Abstract
The role of regulatory T cells (Treg) in maintaining tolerance to self has been intensively scrutinized, particularly since the discovery of Foxp3 as a Treg-specific transcription factor. The BDC2·5NOD transgenic mouse is an excellent model of immunoregulation because it has a very low incidence of diabetes despite a highly autoreactive T-cell repertoire. It has previously been shown that reactivity against islets decreases with age in BDC2·5NOD mice. Here we show that there is a markedly higher frequency of Foxp3+ Treg in the CD4+ subset of 16–20-week-old mice compared with 4- or 8-week-old mice. This phenomenon can be observed in the spleen, thymus, pancreatic draining lymph nodes and the pancreas itself. We show that this early age-related increase in the frequency of Foxp3+ cells does not occur in wild-type NOD, BALB/c or C57BL/6 mice. Further, we show that, in contrast to some reports on Treg in wild-type NOD mice, the suppressive function of BDC2·5NOD Treg from 16- to 20-week-old mice is intact and comparable to that from 4- to 8-week-old mice both in vitro and in vivo. Our data offer insights into the long-term protection of BDC2·5NOD mice from diabetes and an explanation for the age-related decrease in anti-islet responses seen in BDC2·5NOD mice.
Keywords: diabetes, BDC2·5NOD, regulatory T cells, Foxp3, CD25
Introduction
The BDC2·5NOD transgenic mouse expresses a T-cell receptor (TCR) that is specific for an islet autoantigen.1 The TCR DNA is that of an islet-reactive CD4+ clone, BDC2·5, which was identified in wild-type NOD mice.2 The clone is able to transfer diabetes to neonatal NOD mice.3 A recent publication has suggested that the TCR may recognize the glutamic acid decarboxylase (GAD)-65 autoantigen.4 Despite their highly autoreactive repertoire, BDC2·5NOD mice are protected from diabetes5 and are thus an excellent model of dominant immune regulation. Interestingly, these mice appear to become less reactive to islet cell autoantigens as they age. Old mice (aged over 20 weeks) are less susceptible than young mice to cyclophosphamide-induced diabetes.6 In addition, the ability of splenocytes from BDC2·5NOD mice to transfer disease to NOD.severe combined immunodeficiency (scid) recipients declines in an age-dependent manner.7 Splenocytes from 20-week-old mice are far less efficacious in transferring diabetes to NOD.scid recipients than are those from 4-week-old mice.7
The role of regulatory T cells (Treg) in the prevention of autoimmune disease has been emphasized by recent studies of Foxp3. Both mice and humans with mutations in this gene develop widespread autoimmunity early in life.8,9 Expression of the gene is highly enriched within the CD4+ CD25+ regulatory subset10,11 and retroviral transduction of CD4+ CD25– cells with Foxp3 confers regulatory expression upon them.10,12 In wild-type NOD mice, several reports have described an age-related decline in Treg function as contributing to the development of diabetes. Gregori et al. found that splenic CD4+ CD25+ T cells from 16-week-old mice did not behave as suppressor cells either in vitro or in vivo.13 These results were confirmed in a recent study by Chatenoud et al.14. Diabetic NOD mice were shown to re-acquire regulatory activity in their CD4+ CD25+ T-cell population following treatment with anti-CD3 antibody.15 A recent study showed that CD4+ CD25+ T cells in the pancreatic lymph nodes of female but not male NOD mice exhibited an age-related decline in the expression of mRNA coding for Foxp3 and transforming growth factor (TGF)-β116. Nevertheless, it is clear that, in BDC2·5NOD mice, tolerance to islet cell autoantigens appears to strengthen rather than diminish between 4 and 16–20 weeks of age, in contrast to those reports in wild-type NOD mice. Furthermore, reports in other mouse strains have described age-related hyporesponsiveness in the CD4+ subset of T cells, which has been ascribed to increases in the frequency of regulatory T cells.17,18 We therefore examined the frequency and function of Treg from BDC2·5NOD mice of various ages. We found that not only is Treg function intact in vivo and in vitro in old BDC2·5NOD mice but the percentage of peripheral CD4+ CD25+ and CD4+ Foxp3+ regulatory T cells rises in BDC2·5NOD mice in an age-dependent manner in the spleen, thymus, pancreatic lymph nodes (PLNs) and pancreas. These changes occur much earlier than any age-related increases in Treg frequency in other strains and coincide with the time at which splenocytes from the BDC2·5NOD mice become less diabetogenic.
Materials and methods
Mice
BALB/c and C57BL/6 mice were purchased from Harlan (Oxon, UK). NOD, BDC2·5NOD and NOD.scid mice were bred and maintained under barrier conditions in the Biological Services facility of the Department of Pathology, University of Cambridge, Cambridge, UK. They received standard laboratory food and water ad libitum. NOD.scid mice were maintained in microisolator cages with filtered air and handled under sterile conditions in a laminar flow hood. All animal experiments were approved by the Ethical Review Committee of the University of Cambridge.
Antibodies
Commercially available monoclonal antibodies used in this study were fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll protein (PerCP) or biotin conjugates raised against CD4 (RM4-5), CD25 (PC61), CD62L (MEL-14), CD103 (M290), CTLA-4 (UC10), interferon (IFN)-γ (XMG1·2) and interleukin (IL)-10 (JES5–16E3) obtained from BD Biosciences (San Diego, CA). Fc receptors were blocked with a 1/100 dilution of supernatant from the hybridoma 2·4G2 on ice for 30 min except where an anti-rat immunoglobulin was used for secondary staining. The anticlonotype antibody (aBDC, mouse IGg2b) as described in Kanagawa et al.6 was a gift of Dr O. Kanagawa. This antibody was used as a supernatant and detected with FITC anti-mouse IgG2b (Serotec, Kidlington, UK). Anti-glucocorticoid-induced tumour necrosis factor receptor (GITR) (DTA-1, rat IgG2b) was a gift from Professor S. Sakaguchi. It was used as a supernatant and detected with FITC goat anti-rat immunoglobulin (Serotec).
Fluorescence-activated cell sorter (FACS) analysis
Single cell suspensions were made from the spleen and PLNs, red blood cells were lysed using an ammonium chloride buffer and the cells were then re-suspended in FACS buffer [phosphate-buffered saline (PBS), 2% fetal calf serum (FCS) and 0·05% sodium azide (Sigma Aldrich, Poole, UK)]. Fc receptors were blocked with supernatant from the hybridoma 2·4G2 and stained with the appropriate antibodies at the concentrations recommended by the manufacturers for 30 min at 4°. The presence of the transcription factor Foxp3 was detected using a Foxp3 staining set according to the manufacturer's instructions (eBioscience, San Diego, CA). For determination of intracellular cytokine production, cells were restimulated with 500 ng/ml PdBu, 500 ng/ml ionomycin and 1 µg/ml Brefeldin A (all from Sigma-Aldrich) at 37° for 4 hr. Cells were stained for surface markers and fixed overnight in Fix/Perm buffer (eBioscience). Cells were then permeabilized with eBioscience buffer and stained for IL-10, IFN-γ or cytotoxic T-lymphocyte antigen (CTLA)-4. The stained cells were analysed using a FACScan or LSR-2 analyser (Becton Dickinson Biosciences, Erembodegem, Belgium) and CellQuest (Becton Dickinson Biosciences) or flowjo (Tree Star, Inc., Ashland, OR) software.
Analysis of pancreatic infiltrates
Each pancreas was harvested individually and cut into small pieces in cold PBS containing 5% FCS, 56 mm glucose and complete mini protease inhibitors (Roche, Welwyn Garden City, UK) The protease inhibitor cocktail was then washed off using cold sterile PBS (Invitrogen, Paisley, UK). The tissues were incubated in 2 ml of PBS containing 15% FCS, 0·3 mg/ml Liberase CI (Boehringer Mannheim, Ingelheim, Germany) and 10 µg/ml DNAase (Sigma Aldrich) for 10 min at 37°. After digestion, the tissues were washed and cell suspensions were prepared by forcing them through a cell strainer. Suspensions were left to settle for 5 min to remove stromal debris. The supernatants were then harvested. The cells were then washed with PBS and used for FACS analysis.
In vitro Treg assay
CD4+ CD25+ and CD4+ CD25– T cells were isolated using a Regulatory T Cell Isolation Kit (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. CD4+ CD25– T cells were suspended at 5 × 107 cells/ml in PBS with 5 µm 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) and incubated at 37° for 20 min. Cells were washed with PBS and then resuspended in complete medium [IMDM (Gibco, Paisley, UK) supplemented with 10% FCS (Harlan), 100 µg/ml streptomycin (Sigma) and 60 µg/ml penicillin (Sigma)]. Triplicate cultures of the stated number of CFSE-labelled CD4+ CD25– T cells were incubated for 72 hr in round-bottom 96-well plates (Falcon; Becton Dickinson, San Jose, CA) with the stated number of antigen-presenting cells (irradiated, CD4+ T-cell depleted, red cell lysed splenocytes), 1 µg/ml anti-CD3 and the stated number of CD4+ CD25+ T cells. Cells from each well were harvested after 72 hr, stained for CD4 and analysed by flow cytometry.
In vivo transfer experiments with NOD. scid mice
Single cell suspensions were made from the spleens of 4-week-old, 8-week-old or 16–20-week-old BDC2·5NOD mice. CD4+ CD25+ and CD4+ CD25– T cells were isolated using a Regulatory T Cell Isolation Kit (Miltentyi Biotec) according to the manufacturer's instructions. CD4+ CD25+ and CD4+ CD25– T cells were injected intravenously either alone or together at the ratios indicated into 4-week-old or 8-week-old male NOD.scid recipients. The incidence of diabetes was monitored by assessment of glycosuria using Diastix (Bayer, Newbury, UK). Mice were considered diabetic if they tested positive for glycosuria on two consecutive occasions. Typically, there were 5 or 10 mice per group in each experiment.
Statistics
Data were analysed using the GraphPadPrism computer package (GraphPad, San Jose, CA). The Mann–Whitney U-test was used to assess differences between non-parametric data groups. Log rank analysis of Kaplan–Meier survival curves was used to compare time of onset of diabetes between the two treatment regimes. Results were considered to be significant if P < 0·05.
Results
An early age-related increase in the frequency of CD4+ CD25+ cells in spleen and pancreatic lymph nodes of BDC2·5NOD mice
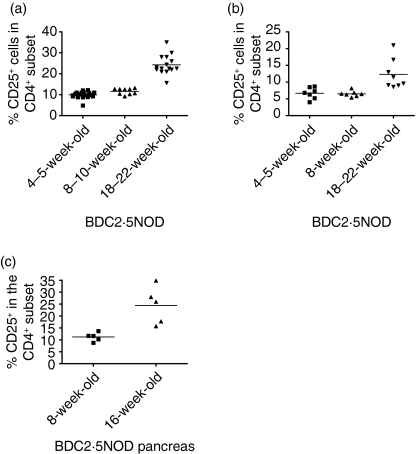
We examined the CD4+ CD25+ population in the spleens, pancreatic lymph nodes and pancreata of old and young BDC2·5NOD mice by FACS analysis (Fig. 1). There was a striking age-related increase in the frequency of splenic CD4+ CD25+ T cells in BDC2·5NOD mice (Fig. 1a). The percentage of the CD4+ subset that expressed CD25 was around 8–10% in 4–5-week-old mice. This increased significantly to 12–13% in 8-week-old mice (P < 0·02). However, in 16–20-week-old mice, the percentage of CD25-expressing cells in the CD4+ subset was over 20%, twofold greater than that observed in 4–5-week-old mice. The difference between 16–20-week-old mice and 4- or 8-week-old mice was highly significant (P < 0·001) in both cases.
Figure 1.
There is an early age-related increase in the frequency of CD25+ cells in the CD4+ subset of BDC2·5NOD mice in the spleen, pancreatic lymph nodes and pancreas. Single cell suspensions from the spleens, pancreatic lymph nodes and pancreata of BDC2·5NOD mice of various ages were co-stained for CD4 and CD25. Results are given as the percentage of CD25+ cells in the CD4+ subset for the spleen (a), pancreatic lymph nodes (b) and pancreas (c)
Pancreatic lymph node cells from old BDC2·5NOD mice also exhibited a higher frequency of CD4+ CD25+ cells than those of young mice (Fig. 1b). Interestingly, in both young (4- and 8-week-old) and older (16–20-week-old) mice, the percentage of CD25+ cells within the CD4+ subset was lower than that observed in the spleen. However, the presence of an age-related rise in the frequency of CD4+ CD25+ cells was consistent between spleen and pancreatic lymph nodes. In both 4–5-week-old mice and 8-week-old mice, the frequency of CD25+ cells within the CD4+ subset was 6–7% (no significant difference in frequency), whereas in 16–20-week-old mice this figure was significantly higher, ranging from 10 to over 20% (P < 0·001). We also noted an age-related increase in the proportion of CD4+ cells that expressed CD25 in the pancreas. The frequency of CD4+ CD25+ cells was significantly higher (P < 0·01) amongst CD4+ cells from pancreatic infiltrates of 20-week-old mice compared with those from 8-week-old mice (Fig. 1c).
The data we obtained on CD25 expression contrast with those obtained by other groups who have examined the frequencies of CD4+ CD25+ cells in other mouse strains. Sakaguchi et al. noted a slight increase in the frequency of CD4+ CD25+ cells with age in C57BL/6 mice, but this occurred over a much longer time frame. Our data were suggestive, but not confirmatory, of an age-related increase in the frequency of Treg in the CD4+ compartment of BDC2·5NOD mice, as expression of CD25 is not an absolute marker of regulatory cells but can represent activated cells too.
An early age-related increase in the CD4+ Foxp3+ populations in the spleens, pancreatic lymph nodes, pancreata and thymi of BDC2·5NOD mice
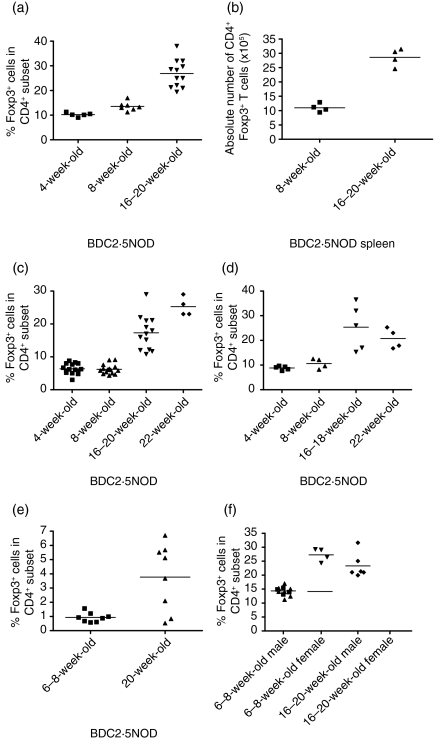
The most specific marker of Treg is the transcription factor Foxp3.10,11 We therefore analysed Foxp3 expression at the single-cell level by FACS analysis of the spleens from 4-week-old, 8-week-old and 16–20-week-old BDC2·5NOD mice (Fig. 2). The proportion of Foxp3+ cells in the CD4+ subset was consistently higher in the spleens of 16–20-week-old mice than in those of 4- or 8-week-old mice (Fig. 2a). In 4-week-old mice, approximately 10% of the CD4+ T cells were Foxp3+, similar to the percentage of CD25+ T cells in the CD4+ subset of such mice. There was a slight and statistically significant increase in the proportion CD4+ cells expressing Foxp3 in 8-week-old mice to around 12–13% (P < 0·01). By contrast, the proportion of Foxp3+ T cells in the CD4+ compartment of older (16–20-week-old) mice was between 25 and 30%, significantly higher than in 4-week-old (P < 0·05) or 8-week-old (P < 0·005) mice. We also examined the absolute numbers of CD4+ and CD4+ Foxp3+ cells in the spleens of 8-week-old and 16-week-old BDC2·5NOD mice. We found that there was no significant difference in the number of CD4+ cells between 8- and 16-week-old BDC2·5NOD mice but that there was a significant increase in the absolute number of CD4+ Foxp3+ cells from around 1 × 106 to around 2·7 × 106 (P < 0·05) (Fig. 2b).
Figure 2.
There is an early age-related increase in the frequency of Foxp3+ cells in the CD4+ subset of BDC2·5NOD mice in the spleen, pancreatic lymph nodes, pancreas and thymus. Single cell suspensions were prepared from the spleens, pancreatic lymph nodes, pancreata and thymi of BDC2·5NOD mice of various ages. They were stained for CD4 and Foxp3. Results are given as the percentage of Foxp3+ cells in the CD4+ subset for the spleen (a) and the absolute number of CD4+ Foxp3+ cells in the spleen (b) The percentage of Foxp3+ cells in the CD4+ subset of pancreatic lymph nodes (c), pancreas (d) and thymus (e) is shown. There are no differences in the relative frequencies of Foxp3+ cells in the CD4+ splenic T-cell subset from male and female BDC2·5NOD mice at 6–8 weeks of age and 16–20 weeks of age (f)
Having established that BDC2·5NOD mice exhibit an early age-related increase in the frequency of CD4+ Foxp3+ cells in the spleen, we sought to establish whether this phenomenon also occurred in the pancreatic lymph nodes and pancreas. In the pancreatic lymph nodes (Fig. 2c), we found that, although the percentage of Foxp3+ cells in the CD4+ subset was lower in the pancreatic lymph nodes than in the spleen in both young and old mice (as we had observed for CD25 expression), the proportion of CD4+ cells that were Foxp3+ was consistently higher in the pancreatic lymph nodes of 16–20-week-old mice compared with young 4-week-old or 8-week-old BDC2·5NOD mice (P < 0·0001 in both cases). There was no significant difference between 4- and 8-week-old mice in the proportion of CD4+ Foxp3+ cells in the CD4+ subset. The same pattern was also observed in the pancreas itself (Fig. 2d). There was no significant difference in the frequency of CD4+ Foxp3+ cells in the pancreata of 4- and 8-week-old mice. However, the frequencies of Foxp3+ cells within the CD4+ subset were significantly higher in 16–18-week-old mice than in 4- and 8-week-old mice (P < 0·05) and in 22-week-old mice than in 4- and 8-week-old mice (P < 0·05).
In order to determine whether the rise in the absolute number and frequency of peripheral Foxp3+ cells amongst the CD4+ subset is a result of increasing thymic export of Treg, we determined the frequency of Foxp3+ Treg in the CD4+ single positive (SP) thymic population of 6–8-week-old and 20-week-old BDC2·5NOD mice. We found that the proportion of Foxp3+ Treg among the CD4SP cells was higher in 20-week-old than in 6–8-week-old BDC2·5NOD mice (P < 0.05) (Fig. 2e).
Experiments in wild-type NOD mice have shown that there are sex-related differences in the Treg function of 16-week-old mice.16 Therefore, we evaluated the frequency of CD4+ Foxp3+ cells in the spleen and the PLNs of 6–8-week-old and 20-week-old male and female BDC2·5NOD mice (Fig. 2f). We found that, in both male and female 6–8-week-old BDC2·5NOD mice, the frequency of CD4+ Foxp3+ cells was approximately 13–15% in the spleen and 4–6% in the PLNs. Furthermore, there was no significant difference between male and female BDC2·5NOD mice at 20 weeks of age. Both sexes exhibited a Treg frequency of approximately 10–14% in the PLNs and 25–30% in the spleen.
No early age-related increase in the frequencies of CD4+ Foxp3+ in BALB/c, C57BL/6 or wild-type NOD mice
One publication has demonstrated an age-related increase in the proportion of CD4+ Foxp3+ cells in wild-type C57BL/6 mice.17 This publication examined the immune systems of very aged mice – comparing 8-week-old mice with those that were 1 or 2 years of age. It did not demonstrate a rise in the frequency of peripheral Treg cells at such a young age as that noted in BDC2·5NOD mice. Therefore, we determined whether this early (by 16 weeks) age-related increase in the frequency of Treg cells is unique to BDC2·5NOD mice or whether it occurs in other strains.
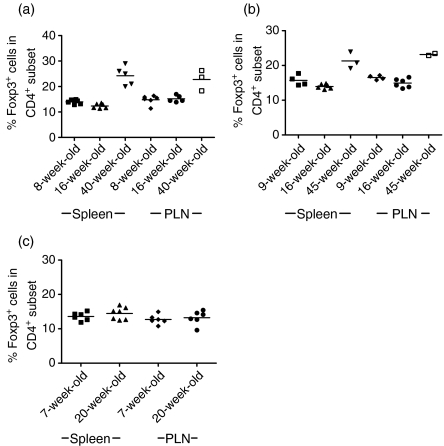
We compared the splenic and PLN frequencies of Foxp3+ cells in 8- and 16 week-old BALB/c, C57BL/6 and wild-type NOD mice with those observed in BDC2·5NOD mice (Fig. 3). We found that only BDC2·5NOD mice exhibit such an early age-related increase in the frequency of Foxp3+ cells in the CD4+ subset. There was no significant difference in the frequencies of Foxp3+ cells in the CD4+ subset observed in the spleens or pancreatic lymph nodes of C57BL/6 (Fig. 3a), BALB/c (Fig. 3b) or NOD (Fig. 3c) mice at 8 and 16 weeks of age. Consistent with the results obtained by Sakaguchi's group in their paper on age-related changes in C57BL/6 mice,17 we found that, in mice approaching 1 year of age, there was an increase in the frequency of Foxp3+ cells within the CD4+ subset.
Figure 3.
C57BL/6, BALB/c and NOD mice do not exhibit the early age-related increase in regulatory T-cell (Treg) frequency noted in BDC2·5NOD mice. Single cell suspensions were made from the spleens and pancreatic lymph nodes cells of C57BL/6, BALB/c and NOD mice of various ages and were stained for the expression of CD4 and Foxp3 according to the protocols detailed in the Materials and methods. Results are given as percentage of Foxp3+ cells in the CD4+ subset in C57BL/6 (a), BALB/ c (b) and NOD (c) mice.
Additionally, we compared the splenic T-cell populations of 16-week-old BDC2·5NOD, BALB/c, C57BL/6 and NOD mice in a parallel experiment. The frequency of splenic CD4+ Foxp3+ cells was significantly higher in 16-week-old BDC2·5NOD mice than in 16-week-old C57BL/6, BALB/c or wild-type NOD mice (P < 0·0005 in all cases).
Expression of the transgenic TCR in young and old BDC2·5NOD mice
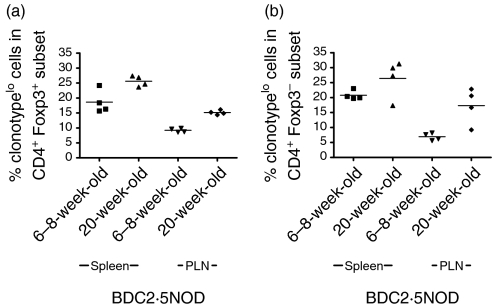
The expression of the Vα1Vβ4 clonotype TCR on T cells from BDC2·5NOD mice can be assessed using a monoclonal antibody directed against the Vα1Vβ4 TCR that was developed by Kanagawa and colleagues6. They showed that, as BDC2·5NOD mice age, a population of CD4+ T cells emerges that expresses low levels of the Vα1Vβ4 clonotype. However, the importance of the Foxp3 gene in Treg biology was not documented at this time. Using monoclonal antibodies against the relevant targets and FACS analysis, we costained for Foxp3 and the Vα1Vβ4 TCR in order to determine whether Foxp3+ cells express the clonotype and whether clonotypelo cells are Foxp3+. Consistent with their data, we found that, when the populations were divided into clonotypehi and clonotypelo, a higher proportion of CD4+ cells were clonotypelo in 20-week-old than in 6–8-week-old mice (data not shown). The Foxp3+ population of cells did express the transgenic TCR. The Foxp3+ population was comprised of both clonotypelo and clonotypehi positive cells. A higher proportion of Foxp3+ cells were clonotypelo in 20-week-old than in 6–8-week-old mice (Fig. 4a). These data were significant in the pancreatic lymph nodes (P < 0·05) but a non-significant trend was also demonstrable in the spleen. In the CD4+ Foxp3– population, there was no significant difference in the percentage of clonotypelo cells in the spleen (Fig. 4b) at different ages but significantly more cells were clonotypelo in the PLNs (P < 0·05).
Figure 4.
Clonotype expression on Foxp3+ regulatory T cells (Treg) in 6–8-week-old and 20-week-old BDC2·5NOD mice. Single cell suspensions were made from the spleens and pancreatic lymph nodes of 6–8- and 20-week-old BDC2·5NOD mice and were stained for the expression of CD4, Foxp3 and the clonotype antibody. The percentage of clonotypelo cells in the CD4+ Foxp3+ population at 6–8 weeks of age and 20 weeks of age (a) and the percentage of clonotypelo cells in the CD4+ Foxp3– population at 6–8 weeks of age and 20 weeks of age (b) are shown.
Phenotype of Treg in old versus young BDC2·5NOD mice
Given that there is an increase in the frequency of CD4+ Foxp3+ cells between young and older BDC2·5NOD mice, we sought to determine whether there were any differences in the surface marker phenotype of these cells. In addition to CD25, other cell surface markers have been postulated to be expressed on Treg.
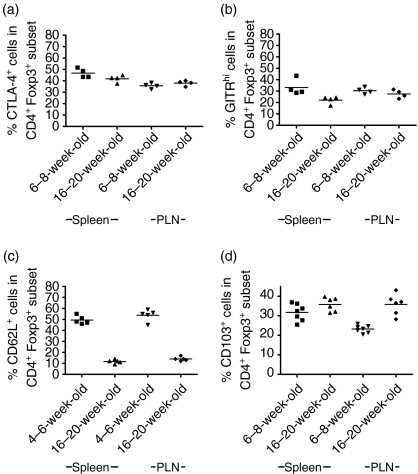
Previous studies have shown that CTLA-4 is preferentially expressed by Treg19,20 and that this molecule may be important in their function.21–23 In BDC2·5NOD mice, blockade of CTLA-4 early in life precipitates autoimmune diabetes.24 Therefore, we evaluated whether expression of this molecule differs between 6–8-week-old and 16–20-week-old BDC2·5NOD mice. We found that expression of CTLA-4 was largely confined to the Foxp3+ subset of cells in mice of both ages. However, we found no significant differences between 6–8-week-old and 16–20-week-old mice in either the geometric mean fluorescence intensity (GMFI) of CTLA-4 expression (data not shown) or the percentage of Foxp3+ cells that were CTLA-4+(Fig. 5a). This was the case in both the spleen and the PLNs. The percentage of Foxp3+ cells that was CTLA-4+ was similar between the spleen and the PLNs.
Figure 5.
Surface marker phenotype of Foxp3+ regulatory T cells (Treg) in 4–8- and 16–20-week-old BDC2·5NOD mice. Single cell suspensions were made from the spleens and pancreatic lymph nodes of 4–8- and 16–20-week-old BDC2·5NOD mice and were stained for the expression of CD4, Foxp3 and (a) cytotoxic T-lymphocyte antigen (CTLA)-4, (b) glucocorticoid-induced tumour necrosis factor receptor (GITR), (c) CD62L or (d) CD103. Results are given as the percentage of the relevant surface marker in the CD4+ Foxp3+ subset.
The GITR molecule10,20,25 has also been proposed as a marker of Treg and has been shown to be expressed at higher levels on CD4+ Foxp3+ cells than on CD4+ Foxp3– cells. We evaluated the surface expression of GITR in both 6–8-week-old and 16–20-week-old BDC2·5NOD mice (Fig. 5b). We found that GITR was expressed at a higher level in the CD4+ Foxp3+ population than in the CD4+ Foxp3– population, consistent with those data from other laboratories. Within the Foxp3+ population of cells, we found that, while the percentage of GITRhi cells was marginally lower in 16–20-week-old than in 6–8-week-old mice (P < 0·05), there was no significant difference in the percentage of GITRhi cells in the PLNs.
We also examined the expression of two other surface markers that have been implicated in Treg function, CD62L and CD103, in young and old BDC2·5 NOD mice. We found that, in the spleens of 16–20 week-old BDC2·5NOD mice, the vast majority of Foxp3+ Treg did not express CD62L, while in 4–6-week-old mice approximately 45% of the splenic Foxp3+ population also expressed CD62L (P < 0·01) (Fig. 5c). These age-related differences in CD62L expression were also apparent in the pancreatic lymph nodes (P < 0·01). Very few CD4+ Foxp3+ cells expressed CD62L in 16–18-week-old NOD mice, despite this being a key molecule governing entry of T cells to secondary lymphoid organs.
The alpha E integrin CD103 has been shown to be expressed on Treg in a number of systems. Some reports have suggested that the CD103+ subset of CD25+ Treg has greater suppressive capacity than the CD103– subset.26,27 We found no significant difference in the expression of CD103 in the spleens of old versus young BDC2·5NOD mice (P = 0·14) but we did observe a significant age-related increase in the percentage of CD103+ cells amongst the Foxp3+ population in the pancreatic lymph nodes (P < 0·005) (Fig. 5d).
Age-related changes in cytokine expression by regulatory T cells
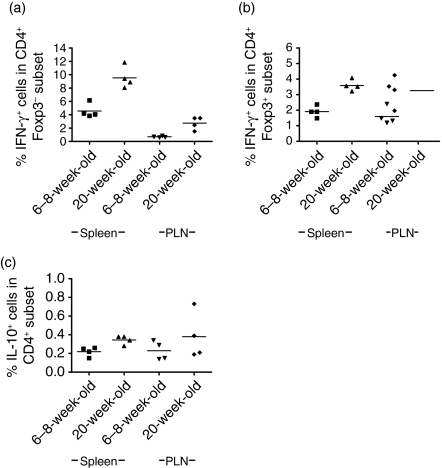
To determine whether there were any differences in the expression of cytokines by BDC2·5NOD Treg from mice of different ages, we co-stained for the presence of intracellular cytokines in both CD4+ Foxp3+ and CD4+ Foxp3– cells. We found that, in response to ex vivo stimulation, CD4+ cells from 20-week-old mice made significantly more IFN-γ than those from 6- to 8-week-old mice (Fig. 6). This phenomenon was particularly noticeable in the CD4+ Foxp3– subset (Fig. 6a), where a significant age-related increase in IFN-γ production was present in both the spleen (P = 0·03) and PLNs (P = 0·03). The CD4+ Foxp3+ population made less IFN-γ than the non-regulatory cells. However, we did note a small but significant age-related increase in the percentage of CD4+ Foxp3+ cells that were IFN-γ+ in the splenic (P =0·03) though not the pancreatic lymph node population (Fig. 6b). As IL-10 plays an important role in diabetes prevention in BDC2·5NOD mice, we also assessed the presence of intracellular IL-10 in T cells following ex vivo stimulation. We found that a much smaller percentage of T cells were IL-10+ than IFN-γ+ and that there was no dramatic increase in IL-10 production as the mice aged (Fig. 6c).
Figure 6.
Age-related changes in cytokine expression by regulatory T cells. Single cell suspensions were made from the spleens and pancreatic lymph nodes of 6–8- and 20-week-old BDC2·5NOD mice. They were stimulated with PdBu and ionomycin to assess ex vivo cytokine secretion. (a, b) The percentage of interferon (IFN)-γ+ cells in the CD4+ Foxp3+ (a) and CD4+ Foxp3– (b) populations at 6–8 weeks of age and 20 weeks of age in spleen and pancreatic draining lymph node (PLNs). (c) The percentage of CD4+ cells expressing interleukin (IL)-10 from 6- to 8-week-old and 20-week-old BDC2·5NOD mice in the spleen and PLNs.
CD4+ CD25+ T cells from old BDC2·5NOD mice exhibit suppressive capabilities in vitro
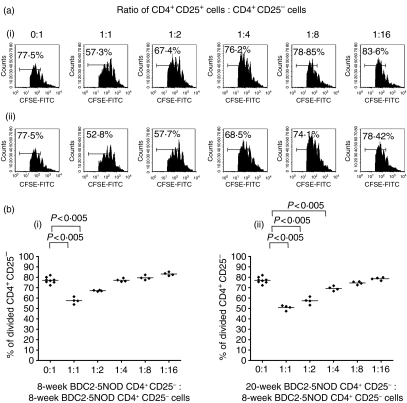
Studies in wild-type NOD mice have shown an age-dependent decrease in Treg function between 8 and 16 weeks of age, while in other strains such as C57BL/6 there are some reports of preservation of function17 and other reports showing a decline of Treg function with age.28 We compared the ability of 8- and 20-week-old CD4+ CD25+ cells to regulate the in vitro responses of CD4+ CD25– cells from 8-week-old BDC2·5NOD mice (Figs 7a and b). CD4+ CD25+ cells from either 8- or 20-week-old BDC2·5NOD mice were able to efficiently suppress the proliferation of 8-week-old BDC2·5NOD CD4+ CD25– cells at a range of ratios.
Figure 7.
CD4+ CD25+ T cells from the spleens of 8-week-old and 20-week-old BDC2·5 NOD mice can suppress proliferation of 8-week-old BDC2·5NOD CD4+ CD25– T cells in vitro. (a) 5 × 104 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE)-labelled CD4+ CD25– T effector cells from an 8-week-old BDC2·5NOD donor together with 2·5 × 105 of their own antigen-presenting cells (APCs) and anti-CD3 (1 µg/ml) were cultured with either no CD4+ CD25+ cells (0:1 ratio) or varying numbers of CD4+ CD25+ cells ranging from 5 × 104 (1:1 ratio) to 3·125 × 103 (1:16 ratio) from either (i) an 8-week-old or (ii) a 20-week-old BDC2·5NOD donor. Representative fluorescence-activated cell sorter (FACS) histogram plots from multiple wells of CFSE and CD4+ cells show a marked reduction of effector T-cell proliferation when regulatory T cells were added at a 1:1 ratio compared with effectors alone irrespective of the source of the regulatory T cells. The suppressive effect of the CD4+ CD25+ T cells was reduced as the ratio of CD4+ CD25+ T cells to CD4+ CD25– T cells decreased in both regulatory T-cell groups. (b) Proliferation data from multiple wells from the above experiment show a significant reduction in 8-week-old effector T-cell proliferation when CD4+ CD25+ cells from either (i) 8-week-old or (ii) 20-week-old BDC2·5NOD mice were added to the cocultures of CD4+ CD25– cells, APCs and anti-CD3.
CD4+ CD25+ T cells from old BDC2·5NOD mice exhibit suppressive capabilities in vivo
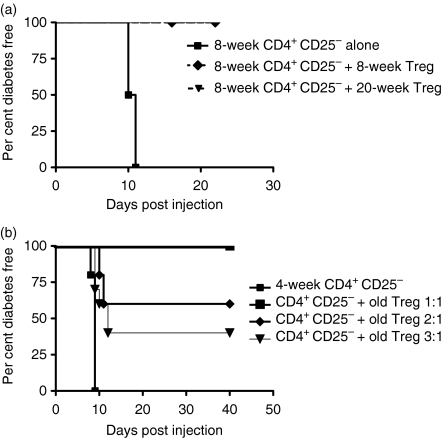
Those papers that demonstrated an age-related loss of in vitro suppression in wild-type NOD mice also showed in vivo defects in Treg activity.13,16 We assessed whether CD4+ CD25+ T cells from 16-week-old BDC2·5NOD mice were capable of suppressing BDC2·5NOD CD4+CD25– T cells in the NOD.scid transfer model. We found that at CD4+ CD25+:CD4+ CD25– ratios of 1:2, Treg from both 8- and 16–20-week-old mice were able completely to prevent disease transfer by CD4+ CD25– cells from 8-week-old BDC2·5NOD mice to 8-week-old male NOD.scid recipients (Fig. 8a) (P < 0·01). We also found that CD4+ CD25+ regulatory cells from 16- to 20-week-old mice could mediate suppression of disease transfer when CD4+ CD25– cells from 4-week-old BDC2·5NOD mice were transferred to NOD.scid recipients (Fig. 8b). Significant suppression was noted even at Treg to T effector cell ratios of 1:3 (P < 0·05).
Figure 8.
CD4+ CD25+ cells from 16- to 20-week-old BDC2·5NOD mice are suppressive in vivo. (a) 2 × 105 CD4+ CD25– cells from 8-week-old BDC2·5NOD mice were transferred to 8-week-old male NOD.scid recipients alone (five mice) or in the presence of 1 × 105 CD4+ CD25+ cells from either 8-week-old or 16–20-week-old BDC2·5NOD mice (five mice in each experiment). (b) 2 × 105 CD4+ CD25– cells from 4-week-old BDC2·5NOD mice were transferred to 4-week-old male NOD.scid recipients alone or in the presence of 2 × 105 (five mice), 1 × 105 (10 mice) or 6·6 × 104 (five mice) CD4+ CD25+ T cells from 16- to 20-week-old BDC2·5NOD mice. Treg, regulatory T cells.
Discussion
The observation that the frequency of Treg cells rises as BDC2·5NOD mice age is intriguing. It is well established that, although CD4+ CD25+ cells account for 5–10% of the peripheral CD4 repertoire in most mouse strains studied, the frequency of this population is plastic and can be increased by many tolerogenic protocols such as anti-CD3,15 anti-CD4029 or anti-tumour necrosis factor (TNF)-α treatment30 and can be decreased by the removal of key costimulatory pathways.31,32 Similarly, it has been shown in mice that the frequency of peripheral Treg rises during pregnancy33 and falls following treatment with pertussis toxin.34 There is an emerging literature on age-related changes in Treg frequency and function in both mice28 and humans.35–38
It had been clear for a number of years that, in certain mouse strains, the ageing process is associated with a degree of hyporesponsiveness amongst CD4+ T cells.18 The mechanisms underlying this were clarified in a paper demonstrating that the proportion of Foxp3+ cells in the CD4+ subset is higher in mice aged 2 years than in 8-week-old mice.17 That report showed only a modest rise in the frequency of CD25+ cells amongst the CD4+ population over this period. The rise in the proportion of the Foxp3+ cells appeared to be largely a result of expansion of the CD25– subset. By contrast, this study shows that the proportion of CD25+ Foxp3+ cells rises with age in BDC2·5NOD mice. Furthermore, we have demonstrated that this age-related increase in the ratio of CD4+ Foxp3+:CD4+ Foxp3– cells occurs much earlier than that observed in other studies. In contrast to other publications that have shown an age-related rise in the frequency of Treg in murine models,39 the rise in the frequency of Foxp3+ cells in BDC2·5NOD mice does not preferentially occur in diseased tissue and is consistent across all tissues studied.
In contrast to studies that have shown increases in Treg frequency with age, it has been suggested that an observed age-related decline in the number of Tregs contributes to the onset of diabetes in wild-type NOD mice.16 One investigation into NOD Treg function reported that the frequency of CD4+ CD25+ was the same in 16-week-old and 8-week-old mice.13 Similar to the results obtained in those studies, we found that the frequency CD4+ CD25+ cells and CD4+ Foxp3+ cells was the same in wild-type NOD mice whether they were 8 or 16–20 weeks old. In contrast to those publications in wild-type NOD mice that showed a decline in the suppressive function of wild-type NOD Treg function with age, we found no deficit in the suppressive behaviour of BDC2·5NOD Treg either in vitro or in vivo. We showed that Treg from 20-week-old mice were as effective as those from 6- to 8-week-old mice in vivo using the NOD.scid transfer system. This experimental system has been widely used to assess in vivo Treg function in both wild-type and BDC2·5NOD mice. It is worth noting, however, that homeostatic expansion of cells is likely to occur in the lymphopenic setting. We therefore cannot exclude the possibility that the suppression seen in these systems may result from competition for niches between the regulatory and effector populations40,41 and may not be analogous to the action of Treg in mice that are not lymphopenic. These lymphopenic systems are widely used, however, and may be a good surrogate marker for Treg function in vivo.
We have at present no clear mechanism for the age-related rise in the peripheral frequency of CD4+ Foxp3+ cells in the periphery of the BDC2·5NOD mouse. Our data showing that the thymic frequency of CD4+ Foxp3+ cells is higher in 20-week-old compared with 6–8-week-old BDC2·5NOD mice indicates that increased thymic export of Treg contributes to the age-related rise in the peripheral frequency of Treg. These findings do not, however, rule out a role for increased peripheral survival, proliferation or conversion of Treg with age.
It is interesting to note that the age-related rise in the peripheral frequency of CD4+ Foxp3+ T cells does not occur in wild-type NOD mice, despite the two animals sharing almost all of their genome. It is tempting to speculate that the restricted T-cell repertoire of the BDC2·5NOD mouse is particularly conducive to the expansion and/or the long-term persistence of Treg. Clearly, the make-up of the T-cell repertoire contributes to the development of Treg in BDC2·5NOD mice because no Treg develop when the mouse is crossed to a NOD.scid or RAG–/– background where endogenous alpha chain rearrangements are prevented (JP, unpublished observations; also Gonzalez et al.42 and Chen et al.43). On this subject, a paper by Kanagawa et al.6 did describe the emergence of a clonotypelo (expressing low levels of the Vα1Vβ4 TCR) population of Treg with age in BDC2·5NOD mice. We found that the increase of this population was also apparent in our colony of BDC2·5NOD mice. We found that the percentages of both Foxp3+ and Foxp3– cells that were clonotypelo increased with age. This clonotypelo population is not therefore exclusively composed of Foxp3+ cells, although the proportion of clonotypelo cells that are Foxp3+ does increase with age. Interestingly, Kanagawa et al.6 did not report an increase in CD4+ CD25+ cells per se in BDC2·5NOD mice. This may be attributable to slight differences between mouse colonies or may relate to the fact that the authors confined their observations to lymph nodes, rather than the spleen, in which the differences in CD25 expression (rather than Foxp3 expression) between old and young mice are not quite as pronounced as those in the spleen.
On the general subject of antigen specificity determining Treg frequency, it is known that the presence and degree of cognate antigen expression in the thymus can affect the Treg to T effector cell ratio observed in the periphery.44,45 Studies in transgenic systems show that Treg can proliferate in response to their cognate antigen in the periphery.46,47 Furthermore, encounter with certain antigens in the right context in the periphery can drive the differentiation of CD4+ CD25– cells into Foxp3-expressing Treg.48,49 It is therefore possible that the BDC2·5NOD Treg antigens are expressed in the thymus or periphery at a level that is particularly conducive to the persistence of a regulatory population (through thymic selection and/or peripheral induction and expansion). By contrast, wild-type NOD Treg can respond to a greater range of antigens because of their diverse T-cell repertoire. The majority of these antigens may not be expressed in a manner favourable to Treg differentiation or expansion as the NOD mouse ages and thus wild-type NOD Treg function could become impaired. In wild-type NOD mice, for instance, which have spontaneous aggressive infiltrates in the pancreas and the lacrimal and salivary glands, some of the Treg antigens may actually be destroyed or their expression altered as a result of ongoing inflammation.
Aside from differences in the range of T-cell specificities, some of the surface markers found on Foxp3+ Treg may differ between wild-type NOD and BDC2·5NOD mice; these differences may in part account for the increased frequency of Treg in BDC2·5NOD mice. A particular set of adhesion molecules on the surface of Treg may enable their preferential extravasation to lymph nodes or tissues where they can receive antigenic stimulation that might provide expansion or survival signals. For instance, the expression of CD62L on the surface of Treg appears important to their efficacy in some systems, including the NOD mouse.50,51 It was shown that, in a NOD.scid transfer system, only the CD62Lhi population were capable of suppressing disease transfer by CD4+ CD25– cells. It was also shown that different surface markers appeared to define the Treg subsets that were responsible for controlling various autoimmune diseases.52 In particular, Alyanakian et al.52 established that depletion of CD62Lhi cells prior to transfer of NOD splenocytes to NOD.scid recipients resulted in diabetes but not gastritis. The importance of CD62Lhi Treg has been emphasized in other experimental systems such as murine models of graft-versus-host disease (GVHD). In these interesting studies, while CD62Llo and CD62Lhi Treg exhibited similar suppressive capabilities in vitro, only CD62Lhi cells were able to efficiently access lymphoid tissues and control GVHD.53 Nevertheless, CD62L does not appear to be so important for access to LN or control of autoimmunity in Treg function in other models,54 including the present one.
We were also intrigued to find that PLN Treg of older BDC2·5NOD mice were more likely to be CD103+ than those in younger mice. CD103+ Treg in some systems are more potent suppressor cells than their CD103– counterparts.26,27 In other studies of age-related changes in the Treg subset, the emergence of CD103+ cells also appears to be important.17,55
In summary, the results presented in this paper show an early age-related increase in the frequency and absolute number of Treg in the BDC2·5NOD mouse. We found that old BDC2·5NOD mice did not exhibit the documented regulatory deficiencies of the wild-type mouse either in vitro or in vivo. These data may explain the relative protection of this animal from disease in the face of its highly autoreactive T-cell repertoire, while the wild-type NOD mouse succumbs to diabetes. Our data may also offer an explanation for other age-related phenomena in the BDC2·5NOD mouse, such as their reduced sensitivity to cyclophosphamide-induced diabetes and the decreased ability of splenocytes from older mice to transfer disease to NOD.scid recipients. Our future work will explore the mechanisms underlying these changes in the CD4+ T-cell subset. Understanding how such effects occur naturally in vivo during the life of an animal may aid our understanding of therapeutic interventions that could boost Treg frequency and efficacy and thus modify or prevent autoimmune tissue destruction.
Acknowledgments
DT was supported by the MB/PhD programme, University of Cambridge, the Jean Shanks foundation and the James Baird fund. RJM is supported by a Wellcome Trust Research Training Fellowship. JMP is supported by the Wellcome Trust.
Glossary
Abbreviations:
- PLN
pancreatic draining lymph node
- Treg
regulatory T cells
References
- 1.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 2.Haskins K, Portas M, Bradley B, Wegmann D, Lafferty K. T-lymphocyte clone specific for pancreatic islet antigen. Diabetes. 1988;37:1444–8. doi: 10.2337/diab.37.10.1444. [DOI] [PubMed] [Google Scholar]
- 3.Haskins K, McDuffie M. Acceleration of diabetes in young NOD mice with a CD4+ islet-specific T cell clone. Science. 1990;249:1433–6. doi: 10.1126/science.2205920. [DOI] [PubMed] [Google Scholar]
- 4.Dai YD, Jensen KP, Lehuen A, Masteller EL, Bluestone JA, Wilson DB, Sercarz EE. A peptide of glutamic acid decarboxylase 65 can recruit and expand a diabetogenic T cell clone, BDC2.5, in the pancreas. J Immunol. 2005;175:3621–7. doi: 10.4049/jimmunol.175.6.3621. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez A, Katz JD, Mattei MG, Kikutani H, Benoist C, Mathis D. Genetic control of diabetes progression. Immunity. 1997;7:873–83. doi: 10.1016/s1074-7613(00)80405-7. [DOI] [PubMed] [Google Scholar]
- 6.Kanagawa O, Militech A, Vaupel BA. Regulation of diabetes development by regulatory T cells in pancreatic islet antigen-specific TCR transgenic nonobese diabetic mice. J Immunol. 2002;168:6159–64. doi: 10.4049/jimmunol.168.12.6159. [DOI] [PubMed] [Google Scholar]
- 7.Phillips JM, Parish NM, Drage M, Cooke A. Cutting edge: interactions through the IL-10 receptor regulate autoimmune diabetes. J Immunol. 2001;167:6087–91. doi: 10.4049/jimmunol.167.11.6087. [DOI] [PubMed] [Google Scholar]
- 8.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 9.Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 10.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 11.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 12.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–7. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 13.Gregori S, Giarratana N, Smiroldo S, Adorini L. Dynamics of pathogenic and suppressor T cells in autoimmune diabetes development. J Immunol. 2003;171:4040–7. doi: 10.4049/jimmunol.171.8.4040. [DOI] [PubMed] [Google Scholar]
- 14.You S, Belghith M, Cobbold S, et al. Autoimmune diabetes onset results from qualitative rather than quantitative age-dependent changes in pathogenic T-cells. Diabetes. 2005;54:1415–22. doi: 10.2337/diabetes.54.5.1415. [DOI] [PubMed] [Google Scholar]
- 15.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–8. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 16.Pop SM, Wong CP, Culton DA, Clarke SH, Tisch R. Single cell analysis shows decreasing FoxP3 and TGFbeta1 coexpressing CD4+CD25+ regulatory T cells during autoimmune diabetes. J Exp Med. 2005;201:1333–46. doi: 10.1084/jem.20042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S. CD4+CD25+Foxp3+ T cells and CD4+CD25–Foxp3+ T cells in aged mice. J Immunol. 2006;176:6586–93. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu J, Moriizumi E. CD4+CD25– T cells in aged mice are hyporesponsive and exhibit suppressive activity. J Immunol. 2003;170:1675–82. doi: 10.4049/jimmunol.170.4.1675. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+) CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+) CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection. CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080–6. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 23.Sansom DM, Walker LS. The role of CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell biology. Immunol Rev. 2006;212:131–48. doi: 10.1111/j.0105-2896.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 24.Luhder F, Hoglund P, Allison JP, Benoist C, Mathis D. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J Exp Med. 1998;187:427–32. doi: 10.1084/jem.187.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+) CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–42. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 26.Lehmann J, Huehn J, de la Rosa M, et al. Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25– regulatory T cells. Proc Natl Acad Sci USA. 2002;99:13031–6. doi: 10.1073/pnas.192162899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banz A, Peixoto A, Pontoux C, Cordier C, Rocha B, Papiernik M. A unique subpopulation of CD4+ regulatory T cells controls wasting disease, IL-10 secretion and T cell homeostasis. Eur J Immunol. 2003;33:2419–28. doi: 10.1002/eji.200324205. [DOI] [PubMed] [Google Scholar]
- 28.Matejuk A, Hopke C, Vandenbark AA, Hurn PD, Offner H. Middle-age male mice have increased severity of experimental autoimmune encephalomyelitis and are unresponsive to testosterone therapy. J Immunol. 2005;174:2387–95. doi: 10.4049/jimmunol.174.4.2387. [DOI] [PubMed] [Google Scholar]
- 29.McGregor CM, Schoenberger SP, Green EA. CD154 is a negative regulator of autoaggressive CD8+ T cells in type 1 diabetes. Proc Natl Acad Sci USA. 2004;101:9345–50. doi: 10.1073/pnas.0402807101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu AJ, Hua H, Munson SH, McDevitt HO. Tumor necrosis factor-alpha regulation of CD4+CD25+ T cell levels in NOD mice. Proc Natl Acad Sci USA. 2002;99:12287–92. doi: 10.1073/pnas.172382999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bour-Jordan H, Salomon BL, Thompson HL, Szot GL, Bernhard MR, Bluestone JA. Costimulation controls diabetes by altering the balance of pathogenic and regulatory T cells. J Clin Invest. 2004;114:979–87. doi: 10.1172/JCI20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 33.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–71. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 34.Chen X, Winkler-Pickett RT, Carbonetti NH, Ortaldo JR, Oppenheim JJ, Howard OM. Pertussis toxin as an adjuvant suppresses the number and function of CD4+CD25+ T regulatory cells. Eur J Immunol. 2006;36:671–80. doi: 10.1002/eji.200535353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dejaco C, Duftner C, Schirmer M. Are regulatory T-cells linked with aging? Exp Gerontol. 2006;41:339–45. doi: 10.1016/j.exger.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Trzonkowski P, Szmit E, Mysliwska J, Mysliwski A. CD4+CD25+ T regulatory cells inhibit cytotoxic activity of CTL and NK cells in humans – impact of immunosenescence. Clin Immunol. 2006;119:307–16. doi: 10.1016/j.clim.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, Nayak L, Moss PA. The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin Exp Immunol. 2005;140:540–6. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bryl E, Witkowski JM. Decreased proliferative capability of CD4(+) cells of elderly people is associated with faster loss of activation-related antigens and accumulation of regulatory T cells. Exp Gerontol. 2004;39:587–95. doi: 10.1016/j.exger.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 39.Scalapino KJ, Tang Q, Bluestone JA, Bonyhadi ML, Daikh DI. Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J Immunol. 2006;177:1451–9. doi: 10.4049/jimmunol.177.3.1451. [DOI] [PubMed] [Google Scholar]
- 40.Barthlott T, Kassiotis G, Stockinger B. T cell regulation as a side effect of homeostasis and competition. J Exp Med. 2003;197:451–60. doi: 10.1084/jem.20021387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stockinger B, Barthlott T, Kassiotis G. T cell regulation: a special job or everyone's responsibility? Nat Immunol. 2001;2:757–8. doi: 10.1038/ni0901-757. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez A, Andre-Schmutz I, Carnaud C, Mathis D, Benoist C. Damage control, rather than unresponsiveness, effected by protective DX5+ T cells in autoimmune diabetes. Nat Immunol. 2001;2:1117–25. doi: 10.1038/ni738. [DOI] [PubMed] [Google Scholar]
- 43.Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+CD25+ Treg cells impinge on autoimmune diabetes. J Exp Med. 2005;202:1387–97. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caton AJ, Cozzo C, Larkin J 3rd, Lerman MA, Boesteanu A, Jordan MS. CD4+ CD25+ regulatory T cell selection. Ann N Y Acad Sci. 2004;1029:101–14. doi: 10.1196/annals.1309.028. [DOI] [PubMed] [Google Scholar]
- 45.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–6. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 46.Cozzo C, Larkin J 3rd, Caton AJ. Cutting edge. self-peptides drive the peripheral expansion of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:5678–82. doi: 10.4049/jimmunol.171.11.5678. [DOI] [PubMed] [Google Scholar]
- 47.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–58. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Boehmer H. Peptide-based instruction of suppressor commitment in naive T cells and dynamics of immunosuppression in vivo. Scand J Immunol. 2005;62(Suppl. 1):49–54. doi: 10.1111/j.1365-3083.2005.01609.x. [DOI] [PubMed] [Google Scholar]
- 49.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–8. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lepault F, Gagnerault MC. Characterization of peripheral regulatory CD4+ T cells that prevent diabetes onset in nonobese diabetic mice. J Immunol. 2000;164:240–7. doi: 10.4049/jimmunol.164.1.240. [DOI] [PubMed] [Google Scholar]
- 51.You S, Slehoffer G, Barriot S, Bach JF, Chatenoud L. Unique role of CD4+CD62L+ regulatory T cells in the control of autoimmune diabetes in T cell receptor transgenic mice. Proc Natl Acad Sci USA. 2004;101(Suppl. 2):14580–5. doi: 10.1073/pnas.0404870101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alyanakian MA, You S, Damotte D, et al. Diversity of regulatory CD4+ T cells controlling distinct organ-specific autoimmune diseases. Proc Natl Acad Sci USA. 2003;100:15806–11. doi: 10.1073/pnas.2636971100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ermann J, Hoffmann P, Edinger M, et al. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 2005;105:2220–6. doi: 10.1182/blood-2004-05-2044. [DOI] [PubMed] [Google Scholar]
- 54.Funatake CJ, Marshall NB, Steppan LB, Mourich DV, Kerkvliet NI. Cutting edge. activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J Immunol. 2005;175:4184–8. doi: 10.4049/jimmunol.175.7.4184. [DOI] [PubMed] [Google Scholar]
- 55.Shimizu J, Moriizumi E. Aging-dependent generation of suppressive CD4+CD25–R123loCD103+ T cells in mice. Eur J Immunol. 2003;33:2449–58. doi: 10.1002/eji.200324040. [DOI] [PubMed] [Google Scholar]