midway between that in the cultured monocytes and the mDCs (GM + IL4-DCs), thus indicating their commitment towards a DC-like phenotype.
Like the conventional GM + IL4-DCs, the GM-Monos had also lost their phagocyte activity considerably, compared to the cultured monocytes. The phagocytic efficacy of the GM-Monos was in between that of the monocytes and GM + IL4-DCs, providing further support for the notion that GM-Monos represent an intermediate phenotype of myeloid cells on the way to commitment towards DCs.
Interestingly, GM-Monos produce predominantly IL-10 and less IL-12 after stimulation, in striking contrast to conventional mDCs, which produce more IL-12 than IL-10. This is in agreement with the report that GM-CSF-induced monocytes produce minimal or no IL-12, although GM-CSF + IL-4-induced DCs produce high levels of IL-12.33 GM-CSF has also been shown to inhibit IL-12 production by the murine Langerhans cells.34 Previous reports also showed that GM-CSF induces the production of IL-10 in vitro from the human monocytes33 and the human monocytic cell line U937.35 Enhanced serum IL-10 was also reported in patients with aggressive non-Hodgkin's lymphoma treated with GM-CSF.36 IL-10 is a cytokine with potent anti-inflammatory activities, capable of inhibiting the production of several proinflammatory cytokines including IL-12 in different cell types.37,38
The expression patterns of TLR-2 and TLR-4 in GM-Monos and conventional mDCs are also intriguing. Although both types of myeloid cells express these TLRs, GM-Monos have higher surface expression of both the TLRs compared to conventional mDCs.
After receiving maturation signals, human GM-Monos acquire a semi-mature-DC-like phenotype, act as anti-inflammatory accessory cells and induce hyporesponsiveness in allogeneic CD4+ T cells in contrast to mature human conventional mDCs. Interestingly, the MLR response of allogeneic CD4+ T cells to mature conventional mDCs was also inhibited by semi-mature autologous GM-Monos. Our data suggest that GM-CSF-driven monocytes may induce anergy in CD4+ T cells at least in part through an IL-10-driven mechanism.
Similar to immature human DC, semi-mature DC-induced T-cell tolerance in mice was found to be dependent on IL-10-producing regulatory T cells.39 IL-10-producing DCs have been identified in Peyer's patches and the liver in mice is associated with induction of either T-cell anergy or the Th2 response.40 Treatment of mouse bone marrow-derived DCs with IL-10 induces differentiation of a distinct subset of IL-10-producing tolerogenic DCs.19 Human GM-Monos generated in vitro produce large amounts of IL-10 and were tolerogenic to CD4+ T cells. Whether anergic CD4+ T cells induced by GM-CSF-driven monocytes have a regulatory function is yet to be determined. The possibility is strengthened by the fact that human GM-Monos induce an IL-10-biased cytokine pattern in tolerized allogeneic CD4+ T cells. Also, the CD4+ CD25+ regulatory T cells in GM-CSF induced suppression of experimental autoimmune thyroiditis in mice has been reported.41
Interestingly, the GM-Monos do not induce tolerance to allogeneic CD8+ T cells, although the magnitude of allostimulation to these cells was marginally lower than that induced by the conventional mDCs. Reduced production of IFN-γ and IL-2 by GM-Mono-driven allogeneic CD8+ T cells also supports their suboptimal activation state. A recent report on simultaneous induction of tolerance of CD4+ T cells and stimulation of CD8+ T cells by semi-mature DCs in mice supports our finding.18 This differential activation pattern of CD4+ and CD8+ T cells by the GM-Monos can be explained by enhanced production of IL-10. IL-10 has been shown to suppress T-cell activation by inhibiting the CD28 costimulatory pathway.42 CD28 costimulation, although very important in TCR-mediated CD4+ T-cell activation, is found to be less important in case of the CD8+ T cells.43,44 Also, the CD28 molecules are expressed on about 50% of the human CD8+ T cells, while about 90% of the CD4+ T cells express CD28 on their surface.45 So, the GM-Mono-derived IL-10 might have dampened the proliferation of the CD4+ T cells by inhibiting the CD28 costimulatory pathway, but could not affect the CD8+ T cells because of the redundancy of this pathway in these cells.
Taken together, our data demonstrate that GM-CSF transforms human monocytes to a cellular phenotype that predominantly produces IL-10 and induces anergy to CD4+ T cells but stimulates CD8+ T cells against alloantigens. Thus this report adds to the growing evidence of a continuous phenotypic spectrum of differentiated human myeloid cells starting from monocytes to mature DCs. It also adds to the growing knowledge about the possible immunomodulatory effects of GM-CSF on human blood cells in addition to its long-known growth-promoting activities. This novel finding about the tolerance-promoting effect of GM-CSF in vitro will be very important in designing rational therapeutic regimens of this cytokine in different clinical settings in humans, making use of its immunomodulatory activities.
Acknowledgments
This study was supported in part by the Council of Scientific and Industrial Research, New Delhi including Grant No. CMM-0002.
Abbreviations
- CBA
cytometric bead array
- DC
dendritic cell
- G-CSF
granulocyte colony-stimulating factor
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- IFN
interferon
- IL
interleukin
- mAb
monoclonal antibody
- mDC
myeloid dendritic cell
- MLR
mixed leucocyte reaction
- PBMC
peripheral blood mononuclear cell
- pDC
plasmacytoid dendritic cell
- Th2
T helper type 2
- TLR
toll-like receptor
- TNF
tumour necrosis factor.
References
- 1.Burgess AW, Camakaris J, Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem. 1977;252:1998–2003. [PubMed] [Google Scholar]
- 2.Burgess AW, Metcalf D. The nature and action of granulocyte–macrophage colony stimulating factors. Blood. 1980;56:947–58. [PubMed] [Google Scholar]
- 3.Cantrell MA, Anderson D, Cerretti DP, et al. Cloning, sequence and expression of a human granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci USA. 1985;82:6250–4. doi: 10.1073/pnas.82.18.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gough NM, Metcalf D, Gough J, Grail D, Dunn AR. Structure and expression of the mRNA for murine granulocyte–macrophage colony stimulatory factor. EMBO J. 1985;4:645–53. doi: 10.1002/j.1460-2075.1985.tb03678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wada H, Noguchi Y, Marino MW, Dunn AR, Old LJ. T cell functions in granulocyte/macrophage colony-stimulating factor deficient mice. Proc Natl Acad Sci USA. 1997;94:12557–61. doi: 10.1073/pnas.94.23.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sylvester RK. Clinical applications of colony-stimulating factors: a historical perspective. Am J Health-Syst Pharm. 2002;59:s6–s12. doi: 10.1093/ajhp/59.suppl_2.S6. [DOI] [PubMed] [Google Scholar]
- 7.Romani N, Reider D, Heuer M, Ebner S, Kampgen E, Eibl B, Niederwieser D, Schuler G. Generation of mature dendritic cells from human blood, an improved method with regard to clinical applicability. J Immunol Meth. 1996;196:137–51. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 8.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 9.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 10.Inaba K, Metlay JP, Crowley MT, Steinman RM. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med. 1990;172:631–40. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanzavecchia A, Sallusto F. The instructive role of dendritic cells on T cell responses: lineages, plasticity and kinetics. Curr Opin Immunol. 2001;13:291–8. doi: 10.1016/s0952-7915(00)00218-1. [DOI] [PubMed] [Google Scholar]
- 12.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 13.Colonna M, Trinchieri G, Liu Y. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–26. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 14.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 15.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–8. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–22. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roncarolo MG, Levings MK, Traversari C. Differentiation of T regulatory cells by immature dendritic cells. J Exp Med. 2001;193:F5–F9. doi: 10.1084/jem.193.2.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleindienst P, Wiethe C, Lutz MB, Brocker T. Simultaneous induction of CD4+ T cell tolerance and CD8 T cell immunity by semimature dendritic dells. J Immunol. 2005;174:3941–7. doi: 10.4049/jimmunol.174.7.3941. [DOI] [PubMed] [Google Scholar]
- 19.Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–17. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 20.Hartung T, Docke WD, Gantner G, Krieger G, Saur A, Stevens P, Volk HD, Wendel A. Effect of granulocyte colony stimulating factor on ex vivo blood cytokine response in human volunteers. Blood. 1995;85:2482–9. [PubMed] [Google Scholar]
- 21.MacDonald KPA, Rowe V, Clouston JK, Welply JK, Kuns RD, Ferrara JLM, Thomas R, Hill GR. Cytokine expanded myeloid precursors function as regulatory antigen-presenting cells and promote tolerance through IL-10-producing regulatory T cells. J Immunol. 2005;174:1841–50. doi: 10.4049/jimmunol.174.4.1841. [DOI] [PubMed] [Google Scholar]
- 22.Rutella S, Bonanno G, Pierelli L, et al. Granulocyte colony-stimulating factor promotes the generation of regulatory DC through induction of IL-10 and IFN-α. Eur J Immunol. 2004;34:1291–302. doi: 10.1002/eji.200324651. [DOI] [PubMed] [Google Scholar]
- 23.Faries MB, Bedrosian I, Xu S, et al. Calcium signaling inhibits interleukin-12 production and activates CD83+ dendritic cells that induce Th2 cell development. Blood. 2001;98:2489–97. doi: 10.1182/blood.v98.8.2489. [DOI] [PubMed] [Google Scholar]
- 24.Young JW, Steinman RM. Accessory cell requirements for the mixed-leukocyte reaction and polyclonal mitogens, as studied with a new technique for enriching blood dendritic cells. Cell Immunol. 1988;111:167–82. doi: 10.1016/0008-8749(88)90061-5. [DOI] [PubMed] [Google Scholar]
- 25.Roy KC, Bandyopadhyay G, Rakshit S, Ray M, Bandyopadhyay S. IL-4 alone without the involvement of GM-CSF transforms human peripheral blood monocytes to a CD1adim, CD83+ myeloid dendritic cell subset. J Cell Sci. 2004;117:3435–45. doi: 10.1242/jcs.01162. [DOI] [PubMed] [Google Scholar]
- 26.Akagawa KS. Functional heterogeneity of colony-stimulating factor-induced human monocyte-derived macrophages. Int J Hematol. 2002;76:27–34. doi: 10.1007/BF02982715. [DOI] [PubMed] [Google Scholar]
- 27.Scott EW, Simon MC, Anastasi J, Singh H. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science. 1994;265:1573–7. doi: 10.1126/science.8079170. [DOI] [PubMed] [Google Scholar]
- 28.Reis e Sousa C, Stahl PD, Austyn JM. Phagocytosis of antigens by Langerhans cells in vitro. J Exp Med. 1993;178:509–19. doi: 10.1084/jem.178.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hornell TM, Beresford GW, Bushey A, Boss JM, Mellins ED. Regulation of the class II MHC pathway in primary human monocytes by granulocyte–macrophage colony-stimulating factor. J Immunol. 2003;171:2374–83. doi: 10.4049/jimmunol.171.5.2374. [DOI] [PubMed] [Google Scholar]
- 30.Lutz MB, Suri RM, Niimi M, Ogilvie AL, Kukutsch NA, Rossner S, Schuler G, Austyn JM. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur J Immunol. 2000;30:1813–22. doi: 10.1002/1521-4141(200007)30:7<1813::AID-IMMU1813>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 31.Anderson KL, Perkin H, Surh CD, Venturini S, Maki RA, Torbett BE. Transcription factor PU.1 is necessary for development of thymic and myeloid progenitor-derived dendritic cells. J Immunol. 2000;164:1855–61. doi: 10.4049/jimmunol.164.4.1855. [DOI] [PubMed] [Google Scholar]
- 32.Guerriero A, Langmuir PB, Spain LM, Scott EW. PU.1 is required for myeloid-derived but not lymphoid-derived dendritic cells. Blood. 2000;95:879–85. [PubMed] [Google Scholar]
- 33.Heystek HC, Mudde GC, Ohler R, Kalthoff S. Granulocyte–macrophage colony-stimulating factor (GM-CSF) has opposing effects on the capacity of monocytes versus monocyte-derived dendritic cells to stimulate the antigen-specific proliferation of a human T cell clone. Clin Exp Immunol. 2000;120:440–7. doi: 10.1046/j.1365-2249.2000.01225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tada Y, Asahina A, Nakamura K, Tomura M, Fujiwara H, Tamaki K. Granulocyte/macrophage colony-stimulating factor inhibits IL-12 production of mouse Langerhans cells. J Immunol. 2000;164:5113–9. doi: 10.4049/jimmunol.164.10.5113. [DOI] [PubMed] [Google Scholar]
- 35.Lehmann MH. Recombinant human granulocyte-macrophage colony-stimulating factor triggers interleukin-10 expression in the monocytic cell line U937. Mol Immunol. 1998;35:479–85. doi: 10.1016/s0161-5890(98)00043-1. [DOI] [PubMed] [Google Scholar]
- 36.Stasi R, Zinzani L, Galieni P, et al. Detection of soluble interleukin-2 receptor and interleukin-10 in the serum of patients with aggressive non-Hodgkin's lymphoma. Identification of a subset at high risk of treatment failure. Cancer. 1994;74:1792–800. doi: 10.1002/1097-0142(19940915)74:6<1792::aid-cncr2820740623>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 37.Moore KW, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–90. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 38.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 39.Verginis P, Li HS, Carayanniotis G. Tolerogenic semimature dendritic cells suppress experimental autoimmune thyroiditis by activation of thyroglobulin-specific CD4+CD25+ T cells. J Immunol. 2005;174:7433–9. doi: 10.4049/jimmunol.174.11.7433. [DOI] [PubMed] [Google Scholar]
- 40.Iwasaki A, Kelsall BL. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med. 1999;190:229–39. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gangi E, Vasu C, Cheatem D, Prabhakar SB. IL-10-producing CD4+CD25+ regulatory T cells play a critical role in granulocyte–macrophage colony-stimulating factor-induced suppression of experimental autoimmune thyroiditis. J Immunol. 2005;174:7006–13. doi: 10.4049/jimmunol.174.11.7006. [DOI] [PubMed] [Google Scholar]
- 42.Akdis CA, Joss A, Akdis M, Faith A, Blaser K. A molecular basis for T cell suppression by IL-10: CD28-associated IL-10 receptor inhibits CD28 tyrosine phosphorylation and phosphatidylinositol-3-kinase binding. FASEB J. 2000;14:1666–8. doi: 10.1096/fj.99-0874fje. [DOI] [PubMed] [Google Scholar]
- 43.Goldstein JS, Chen T, Brunswick M, Mostowsky H, Kozlowsky S. Purified MHC class I and peptide complexes activate naïve CD8+ T cells independently of the CD28/B7 and LFA-1/ICAM-1 costimulatory interactions. J Immunol. 1998;160:3180–7. [PubMed] [Google Scholar]
- 44.Wang B, Maile R, Greenwood R, Collins E, Frelinger J. Naive CD8+ T cells do not require costimulation for proliferation and differentiation into cytotoxic effector cells. J Immunol. 2000;164:1216–22. doi: 10.4049/jimmunol.164.3.1216. [DOI] [PubMed] [Google Scholar]
- 45.Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signaling. Nat Rev Immunol. 2003;3:939–51. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]