Abstract
Immunomodulatory dendritic cells (DCs) that induce antigen-specific T-cell tolerance upon in vivo adoptive transfer are promising candidates for immunotherapy of autoimmune diseases. The feasibility of such a strategy has recently proved its efficacy in animal models of allotransplantation and experimental allergic encephalitis, but the effect in inflammatory bowel disease has not yet been demonstrated. In severe combined immunodeficient (SCID) mice, adoptively transferred CD4+ CD25– T cells repopulate the lymphoid tissues and lead to development of chronic colitis characterized by CD4+ T-cell proliferation against enterobacterial extract in vitro. In this model, we adoptively transferred in-vitro-generated bone-marrow-derived DCs exposed to interleukin-10 (IL-10) and an enterobacterial extract. We show that these cells are CD11c positive with intermediate expression of CD40, CD80 and CD86 and have a diminished secretion of IL-6, IL-12 p40/70, tumour necrosis factor-α and keratinocyte-derived chemokine (KC) compared to DCs treated with enterobacterial extract alone. In vivo, these cells prevented weight loss in SCID mice adoptively transferred with CD4+ CD25– T cells, resulted in a lower histopathology colitis score and tended to result in higher serum levels of IL-1α, IL-10, IL-12, IL-13, IL-17, KC and monokine induced by interferon-gamma (MIG). These data underscore the potential of using immunomodulatory DCs to control inflammatory bowel disease and demonstrate its potential use in future human therapeutic settings.
Keywords: colitis, dendritic cells, interleukin-10, severe combined immunodeficiency, tolerance
Introduction
Dendritic cells (DCs) are thought to exist in at least three distinctive stages. Immature DCs are located in peripheral tissues and prepared to take up antigens. When DCs are activated, e.g. by pathogen-associated molecular patterns (PAMPs) from microbial products, they mature and migrate to the regional lymph node. Here they express high levels of major histocompatibility complex (MHC) II, costimulatory molecules and inflammatory cytokines such as interleukin-12 (IL-12) p70. This mature phenotype of DCs is associated with T-cell priming and initiation of an adaptive immune response.1 In the absence of PAMPs the immature DCs may take up autologous material from dying cells and tissue debris and develop into semi-mature DCs, which express intermediate levels of MHC class II and costimulatory molecules and secrete only low amounts of inflammatory cytokines. These cells are also able to migrate to the regional lymph nodes, but instead of immunity they induce T-cell tolerance for the autoantigens they are presenting and/or they may activate regulatory T cells, which complement the tolerogenic environment.1,2 The in vitro generation of such tolerogenic DCs for therapeutic use is obviously a major focus in autoimmunity research, and stimulation of immature DCs with IL-10,3 tumour necrosis factor-α (TNF-α),2 transforming growth factor-β1 (TGF-β1)4 and soluble CD835 has proved successful in a number of disease models.
In severe combined immunodeficient (SCID) mice, adoptively transferred CD4+ T cells repopulate the lymphoid tissues, including the gut-associated lymphoid tissues, and may lead to the development of chronic colitis.6 If assayed ex vivo, the transferred CD4+ T cells have been shown to proliferate vigorously when exposed to enterobacterial extract in vitro.7 Likewise, CD4+ T cells depleted of CD25+ regulatory T cells obtained from normal mice proliferate vigorously upon stimulation with enterobacterial extract in vitro, and this proliferation is restricted by MHC class II presentation, CD4 ligation and antigen processing by antigen-presenting cells.8,9 T-cell reactivity against enterobacterial antigens has also been demonstrated in patients with inflammatory bowel disease10,11 and it is therefore generally accepted that T-cell reactivity against commensal flora in the intestine is important for the initiation and maintenance of inflammatory bowel diseases.
Whereas mature immunogenic DCs have been tested thoroughly in clinical cancer vaccine trials, the potential of semi-mature tolerogenic DCs to suppress immune reactivity has only recently been demonstrated in murine models of allotransplantation,12–14 experimental autoimmune encephalomyelitis (EAE),2,5 myasthenia gravis4 and type 1 diabetes,15 and the effects of tolerogenic DCs in inflammatory bowel disease have not yet been demonstrated. In this report, we investigated the disease-ameliorating effect of adoptively transferred DCs cultured in IL-10 and pulsed with an enterobacterial extract before transfer in the SCID model of inflammatory bowel disease.
Materials and methods
Mice
Conventional 6- to 8-week-old female BALB/c mice and SCID mice were purchased from Taconic Europe (Ry, Denmark) and kept under controlled microbial conditions at the local animal facility of the Panum Institute. All animal experiments were performed in accordance with the permissions obtained from the Animal Ethical Committee, Copenhagen, Department of Justice.
Generation of bone marrow DCs
Dendritic cells were generated from bone marrow cells derived from BALB/c mice. Bone marrow cells from femurs and tibias were washed and cultured overnight in six-well plates (TPP, Trasadingen, Schwitzerland) at 2 × 106 cells/ml in 3 ml culture medium (CM)/well. Culture medium was RPMI-1640 with Glutamax supplemented with 10% fetal calf serum (FCS; Harlan Sera-Laboratory Ltd, Hillcrest, UK) and antibiotics. The next day, non-adherent cells were harvested and resuspended in CM containing 10 ng/ml granulocyte–macrophage colony-stimulating factor (GM-CSF) plus 20 ng/ml IL-4 (both from Peprotech, Rocky Hill, NJ) and cultured at 1 × 106 cells/ml in 3 ml CM/well. Fresh cytokines and medium were added on day 3. For different subsets of DCs, 40 ng/ml IL-10 (Peprotech) was added on day 6, 133 μg/ml enterobacterial extract on day 7 in different combinations and cells were harvested on day 8 using phosphate-buffered saline (PBS)/Trypsin/ethylenediaminetetraacetic acid solution (Gibco, Paisley, UK).
Induction of colitis
CD4+ T cells were isolated from the spleens of BALB/c mice with anti-CD4 Dynabeads and Detach-a-Bead (Dynal, Oslo, Norway) according to the manufacturer's instructions. These cells were subsequently depleted of CD25+ cells with phycoerythrin-conjugated anti-CD25 antibody (cat no. 130-091-013) and anti-phycoerythrin microbeads (cat. no. 130-048-801) kit from MACS Miltenyi (Bergisch Gladbach, Germany). CD4+ CD25– T cells were resuspended in PBS/1% bovine serum albumin (BSA) and transferred intraperitoneally (i.p.) to SCID mice, 3 × 105/mouse. Mice treated with DCs received 0·3 × 106 to 1 × 106 DC i.p. 2–3 hr in advance. The mice were then monitored once or twice a week for weight loss, loose stools, bloody diarrhoea and rectal prolapse.
Immunohistochemistry
For histological examinations, the distal 2 cm of rectum was fixed in 4% paraformaldehyde. The samples were embedded in paraffin, cut by serial microtomy and stained with haematoxylin & eosin. The specimens were all examined in a blinded fashion by one pathologist. Histopathological changes were evaluated as described previously:16 score 0, no signs of inflammation; score 1, slight infiltrations in lamina propria by mononuclear cells; scores 0–1 are present in non-transplanted SCID mice; score 2, moderate mononuclear cell infiltration of lamina propria, increased mitotic activity in the epithelial layer; score 3, as in 2 but patchy mononuclear cell infiltration in the submucosal layer, elongation of the crypts with depletion of goblet cells; score 4, as in 3, but some crypt destruction and epithelial metaplasia; score 5, as in 4, but including increased crypt destruction and microabscesses, polymorphonuclear infiltration of epithelial layer and circumscript mononuclear cell infiltration in the submucosal layer, epithelial ulcerative lesions.
Enterobacterial extract
The extract was prepared by removing the faecal content of the colon and caecum into ice-cold PBS followed by sonification, centrifugation, filtration and protein measurement as described previously.7 The extract was kept at − 80° until use.
CD4+ T-cell proliferation
Splenocytes (8 × 106 cells/well in 2 ml CM in 24-well plates) from BALB/c mice were pulsed overnight with 200 μg/ml enterobacterial extract, irradiated and used as antigen-presenting cells as previously described.7 Proliferation experiments were performed as quadruplicates in a 96-well round-bottomed microtitre plates with 105 antigen-presenting cells and 105 CD4+ T cells from different groups of SCID mice. The cells were cultured for 5 days including 18 hr incorporation of 0·5 μCi/well [3H]thymidine (Amersham, Little Chalfont, UK) for the measurement of proliferation.
Measurement of cytokines
Murine fibroblast growth factor basic, GM-CSF, interferon-γ, IL-1-α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p40/p70, IL-13, IL-17, interferon-inducible protein-10 (IP-10), keratinocyte-derived chemokine (KC), monocyte chemotactic protein-1 (MCP-1), monokine induced by interferon-gamma (MIG), macrophage inflammatory protein-1α (MIP-1α), TNF-α and vascular endothelial growth factor basic were measured in serum from different groups of mice and in the supernatants of DCs with a Cytokine 20-Plex for Luminex (Biosource Cat. No LMC0006) according to the manufacturer's protocol. Measurement was carried out using the Luminex 100 IS (Luminexcorp, Austin, TX). More than 100 events were acquired per bead set. StarStation version 2·0 software (Applied Cytometry Systems, Sheffield, UK) was used for cytokine quantification analysis.
Statistics
Significant differences between sample means were determined with the two-tailed Student's t-test for independent samples and results were considered significant when P < 0·05. A two-way analysis of variance (anova) was used to determine significant differences between weight curves of untreated and treated SCID mice. Significant differences between histopathology scores were determined with the Mann–Whitney U-test.
Fluorescence-activated cell sorter analysis
Bone marrow DCs were harvested as described above and stained for 1 hr in PBS with 0·25% BSA, using biotin-coupled antibodies against CD11c (HL3), phycoerythrin-coupled antibodies against CD11b (M1/70), CD40 (3/23), I-A/E (M5/114.15.2) or fluorescein isothiocyanate-coupled antibodies against CD80 (16-10A1) or CD86 (GL1) (all from BD Pharmingen, Erembodegem, Belgium). Subsequently, cells were washed once and stained with streptavidin-conjugated allophycocyanin for 30 min. After a final wash, cells were analysed on a FACSCalibur (BD Pharmingen) and data were analysed using BD Diva software. At least 5000 cells were collected, using a live gate based on forward and side scatter properties.
Results
DC phenotype
Immature in-vitro-generated DCs were treated with IL-10 and pulsed with enteroantigen at day 6 and 7 of culture, or left untreated, and subsequently co-injected with CD4+ CD25– T cells into SCID mice. Untreated DCs consisted of approximately 70% CD11c-positive cells and had the phenotype of immature DCs with low expression of costimulatory molecules and MHC class II. The expression of the costimulatory molecules CD40, CD80 and CD86, as well as MHC class II molecules, was up-regulated after the addition of enterobacterial extract (Fig. 1), demonstrating an activating function of the extract on DC phenotype. Preincubation with IL-10 before the addition of enterobacterial extract resulted in a somewhat lower expression of MHC class II, whereas DCs treated with IL-10 alone showed a phenotype resembling that of untreated immature DCs (Fig. 1).
Figure 1.
DC phenotype. Bone marrow DCs were stimulated as indicated and harvested on day 8. Flow cytometric analysis of cells stained by fluorochrome-conjugated monoclonal antibodies was performed as indicated in the Materials and methods. Bone marrow DCs stimulated with IL-10 and/or enterobacterial extract (Extr) stained for (a): CD11c and CD11b and (b): CD11c and CD80, CD86, CD40, I-A/E, or isotype control. All histograms in (b) are gated on CD11c+ cells. One representative experiment of two performed is shown.
DC cytokine secretion
We next tested the secretion of T helper type 1 (Th1)/Th2 cytokines and proinflammatory chemokines from DCs treated with IL-10, enterobacterial extract or both. Exposure of DCs to enteroantigen induced secretion of IL-1α, IL-6, IL-12 p40/70 and TNF-α as well as of KC and MCP-1, and the addition of IL-10 led to a partial inhibition of enteroantigen-induced IL-6, IL-12 p40/70, TNF-α and KC secretion, but not of IL-1α and MCP-1 secretion (Fig. 2).
Figure 2.
DC cytokine secretion. Bone marrow DCs were stimulated as indicated and supernatant was harvested on day 8. Supernatants were then analysed with a Cytokine 20-Plex for cytokine content. One representative experiment of two performed is shown.
IL-10-treated DCs pulsed with enterobacterial antigen ameliorate colitis development
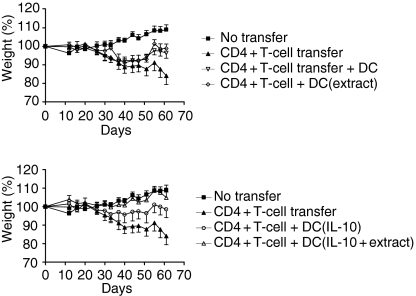
As shown previously, adoptive transfer of CD4+ CD25– cells into SCID mice leads to chronic colitis with weight loss, loose stools, bloody diarrhoea and rectal prolapse.6,7 We generated DCs in the presence of IL-10 and pulsed these with enterobacterial extract. The DCs were injected i.p. into SCID mice 2–3 hr before the adoptive transfer of CD4+ CD25– T cells. The body weight curves are shown in Fig. 3. Injection of untreated DCs or DCs pulsed with enterobacterial extract did not significantly prevent the weight loss normally seen after injection of CD4+ CD25– T cells (Fig. 3a). In contrast, a single injection of 0·3 × 106 DCs treated with IL-10 and exposed to enterobacterial extract completely prevented weight loss (Fig. 3b), and only this treatment group was significantly different from the group that received CD4+ CD25– T cells only (two-way anova, P = 0·025). In contrast, the group that received DCs generated with IL-10 only was not significantly different from either the group that received CD4+ CD25– T cells only or from the negative control (Fig. 3b).
Figure 3.
IL-10-treated DCs pulsed with enterobacterial antigen ameliorate weight loss in SCID mice adoptively transferred with CD4+ CD25– T cells. Bone marrow DCs were stimulated as indicated, harvested on day 8 and injected i.p. into SCID mice before CD4+ CD25– T-cell transfer. Mouse weight was registered twice per week, and mice were killed around day 60. Data represents mean ± SEM of mice in each treatment group (n = 8) in one representative experiment out of two.
Histopathology
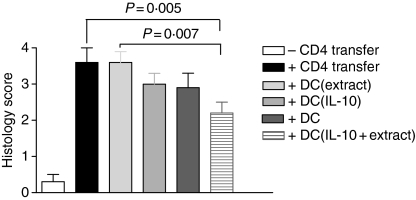
Figure 4 shows the colitis scores of the six experimental groups. The histology scores in all transplanted mice were between 2·2 and 3·6. The group of mice (n = 8) co-injected with DCs treated with IL-10 and exposed to enterobacterial extract showed the lowest colitis score, which was found to be significantly below the scores of the groups of mice injected only with CD4+ CD25– T cells (n = 8) and the group injected with DCs exposed to enterobacterial extract without IL-10 pretreatment (n = 7). Mice that received untreated DCs or DCs treated with IL-10 likewise had a lower mean histopathology score but this was not significant.
Figure 4.
Histology score in SCID mice. Bone marrow DCs were stimulated as indicated, harvested on day 8 and injected i.p. into SCID mice before CD4+ CD25– T-cell transfer. When mice were killed around day 60, the distal 2 cm of rectum was stained with haematoxylin & eosin and a histopathology score was calculated. One representative experiment of two performed is shown.
T-cell proliferation against enterobacterial extract ex vivo
CD4+ T cells obtained from the colitic mice were exposed to enteroantigen ex vivo for 4–5 days, and their proliferation was measured using [3H]thymidine incorporation. Compared with the proliferation of T cells obtained from mice transplanted with CD4+ CD25– T cells alone, the proliferation of cells from mice co-injected with IL-10-treated DCs pulsed with enterobacterial extract was significantly reduced. However, proliferation values were also significantly diminished in T cells from mice injected with IL-10-treated DCs, or with DCs exposed to enterobacterial antigen alone (Fig. 5).
Figure 5.
CD4+ T-cell proliferation. CD4+ T cells from different groups of SCID mice were mixed with irradiated enterobacterial extract pulsed spleen cells and cultured for 5 days including 18 hr incorporation of [3H]thymidine for the measurement of proliferation. Data are presented as mean ± SEM counts/min (c.p.m.) in one representative experiment.
Serum levels of cytokines in normal and colitic mice
The serum cytokine profile of colitic mice co-injected with DCs was analysed by multiplex assay 60 days after T-cell transfer. Mice injected with only CD4+ CD25– cells had increased levels of IL-1α, IL-10, IL-12, IL-13, IL-17, KC and MIG, and similar serum levels of IL-1β compared with normal SCID mice (Fig. 6). In general, the cytokine levels induced by injection of CD4+ CD25– T cells were not increased by co-injection with DCs. However, there was a non-significant tendency towards higher cytokine levels in mice injected with DCs pulsed with IL-10 plus enterobacterial extract (Fig. 6). No IFN-γ, IL-2, IL-4, IL-5, IL-6, fibroblast growth factor basic, TNF-α, GM-CSF, IP-10, MCP-1 or MIP-1α was detected (data not shown).
Figure 6.
Serum cytokines and chemokines. Bone marrow DCs were stimulated as indicated, harvested on day 8, and injected i.p. into SCID mice before CD4+ CD25– T-cell transfer. When mice were killed around day 60, an eye bleed was performed, and serum was obtained from individual mice. The serum was then analysed with a Cytokine 20-Plex for cytokine/chemokine content. Data represent mean ± SEM of individual mice (n = 8) in different treatment groups in one representative experiment out of two performed.
Discussion
There is evidence that the function of DCs is related to their state of maturation, and it has been repeatedly demonstrated that mature DCs are immunogenic, whereas immature or semi-mature DCs are less immunogenic or may even display a tolerogenic potential.17,18 When applied in murine therapeutic models, DCs with a tolerogenic potential can induce T-cell anergy and regulatory T-cell activity leading to antigen-specific tolerance.2,12–14,19,20 The therapeutic effect of such tolerogenic DCs has been tested in murine models of allotransplantation2,12–14 and of autoimmune disorders such as EAE,2,5 myasthenia gravis4 and diabetes.15 Most likely as a result of a lack of a defined antigen (auto- or bacterial) in murine models of colitis, the effect of such tolerogenic DCs in disease prevention has not been tested previously. In this paper we have used enterobacterial extract as a source of antigen, and have tested the ability of tolerogenic DCs pulsed with enterobacterial extract to prevent the induction of colitis in SCID mice after transfer of CD4+ CD25– T cells.
IL-10 pretreatment of both human and murine DCs is a well-established way to induce tolerogenic DCs, which can inhibit CD4+ Th1, CD4+ Th2 and CD8+ T-cell responses in an antigen-specific manner.3,19,21,22 Tolerogenic DCs are thought to mediate their function through the release of anti-inflammatory cytokines (IL-10, TGF-β), the down-regulation of proinflammatory cytokines (IL-6, IL-12, TNF-α) and down-modulation of surface costimulatory molecule expression,3,22–25 as well as by up-regulation of inhibitory immunoglobulin-like transcripts 3 and 4.26 Exposure to the enterobacterial extract used in this report clearly delivers a maturation signal to DCs, as shown by up-regulation of CD80 and CD86 as well as CD40, and MHC class II molecules, and the increased secretion of IL-1α, IL-6, IL-12 p40/70 and TNF-α. In contrast, pretreatment with IL-10 + enteroantigen resulted in lower expression of these surface molecules and decreased secretion of IL-6, IL-12 p40/70 and TNF-α. Thus, these IL-10-treated DCs express only low levels of costimulatory molecules in the absence of proinflammatory cytokines and thus resemble tolerogenic DCs that have been used in other studies.2,12–14,19,20
The application of tolerogenic DCs in autoimmune disorders such as EAE has the advantage of a defined antigen and the definition of MHC-binding peptides from such antigens. In the SCID transfer model of colitis, no single well-defined antigen has been discovered except for Helicobacter species, which in certain models have been associated with the immune pathology of colitis.27,28 As in the murine models, inflammatory bowel disease in humans is also believed to be driven by an abnormal immune response against the normal intestinal flora.29 In the present study we used an enterobacterial extract and we know from previous studies that the yet undefined antigens from this extract are processed and presented via MHC class II molecules.8,9
The DC-induced amelioration of colitis observed in the present study appears to be antigen-specific because DCs had to be pulsed with both IL-10 and enterobacterial extract to give significant protection from disease. Injection of the IL-10-treated and extract-pulsed DCs prevented the weight loss seen in SCID mice after CD4+ CD25– transfer and to some extent also ameliorated intestinal inflammation. We have previously shown that treatment with tolerogenic DCs generated in FCS induces a CD4+ T-cell-dependent Th2 response to FCS-derived antigens including BSA and thereby enhances systemic production of IL-4, IL-5 and IL-10. Also, the effect of tolerogenic DCs generated in FCS in the non-obese diabetic (NOD) mouse has, in part, been the result of antigen-unspecific responses against FCS.30–33 Induction of such an immunosuppressive environment seems to be pivotal in the prevention of disease in the SCID transfer model, because the use of tolerogenic DCs generated in an autologous manner had no effect on weight loss. However, there was some effect of these DCs on histopathology (data not shown), and it is therefore likely that the FCS-specific immunosuppressive T-cell response primarily ameliorates the effects of the general weight loss inducing ‘cytokine storm’ generated in mice after CD4+ CD25– transfer, whereas a smaller subset of tolerogenic enteroantigen-specific T cells are induced by the treatment and interferes with gut pathology.
The injection of IL-10-treated, enteroantigen-pulsed DCs led to an increase in the levels of IL-10, IL-1α, IL-13, IL-17 and IL-12p40/70, thus resulting in an overall dominance of anti-inflammatory cytokines. Interestingly, IL-17 is secreted by a subset of pathogenic CD4+ T cells;34,35 in some autoimmune diseases, e.g. EAE,36 but in spite of this, neutralization of this cytokine in an inflammatory bowel disease model has been shown to aggravate disease.37 Clearly the final outcome is probably antigen-specific, because treatment of mice with unpulsed immature DCs led to the same serum cytokine profile as in protected mice, but to aggravated disease.
In conclusion, our data show that DCs pretreated with IL-10 and pulsed with enterobacterial extract can prevent the weight loss observed in SCID mice after CD4+ CD25– T-cell transfer, and ameliorate the immunopathology of the gut wall. DC-induced disease amelioration is positively correlated with decreased T-cell proliferation against the enterobacterial extract and with a systemic dominance of anti-inflammatory cytokines such as IL-10 and IL-13.
Acknowledgments
This work was supported by grants from ‘Colitis-Crohn fonden’, ‘DADES fond’, ‘The Danish Research Agency’, ‘Augustinusfonden’ and ‘Lægeforeningens Forskningsfond’. We would also like to thank Ane Rulykke and Trine Lind Devantier for excellent technical performance.
References
- 1.Gad M, Claesson MH, Pedersen AE. Dendritic cells in peripheral tolerance and immunity. APMIS. 2003;111:766–75. doi: 10.1034/j.1600-0463.2003.11107808.x. [DOI] [PubMed] [Google Scholar]
- 2.Menges M, Rössner S, Voigtländer C, et al. Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. J Exp Med. 2002;195:15–21. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haase C, Jorgensen TN, Michelsen BK. Both exogenous and endogenous interleukin-10 affects the maturation of bone-marrow-derived in vitro and strongly influences T cell priming in vivo. Immunology. 2002;107:489–99. doi: 10.1046/j.1365-2567.2002.01529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yarilin D, Duan R, Huang YM, Xiao BG. Dendritic cells exposed in vitro to TGF-beta1 ameliorate experimental autoimmune myastenia gravis. Clin Exp Immunol. 2002;127:214–19. doi: 10.1046/j.1365-2249.2002.01748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zinser E, Lechmann M, Golka A, Lutz MB, Steinkasserer A. Prevention and treatment of experimental autoimmune encephalomyelitis by soluble CD83. J Exp Med. 2004;200:345–51. doi: 10.1084/jem.20030973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claesson MH, Bregenholt S, Bonhagen K, et al. Colitis-inducing potency of CD4+ T cells in immunodeficient, adoptive hosts depends on their state of activation, IL-12 responsiveness, and CD45RB surface phenotype. J Immunol. 1999;162:3702–10. [PubMed] [Google Scholar]
- 7.Brimnes J, Reimann J, Nissen M, Claesson M. Enteric bacterial antigens activate CD4(+) T cells from scid mice with inflammatory bowel disease. Eur J Immunol. 2001;31:23–31. doi: 10.1002/1521-4141(200101)31:1<23::aid-immu23>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Gad M, Pedersen AE, Kristensen NN, Kury E, Claesson MH. Demonstration of strong enterobacterial reactivity of CD4+CD25– T cells from conventional and germ-free mice which is counter-regulated by CD4+CD25+ T cells. Eur J Immunol. 2004;34:695–704. doi: 10.1002/eji.200324394. [DOI] [PubMed] [Google Scholar]
- 9.Gad M, Lundsgaard D, Kjellev S, et al. Reactivity of naive CD4+CD25– T cells against gut microflora in healthy mice. Int Immunol. 2006;18:817–25. doi: 10.1093/intimm/dxl018. [DOI] [PubMed] [Google Scholar]
- 10.Duchmann R, May E, Heike M, Knolle P, Neurath M, Meyer zum Buschenfelde KH. T cell specificity and cross reactivity towards enterobacteria, bacteroides, bifidobacterium, and antigens from resident intestinal flora in humans. Gut. 1999;44:812–18. doi: 10.1136/gut.44.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duchmann R, Neurath M, Meyer zum Buschenfelde KH. Responses to self and non-self intestinal microflora in health and inflammatory bowel disease. Res Immunol. 1997;148:589–94. doi: 10.1016/s0923-2494(98)80154-5. [DOI] [PubMed] [Google Scholar]
- 12.Lutz MB, Suri RM, Niimi M, et al. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation-resistant and prolong allograft survival in vivo. Eur J Immunol. 2000;30:1813–22. doi: 10.1002/1521-4141(200007)30:7<1813::AID-IMMU1813>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Lutz MB, Kukutsch NA, Menges M, Rössner S, Schuler G. Culture of bone marrow cells in GM-CSF plus high doses of lipopolysaccharide generates exclusively immature dendritic cells which induce alloantigen-specific CD4 T cell anergy in vitro. Eur J Immunol. 2000;30:1048–52. doi: 10.1002/(SICI)1521-4141(200004)30:4<1048::AID-IMMU1048>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 14.Fu F, Li Y, Qian S, et al. Costimulatory molecule-deficient dendritic cell progenitors (MHC class II+CD80dim, CD86–) prolong cardiac allograft survival in nonimmunosuppressed recipients. Transplantation. 1996;62:659–65. doi: 10.1097/00007890-199609150-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steptoe RJ, Ritchie JM, Jones LK, Harrison LC. Autoimmune diabetes is suppressed by transfer of proinsulin-encoding Gr-1+ myeloid progenitor cells that differentiate in vivo into resting dendritic cells. Diabetes. 2005;54:434–42. doi: 10.2337/diabetes.54.2.434. [DOI] [PubMed] [Google Scholar]
- 16.Moller PL, Paerregaard A, Gad M, Kristensen NN, Claesson MH. Colitic scid mice fed Lactobacillus spp. show an ameliorated gut histopathology and an altered cytokine profile by local T cells. Inflamm Bowel Dis. 2005;11:814–19. doi: 10.1097/01.mib.0000175906.77340.15. [DOI] [PubMed] [Google Scholar]
- 17.O'Neill DW, Adams S, Bhardwaj N. Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood. 2004;104:2235–46. doi: 10.1182/blood-2003-12-4392. [DOI] [PubMed] [Google Scholar]
- 18.Adams S, O'Neill DW, Bhardwaj N. Recent advances in dendritic cell biology. J Clin Immunol. 2005;25:177–88. doi: 10.1007/s10875-005-4086-2. [DOI] [PubMed] [Google Scholar]
- 19.Steinbrink K, Wolf M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–80. [PubMed] [Google Scholar]
- 20.Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145–55. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 21.Steinbrink K, Jonuleit H, Muller G, Schuler G, Knop J, Enk AH. Interleukin-10-treated human dendritic cells induce a melanoma-antigen specific anergy in CD8(+) T cells resulting in a failure to lyse tumor cells. Blood. 1999;93:1634–42. [PubMed] [Google Scholar]
- 22.Yang AS, Lattime EC. Tumor-induced interleukin 10 suppresses the ability of splenic dendritic cells to stimulate CD4 and CD8 T-cell responses. Cancer Res. 2003;63:2150–7. [PubMed] [Google Scholar]
- 23.Harizi H, Norbert G. Inhibition of IL-6, TNF-alpha, and cyclooxygenase-2 protein expression by prostaglandin E2-induced IL-10 in bone marrow-derived dendritic cells. Cell Immunol. 2004;228:99–109. doi: 10.1016/j.cellimm.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharyya S, Sen P, Wallet M, Long B, Baldwin AS, Jr, Tisch R. Immunoregulation of dendritic cells by IL-10 is mediated through suppression of the PI3K/Akt pathway and of IkappaB kinase activity. Blood. 2004;104:1100–9. doi: 10.1182/blood-2003-12-4302. [DOI] [PubMed] [Google Scholar]
- 25.McBride JM, Jung T, de Vries JE, Aversa G. IL-10 alters DC function via modulation of cell surface molecules resulting in impaired T-cell responses. Cell Immunol. 2002;215:162–72. doi: 10.1016/s0008-8749(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 26.Manavalan JS, Rossi PC, Vlad G, Piazza F, Yarilana A, Cortesini R, et al. High expression of ILT3 and ILT4 is a general feature of tolerogenic dendritic cells. Transpl Immunol. 2003;11:245–58. doi: 10.1016/S0966-3274(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 27.Schomer NH, Dangler CA, Schrenzel MD, Fox JG. Helicobacter bilis-induced inflammatory bowel disease in scid mice with defined flora. Infect Immunol. 1997;65:4858–64. doi: 10.1128/iai.65.11.4858-4864.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franklin CL, Riley LK, Livingston RS, et al. Enteric lesions in SCID mice infected with ‘Helicobacter typhlonicus’, a novel urease-negative Helicobacter species. Laboratory Anim Sci. 1999;49:496–505. [PubMed] [Google Scholar]
- 29.Heller F, Duchmann R. Intestinal flora and mucosal immune responses. Int J Med Microbiol. 2003;293:77–86. doi: 10.1078/1438-4221-00246. [DOI] [PubMed] [Google Scholar]
- 30.Papaccio G, Nicoletti F, Pisanti FA, Bendtzen K, Galdieri M. Prevention of spontaneous autoimmune diabetes in NOD mice by transferring in vitro antigen-pulsed syngeneic dendritic cells. Endocrinology. 2000;141:1500–5. doi: 10.1210/endo.141.4.7437. [DOI] [PubMed] [Google Scholar]
- 31.Feili-Hariri M, Dong X, Alber SM, Watkins SC, Salter RD, Morel PA. Immunotherapy of NOD mice with bone marrow-derived dendritic cells. Diabetes. 1999;48:2300–8. doi: 10.2337/diabetes.48.12.2300. [DOI] [PubMed] [Google Scholar]
- 32.Feili-Hariri M, Falkner DH, Morel PA. Regulatory Th2 response induced following adoptive transfer of dendritic cells in prediabetic NOD mice. Eur J Immunol. 2002;32:2021–30. doi: 10.1002/1521-4141(200207)32:7<2021::AID-IMMU2021>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 33.Haase C, Ejrnaes M, Juedes AE, Wolfe T, Markholst H, von Herrath MG. Immunomodulatory dendritic cells require autologous serum to circumvent nonspecific immunosuppressive activity in vivo. Blood. 2005;106:4225–33. doi: 10.1182/blood-2005-03-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mangan PR, Harrington LE, O'Quinn DB, et al. Transforming growth factor-beta induces development of the T(H) 17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 35.Betelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 36.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110:55–62. doi: 10.1016/j.clim.2003.09.013. [DOI] [PubMed] [Google Scholar]