Abstract
The family of lipid kinases termed phosphoinositide-3-kinase (PI3K) is known to contribute at multiple levels to innate and adaptive immune responses, and is hence an attractive target for drug discovery in inflammatory and autoimmune disease, including respiratory diseases. The development of isoform-selective pharmacological inhibitors, targeted gene manipulation and short interfering RNA (siRNA) target validation have facilitated a better understanding of the role that each member of this family of kinases plays in the physiology and pathology of the respiratory system. In this review, we will evaluate the evidence for the roles of specific PI3K isoforms in the lung and airways, and discuss their potential as targets for novel drug therapies.
Keywords: PI3K, lung disease, asthma, inflammation
The defining characteristics of chronic respiratory diseases include inflammatory cell recruitment, inflammatory mediator expression, tissue remodelling, and altered airway smooth muscle contraction. Central to these events is a complex interplay between receptor signalling and downstream lipid and protein kinases. One of the main families of kinases involved in these processes is phosphoinositide 3-kinase (PI3K). As respiratory diseases are very common1,2 and novel drugs are required to cover the unmet needs of patients, recent advances point to the modulation of PI3K as a possible therapeutic target. In this article, we review the role that PI3K plays in lung diseases and the possible beneficial effects of targeting PI3K activity.
The PI3K signalling pathway: an overview
Phosphatidylinositol 3-kinase was initially postulated as a cancer target because it was initially co-purified with oncoproteins in 1987.3 Since then it has been implicated as a potential drug target in other disease settings, reflecting its essential roles in many of our basic homeostatic mechanisms, including cell differentiation, growth, metabolism and immune function.
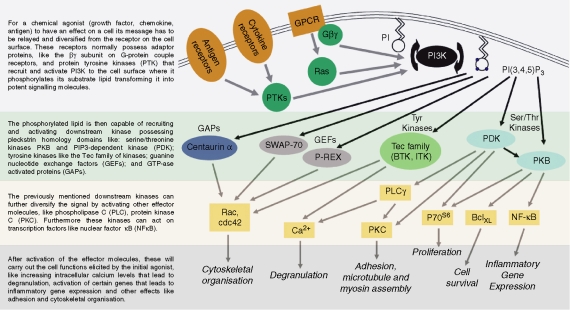
The term PI3K is applied to a family of kinases that phosphorylates the D-3 position of the inositol ring of target lipids; Class I PI3Ks phosphorylate PI(4,5)P2 to produce PI(3,4,5)P3, a critical second messenger that propagates intracellular signals through a host of downstream protein mediators.4 PI(3,4,5)P3 acts as a docking site at the plasma membrane, recruiting and activating proteins containing phospholipid-binding domains. These downstream PI3K effectors include protein kinases that promote cell growth, survival and proliferation (such as Akt, PDK1, and the Tec family kinases); GAPs and GEFs (such as P-Rex5 and Swap706) that regulate the GTPases mediating cell motility and membrane trafficking; and scaffolding proteins (Gab2, etc.) that nucleate the assembly of key signalling complexes.7,8 As highlighted in Fig. 1, PI3K is therefore a hub through which many signals are relayed before being specialized into secondary streams of signalling, and thus the therapeutic challenge is to achieve control of pathological activation without detrimental off-target effects. Specific targeting of individual PI3K isoforms may provide the key to understanding and controlling PI3K in disease.
Figure 1.
PI3K as a central hub in signalling in inflammation and homeostasis. This figure aims to be representative of the main kinases and proteins involved in different cell functions.
As seen on Table 1, the PI3K family is divided into three classes based on their different isoform structure and substrate specificity. These differences, coupled with differences in their distribution, allow PI3K isoforms to mediate distinct functions (a list of which also appears in Table 1). Class I PI3K is the most studied of the PI3K classes and the majority of research focuses on these isoforms. Comparatively little is known about Class II PI3Ks, aside from their suspected role in clathrin-associated vesicle trafficking,9 partly because Class II PI3K-C2α is refractory to inhibition by wortmannin and LY294002,10 the most widely used broad-spectrum inhibitors of PI3K. Therefore, use of these compounds (LY294002, wortmannin) has probably underestimated the potential roles for this isoform. Finally, class III PI3K produce an apparently non-signalling inositol lipid, although class III PI3K has also been recently suggested to be important in Toll-like receptor (TLR) signalling.11
Table 1.
Main characteristics of the members of the PI3K family
| Class | Subclass | Catalytic subunit | Adaptor/ Regulatory subunits | Regulated by | Distribution | In vitro lipid products | Functions for selective inhibitors | Organization with licences |
|---|---|---|---|---|---|---|---|---|
| I | A | p110α | p85α, p85β, | RTKa | Ubiquitous | PI(3)Pb, | Developmentc14, | Eli Lilly |
| p55α, p55γ, p50α | PI(3,4)P2d, | Human cancere116, | Iconix | |||||
| PI(3,4,5)P3f | Myocardial contractility117 | Echelon | ||||||
| Astra Zeneca | ||||||||
| Wyeth | ||||||||
| PIramed | ||||||||
| Moreg | ||||||||
| p110β | p85α, p85β, | RTKa, | Ubiquitous | PI(3)Pb, | Developmentc15, | Kinacia | ||
| p55α, p55γ, p50α | GPCRh | PI(3,4)P2d | Insulin signalling118, | |||||
| PI(3,4,5)P3f | Motility119, | |||||||
| Phagocytosis120, | ||||||||
| Thrombus formation121, | ||||||||
| p110δ | p85α, p85β, | RTKa | Leucocytesi | PI(3)Pb, | Immunity24, | ICOS | ||
| p55α, p55γ, p50α | PI(3,4)P2d, | Cytokine receptor signalling122, | PIramed | |||||
| PI(3,4,5)P3f | B-cell development24, | |||||||
| B-cell migration38, | ||||||||
| T-cell development123, | ||||||||
| Cell proliferation124, | ||||||||
| B- and T-cell antigenreceptor signalling24, | ||||||||
| Smooth muscle tone andhypertension125, | ||||||||
| Neutrophil migration and burst40 | ||||||||
| B | p110γ | p101, p87PIKAP | GPCRh | Myeloidi | PI(3)Pb, | Thymocyte development126, | Novartis | |
| PI(3,4)P2d, | T cell development123, | Bayer | ||||||
| PI(3,4,5)P3f | T cell migration38, | Pfizer | ||||||
| Neutrophil migration126, | Serono | |||||||
| Macrophage and dendritic cell migration37, | Targegen | |||||||
| Mast cell degranulation29, | Calbiochem | |||||||
| Neutrophil burst40, | ||||||||
| Insulin secretion127, | ||||||||
| Myocardial contractility117 | ||||||||
| II | C2α | Clathrin | RTKa | Widespread | PI(3)Pb, | Vesicle trafficking9, | ||
| GPCRh, IRj | PI(3,4)P2d | Insulin signalling128, | ||||||
| Cell survival129, | ||||||||
| Vascular smooth musclecontraction130 | ||||||||
| C2β | Clathrin | RTKa, | Widespread | PI(3)Pb, | Vesicle trafficking131, | |||
| GPCRh, IRj | PI(3,4)P2d | Cell migration132,133, | ||||||
| Liver growth134 | ||||||||
| C2γ | Clathrin | ? | Hepatic | PI(3)Pb, | Liver regeneration135 | |||
| PI(3,4)P2d | ||||||||
| III | Vps34p | Vps15p | TLRk, constitutive | Ubiquitous | PI(3)Pb | Toll-like receptor signalling136, | ||
| Receptor-independent membranetrafficking137 |
Receptor tyrosine kinase
phosphatidyl inositol-3-monophosphate
deletion associated with defective mouse embryonic proliferation and death
phosphatidyl inositol-3,4-bisphosphate
mutated/amplified in human cancer
phosphatidyl inositol 3,4,5-trisphosphate
due to the high frequency of PIK3CA mutations in human cancer, extensive research into selective inhibitors for this isoform have been carried out by many other institutions (Yamanouchi, Xinxiang Medical College, Semafore, Chiron, Zenyaku Kogyo, Boehringer, University of British Columbia)
G-protein coupled receptors
mainly, but also expressed in endothelial cells and others
integrin receptors
Toll-like receptors.
Class I PI3K functions
Elucidation of the precise functions of different classes of PI3K has been a difficult process. The primary pharmacological tools to determine PI3K function, the inhibitors wortmannin and LY294002, have broad specificity across PI3K isoforms.12 These compounds also inhibit enzymes that are functionally associated with PI3K, like the mammalian target of rapamycin and myosin light-chain kinase.13 With high interest within the pharmaceutical industry, second-generation isoform-specific inhibitors are now being generated (Table 1). The use of these inhibitors, as well as molecular and gene-targeting technology, have begun to give us a more profound insight into the different roles of each one of the members of the PI3K family. The ubiquitously expressed p110α and p110β catalytic subunits have been particularly difficult to study individually, because knockout mice have proven to be lethal at an embryonic stage, suggesting a role for these isoforms in cell proliferation during development.14,15 Nevertheless, studies using heterozygous animals and isoform-specific antibodies have yielded some results, as seen in Table 1. Most progress has been made regarding the p110γ and p110δ isoforms as outlined below.
p110γ and p110δ are key signals in the innate and adaptive immune responses
Both p110γ and p110δ are expressed predominantly (but not exclusively) in leucocytes, leading to speculation that these isoforms are the dominant isoforms involved in PI3K-mediated signalling of both the innate and adaptive immune responses.16 Indeed, PI3K is activated by different TLRs in eosinophils,17 macrophages,18 neutrophils19 and dendritic cells.20 However, PI3Ks are implicated in the negative regulation of TLR-induced interleukin-12 (IL-12) and interferon-γ production.20,21 Hence, gene targeting of class IA isoforms results in defective clearance of intestinal nematodes [a T helper type 2 (Th2) response] but improved resistance to Leishmania infection (a Th1-dependent response).20,22
For adaptive immunity, both p110δ–/– mice23 and mice expressing an inactive form of the p110δ catalytic subunit24 display impaired B-cell and T-cell antigen receptor signalling. Of specific importance to respiratory disease, it has been shown recently that B-cell receptor-mediated antigen presentation is dependent on p110δ activity.25 Furthermore, analysis of mice expressing a catalytically inactive form of the p110δ isoform has revealed a pivotal role for this isoform in CD28-costimulated T-cell clonal expansion and differentiation26 as well as function of CD4+ CD25+ Foxp3+ regulatory T cells.27 Finally, PI3K isoforms have also been demonstrated to play key roles in mast cell function, which are linked to both innate and adaptive responses and will be discussed in further detail later.28,29
p110γ and p110δ are the predominant isoforms involved in leucocyte migration
The ordered directional migration of leucocytes is a key process fundamental to constitutive immune surveillance and immune responses. Macrophages and neutrophils from p110γ–/– mice display reduced migratory capacity in response to several chemoattractants including chemokines.30 The p110γ isoform also mediates T-cell migration both in vitro and in vivo although its involvement is context-dependent and often depends on the activation/differentiation status of the T cell.31–34 More recent evidence indicates that while 3′-phosphoinositide lipids accumulate in a p110γ-dependent fashion at the up-gradient leading edge, this signal is not required for efficient gradient sensing or gradient-biased movement.35,36 Rather, p110γ appears to be a critical determinant of overall cell motility rather than directional aspects of migration. Possibly as a consequence of impaired migratory capacity, p110γ–/– mice exhibit defects in T-cell development and activation. These mice also show a defective capacity to mount contact hypersensitivity and delayed-type hypersensitivity reactions.37 T-cell migration is largely unaffected by p110δ mutation,38 suggesting that the p110γ isoform is the predominant isoform regulating migration in this cell type.
In contrast with the T-cell effects described above, B-cell migration to chemokines is not significantly affected by p110γ deficiency, but is strikingly impaired in mice expressing a catalytically inactive form of the p110δ isoform.19,20 In this regard, analysis of p110δ-deficient B cells showed a defect in B-cell chemotaxis to CXCL13, while responses to CCR7 and CXCR4 ligands were less affected. Similarly, in vivo administration of the novel p110δ-specific inhibitor IC87114 reduces neutrophil tethering and increases rolling velocities on cytokine-activated microvessels.39
The p110δ–p110γ partnership
As p110δ and p110γ share similar patterns of distribution within immune cells, they interact to accomplish certain functions within these cells. N-formyl-methionyl-leucyl-phenylalanine stimulation of tumour necrosis factor-α (TNF-α)-primed human neutrophils results in a biphasic activation of PI3K. The first phase depends on p110γ, while the second phase is largely dependent on p110δ. The second phase of PI3K activation is increased by TNF-α priming and it regulates the activation of reactivate oxygen species production. However, this p110δ-dependent second-phase PI3K activity requires the p110γ-dependent first phase.40 Similarly, in p110δ/p110γ–/– mice, the activity of both these isoforms has been found to be essential for thymocyte survival as well as T-cell development and production.41,42 Furthermore, as reviewed further on, this p110δ–p110γ partnership also exists in parenchymal cells (i.e. specialized tissue cells of an organ, like lung epithelial and endothelial cells), where these isoforms interact to express adhesion molecules.43 In short, both p110δ and p110γ represent exciting therapeutic targets in specific inflammatory conditions because blockade of their activity is likely to influence immune function at multiple levels.
Importance of PI3K in immune cell function and relevance to lung disease
Within chronic respiratory diseases, asthma and chronic obstructive pulmonary disease (COPD) are the most common clinical entities; while acute respiratory distress syndrome and lung fibrotic diseases represent a significant therapeutic challenge. A summary of PI3K research into the pathophysiology of lung disease can be found in Table 2.
Table 2.
PI3K, and its individual isoforms, as targets in respiratory disease research
| Target | Disease | Model | Research tool | Targeting impact | Refs |
|---|---|---|---|---|---|
| PI3K | ALI-ARDS | Endotoxin-induced (in vitro) | Inhibitor | Reduced expression of IL-1β and TNF-α in cultured neutrophils | 46 |
| Endotoxin-Induced (in vivo) | Inhibitor | No reduction in IL-6 or CXCL2 levels | 54 | ||
| Endotoxin-Induced (in vivo) | Inhibitor | Decreased oedema, neutrophil infiltration and inflammatory cytokine expression | 46 | ||
| Overventilation (ex vivo) | Inhibitor | Attenuated filtration coefficients in isolated lungs | 55 | ||
| Overventilation (in vivo) | Inhibitor | Diminished IL-6 and CXCL2 release | 54 | ||
| HCl lung instillation (in vivo) | Inhibitor | Reduced neutrophil recruitment to lungs | 60 | ||
| COPD | IL-1β-induced mucin secretion (in vitro) | Inhibitor | Attenuated mucin secretion in cultured airway epithelial cells | 95 | |
| Chemotaxis assay (in vitro) | Inhibitor | Inhibited chemotactic responses to a CXCR3 ligand in cultured airway epithelial cells | 138 | ||
| Nicotine-induced survival and transformed phenotype (in vitro) | Inhibitor | Prevented transformed phenotype and enhanced survival in human airway epithelial cells | 87 | ||
| Asthma | IL-13-induced mucus secretory phenotype (in vitro) | Inhibitor | Reduced mucus hypersecretory phenotype and goblet cell metaplasia in bronchial epithelial cell cultures | 88 | |
| Thrombin-, EGF-induced and PDGF-induced (in vitro) | Inhibitor | Reduced proliferation of cultured airway smooth muscle cells | 101, 102, 104 | ||
| Long-term serum deprivation (in vitro) | Inhibitor | Inhibited the transformation of cultured airway myocytes into a contractile phenotype | 96 | ||
| Methacoline-induced (in vitro) | Inhibitor | Partially attenuated contraction in cultured airway smooth muscle strips | 99 | ||
| Ovalbumin-induced (in vitro) | Inhibitor | Decreased bronchial contraction in isolated bronchial tissue | 98 | ||
| Ovalbumin-induced (in vivo) | Inhibitor | Diminished levels of interleukins (4, 5 and 13), lung tissue eosinophilia, airway mucus production and AHR | 71, 72 | ||
| Ovalbumin-induced (in vivo) | Inhibitor | No improvement in lung eosinophilia or in AHR to acetylcholine, attenuated eosinophil levels and degranulation in BAL fluid, and AHR to histamine | 78, 80 | ||
| p85α | ALI-ARDS | TNF-α-induced (in vitro) | Blocking protein (TAT construct) | Attenuated reactive oxygen species production in primary granulocytes | 59 |
| TNF-α-induced (in vivo) | Blocking protein (TAT construct) | Reduced microvascular injury and granulocyte recruitment to the lung | 59 | ||
| Asthma | Ovalbumin-induced (in vivo) | Gene disruption | Decreased secretion of interleukins (4 and 5), eosinophil and lymphocyte lung infiltration, and AHR | 73 | |
| p110 | Asthma | Thrombin-, EGF-induced (in vitro) | Gene disruption | Attenuated proliferation of cultured airway smooth muscle cells | 106 |
| p110δ | ALI-ARDS | i.p. E. coli challenge (in vivo) | Gene disruption | No protection against disease model | 61 |
| Endotoxin-Induced (in vivo) | Inhibitor | Reduced lung neutrophil accumulation | 39, 46 | ||
| Endotoxin-Induced (in vivo) | Gene disruption | Diminished neutrophils recruitment, pulmonary levels of IL-1β and TNF-α | 46 | ||
| Endotoxin-Induced (in vivo) | Gene disruption | Reduced neutrophil recruitment | 43 | ||
| COPD | Intranasal chemokine instillation (in vivo) | Gene disruption | No protection against disease model | 67 | |
| Asthma | Allergen-IgE-induced (in vitro) | Gene disruption and inhibitor | Inhibited degranulation and cytokine release in cultured mast cells | 28 | |
| Ovalbumin-induced (in vivo) | Inhibitor | Diminished levels of interleukins (4, 5 and 13), leucocyte infiltration to the lungs, and IgE and LTC4 release | 74 | ||
| Ovalbumin-induced (in vivo) | Haemopoietic cell-restricted gene disruption | Reduced levels of type 2 cytokines, eosinophil recruitment to the lung, airway inflammation and mucus production | 75 | ||
| p110γ | ALI-ARDS | Overventilation (ex vivo) | Gene disruption | Attenuated levels of histological indices and levels of PKB phosphorylation in isolated lungs | 56 |
| Endotoxin-induced (in vivo) | Gene disruption | Decreased neutrophil recruitment to the lung | 43 | ||
| COPD | Chemotaxis assay (in vitro) | Gene disruption | Reduced chemotaxis to CXCR2 ligands in neutrophils | 67 | |
| Intranasal chemokine instillation (in vivo) | Gene disruption | Diminished neutrophil accumulation in the lung | 67 | ||
| Asthma | Allergen-IgE-adenosine- induced (in vitro) | Gene disruption | Partially attenuated degranulation and cytokine release in cultured mast cells | 29 |
AHR, airway hyperresponsiveness; ALI-ARDS, acute lung injury–adult respiratory distress syndrome; BAL, bronchoalveolar lavage; COPD, chronic obstructive pulmonary disease; EGF, epidermal growth factor; IgE, immunoglobulin E; IL-6, interleukin-6; LTC4, leukotriene C4; PDGF, platelet-derived growth factor; TNF-α, tumour necrosis factor-α.
Acute lung injury (ALI) and adult respiratory distress syndrome (ARDS)
The importance of ALI-ARDS has recently been highlighted by a European multicentre epidemiological survey, in which the occurrence in intensive-care units approximates 7% of all admissions and 15% of patients receiving more than 24 hr of mechanical ventilation. Overall, the hospital mortality of patients diagnosed with ARDS was close to 55%.44 ALI-ARDS is characterized by increased expression of proinflammatory cytokines, development of interstitial pulmonary oedema and neutrophil accumulation in the lungs45,46 that cannot be explained by, but may coexist with, left atrial or pulmonary capillary hypertension.
The distinction between ALI and ARDS is the degree of hypoxaemia; ARDS represents that subset of patients at the severe end of the spectrum of ALI47 who are characterized by a diffuse inflammation of their lung parenchyma. ALI-ARDS is caused by an uncontrolled systemic inflammatory response resulting from clinical events including major surgery, trauma, multiple transfusions, severe burns, pancreatitis, sepsis and prolonged mechanical ventilation.48 The major animal models for ALI-ARDS replicate more closely the events of these latter two causes, as intratracheal instillation of lipopolysaccharide (LPS) or mechanical overventilation of the lungs is commonly used.49,50 The initial insult on the lung tissue results in the release of inflammatory mediators by local macrophages, epithelial and endothelial cells. This array of chemical agents, in conjunction with surface expression of adhesion molecules by parenchymal cells, attracts neutrophils and T lymphocytes into the lungs, further amplifying the inflammation, creating a positive feedback loop in which inflammation leads to more inflammation, leading inexorably to tissue destruction and fibrosis.50
PI3K and ALI-ARDS
In isolated lungs, ventilation with increased volumes or pressures, termed overventilation, elicits local and systemic concentrations of proinflammatory mediators51 to an extent that is comparable with that achieved by bacterial endotoxins.52 Various studies have identified ventilation with high distending volumes/pressures as a potent physical force that activates PI3K53,54 with overventilation causing a much stronger activation of protein kinase B (PKB) in lung homogenates than that seen with LPS instillation.54
Recently two publications have emphasized the role of PI3K in ALI-ARDS models. Miyahara et al. showed that LY294002-pretreatment in isolated lungs attenuated ventilation-induced lung injury, as measured by filtration coefficients.55 Moreover, Lioetti et al. demonstrated that overventilated isolated lungs from p110γ–/– mice possessed diminished levels of histological indices of lung injury and of PKB phosphorylation compared to wild-type56 suggesting this PI3K isoform as a possible drug target within ventilation-induced ALI-ARDS.
As is the case for most inflammatory diseases, the main focus of research in ALI-ARDS is maintained in immune cells. Prolonged exposure to inflammatory mediators, for example in sepsis, is considered one of the main underlying mechanisms in ARDS.57 As a consequence of an over-reactive immunological response to infection, circulating leucocytes become activated with an increased fraction moving into the pulmonary vessels. As the inflammation continues, leucocytes migrate into the lung tissue and increase endothelial permeability, generating pulmonary oedema and impaired gas exchange function, hence the beginning of ALI-ARDS.58 In the case of endotoxin-induced ARDS models, Yum et al. found that in vitro exposure of neutrophils to endotoxin resulted in phosphorylation of PKB, activation of nuclear factor-κB (NF-κB) and expression of the proinflammatory cytokines IL-1β and TNF-α through PI3K-dependent pathways. In vivo, endotoxin administration to mice resulted in the activation of PI3K and PKB in neutrophils that accumulated in the lungs.46 Furthermore, inhibition of PI3K leading to a blockade of NF-κB activation, decreased endotoxin-induced oedema, neutrophil infiltration and proinflammatory cytokine expression in the lungs.46 In a study using a TNF-α-induced model of lung inflammation, mice expressing a dominant negative form of the PI3K p85 regulatory protein showed reduced microvascular injury and decreased recruitment of granulocytes to the lungs.59 Moreover, they demonstrated that granulocytes treated with LY294002 or expressing the dominant negative p85 construct were prevented from oxidant production in response to TNF-α.59 In an in vivo mouse model of gastric acid aspiration-induced ALI, treatment with presqualene diphosphate (a novel structural mimetic of an anti-inflammatory membrane lipid) inhibits PI3K activity and reduces lung neutrophil recruitment compared with the results from control groups.60
There are few studies looking at the specific roles of each of the PI3K isoforms in ALI-ARDS models. Puri et al. using the Class IA p110δ-selective inhibitor IC87114, showed a significant reduction in neutrophil accumulation in an endotoxin-induced model of acute lung injury.39 Moreover, the severity of endotoxaemia-induced ALI was significantly diminished in p110γ–/– mice.46 The extent of injury (quantified by lung oedema, nuclear translocation of NF-κB, neutrophil recruitment, and pulmonary levels of IL-1β and TNF-α) was significantly reduced in p110γ–/– mice compared with wild-type mice.46 However, in another model, where lung neutrophil recruitment and vascular injury was bacteraemia-induced (i.e. by intraperitoneal Escherichia coli challenge) instead of endotoxin-induced, p110γ–/– mice presented higher levels of leucocyte accumulation in the lung, as well as greater microvascular permeability, resulting in lung oedema. These changes were only significant for the first hour after E. coli injection, thereafter pathological events were similar in both groups (p110γ–/– and wild-type) mice.61 Although a possible explanation for this increased infiltration of leucocytes could be the neutrophilia previously observed in p110γ–/– mice62 the authors associated these unfavourable events in lung inflammation with increased expression of CD47 and integrin β3 by p110γ–/– leucocytes.61 These results show that the role of PI3K, particularly p110γ, in septicaemia-related neutrophil activation (including that involved in ALI-ARDS) is still unclear.
Chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease is one of the most important causes of impaired respiratory function. It is defined by irreversible, progressive airflow limitation associated with an abnormal inflammatory response of the lungs to certain particles or gases, primarily those found in tobacco smoke. COPD includes different clinical entities, most prominently chronic bronchitis and emphysema.63 The cell types that are characteristically involved within the pathogenesis of COPD are CD8+ T cells, macrophages and neutrophils with certain lung parenchymal cells (such as epithelial cells) also activated.64 There are many different animal models for COPD, the most common being elastase instillation and cigarette-smoke exposure, with gene-targeting techniques quickly gaining widespread acceptance and usage.65
PI3K and COPD
Unfortunately, no studies have reported the responses of PI3K gene-targeted animals in COPD models. Nevertheless, there are certain studies that evaluate the potential importance of PI3K within this pathology. Neutrophil recruitment into sites of inflammation is central to the pathology of several disease states, including COPD.66 Thomas et al. observed that p110γ–/– neutrophils in vitro presented reduced chemotaxis towards keratinocyte-derived chemokine (KC, the mouse orthologue of human CXCL1) and macrophage inflammatory protein-2 (MIP-2 or CXCL2), both chemokines signalling through the CXCR2 receptor. Furthermore, p110γ–/– mice showed reduced accumulation of neutrophils following intranasal instillation in vivo; whereas mice possessing a p110δ kinase-dead mutation showed no inhibition in either scenario.67
Asthma
Asthma, a chronic disease characterized by airway hyperreactivity, inflammation and remodelling, occurs in 5–8% of the US population and is an extraordinarily common cause of pulmonary impairment worldwide.68 Bronchial asthma is characterized by airway eosinophilia, goblet cell hyperplasia with mucus hypersecretion, and hyperresponsiveness to inhaled allergens and to non-specific stimuli69. In atopic individuals, T cells mature preferentially towards the Th2 subtype after exposure to allergen on the surface of antigen-presenting cells. These cells, through the production of a diverse array of inflammatory mediators, recruit and activate mast cells, granulocytes, B cells and local cells (primarily airway epithelial and smooth muscle cells); leading to the pathological events that define this disease.70
PI3K and asthma
There is an important therapeutic effect of PI3K inhibitors in asthma models of disease. Initial reports established that the greatly increased levels of IL-4 and IL-5 achieved 72 hr after OVA inhalation could be significantly reduced by the intratracheal administration of PI3K inhibitors (wortmannin or LY294002).71 Subsequently, it was shown that intratracheal administration of LY294002 significantly inhibited most of the pathological characteristics of the mouse asthma model, like eosinophil counts, eotaxin levels, IL-5 and IL-13 in bronchoalveolar lavage fluid (BAL). Furthermore, lung tissue eosinophilia, airway mucus production and airway hyperresponsiveness to inhaled methacholine were all significantly suppressed.72
Accordingly, both PI3K and, consequently, PKB activities are increased significantly after allergen challenge in murine models of asthma. Although the activity of PKB was shown to be sensitive to pretreatment with broad-spectrum PI3K inhibitors71 these studies could not distinguish among the various forms of PI3K. It was not until Myou et al. examined the effects of dysfunctional p85 in the context of an asthma animal model that it was clearly demonstrated that Class IA PI3K play key roles in pulmonary infiltration of lymphocytes and eosinophils, antigen-induced airway inflammation and hyperresponsiveness (including increase in mucus-containing epithelial cells), and Th2 cytokine production (IL-5 and IL-4) in BAL. Although p85 blockage reduced the secretion of Th2 cytokines, it had no effect on the BAL secretion of the Th1 cytokine, interferon-γ. These data suggest that PI3K Class IA regulates, in part, the balance between Th1 and Th2 responses.73 A study looking specifically at the role of p110δ in Th1 and Th2 differentiation, found that T cells obtained from p110δ kinase-dead mice had impaired differentiation towards both of these subtypes.26
Class IA p110δ has been implicated as a key mediator in the asthmatic response. p110δ silencing in vitro, either genetically or pharmacologically, has been shown to inhibit mast cell allergen-immunoglobulin E (IgE)-induced degranulation and cytokine release.28 Recently, Lee et al. using intratracheal administration of the p110δ-specific inhibitor, IC87114, in a mouse asthma model, were able to diminish leucocyte, eosinophil, neutrophil and lymphocyte accumulation in the lungs, as well as attenuate Th2 cytokine levels (IL-4, IL-5 and IL-13). This compound also reduced IgE and leukotriene C4 release into the airways.74 A recent paper, where a haemopoietic cell-restricted p110δ gene disruption was carried out, showed that p110δ-inactivated mice when challenged with ovalbumin had lower levels of type 2 cytokines, attenuated airway inflammation and reduced mucus production and eosinophil recruitment to the lung. Furthermore, the response of the p110δ-inactivated mice to inhaled methacholine was also reduced.75 These studies highlight the therapeutic potential of targeting p110δ in the context of asthma.
With regard to Class IB PI3K, p110γ knockout mice have demonstrated partial protection from anaphylaxis following intradermal injection of IgE, adenosine and allergen.29 The lack of responsiveness of p110γ-knockout mice to adenosine is particularly interesting because a role for this mediator in asthma and COPD has long been advocated. Several different G-protein-coupled receptors for adenosine have been found; the main receptor involved in adenosine-induced mast cell degranulation, and consequent bronchoconstriction, is the A2B receptor.76 Levels of adenosine are increased in the BAL of asthmatics and local delivery of AMP induces bronchoconstriction in asthmatics, but not normal individuals.77
The eosinophil response appears to be a critical feature in asthma. Many inflammatory mediators activate eosinophils via signal transduction pathways involving the enzyme PI3K.78 Furthermore, PI3K has also been shown to be essential in the migration of eosinophils caused by a number of chemoattractants. Specifically, Palframan et al. reported that wortmannin inhibited IL-5-induced release of eosinophils from perfused bone marrow, as well as eosinophil chemokinesis in vitro.79 Similarly, Tigani et al. showed that wortmannin given at high concentrations inhibits the increased number of eosinophils and eosinophil peroxidase activity in the BAL of ovalbumin-challenged animals.80 Considerable controversy remains regarding the relationship between bronchial eosinophilic inflammation and airway hyperresponsiveness, but it is believed that eosinophils degranulate to release toxic granule proteins and that these products can cause airway hyperresponsiveness.81 In allergen-induced lung inflammation, LY294002 completely failed to prevent the increase in eosinophil influx into the lung, but significantly attenuated the increase in the BAL index of bronchial eosinophil degranulation and airway hyperresponsiveness to histamine, but not to acetylcholine. This might suggest that it is the degranulation of eosinophils in the lungs, rather than their mere accumulation, that is important for the development of allergen-induced airway hyperresponsiveness to histamine.78 Others have reported a similar dissociation between airway hyperresponsiveness and pulmonary eosinophilia in allergen-sensitized and challenged guinea pigs.82–84 However, there is some evidence to suggest that BAL eosinophil count in asthmatic patients has often positively correlated with the magnitude of airway hyperresponsiveness.85,86
Evidence that PI3K isoforms expressed in lung parenchymal cells contribute to airway disease
Acute lung injury and adult respiratory distress syndrome
Several studies highlight the importance of parenchymal cell PI3K in lung disease. It was initially demonstrated that overventilation elicits PI3K-dependent activation of PKB in pulmonary endothelial cells, leading to production of nitric oxide.53 This same group later showed that in isolated perfused mouse lungs, overventilation causes, in a PI3K-dependent manner, nuclear translocation of NF-κB.54 Consequently, expression of I-κBβ, a NF-κB inhibitor, is diminished in alveolar cells of overventilated lungs. This effect can be reversed by inhibition of PI3K, suggesting that PI3K leads to phosphorylation and degradation of I-κBβ proteins and hence the activation NF-κB. Furthermore, this LY294002-mediated PI3K blockade inhibits NF-κB nuclear translocation, impairing the production of IL-6 and CXCL2 in an overventilation (but not endotoxin-induced) ARDS model.54 The selectivity of PI3K activation for ventilation injury suggests that it may be possible to reduce some of the side-effects of ventilation without causing severe immune suppression.
In some systems endothelial, rather than leucocyte, p110δ and p110γ may play a predominant role. Using an endotoxaemia-induced model of ALI, reconstituting p110γ–/– mice with wild-type neutrophils does not fully restore lung neutrophil recruitment, and the resistance of neutrophil recruitment to wild-type reconstitution is even more pronounced in double (p110δ and p110γ) knockout mice.43In vitro experiments reveal that endothelial p110δ and p110γ activities are required for selectin-dependent adhesion of neutrophils to the endothelium, demonstrating that both PI3K subclasses (IA and IB) are needed in the endothelium to recruit neutrophils efficiently to the inflamed lung. This highlights the fact that PI3K in parenchymal cells, and not just in leucocytes, plays an important part in inflammation in the lung.
Chronic obstructive pulmonary disease
Nicotine, the addictive chemical found in tobacco cigarettes and a precursor of certain carcinogens, activates PI3K and leads to increased phosphorylation levels of its downstream mediators PKB, GSK-3 and others, in non-immortalized human airway epithelial cells.87 This activation leads to enhanced survival and partially induces a transformed phenotype, reminiscent of premalignant lesions typically found in patients with smoking-associated COPD. This information highlights the fact that some of the chemicals found in cigarette smoke activate PI3K in lung parenchymal cells, and that this interaction mediates alterations in the cells that could have consequences in the establishment not only of COPD but also of lung cancer.
Furthermore, when the epithelium is persistently exposed to inflammatory mediators in COPD and asthma, it can change phenotype to a mucus-hypersecreting metaplasia, called goblet cell. This response is a basic innate host defence to enhance mucus production and, thus, the removal of harmful stimuli from the lungs.88 Various cytokines and inflammatory mediators stimulate mucus hypersecretion, directly or indirectly.89,90 Mucin secretion is activated by TNF-α,91 IL-1β92 and LPS93 by up-regulating expression of the mucin genes. Among the cytokines that have various inflammatory functions in acute and chronic upper respiratory tract infection, IL-1β is related to the pathogenesis of respiratory tract infection in upper airway diseases such as COPD and asthma.94 When epithelial cells were pretreated with a PI3K inhibitor, IL-1β-induced MUC2 gene expression and mucin secretion were attenuated.95 Furthermore, IL-13, another cytokine shown to play an important role in airway disease, was demonstrated to induce a mucus hypersecretory phenotype via the mitogen-activated protein kinase (MAPK) and PI3K pathways.88
Asthma
Airway smooth muscle (ASM) cells play a prominent role in the perpetuation of airway inflammation. ASM cells under chronic inflammatory conditions have the capacity to contract more intensely and for longer periods of time than normal ASM cells, further restricting airflow to the lungs.96 When airway mast cells degranulate as a result of their interaction with allergens, toxins and other autocrine factors, they liberate chemical mediators which cause ASM to contract and further restrict airflow to the lungs.97 Histamine and peptidoleukotrienes are the two major mast cell-derived mediators responsible for the anaphylactic contraction of airways isolated from both humans and guinea pigs. Treatment with PI3K and/or MAPK inhibitors has been shown to attenuate the anaphylactic bronchial contraction and facilitate the relaxation of constricted airways,98 implying a role for these kinases in the activity of mast cell-derived mediators in the contraction of ASM cells. Furthermore, in vitro tests have shown that PI3K plays a role in regulating contraction99 and migration100 in ASM cells, as well as in the transformation of ASM cells into the contractile phenotype observed in asthma.96
ASM cells also play an important role in the induction of the chronic features of airway remodelling that occur in asthma, because when these cells grow either in size and/or number they limit the flow of air into the lungs by trapping the air already contained in the lungs.97 Many different factors, including cytokines, growth factors, inflammatory mediators, contractile agonists, and extracellular matrix proteins induce ASM proliferation. These mitogens can be divided into two broad groups: those that activate receptors with intrinsic protein tyrosine kinase activity (e.g. epidermal growth factor, EGF) and those that activate G-protein-coupled receptors (e.g. thrombin).101 PI3K kinase inhibitors reduce ASM proliferation induced by thrombin,101,102 EGF,101 transforming growth factor-β103 and platelet-derived growth factor (PDGF)104. It is important to note that when the inhibitors were added 6 hr after the cells were stimulated with certain mitogens, proliferation was no longer inhibited, suggesting that PI3K may be more important in modulating the early signals that lead to proliferation in ASM.105
Despite PI3K playing an important role in modulating mitogen-induced smooth muscle proliferation, the repertoire of PI3K isoforms expressed and their attendant functions have not been well studied in ASM cells. It is known that human ASM cells express class IA, and class II PI3K but not the class IB isoform.106 Transient expression of constitutively active class IA PI3K human ASM cells was associated with activation of the cyclin D1 promoter,107 induction of DNA synthesis and generation of ASM cell growth. Interestingly, inhibition of class IA PI3K does not completely inhibit mitogen-induced DNA synthesis.106 The incomplete inhibition of mitogen-induced proliferative responses in human ASM cells suggests that although class IA PI3K may play a major role in myocyte growth, other pathways are also likely to modulate this event. Some have suggested that mitogens act via their receptors to activate two parallel signalling pathways in ASM cells, namely extracellular signal-regulated kinase (ERK) and the PI3K pathways, each pathway being crucial in obtaining the proliferative response,68 although this may be mitogen-specific108.
The downstream targets of PI3K that are associated with promoting protein synthesis and accumulation include pp70/85-kDa S6 kinases, collectively referred to as pp70S6k.109 In numerous cell types, PI3K has been shown to be an important activator of pp70S6k in response to serum and growth factors.110 It has been demonstrated that inhibition of pp70S6k attenuates human ASM proliferation.101 Recently, a critical role for PI3K-dependent pp70S6k activation following induction of DNA synthesis by PDGF in bovine tracheal smooth muscle cells was demonstrated.111 Furthermore, Halayko et al. have provided primary evidence that PI3K activation of pp70S6k in airway myocytes leads to the accumulation of contractile apparatus proteins and to differentiation and growth of ASM cells.96 Moreover, it has been shown that expression of a constitutively active p110 results in activation of pp70S6k, and expression of a dominant negative mutant p85 blocks EGF and thrombin-induced pp70S6k activation and DNA synthesis,101 clearly indicating a role for Class IA PI3K in pp70S6k activation and thrombin and EGF-induced ASM cell proliferation.
The way forward: making the choice between isoform specificity and global inhibition?
The recent advent of clinical trials using small molecule inhibitors against p38 MAPK for use in patients with a diverse array of inflammatory pathologies, like Crohn's disease,112,113 and the use of RX-0201, an antisense oligonucleotide that directly blocks PKB signalling, as a treatment for cancer in a phase I clinical trial (http://www.rexahn.com), would predict that the future for small molecule inhibitors for signalling molecules is very promising, with some speculating that inhibitors targeting PI3K Class IB p110γ could be the anti-inflammatory drug of the future.114
Some companies, like Semafore Pharmaceuticals Inc. (Indianapolis, IN), believe that a pan-PI3K inhibitor, like their lead compound SF1126, could prove to be useful against tumour development in cancer therapy; while others, like TargeGen, Inc. (San Diego, CA), with their selective PI3Kδ/γ inhibitor TG100-115, which is about to begin clinical trials for the treatment of acute myocardial infarction (ClinicalTrials.gov Identifier: NCT00103350), believe specificity is the correct approach to delivering kinase inhibitors in the clinic. Whether PI3K inhibitors, either isoform-selective or broad-spectrum, eventually prove to be sufficiently effective as anti-inflammatory agents is a question that only time and research will answer.115
In respiratory diseases, a wide variety of therapeutic options are available but better drugs are required. As seen in this review, a p110δ-specific inhibitor seems to offer the most potential as a therapeutic target in respiratory disease. This inhibitor could offer the opportunity of reducing Th2 responses without severely affecting Th1-mediated immunity, which would prove highly beneficial to the allergic patient. Furthermore, the effects of p110δ silencing on mucus production, mast cell degranulation and leucocyte recruitment are all very positive effects that an inhibitor could have in the clinic. For better or for worse, in the pharmaceutical industry the race to develop therapeutic prototypes that target PI3K is well underway. Meanwhile, basic research continues to elucidate the possible advantages and disadvantages of using isoform selective versus pan-isoform inhibitors in therapeutic settings.
References
- 1.Lugogo NL, Kraft M. Epidemiology of asthma. Clin Chest Med. 2006;27:1–15. doi: 10.1016/j.ccm.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Avecillas JF, Freire AX, Arroliga AC. Clinical epidemiology of acute lung injury and acute respiratory distress syndrome. Incidence, diagnosis and outcomes. Clin Chest Med. 2006;27:549–57. doi: 10.1016/j.ccm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Whitman M, Downes CP, Keeler M, Keller T, Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988;332:644–6. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]
- 4.Rameh LE, Cantley LC. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274:8347–50. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 5.Welch HC, Coadwell WJ, Ellson CD, et al. P-Rex1, a PtdIns (3,4,5)P3- and Gbetagamma-regulated guanine–nucleotide exchange factor for Rac. Cell. 2002;108:809–21. doi: 10.1016/s0092-8674(02)00663-3. [DOI] [PubMed] [Google Scholar]
- 6.Shinohara M, Terada Y, Iwamatsu A, et al. SWAP-70 is a guanine-nucleotide-exchange factor that mediates signalling of membrane ruffling. Nature. 2002;416:759–63. doi: 10.1038/416759a. [DOI] [PubMed] [Google Scholar]
- 7.Parry RV, Riley JL, Ward SG. Signalling to suit function: tailoring phosphoinositide 3-kinase during T-cell activation. Trends Immunol. 2007;28:161–8. doi: 10.1016/j.it.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Vanhaesebroeck B, Leevers SJ, Ahmadi K, et al. Synthesis and function 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 9.Domin J, Gaidarov I, Smith MEK, Keen JH, Waterfield MD. The class II phosphoinositide 3-kinase PI3K-C2alpha is concentrated in the trans-Golgi network and present in clathrin-coated vesicles. J Biol Chem. 2000;275:11943–50. doi: 10.1074/jbc.275.16.11943. [DOI] [PubMed] [Google Scholar]
- 10.Domin J, Pages F, Volinia S, et al. Cloning of a human phosphoinositide 3-kinase with a C2 domain that displays reduced sensitivity to the inhibitor wortmannin. Biochem J. 1997;326:139–47. doi: 10.1042/bj3260139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo CC, Lin WT, Liang CM, Liang SM. Class I and III phosphatidylinositol 3′-kinase play distinct roles in TLR signaling pathway. J Immunol. 2006;176:5943–9. doi: 10.4049/jimmunol.176.10.5943. [DOI] [PubMed] [Google Scholar]
- 12.Finan PM, Thomas MJ. PI 3-kinase inhibition: a therapeutic target for respiratory disease. Biochem Soc Trans. 2004;32:378–82. doi: 10.1042/bst0320378. [DOI] [PubMed] [Google Scholar]
- 13.Ward S, Sotsios Y, Dowden J, Bruce I, Finan P. Therapeutic potential of phosphoinositide 3-kinase inhibitors. Chem Biol. 2003;10:207–13. doi: 10.1016/s1074-5521(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 14.Bi L, Okabe I, Bernard DJ, Wynshaw-Boris A, Nussbaum RL. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110alpha subunit of phosphoinositide 3-kinase. J Biol Chem. 1999;274:10963–8. doi: 10.1074/jbc.274.16.10963. [DOI] [PubMed] [Google Scholar]
- 15.Bi L, Okabe I, Bernard DJ, Nussbaum RL. Early embryonic lethality in mice deficient in the p110beta catalytic subunit of PI 3-kinase. Mamm Genome. 2002;13:169–72. doi: 10.1007/BF02684023. [DOI] [PubMed] [Google Scholar]
- 16.Chantry D, Vojtek A, Kashishian A, et al. p110delta, a novel phosphatidylinositol 3-kinase catalytic subunit that associates with p85 and is expressed predominantly in leukocytes. J Biol Chem. 1997;272:19236–41. doi: 10.1074/jbc.272.31.19236. [DOI] [PubMed] [Google Scholar]
- 17.Wong CK, Cheung PF, Ip WK, Lam CW. Intracellular signaling mechanisms regulating Toll-like receptor-mediated activation of eosinophils. Am J Respir Cell Mol Biol. 2007 doi: 10.1165/rcmb.2006-0457OC. in press. [DOI] [PubMed] [Google Scholar]
- 18.Yang CS, Song CH, Lee JS, et al. Intracellular network of phosphatidylinositol 3-kinase, mammalian target of the rapamycin/70 kDa ribosomal S6 kinase 1, and mitogen-activated protein kinase pathways for regulating mycobacteria-induced IL-23 expression in human macrophages. Cell Microbiol. 2006;8:1158–71. doi: 10.1111/j.1462-5822.2006.00699.x. [DOI] [PubMed] [Google Scholar]
- 19.Strassheim D, Asehnoune K, Park JS, et al. Phosphoinositide 3-kinase and Akt occupy central roles in inflammatory responses of Toll-like receptor 2-stimulated neutrophils. J Immunol. 2004;172:5727–33. doi: 10.4049/jimmunol.172.9.5727. [DOI] [PubMed] [Google Scholar]
- 20.Fukao T, Tanabe M, Terauchi Y, et al. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–81. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 21.Yang CS, Lee JS, Jung SB, et al. Differential regulation of interleukin-12 and tumour necrosis factor-alpha by phosphatidylinositol 3-kinase and ERK 1/2 pathways during Mycobacterium tuberculosis infection. Clin Exp Immunol. 2006;143:150–60. doi: 10.1111/j.1365-2249.2005.02966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukao T, Yamada T, Tanabe M, et al. Selective loss of gastrointestinal mast cells and impaired immunity in PI3K-deficient mice. Nat Immunol. 2002;3:295–304. doi: 10.1038/ni768. [DOI] [PubMed] [Google Scholar]
- 23.Clayton E, Bardi G, Bell SE, et al. A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation. J Exp Med. 2002;196:753–63. doi: 10.1084/jem.20020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okkenhaug K, Bilancio A, Farjot G, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–4. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 25.Al-Alwan MM, Okkenhaug K, Vanhaesebroeck B, Hayflick JS, Marshall AJ. Requirement for phosphoinositide 3-kinase p110delta signaling in B cell antigen receptor-mediated antigen presentation. J Immunol. 2007;178:2328–35. doi: 10.4049/jimmunol.178.4.2328. [DOI] [PubMed] [Google Scholar]
- 26.Okkenhaug K, Patton DT, Bilancio A, Garcon F, Rowan WC, Vanhaesebroeck B. The p110delta isoform of phosphoinositide 3-kinase controls clonal expansion and differentiation of Th cells. J Immunol. 2006;177:5122–8. doi: 10.4049/jimmunol.177.8.5122. [DOI] [PubMed] [Google Scholar]
- 27.Patton DT, Garden OA, Pearce WP, et al. Cutting edge: the phosphoinositide 3-kinase p110 delta is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177:6598–602. doi: 10.4049/jimmunol.177.10.6598. [DOI] [PubMed] [Google Scholar]
- 28.Ali K, Bilancio A, Thomas M, et al. Essential role for the p110delta phosphoinositide 3-kinase in the allergic response. Nature. 2004;431:1007–11. doi: 10.1038/nature02991. [DOI] [PubMed] [Google Scholar]
- 29.Laffargue M, Calvez R, Finan P, et al. Phosphoinositide 3-kinase gamma is an essential amplifier of mast cell function. Immunity. 2002;16:441–51. doi: 10.1016/s1074-7613(02)00282-0. [DOI] [PubMed] [Google Scholar]
- 30.Sasaki T, Irie-Sasaki J, Jones RG, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–6. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 31.Camps M, Ruckle T, Ji H, et al. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11:936–43. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- 32.Cronshaw DG, Owen C, Brown Z, Ward SG. Activation of phosphoinositide 3-kinases by the CCR4 ligand macrophage-derived chemokine is a dispensable signal for T lymphocyte chemotaxis. J Immunol. 2004;172:7761–70. doi: 10.4049/jimmunol.172.12.7761. [DOI] [PubMed] [Google Scholar]
- 33.Curnock AP, Sotsios Y, Wright KL, Ward SG. Optimal chemotactic responses of leukemic T cells to stromal cell-derived factor-1 requires the activation of both class IA and IB phosphoinositide 3-kinases. J Immunol. 2003;170:4021–30. doi: 10.4049/jimmunol.170.8.4021. [DOI] [PubMed] [Google Scholar]
- 34.Medina-Tato DA, Watson ML, Ward SG. Leukocyte navigation mechanisms as targets in airway diseases. Drug Discov Today. 2006;11:866–79. doi: 10.1016/j.drudis.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Ferguson GJ, Milne L, Kulkarni S, et al. PI(3) Kgamma has an important context-dependent role in neutrophil chemokinesis. Nat Cell Biol. 2007;9:86–91. doi: 10.1038/ncb1517. [DOI] [PubMed] [Google Scholar]
- 36.Nishio M, Watanabe K, Sasaki J, et al. Control of cell polarity and motility by the PtdIns (3,4,5)P3 phosphatase SHIP1. Nat Cell Biol. 2007;9:36–44. doi: 10.1038/ncb1515. [DOI] [PubMed] [Google Scholar]
- 37.Del PA, Vermi W, Dander E, et al. Defective dendritic cell migration and activation of adaptive immunity in PI3Kgamma-deficient mice. EMBO J. 2004;23:3505–15. doi: 10.1038/sj.emboj.7600361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reif K, Okkenhaug K, Sasaki T, Penninger JM, Vanhaesebroeck B, Cyster JG. Cutting edge: differential roles for phosphoinositide 3-kinases, p110gamma and p110delta, in lymphocyte chemotaxis and homing. J Immunol. 2004;173:2236–40. doi: 10.4049/jimmunol.173.4.2236. [DOI] [PubMed] [Google Scholar]
- 39.Puri KD, Doggett TA, Douangpanya J, et al. Mechanisms and implications of phosphoinositide 3-kinase delta in promoting neutrophil trafficking into inflamed tissue. Blood. 2004;103:3448–56. doi: 10.1182/blood-2003-05-1667. [DOI] [PubMed] [Google Scholar]
- 40.Condliffe AM, Davidson K, Anderson KE, et al. Sequential activation of class IB and class IA PI3K is important for the primed respiratory burst of human but not murine neutrophils. Blood. 2005;106:1432–40. doi: 10.1182/blood-2005-03-0944. [DOI] [PubMed] [Google Scholar]
- 41.Swat W, Montgrain V, Doggett TA, et al. Essential role of PI3Kdelta and PI3Kgamma in thymocyte survival. Blood. 2006;107:2415–22. doi: 10.1182/blood-2005-08-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webb LM, Vigorito E, Wymann MP, Hirsch E, Turner M. Cutting edge: T cell development requires the combined activities of the p110gamma and p110delta catalytic isoforms of phosphatidylinositol 3-kinase. J Immunol. 2005;175:2783–7. doi: 10.4049/jimmunol.175.5.2783. [DOI] [PubMed] [Google Scholar]
- 43.Puri KD, Doggett TA, Huang CY, et al. The role of endothelial PI3Kgamma activity in neutrophil trafficking. Blood. 2005;106:150–7. doi: 10.1182/blood-2005-01-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brun-Buisson C, Minelli C, Bertolini G, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med. 2004;30:51–61. doi: 10.1007/s00134-003-2022-6. [DOI] [PubMed] [Google Scholar]
- 45.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 46.Yum HK, Arcaroli J, Kupfner J, et al. Involvement of phosphoinositide 3-kinases in neutrophil activation and the development of acute lung injury. J Immunol. 2001;167:6601–8. doi: 10.4049/jimmunol.167.11.6601. [DOI] [PubMed] [Google Scholar]
- 47.Bernard GR, Artigas A, Brigham KL et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 48.Puneet P, Moochhala S, Bhatia M. Chemokines in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2005;288:L3–L15. doi: 10.1152/ajplung.00405.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimabukuro DW, Sawa T, Gropper MA. Injury and repair in lung and airways. Crit Care Med. 2003;31:S524–S531. doi: 10.1097/01.CCM.0000081437.06466.B3. [DOI] [PubMed] [Google Scholar]
- 50.Belperio JA, Keane MP, Lynch JP, III, Strieter RM. The role of cytokines during the pathogenesis of ventilator-associated and ventilator-induced lung injury. Semin Respir Crit Care Medical. 2006;27:350–64. doi: 10.1055/s-2006-948289. [DOI] [PubMed] [Google Scholar]
- 51.von Bethmann AN, Brasch F, Nusing R, et al. Hyperventilation induces release of cytokines from perfused mouse lung. Am J Respir Crit Care Med. 1998;157:263–72. doi: 10.1164/ajrccm.157.1.9608052. [DOI] [PubMed] [Google Scholar]
- 52.Held HD, Boettcher S, Hamann L, Uhlig S. Ventilation-induced chemokine and cytokine release is associated with activation of nuclear factor-kappaB and is blocked by steroids. Am J Respir Crit Care Med. 2001;163:711–16. doi: 10.1164/ajrccm.163.3.2003001. [DOI] [PubMed] [Google Scholar]
- 53.Kuebler WM, Uhlig U, Goldmann T, et al. Stretch activates nitric oxide production in pulmonary vascular endothelial cells in situ. Am J Respir Crit Care Med. 2003;168:1391–8. doi: 10.1164/rccm.200304-562OC. [DOI] [PubMed] [Google Scholar]
- 54.Uhlig U, Fehrenbach H, Lachmann RA, et al. Phosphoinositide 3-OH kinase inhibition prevents ventilation-induced lung cell activation. Am J Respir Crit Care Med. 2004;169:201–8. doi: 10.1164/rccm.200303-343OC. [DOI] [PubMed] [Google Scholar]
- 55.Miyahara T, Hamanaka K, Weber DS, Drake D, Anghelescu M, Parker JC. Phosphoinositide 3-kinase Src and Akt modulate acute ventilation induced vascular permeability increases in mouse lungs. Am J Physiol Lung Cell Mol Physiol. 2007 doi: 10.1152/ajplung.00279.2005. in press. [DOI] [PubMed] [Google Scholar]
- 56.Lionetti V, Lisi A, Patrucco E, et al. Lack of phosphoinositide 3-kinase-gamma attenuates ventilator-induced lung injury. Crit Care Med. 2006;34:134–41. doi: 10.1097/01.ccm.0000190909.70601.2c. [DOI] [PubMed] [Google Scholar]
- 57.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202:145–56. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- 58.Weinacker AB, Vaszar LT. Acute respiratory distress syndrome: physiology and new management strategies. Annu Rev Med. 2001;52:221–37. doi: 10.1146/annurev.med.52.1.221. [DOI] [PubMed] [Google Scholar]
- 59.Gao XP, Zhu X, Fu J, Liu Q, Frey RS, Malik AB. Blockade of class IA phosphoinositide 3-kinase in neutrophils prevents NADPH oxidase activation- and adhesion-dependent inflammation. J Biol Chem. 2007;282:6116–25. doi: 10.1074/jbc.M610248200. [DOI] [PubMed] [Google Scholar]
- 60.Bonnans C, Fukunaga K, Keledjian R, Petasis NA, Levy BD. Regulation of phosphatidylinositol 3-kinase by polyisoprenyl phosphates in neutrophil-mediated tissue injury. J Exp Med. 2006;203:857–63. doi: 10.1084/jem.20052143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ong E, Gao XP, Predescu D, Broman M, Malik AB. Role of phosphatidylinositol 3-kinase-gamma in mediating lung neutrophil sequestration and vascular injury induced by E. coli sepsis. Am J Physiol Lung Cell Mol Physiol. 2005;289:L1094–L1103. doi: 10.1152/ajplung.00179.2005. [DOI] [PubMed] [Google Scholar]
- 62.Hirsch E, Katanaev VL, Garlanda C, et al. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–53. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 63.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD. A summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 64.Ulbrich H, Eriksson EE, Lindbom L. Leukocyte and endothelial cell adhesion molecules as targets for therapeutic interventions in inflammatory disease. Trends Pharmacol Sci. 2003;24:640–7. doi: 10.1016/j.tips.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 65.Shapiro SD. Animal models for COPD. Chest. 2000;117:223S–7S. doi: 10.1378/chest.117.5_suppl_1.223s. [DOI] [PubMed] [Google Scholar]
- 66.Barnes PJ. Chronic obstructive pulmonary disease *12: New treatments for COPD. Thorax. 2003;58:803–8. doi: 10.1136/thorax.58.9.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thomas MJ, Smith A, Head DH, et al. Airway inflammation: chemokine-induced neutrophilia and the class I phosphoinositide 3-kinases. Eur J Immunol. 2005;35:1283–91. doi: 10.1002/eji.200425634. [DOI] [PubMed] [Google Scholar]
- 68.Ammit AJ, Panettieri RA., Jr Invited review: the circle of life: cell cycle regulation in airway smooth muscle. J Appl Physiol. 2001;91:1431–7. doi: 10.1152/jappl.2001.91.3.1431. [DOI] [PubMed] [Google Scholar]
- 69.Kay AB. Asthma and inflammation. J Allergy Clin Immunol. 1991;87:893–910. doi: 10.1016/0091-6749(91)90408-g. [DOI] [PubMed] [Google Scholar]
- 70.Heijink IH, Van Oosterhout AJ. Targeting T cells for asthma. Curr Opin Pharmacol. 2005;5:227–31. doi: 10.1016/j.coph.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 71.Kwak YG, Song CH, Yi HK, et al. Involvement of PTEN in airway hyperresponsiveness and inflammation in bronchial asthma. J Clin Invest. 2003;111:1083–92. doi: 10.1172/JCI16440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duan W, Aguinaldo Datiles AM, Leung BP, Vlahos CJ, Wong WS. An anti-inflammatory role for a phosphoinositide 3-kinase inhibitor LY294002 in a mouse asthma model. Int Immunopharmacol. 2005;5:495–502. doi: 10.1016/j.intimp.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 73.Myou S, Leff AR, Myo S, et al. Blockade of inflammation and airway hyperresponsiveness in immune-sensitized mice by dominant-negative phosphoinositide 3-kinase-TAT. J Exp Med. 2003;198:1573–82. doi: 10.1084/jem.20030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee KS, Lee HK, Hayflick JS, Lee YC, Puri KD. Inhibition of phosphoinositide 3-kinase delta attenuates allergic airway inflammation and hyperresponsiveness in murine asthma model. FASEB J. 2006;20:455–65. doi: 10.1096/fj.05-5045com. [DOI] [PubMed] [Google Scholar]
- 75.Nashed BF, Zhang T, Al-Alwan M, et al. Role of the phosphoinositide 3-kinase p110delta in generation of type 2 cytokine responses and allergic airway inflammation. Eur J Immunol. 2007;37:416–24. doi: 10.1002/eji.200636401. [DOI] [PubMed] [Google Scholar]
- 76.van den Berg M, Hylkema MN, Versluis M, Postma DS. Role of adenosine receptors in the treatment of asthma and chronic obstructive pulmonary disease: recent developments. Drugs R & D. 2007;8:13–23. doi: 10.2165/00126839-200708010-00002. [DOI] [PubMed] [Google Scholar]
- 77.Fozard JR. The case for a role for adenosine in asthma: almost convincing? Curr Opin Pharmacol. 2003;3:264–9. doi: 10.1016/s1471-4892(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 78.Ezeamuzie CI, Sukumaran J, Philips E. Effect of wortmannin on human eosinophil responses in vitro and on bronchial inflammation and airway hyperresponsiveness in guinea pigs in vivo. Am J Respir Crit Care Med. 2001;164:1633–9. doi: 10.1164/ajrccm.164.9.2101104. [DOI] [PubMed] [Google Scholar]
- 79.Palframan RT, Collins PD, Severs NJ, Rothery S, Williams TJ, Rankin SM. Mechanisms of acute eosinophil mobilization from the bone marrow stimulated by interleukin 5. The role of specific adhesion molecules and phosphatidylinositol 3-kinase. J Exp Med. 1998;188:1621–32. doi: 10.1084/jem.188.9.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tigani B, Hannon JP, Mazzoni L, Fozard JR. Effects of wortmannin on airways inflammation induced by allergen in actively sensitised Brown Norway rats. Eur J Pharmacol. 2001;433:217–23. doi: 10.1016/s0014-2999(01)01515-1. [DOI] [PubMed] [Google Scholar]
- 81.Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol. 2000;105:651–63. doi: 10.1067/mai.2000.105712. [DOI] [PubMed] [Google Scholar]
- 82.Ishida K, Thomson RJ, Beattie LL, Wiggs B, Schellenberg RR. Inhibition of antigen-induced airway hyperresponsiveness, but not acute hypoxia nor airway eosinophilia, by an antagonist of platelet-activating factor. J Immunol. 1990;144:3907–11. [PubMed] [Google Scholar]
- 83.Matsuse T, Thomson RJ, Chen XR, Salari H, Schellenberg RR. Capsaicin inhibits airway hyperresponsiveness but not lipoxygenase activity or eosinophilia after repeated aerosolized antigen in guinea pigs. Am Rev Respir Dis. 1991;144:368–72. doi: 10.1164/ajrccm/144.2.368. [DOI] [PubMed] [Google Scholar]
- 84.Heuer HO, Wenz B, Jennewein HM, Urich K. Dissociation of airway responsiveness and bronchoalveolar lavage (BAL) cell composition in sensitized guinea-pigs after daily inhalation of ovalbumin. Clin Exp Allergy. 1994;24:682–9. doi: 10.1111/j.1365-2222.1994.tb00973.x. [DOI] [PubMed] [Google Scholar]
- 85.Durham SR, Kay AB. Eosinophils, bronchial hyperreactivity and late-phase asthmatic reactions. Clin Allergy. 1985;15:411–18. doi: 10.1111/j.1365-2222.1985.tb02290.x. [DOI] [PubMed] [Google Scholar]
- 86.Foresi A, Bertorelli G, Pesci A, Chetta A, Olivieri D. Inflammatory markers in bronchoalveolar lavage and in bronchial biopsy in asthma during remission. Chest. 1990;98:528–35. doi: 10.1378/chest.98.3.528. [DOI] [PubMed] [Google Scholar]
- 87.West KA, Brognard J, Clark AS, et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Atherton HC, Jones G, Danahay H. IL-13-induced changes in the goblet cell density of human bronchial epithelial cell cultures: MAP kinase and phosphatidylinositol 3-kinase regulation. Am J Physiol Lung Cell Mol Physiol. 2003;285:L730–L739. doi: 10.1152/ajplung.00089.2003. [DOI] [PubMed] [Google Scholar]
- 89.Temann UA, Prasad B, Gallup MW, et al. A novel role for murine IL-4 in vivo: induction of MUC5AC gene expression and mucin hypersecretion. Am J Respir Cell Mol Biol. 1997;16:471–8. doi: 10.1165/ajrcmb.16.4.9115759. [DOI] [PubMed] [Google Scholar]
- 90.Li JD, Feng W, Gallup M, et al. Activation of NF-kappaB via a Src-dependent Ras-MAPK-pp90rsk pathway is required for Pseudomonas aeruginosa-induced mucin overproduction in epithelial cells. Proc Natl Acad Sci USA. 1998;95:5718–23. doi: 10.1073/pnas.95.10.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Levine SJ, Larivee P, Logun C, Angus CW, Ognibene FP, Shelhamer JH. Tumor necrosis factor-alpha induces mucin hypersecretion and MUC-2 gene expression by human airway epithelial cells. Am J Respir Cell Mol Biol. 1995;12:196–204. doi: 10.1165/ajrcmb.12.2.7865217. [DOI] [PubMed] [Google Scholar]
- 92.Kim YD, Kwon EJ, Kwon TK, Baek SH, Song SY, Suh JS. Regulation of IL-1beta-mediated MUC2 gene in NCI-H292 human airway epithelial cells. Biochem Biophys Res Commun. 2000;274:112–16. doi: 10.1006/bbrc.2000.3107. [DOI] [PubMed] [Google Scholar]
- 93.Li JD, Dohrman AF, Gallup M, et al. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc Natl Acad Sci USA. 1997;94:967–72. doi: 10.1073/pnas.94.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shelhamer JH, Levine SJ, Wu T, Jacoby DB, Kaliner MA, Rennard SINIH conference. Airway inflammation. Ann Intern Med. 1995;123:288–304. doi: 10.7326/0003-4819-123-4-199508150-00008. [DOI] [PubMed] [Google Scholar]
- 95.Kim YD, Jeon JY, Woo HJ, et al. Interleukin-1beta induces MUC2 gene expression and mucin secretion via activation of PKC-MEK/ERK, and PI3K in human airway epithelial cells. J Korean Med Sci. 2002;17:765–71. doi: 10.3346/jkms.2002.17.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Halayko AJ, Kartha S, Stelmack GL, et al. Phosphatidylinositol-3 kinase/mammalian target of rapamycin/p70S6K regulates contractile protein accumulation in airway myocyte differentiation. Am J Respir Cell Mol Biol. 2004;31:266–75. doi: 10.1165/rcmb.2003-0272OC. [DOI] [PubMed] [Google Scholar]
- 97.King GG, Pare PD, Seow CY. The mechanics of exaggerated airway narrowing in asthma: the role of smooth muscle. Respir Physiol. 1999;118:1–13. doi: 10.1016/s0034-5687(99)00076-6. [DOI] [PubMed] [Google Scholar]
- 98.Tsang F, Fred Wong WS. Inhibitors of tyrosine kinase signaling cascade attenuated antigen challenge of guinea-pig airways in vitro. Am J Respir Crit Care Med. 2000;162:126–33. doi: 10.1164/ajrccm.162.1.9908105. [DOI] [PubMed] [Google Scholar]
- 99.Gosens R, Bromhaar MM, Tonkes A, et al. Muscarinic M3 receptor-dependent regulation of airway smooth muscle contractile phenotype. Br J Pharmacol. 2004;141:943–50. doi: 10.1038/sj.bjp.0705709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Day RM, Lee YH, Park AM, Suzuki YJ. Retinoic acid inhibits airway smooth muscle cell migration. Am J Respir Cell Mol Biol. 2006;34:695–703. doi: 10.1165/rcmb.2005-0306OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Krymskaya VP, Penn RB, Orsini MJ, et al. Phosphatidylinositol 3-kinase mediates mitogen-induced human airway smooth muscle cell proliferation. Am J Physiol. 1999;277:L65–L78. doi: 10.1152/ajplung.1999.277.1.L65. [DOI] [PubMed] [Google Scholar]
- 102.Tsang F, Choo HH, Dawe GS, Wong WS. Inhibitors of the tyrosine kinase signaling cascade attenuated thrombin-induced guinea pig airway smooth muscle cell proliferation. Biochem Biophys Res Commun. 2002;293:72–8. doi: 10.1016/S0006-291X(02)00170-5. [DOI] [PubMed] [Google Scholar]
- 103.Goldsmith AM, Bentley JK, Zhou L, et al. Transforming growth factor-beta induces airway smooth muscle hypertrophy. Am J Respir Cell Mol Biol. 2006;34:247–54. doi: 10.1165/rcmb.2005-0166OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Walker TR, Moore SM, Lawson MF, Panettieri RA, Jr, Chilvers ER. Platelet-derived growth factor-BB and thrombin activate phosphoinositide 3-kinase and protein kinase B. Role in mediating airway smooth muscle proliferation. Mol Pharmacol. 1998;54:1007–15. doi: 10.1124/mol.54.6.1007. [DOI] [PubMed] [Google Scholar]
- 105.Hirst SJ, Walker TR, Chilvers ER. Phenotypic diversity and molecular mechanisms of airway smooth muscle proliferation in asthma. Eur Respir J. 2000;16:159–77. doi: 10.1034/j.1399-3003.2000.16a28.x. [DOI] [PubMed] [Google Scholar]
- 106.Krymskaya VP, Ammit AJ, Hoffman RK, Eszterhas AJ, Panettieri RA., Jr Activation of class IA PI3K stimulates DNA synthesis in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1009–L1018. doi: 10.1152/ajplung.2001.280.5.L1009. [DOI] [PubMed] [Google Scholar]
- 107.Orsini MJ, Krymskaya VP, Eszterhas AJ, Benovic JL, Panettieri RA, Jr, Penn RB. MAPK superfamily activation in human airway smooth muscle: mitogenesis requires prolonged p42/p44 activation. Am J Physiol. 1999;277:L479–L488. doi: 10.1152/ajplung.1999.277.3.L479. [DOI] [PubMed] [Google Scholar]
- 108.Roche S, Koegl M, Courtneidge SA. The phosphatidylinositol 3-kinase α is required for DNA synthesis induced by some, but not all, growth factors. PNAS. 1994;91:9185–9. doi: 10.1073/pnas.91.19.9185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Halayko AJ, Kartha S, Stelmack GL, et al. Phosphatidylinositol-3 kinase/mammalian target of rapamycin/p70S6K regulates contractile protein accumulation in airway myocyte differentiation. Am J Respir Cell Mol Biol. 2004;31:266–75. doi: 10.1165/rcmb.2003-0272OC. [DOI] [PubMed] [Google Scholar]
- 110.Chung J, Grammer TC, Lemon KP, Kazlauskas A, Blenis J. PDGF- and insulin-dependent pp70S6k activation mediated by phosphatidylinositol-3-OH kinase. Nature. 1994;370:71–5. doi: 10.1038/370071a0. [DOI] [PubMed] [Google Scholar]
- 111.Scott PH, Belham CM, al-Hafidh J, et al. A regulatory role for cAMP in phosphatidylinositol 3-kinase/p70 ribosomal S6 kinase-mediated DNA synthesis in platelet-derived-growth-factor-stimulated bovine airway smooth-muscle cells. Biochem J. 1996;318:965–71. doi: 10.1042/bj3180965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schreiber S, Feagan B, D'Haens G, et al. Oral p38 mitogen-activated protein kinase inhibition with BIRB 796 for active Crohn's disease: a randomized, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4:325–34. doi: 10.1016/j.cgh.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 113.Dominguez C, Powers DA, Tamayo N. p38 MAP kinase inhibitors: many are made, but few are chosen. Curr Opin Drug Discov Devel. 2005;8:421–30. [PubMed] [Google Scholar]
- 114.Ruckle T, Schwarz MK, Rommel C. PI3Kγ inhibition: towards an ‘aspirin of the 21st century’? Nat Rev Drug Discov. 2006;5:903–18. doi: 10.1038/nrd2145. [DOI] [PubMed] [Google Scholar]
- 115.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 116.Lee JW, Soung YH, Kim SY, et al. PIK3CA gene is frequently mutated in breast carcinomas and hepatocellular carcinomas. Oncogene. 2005;24:1477–80. doi: 10.1038/sj.onc.1208304. [DOI] [PubMed] [Google Scholar]
- 117.Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37:449–71. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 118.Brachmann SM, Ueki K, Engelman JA, Kahn RC, Cantley LC. Phosphoinositide 3-kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice. Mol Cell Biol. 2005;25:1596–607. doi: 10.1128/MCB.25.5.1596-1607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yip SC, El-Sibai M, Hill KM, et al. Over-expression of the p110beta but not p110alpha isoform of PI 3-kinase inhibits motility in breast cancer cells. Cell Motil Cytoskeleton. 2004;59:180–8. doi: 10.1002/cm.20032. [DOI] [PubMed] [Google Scholar]
- 120.Leverrier Y, Okkenhaug K, Sawyer C, Bilancio A, Vanhaesebroeck B, Ridley AJ. Class I phosphoinositide 3-kinase p110beta is required for apoptotic cell and Fcgamma receptor-mediated phagocytosis by macrophages. J Biol Chem. 2003;278:38437–42. doi: 10.1074/jbc.M306649200. [DOI] [PubMed] [Google Scholar]
- 121.Jackson SP, Schoenwaelder SM, Goncalves I, et al. PI 3-kinase p110beta: a new target for antithrombotic therapy. Nat Med. 2005;11:507–14. doi: 10.1038/nm1232. [DOI] [PubMed] [Google Scholar]
- 122.Bilancio A, Okkenhaug K, Camps M, et al. Key role of the p110delta isoform of PI3K in B-cell antigen and IL-4 receptor signaling: comparative analysis of genetic and pharmacologic interference with p110delta function in B cells. Blood. 2006;107:642–50. doi: 10.1182/blood-2005-07-3041. [DOI] [PubMed] [Google Scholar]
- 123.Webb LM, Vigorito E, Wymann MP, Hirsch E, Turner M. Cutting edge: T cell development requires the combined activities of the p110gamma and p110delta catalytic isoforms of phosphatidylinositol 3-kinase. J Immunol. 2005;175:2783–7. doi: 10.4049/jimmunol.175.5.2783. [DOI] [PubMed] [Google Scholar]
- 124.Sujobert P, Bardet V, Cornillet-Lefebvre P, et al. Essential role for the p110delta isoform in phosphoinositide 3-kinase activation and cell proliferation in acute myeloid leukemia. Blood. 2005;106:1063–6. doi: 10.1182/blood-2004-08-3225. [DOI] [PubMed] [Google Scholar]
- 125.Northcott CA, Hayflick J, Watts SW. Upregulated function of phosphatidylinositol-3-kinase in genetically hypertensive rats: a moderator of arterial hypercontractility. Clin Exp Pharmacol Physiol. 2005;32:851–8. doi: 10.1111/j.1440-1681.2010.04276.x. [DOI] [PubMed] [Google Scholar]
- 126.Sasaki T, Irie-Sasaki J, Jones RG, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–6. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 127.MacDonald PE, Joseph JW, Yau D, et al. Impaired glucose-stimulated insulin secretion, enhanced intraperitoneal insulin tolerance, and increased beta-cell mass in mice lacking the p110gamma isoform of phosphoinositide 3-kinase. Endocrinology. 2004;145:4078–83. doi: 10.1210/en.2004-0028. [DOI] [PubMed] [Google Scholar]
- 128.Soos MA, Jensen J, Brown RA, O'Rahilly S, Shepherd PR, Whitehead JP. Class II phosphoinositide 3-kinase is activated by insulin but not by contraction in skeletal muscle. Arch Biochem Biophys. 2001;396:244–8. doi: 10.1006/abbi.2001.2587. [DOI] [PubMed] [Google Scholar]
- 129.Kang S, Song J, Kang J, et al. Suppression of the alpha-isoform of class II phosphoinositide 3-kinase gene expression leads to apoptotic cell death. Biochem Biophys Res Commun. 2005;329:6–10. doi: 10.1016/j.bbrc.2005.01.091. [DOI] [PubMed] [Google Scholar]
- 130.Wang Y, Yoshioka K, Azam MA, et al. Class II phosphoinositide 3-kinase alpha-isoform regulates Rho, myosin phosphatase and contraction in vascular smooth muscle. Biochem J. 2006;394:581–92. doi: 10.1042/BJ20051471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wheeler M, Domin J. The N-terminus of phosphoinositide 3-kinase-C2beta regulates lipid kinase activity and binding to clathrin. J Cell Physiol. 2006;206:586–93. doi: 10.1002/jcp.20507. [DOI] [PubMed] [Google Scholar]
- 132.Domin J, Harper L, Aubyn D, et al. The class II phosphoinositide 3-kinase PI3K-C2beta regulates cell migration by a PtdIns3P dependent mechanism. J Cell Physiol. 2005;205:452–62. doi: 10.1002/jcp.20478. [DOI] [PubMed] [Google Scholar]
- 133.Katso RM, Pardo OE, Palamidessi A, et al. Phosphoinositide 3-kinase C2beta regulates cytoskeletal organization and cell migration via Rac-dependent mechanisms. Mol Biol Cell. 2006;17:3729–44. doi: 10.1091/mbc.E05-11-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sindic A, Crljen V, Matkovic K, Lukinovic-Skudar V, Visnjic D, Banfic H. Activation of phosphoinositide 3-kinase C2beta in the nuclear matrix during compensatory liver growth. Adv Enzyme Regul. 2006;46:280–7. doi: 10.1016/j.advenzreg.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 135.Ono F, Nakagawa T, Saito S, et al. A novel class II phosphoinositide 3-kinase predominantly expressed in the liver and its enhanced expression during liver regeneration. J Biol Chem. 1998;273:7731–6. doi: 10.1074/jbc.273.13.7731. [DOI] [PubMed] [Google Scholar]
- 136.Kuo CC, Lin WT, Liang CM, Liang SM. Class I and III phosphatidylinositol 3′-kinase play distinct roles in TLR signaling pathway. J Immunol. 2006;176:5943–9. doi: 10.4049/jimmunol.176.10.5943. [DOI] [PubMed] [Google Scholar]
- 137.Lindmo K, Stenmark H. Regulation of membrane traffic by phosphoinositide 3-kinases. J Cell Sci. 2006;119:605–14. doi: 10.1242/jcs.02855. [DOI] [PubMed] [Google Scholar]
- 138.Shahabuddin S, Ji R, Wang P, et al. CXCR3 chemokine receptor-induced chemotaxis in human airway epithelial cells: role of p38 MAPK and PI3K signaling pathways. Am J Physiol Cell Physiol. 2006;291:C34–C39. doi: 10.1152/ajpcell.00441.2005. [DOI] [PubMed] [Google Scholar]