Abstract
Nasal carriage of Staphylococcus aureus is an important source of nosocomial infection and community-acquired methicillin-resistant S. aureus (MRSA). Previous studies by our laboratory revealed that nasal carriage of S. aureus is accompanied by subclinical inflammation, which is insufficient to prevent colonization, and that carriage might also be a result of adaptation and selection of certain S. aureus strains to the host's nasal environment. In the present study we observed that a carrier strain of S. aureus preferentially colonizes and attaches to nasal epithelial cells (NEC) compared to a non-carrier S. aureus strain. Conversely, when naive NEC were pretreated with interleukin-1β for 24 hr, the growth and attachment of the carrier strain of S. aureus were significantly reduced in comparison to the non-carrier strain, emphasizing the pivotal role played by host innate immunity in the initial events of nasal carriage. While both strains up-regulated the expression of the pattern recognition receptor, Toll-like receptor 2 (TLR2), NEC exposed to the nasal carrier strain had a 4-hr delay in TLR2 expression compared with NEC exposed to non-carrier S. aureus. Moreover, even after 20 hr of colonization the expression of two principal epithelial antimicrobial peptides, human β-defensin-2 and human β-defensin-3, was negligibly induced in NEC exposed to the nasal carrier strain of S. aureus in comparison to the non-carrier strain. These results suggest that carrier strains of S. aureus retain a competitive advantage over non-carrier strains by delaying the host's innate response to epithelial colonization and infection.
Keywords: defensins, immunomodulation, nasal carriage, Staphylococcus, Toll-like receptors
Introduction
Staphylococcus aureus, a major cause of nosocomial and community-acquired infection, is carried in the nasal passages of approximately a quarter of healthy humans.1–3 Frequently, individuals harbour S. aureus strains that are proving increasingly resistant to powerful conventional antibiotics such as methicillin, with certain strains exhibiting reduced sensitivity to vancomycin.4,5 To successfully colonize the nasal passageway, S. aureus must overcome mechanical clearance mechanisms, the innate antibacterial host nasal environment and then attach to the nasal epithelium. In carriers, S. aureus predominantly colonizes the moist squamous epithelium on the septum adjacent to the nasal ostium.6 This region is devoid of cilia and subepithelial glands, and thus its protection is mostly derived from the overlying fluid and innate factors from epithelia.
The innate immune system, which consists of physiological barriers, pathogen recognition receptors, humoral effectors and innate immune cells, plays a principal role in the elimination of these colonizing pathogens. Nasal epithelial cells (NEC) detect S. aureus through germline-encoded pattern recognition receptors, known as Toll-like receptors (TLRs), either alone or in concert with other receptors.7,8 The nasal mucosa can also respond directly to bacterial challenge through the elaboration of cationic polypeptides, which are responsible for the majority of antibacterial activity of nasal fluid.9 The quantitatively most abundant antimicrobial polypeptides, lysozyme, lactoferrin and secretory leukoprotease inhibitor, contribute to this activity,9 although certain S. aureus strains are reportedly resistant to both lysozyme and lactoferrin.10
Cationic antibacterial peptides called defensins are secreted by the epithelia and are present within the overlying nasal fluid. Defensins are a group of highly conserved cationic proteins, which exhibit broad-spectrum activity against bacteria, fungi and some enveloped viruses. Epithelia express three β-defensins: human β-defensin 1 (HBD-1), HBD-2 and HBD-3. HBD-1 is constitutively expressed at very low levels in the airways, while HBD-2 has been shown to be up-regulated upon infection.11 HBD-2 can be induced through activation of the pathogen recognition receptor TLR2, and can chemotactically recruit neutrophils to the site of infection.12,13 HBD-3 is also up-regulated during bacterial challenge and, unlike HBD-1 and HBD-2, is effectively antimicrobial against S. aureus in vitro.14–16
We previously observed that the antibacterial activity of nasal fluid from S. aureus carriers was defective in killing carrier, but not laboratory (non-carrier), strains of S. aureus.6 Compared to healthy patients and individuals with acute rhinitis, nasal fluid from carriers contained elevated levels of HBD-2 as well as human neutrophil peptides 1–3, markers of epithelium-mediated and neutrophil-mediated inflammation, respectively. While persistent nasal colonization by S. aureus in vivo elicited a subclinical immune response, the host's natural armamentarium was not sufficient to prevent colonization.6
In the current report, we extended these studies by examining the nasal epithelia's host response to infection with highly characterized carrier and non-carrier strains of S. aureus.6,17 We reveal that the nasal carrier strain of S. aureus displayed significantly greater growth and attachment to naive NEC than the non-carrier strain. Conversely, interleukin-1β (IL-1β) -activated NEC cells were able to completely prevent the growth and colonization of carrier S. aureus, while the non-carrier strain was significantly less affected. In stark contrast to the non-carrier strain, the carrier strain of S. aureus delayed the expression of TLR2 by 4 hr, and prevented the expression of both HBD-2 and HBD-3 from NEC. Collectively, these data suggest that while carrier S. aureus remains sensitive to the innate immune effectors of activated nasal epithelia, carrier strains probably suppress the innate epithelial host response long enough to enable colonization of the nasal mucosa.
Materials and methods
Bacterial strains and culture conditions
Staphylococcus aureus (SA) D30 was isolated from the anterior nares of a healthy donor. It has been extensively characterized6,17 and served as the carrier strain in experiments herein. The non-carrier strain S. aureus 930918-3 and S. epidermidis were kindly provided by Dr Robert I. Lehrer, UCLA.18 Bacteria were grown on trypticase soy agar (TSA; Bacto™, Becton, Dickinson and Company, Sparks, MD) and subcultured in tryptic soy broth (TSB; Bacto™) from which stocks were prepared. For all experiments, snap-frozen (− 80°) stocks were thawed rapidly and cultured at 37° for 2 hr in 50 ml of sterile prewarmed TSB. The inoculum used in NEC experiments was estimated by measuring the absorbance at 625 nm of a washed bacterial suspension in phosphate-buffered saline (PBS; Mediatech Inc., Herndon, VA). An optical density at 625 nm of 0·1 was estimated to have 2·0 × 107 colony-forming units (CFU)/ml. Inocula were subsequently quantified by plating serial dilutions on TSA and counting CFU following 18 hr incubation at 37°.
Organotypic NEC culture and stimulation
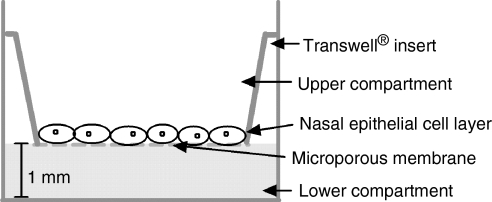
Human NEC (RPMI 2650, American Type Culture Collection, Manassas, VA) were cultivated on porous collagen-coated cell culture inserts (Transwell COL, 24-mm diameter, polyester, 0·4-μm pore size, Corning Inc, NY; Fig. 1). Cell culture medium consisted of Dulbecco's modified Eagle's minimal essential medium (DMEM; Mediatech Inc) with high glucose (4·5 g/l) and sodium pyruvate (110 mg/l), supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), l-glutamine (584 mg/l) supplied by Irvine Scientific (Santa Ana, CA) and 10% (v/v) fetal bovine serum (Gemini Bio-Products, West Sacramento, CA). Cells were cultivated for 4 days in 5% CO2 at 37° until confluence was achieved, the medium being replaced every second day. On the fourth day the organotypic cell layer was exposed to the air–liquid interphase and supplied with antibiotic-free DMEM for an additional 4 days. For experimental procedures, organotypic cell layers were inoculated with 20–40 bacteria and incubated for 4–20 hr at 37° in 5% CO2. Using the Trypan blue exclusion method, viability of NEC after incubation with both strains of S. aureus and with S. epidermidis was greater than 95%. Bacterial growth was assessed by washing the apical surface of NEC thrice with 0·5 ml of sterile prewarmed (37°) PBS, incubating serial dilutions of this on TSA plates, and counting CFU after 18 hr of incubation (37°, 5% CO2). Bacterial attachment was assessed in a similar manner except that washed NEC were sonicated (three 10-second bursts) and the resultant homogenate was treated as above.
Figure 1.
Transwell cell culture growth system consisting of a microporous membrane suspended above cell culture media to enable the growth of NEC at the air–liquid interphase.
RNA isolation and cDNA synthesis
Total RNA was extracted from NEC utilizing TRIzol reagent (Invitrogen, Carlsbad, CA).19 For each condition, extracts from three separate wells were pooled to reduce intraexperimental variability. Contaminating genomic DNA was eliminated using TURBO DNA-free™ (Ambion Inc, Austin, TX) according to the manufacturer's instructions. Subsequent synthesis of cDNA from 200 ng of purified RNA was performed using iScript (Bio-Rad, Hercules, CA) in a total reaction volume of 25 μl, following manufacturer's instructions.
Protein isolation and purification
After the mRNA had been extracted using TRIzol reagent, DNA was precipitated from the interphase by the addition of 100% ethanol, incubated at room temperature for 2–3 min, and centrifuged at 2000–3000 g for 5 min at 4°. The phenol/ethanol supernatant was removed (400 μl) and added to 750 μl isopropanol (Fisher Chemicals, Fairlawn, NJ) in a microcentrifuge tube. Samples were stored at room temperature for 10 min and subsequently centrifuged at 12 000 g for 10 min at 4° to sediment the protein precipitate. The supernatant was then removed and the protein pellet was washed with 0·3 m guanidine hydrochloride (Invitrogen), incubated for 20 min at room temperature and then centrifuged at 7500 g for 5 min at 4°. This step was repeated three times. After the final wash the pellet was vortexed in 75% ethanol, incubated for 20 min at room temperature, and then centrifuged at 7500 g for 5 min at 4°. The pellet was then vacuum dried and dissolved in 1 ml 1% sodium dodecyl sulphate (Sigma, St Louis, MO). Insoluble material was sedimented by centrifugation and the supernatant was transferred to another tube. This protein was used to quantify total protein content and for HBD-2/3 enzyme-linked immunosorbent assay (ELISA).
Quantitative real-time PCR (qPCR) of HBD-2/3 and TLR2
HBD-2, HBD-3 and TLR2 were analysed by qPCR using iQ™ SYBR Green Supermix (Bio-Rad). Each reaction contained 12·5 μl Real-Time SYBR Green PCR Master Mix, 1·0 μl (300–800 ng/ml) First Strand cDNA template and 1·0 μl (0·4 μm final concentration) of forward and reverse primers, to a final volume of 25 μl with molecular grade water. Primers for HBD-2 and HBD-3 were supplied by SuperArray (SuperArray Bioscience Corporation, Frederick, MD). HBD-2 product size was 92 base pairs (bp; Accession no. NM_004942.2), while the HBD-3 product size was 72 bp (Accession no. NM_018661.2). TLR2 was amplified using sense primer (5′-GCCAAAGTCTTGATTGATTGG-3′) and antisense primer (5′-TTGAAGTTCTCCAGCTCCTG-3′).20 Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) used as a control was amplified using sense primer (5′-TGGTATCGTGGAGGACTC-3′) and antisense primer (5′-AGTAGAGGCAGGGATGATG-3′). The qPCR was performed on a My iQ™ single-colour real-time PCR Detection System (Bio-Rad) using 96-well PCR plate reactions in triplicate with controls in the presence and absence of template. GAPDH was used to normalize the levels of cDNA. Amplification of HBD-3 was performed at one cycle of 95° for 15 min and then 40 cycles of (denaturation at 95° for 30 seconds; annealing at 55° for 30 seconds; and extension at 72° for 30 seconds), HBD-2 amplification was performed at one cycle at 95° for 15 min and then 40 cycles of (denaturation at 95° for 15 seconds; annealing at 55° for 20 seconds), and amplification of TLR2 was performed at one cycle of 95° for 15 min and then 40 cycles of (denaturation at 95° for 30 seconds; annealing at 54·3° for 30 seconds; and extension at 72° for 5 seconds). Data were analysed by MyiQ™ single-colour real-time PCR Detection System software (Bio-Rad). Products from qPCR reactions were subcloned into pCR II-TOPO Vector (Invitrogen) using TOPO TA Cloning kit Dual promoter (Invitrogen) and One Shot TOP 10 chemically competent cells (Invitrogen), and sequenced using standard methods.
Quantification of HBD-2/3 by ELISA
Both NEC cellular components and supernatants from NEC cultures were analysed for the presence of HBD-2 and HBD-3 by commercial ELISA (PeproTech, Rocky Hill, NJ). For HBD-2, a BD Falcon Microtest™ 96-well ELISA plate (BD Biosciences, Bedford, MA) was coated with 0·25 μg/ml antigen-affinity purified goat anti-HBD-2 (100 μl) and the plate was sealed and incubated overnight at room temperature. Wells were then washed using wash buffer (0·05% Tween-20; Fisher Scientific, Pittsburgh, PA) in PBS and inverted to remove the residual buffer. Wells were then blocked using 1% bovine serum albumin (Sigma) in PBS for 1 hr at room temperature. The wells were then washed again in wash buffer and the samples and standard HBD-2 were added to the wells. Standards were diluted using standard diluent (0·05% Tween-20, 0·1% bovine serum albumin in PBS). The plates were incubated at room temperature for 2 hr and subsequently washed four times with wash buffer. The detection antibody, biotinylated antigen affinity purified goat anti-HBD-2, was added at a concentration of 0·5 μg/ml (100 μl/well) and incubated for 2 hr at room temperature. The wells were then washed four times in wash buffer and avidin peroxidase (100 μl) at a concentration of 1 : 2000 (with standard diluent) was added, incubated for 30 min at room temperature, and washed four times with wash buffer. Liquid chromogenic substrate, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (Sigma) (100 μl), was added to each well and incubated at room temperature for colour development. The ELISA plate was read using a 96-well plate reader (SpectraMax; Molecular Devices, Sunnyvale, CA) at 405 nm with a wavelength correction set at 650 nm at 5-minute intervals for 15 min. The sensitivity of the ELISA for HBD-2 was 8–1000 pg/ml.
For HBD-3 the ELISA quantification procedure was the same except that capture antibody was used at a concentration of 3 μg/ml, the detection antibody was used at a concentration of 0·25 μg/ml and the plate was read at 5-minute (at 405 nm) intervals for 35 min. The limits of sensitivity for the detection of HBD-3 were 62–4000 pg/ml. Readings for both peptides were obtained in triplicate for three to five independent experiments. Levels of the peptide were compared to the total levels of protein using the bicinchoninic acid method (Pierce Biotechnology, Rockford, IL) according to the manufacturer's instructions and adjusted. Levels of peptide were then subtracted from the basal levels for each time-point.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 4 software (Hearne Scientific Software Pty. Ltd, Melbourne, VIC, Australia). For the analysis of growth and attachment, a minimum of six independent experiments were performed with a minimum of three replicates for each experiment. Numerical values for CFU were transformed to log10 values to enhance the normal distribution. A Student's t-test with a two-tailed distribution was used to analyse the data, assuming two-sample unequal variance (heteroscedastic) or two-sample equal variance (homoscedastic) wherever appropriate.
The measurement of gene expression from bacterially colonized NEC was compared with untreated cells. The difference in expression levels was calculated using the delta-delta cycle threshold (ΔΔCt) method for relative quantification on a triplicate of experiments;21 this was repeated a minimum of four times and analysed using the Student's t-test in GraphPad Prism 4 software. For the analysis of HBD-2/HBD-3 peptides by ELISA, a one tailed heteroscedastic distribution was assumed.
Results
The carrier strain of S. aureus displays greater growth and attachment to NEC than the non-carrier strain
We previously determined that colonization of the host by nasal carrier strains of S. aureus was determined by an adaptation to certain donors' permissive nasal secretions.6 Moreover, nasal colonization is probably multifactorial, encompassing determinants from both the host and the bacteria. Herein, we examined the latter: whether strains isolated from the nose of a carrier exhibited properties that influenced colonization of nasal mucosa. Strains used were S. aureus D30, a nasal carrier strain; S. aureus 930918, a genetically similar non-carrier strain (A.M.C., unpublished genome sequence data); and S. epidermidis, which is part of the normal nasal and epidermal microbiota. For all experiments, NEC layers were inoculated with 38 ± 4 (mean ± SEM) bacteria and incubated at 37° for 4 hr in 5% CO2. Bacterial growth and attachment were assessed as outlined in the Materials and methods.
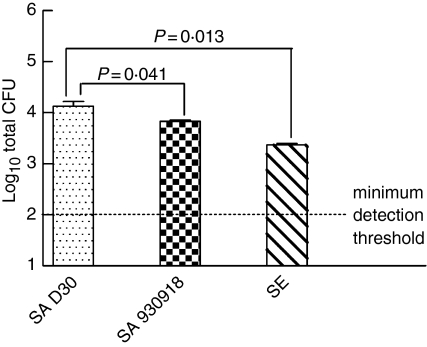
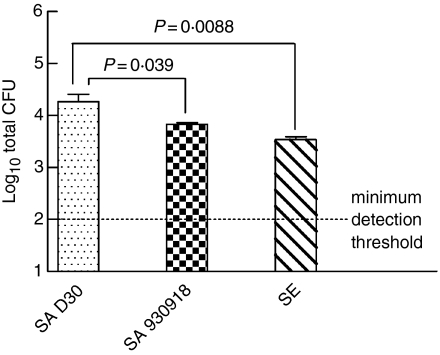
Following the 4-hr incubation, we observed that there was significantly greater growth of the carrier strain in comparison with the non-carrier strain (Fig. 2; P = 0·041, n = 12 carrier S. aureus, n = 8 non-carrier S. aureus) or S. epidermidis (P = 0·013, n = 11 S. epidermidis). When the attachment of bacteria to NEC was assessed, the carrier strain of S. aureus exhibited significantly greater attachment to NEC than the non-carrier strain (Fig. 3; P = 0·039, n = 12 for carrier strain S. aureus and n = 8 for non-carrier strain S. aureus) or S. epidermidis (P = 0·0088, n = 11 for S. epidermidis). The greater growth and attachment of the carrier strain of S. aureus to NEC provides evidence of a superior adaptive colonizing stratagem.
Figure 2.
The carrier strain of S. aureus displays greater growth on NEC than the non-carrier strain. Nasal epithelial cell layers, which had been cultured at the air–liquid interphase in antibiotic-free medium, were inoculated with 38 ± 4 (mean ± SEM) bacteria/well to assess bacterial growth dynamics. The organisms used were S. aureus D30 (SA D30), the carrier strain; S. aureus 930918 (SA 930918), the non-carrier strain; and S. epidermidis (SE), the control strain. The apical surface of NEC layers was washed after 4 hr and bacteria were enumerated and expressed as colony-forming units (CFU). The results represent the mean ± SEM of quadruplicate experiments.
Figure 3.
The carrier strain of S. aureus displays greater attachment to NEC than the non-carrier strain. Nasal epithelial cell layers, which had been cultured at the air–liquid interphase in antibiotic-free medium, were inoculated with 38 ± 4 (mean ± SEM) bacteria/well to assess bacterial growth dynamics. The organisms used were S. aureus D30 (SA D30), S. aureus 930918 (SA 930918), and S. epidermidis (SE). The apical surface of the NEC layers was washed after 4 hr and then sonicated to detach bacteria, which were subsequently enumerated on TSA. The results are expressed as CFU and represent the mean ± SEM of quadruplicate experiments.
The growth and attachment of the carrier strain of S. aureus is significantly reduced in comparison to the non-carrier strain by prestimulation of NEC with IL-1β
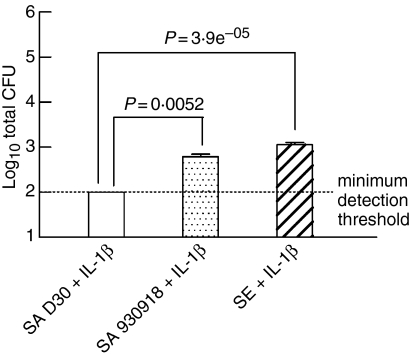
Previously, we determined that the antimicrobial activity of nasal fluid from carriers was defective in the killing of endogenous S. aureus and nasal carrier strains but not laboratory strains of S. aureus.6 To examine the possibility that S. aureus attenuates the innate immune response to attach and proliferate, NEC layers were prestimulated by the addition of physiologically relevant concentrations of IL-1β (10 ng/ml) 24 hr before the addition of colonizing bacteria. Under these conditions it was observed that the nasal carrier strain of S. aureus showed a significant reduction in growth on NEC in comparison to the non-carrier strain (Fig. 4; P = 0·0052, n = 6 for carrier strain S. aureus and n = 16 for non-carrier strain S. aureus) or in comparison with S. epidermidis (P = 3·9 × 10−5, n = 12 for S. epidermidis). Note that prestimulating NEC with IL-1β had the least effect on the growth of S. epidermidis, possibly because it is indigenous and non-pathogenic, and thus less immunostimulatory.
Figure 4.
The growth of the carrier strain of S. aureus is significantly reduced in comparison to the non-carrier strain by prestimulation of NEC with IL-1β. Nasal epithelial cell layers, which had been cultured at the air–liquid interphase in an antibiotic-free medium, were prestimulated with IL-1β (10 ng/ml). These were inoculated with 38 ± 4 (mean ± SEM) bacteria/well to assess their growth dynamics. The organisms used were S. aureus D30 (SA D30), S. aureus 930918 (SA 930918), and S. epidermidis (SE). The apical surface of NEC layers was washed after 4 hr and then sonicated to detach bacteria, which were subsequently enumerated on TSA. The results represent the mean ± SEM of quadruplicate experiments.
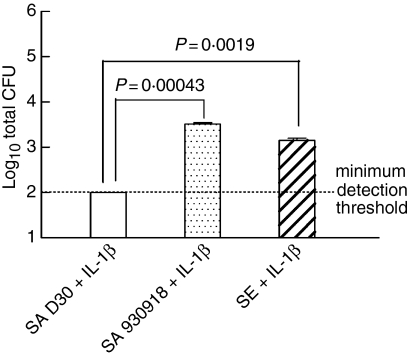
We observed a similar trend when bacterial attachment to NEC was quantified. There was a highly significant reduction in the attachment of the carrier strain of S. aureus to NEC in comparison with the non-carrier strain (Fig. 5; P = 0·00043, n = 6 for the carrier strain S. aureus and n = 16 for the non-carrier strain S. aureus) or in comparison to S. epidermidis (P = 0·0019, n = 12 for S. epidermidis). Both results indicate that the carrier strain of S. aureus remains susceptible to the innate immune response and that prestimulation of NEC with IL-1β may be crucial in the prevention of colonization by nasal carrier strains of S. aureus. In aggregate, these results prompted us to investigate whether carrier strains of S. aureus have the potential to suppress the innate host response of NEC to S. aureus colonization.
Figure 5.
The attachment of the carrier strain of S. aureus is significantly reduced in comparison to the non-carrier strain by prestimulation of NEC with IL-1β. Nasal epithelial cell layers, which had been cultured at the air–liquid interphase in an antibiotic-free medium, were prestimulated with 10 ng/ml IL-1β. The cell layers were inoculated with 38 ± 4 (mean ± SEM) bacteria/well to assess their growth dynamics. The organisms used were S. aureus D30 (SA D30), S. aureus 930918 (SA 930918), and S. epidermidis (SE). The apical surface of NEC layers was washed after 4 hr and then sonicated to detach bacteria, which were subsequently enumerated on TSA. The results represent the mean ± SEM of quadruplicate experiments.
Since it was observed at the cellular level that the carrier strain of S. aureus preferentially colonized and attached to NEC over its non-carrier counterpart, we investigated key molecular markers of innate immune up-regulation using qPCR and ELISA. In all the remaining experiments, NEC layers were inoculated with bacteria for periods of 0, 4, 8 and 20 hr with 20 ± 1 (mean ± SEM) bacteria for q-PCR and 57 ± 13 (mean ± SEM) bacteria for ELISA. Thereafter, the relative expression levels of TLR2, HBD-2 and HBD-3 were quantified.
The up-regulation of TLR2 is delayed following colonization with carrier S. aureus
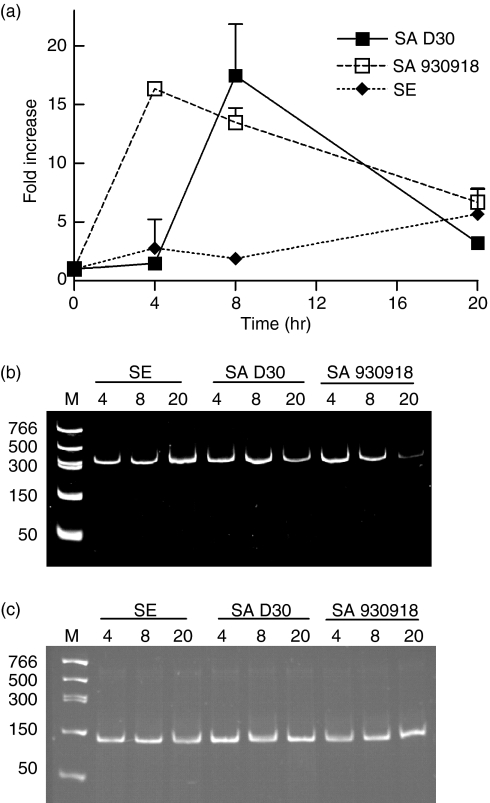
The detection of pathogenic colonization on host NEC was assessed by determining the expression of TLR2. While both strains of S. aureus up-regulated TLR2 to the same degree (Fig. 6), the carrier strain delayed TLR2 induction by approximately 4 hr. As expected, S. epidermidis only marginally induced the expression of TLR2 (Fig. 6). Although TLR2 is up-regulated by both carrier and non-carrier strains of S. aureus, the delay of TLR2 induction by the carrier strain might serve as a necessary foothold by which this strain can initiate colonization of nasal epithelia.
Figure 6.
The up-regulation of TLR2 is delayed following colonization with the carrier strain of S. aureus. (a) NEC layers were cultured at the air–liquid interphase in an antibiotic-free medium and inoculated with 20 ± 1 (mean ± SEM) bacteria/well for a period of up to 20 hr. The organisms used were S. aureus D30 (SA D30), the carrier strain; S. aureus 930918 (SA 930918), the non-carrier strain; and S. epidermidis (SE), the control strain. Expression of TLR2 mRNA from NEC was assessed by real-time quantitative RT-PCR (qPCR). GAPDH was used to normalize cDNA input. The results represent mean ± SEM of four separate experiments. (b,c) Representative acrylamide gel (0·5 μg/ml ethidium bromide) of TLR2 (b) and GAPDH (c) qPCR products visualized under UV after 40 rounds of amplification to verify that the qPCR reactions amplified only the products of interest.
The carrier strain of S. aureus down-regulates the expression of HBD-2 from NEC
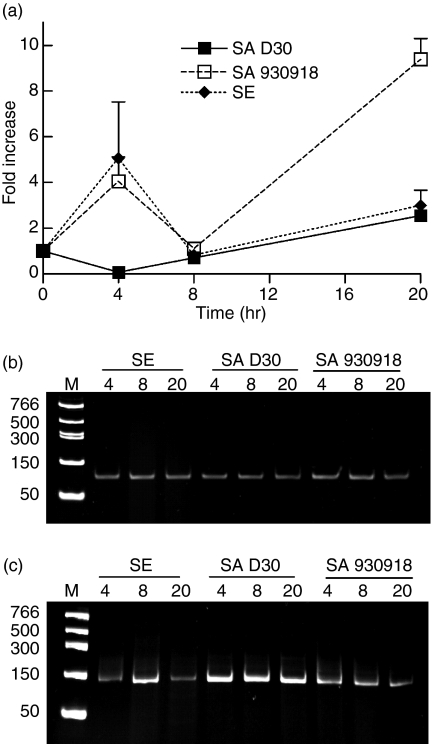
Defensins are a component of the antimicrobial (poly)peptide fraction of nasal fluid.9 Consequently we measured relative expression levels of HBD-2 and HBD-3 mRNA and peptide from NEC inoculated with bacteria over a 20-hr time period by qPCR and ELISA. Colonization of NEC with the non-carrier strain of S. aureus significantly elevated levels of expression of HBD-2 after 4 hr compared with the carrier strain (Fig. 7; P = 0·032, n = 4). Interestingly, the same trend was also observed for S. epidermidis (P = 0·0089, n = 4). However, after 20 hr of incubation, the expression of HBD-2 was elevated in NEC treated with the non-carrier strain of S. aureus compared with the carrier strain (P = 0·0049, n = 4), whereas the expression of HBD-2 in the S. epidermidis condition returned to baseline. The quantitative measurement of HBD-2 peptide by ELISA yielded values that were below the threshold of detection (8 pg/ml). However, the mRNA data mirror the previous observation pertaining to TLR2, in that the carrier strain of S. aureus suppressed the expression of HBD-2. As it has been reported that the antimicrobial peptide HBD-2 can be induced through TLR2,12,13 a delay in TLR2 expression might also translate to a delay in HBD-2 expression. However, the near absence of HBD-2 would suggest that additional evasion strategies might be employed by the carrier strain of S. aureus.
Figure 7.
The carrier strain of S. aureus down-regulates the expression of HBD-2 from NEC. (a) NEC layers were cultured at the air–liquid interphase in an antibiotic-free medium and inoculated with 20 ± 1 (mean ± SEM) bacteria/well for a period of up to 20 hr. The organisms used were S. aureus D30 (SA D30), the carrier strain; S. aureus 930918 (SA 930918), the non-carrier strain; and S. epidermidis (SE), the control strain. Expression of HBD-2 mRNA from NEC was assessed by qPCR. GAPDH was used to normalize cDNA input. The results represent mean ± SEM of four separate experiments. (b,c) Representative acrylamide gel (0·5 μg/ml ethidium bromide) of HBD-2 (b) and GAPDH (c) qPCR products visualized under UV after 40 rounds of amplification to verify that the qPCR reactions amplified only the products of interest.
The carrier strain of S. aureus down-regulates the expression of HBD-3 from NEC
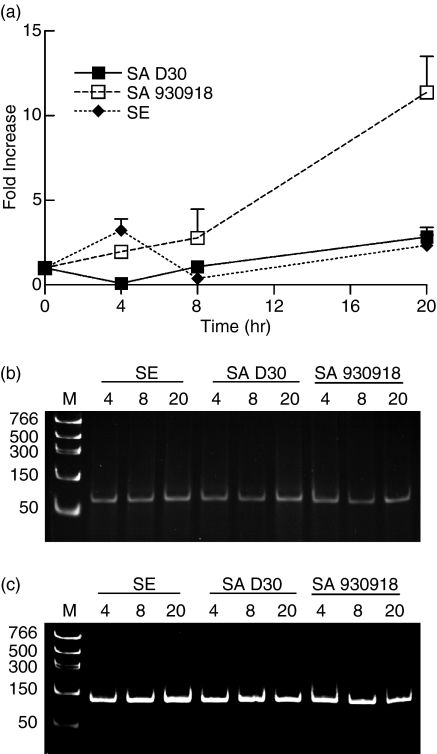
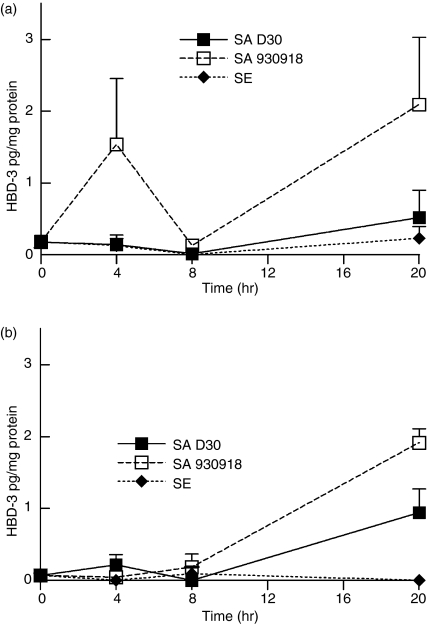
While HBD-2 is largely ineffective against S. aureus in vitro, HBD-3 is very active against many S. aureus strains.15 We therefore measured the expression levels of HBD-3 from NEC layers incubated with carrier and non-carrier strains of S. aureus. The results paralleled the expression of HBD-2: after a 4-hr incubation, there was a significantly lower expression of HBD-3 in NEC incubated with the carrier strain of S. aureus compared with those incubated with the non-carrier strain (P = 0·012, n = 4 for carrier and non-carrier S. aureus; Fig. 8). Most interestingly, after 20 hr of incubation there was a nine-fold increase in HBD-3 expression from NEC incubated with the non-carrier strain of S. aureus compared with those incubated with the carrier strain (P = 0·024, n = 4 for both strains of bacteria; Fig. 8). As with HBD-2, the level of HBD-3 mRNA did not rise significantly above baseline in NEC incubated with the carrier strain of S. aureus.
Figure 8.
The carrier strain of S. aureus down-regulates the expression of HBD-3 from NEC. (a) NEC layers were cultured at the air–liquid interphase in an antibiotic-free medium and inoculated with 20 ± 1 (mean ± SEM) bacteria/well for a period of up to 20 hr. The organisms used were S. aureus D30 (SA D30), the carrier strain; S. aureus 930918 (SA 930918), the non-carrier strain; and S. epidermidis (SE), the control strain. Expression of HBD-3 mRNA from NEC was assessed by qPCR. GAPDH was used to normalize cDNA input. The results represent means ± SEM of four separate experiments. (b,c) Representative acrylamide gel (0·5 μg/ml ethidium bromide) of HBD-3 (b) and GAPDH (c) qPCR products visualized under UV after 40 rounds of amplification to verify that the qPCR reactions amplified only the products of interest.
When HBD-3 peptide levels were measured using ELISA, a pattern emerged similar to that of HBD-3 mRNA. In the cellular supernatant, it was observed that the non-carrier strain expressed higher levels of HBD-3 than the carrier strain (P = 0·038, n = 3 for carrier and non-carrier S. aureus) and higher levels than S. epidermidis (P = 0·005, n = 3; Fig. 9b). For NEC levels of HBD-3, the difference between the carrier strain and the non-carrier strain at 20-hr incubation was not statistically significant (P = 0·089, n = 5 for carrier and non-carrier strains of S. aureus; Fig. 9a). In aggregate, these data indicate that the carrier strain of S. aureus had a greater ability to subvert innate immune effector mechanisms than non-carrier or other strains of Staphylococcus.
Figure 9.
The carrier strain of S. aureus down-regulates HBD-3 from NEC. NEC layers were cultured at the air–liquid interphase in an antibiotic-free medium and inoculated with 57 ± 13 (mean ± SEM) bacteria/well for a period of up to 20 hr. HBD-3 peptide from NEC was assessed by ELISA, for both the cells (a) and the supernatant (b). The organisms used were S. aureus D30 (SA D30), the carrier strain; S. aureus 930918 (SA 930918), the non-carrier strain; and S. epidermidis (SE), the control strain. The results represent means ± SEM of three to five separate experiments.
Discussion
Our results indicate that the nasal carrier strain of S. aureus has significantly greater attachment and proliferation capabilities on NEC layers than the non-carrier strain or even S. epidermidis. Although the nasal carrier strain of S. aureus stimulated delayed expression of TLR2, the subsequent expression of antimicrobial and chemotactic defensin peptides HBD-2 and HBD-3 was nearly absent as compared with the non-carrier strain. These are logical extensions of our previous studies, which observed that nasal secretions from carriers supported the growth of nasal carrier strains to a greater extent than non-carrier strains.6 Those studies were conducted on human subjects whose nasal mucosae were in contact with S. aureus for many months or years, and therefore the nasal epithelial cells were not naive. In contrast, the colonization experiments presented here defined the initial colonization process of carrier strains of S. aureus on naive NEC cells. The overall trend of our data support the hypothesis that the carrier strain of S. aureus displays preferential colonization and attachment capabilities in comparison to the non-carrier strains on NEC layers.
One of the most surprising differences in the growth and attachment characteristics of the carrier strain versus the non-carrier strain was that the stimulation of NEC by IL-1β had a greater effect on the attachment of the carrier strain than it did on the non-carrier strain and the control organism, S. epidermidis. That the stimulation of innate immunity did not affect S. epidermidis is understandable from the standpoint that endogenous microbiota might have evolved mechanisms to coexist on the nasal epithelial surface.17 However, the observation that IL-1β affects the attachment of the nasal carrier strain of S. aureus more than the non-carrier strain suggests that the carrier strain might evade an IL-1β or an IL-1β-mediated pathway. It is well known that S. aureus uses multiple strategies to attach to their hosts,22–26 our studies suggest an additional strategy of immunoevasion by suppressing pathways leading to innate immune activation.
One of the principal physiological down-regulating molecules of IL-1β is IL-1 receptor antagonist (IL-1Ra). This molecule has been reported to be a key element in the immunoevasion of innate immunity in certain parasitic infections.27 In addition, researchers who have used a similar air–liquid interphase epithelial cell culture system and similar numbers of infective bacteria to our experiments have also reported that IL-1β restricts the growth of bacteria.13 Indeed it has been noted that epithelial cells use IL-1R and myeloid differentiation antigen (MyD88) signalling to promote neutrophil recruitment28 but that MyD88, which is also a component of the TLR2-mediated signalling cascade, inhibits the IL-1-induced inflammatory response.29 While these findings are consistent with our results, they also raise other questions, for example whether there is a mechanism of attachment specific to the carrier strain of S. aureus or whether S. aureus relies on a transient down-regulation of innate immune effectors to provide a selective advantage in attachment to nasal epithelial cells.
We observed that both strains of S. aureus reached similar levels of expression of TLR2, one of the principal pattern recognition receptors of Gram-positive bacteria, but that the carrier strain appeared to promote a 4-hr delay in the up-regulation of this receptor in NEC. This strategy may enable the carrier strain of S. aureus a significant window of opportunity in which to evade the host's innate immune mechanisms. Induction of TLR2 has also been shown by other investigators to stimulate the production of HBD-2 in keratinocytes and epithelial cells12 but this peptide is not appreciably active against S. aureus. As HBD-2 has other known functions such as signalling neutrophil recruitment,12,13 its down-regulation by carrier strains of S. aureus may have implications apart from direct antimicrobial activity. More importantly, we observed a down-regulation of HBD-3 expression. HBD-3 is one of the more effective anti-staphylococcal peptides,15 which has previously been shown to be up-regulated during S. aureus colonization.16,30
Although the innate immune system relies upon diverse strategies for the detection and elimination of pathogens, nasal carrier strains of S. aureus have evolved a range of immunoevasion techniques to become successful pathogens. The carrier strain of S. aureus in these investigations had an ability to delay the recognition of its pathogenicity through TLR2, and possibly other receptors, and to prevent the up-regulation of epithelial defensin thus effectively evading the host's innate response to colonization. This critically delayed immune response would be advantageous to pathogens such as the strains of S. aureus specific to nasal carriers, enabling sufficient time to attach and colonize the epithelial surface. The sensitivity of the carrier strain to prestimulated epithelial cells may also provide a therapeutic answer to the nasal carriage of S. aureus.
Acknowledgments
The authors thank Dr Amy Liese Cole and Nicholas Felts for their expert technical assistance with this project. These studies were supported by grant AI060753 from the National Institutes of Health.
Abbreviations
- CFU
colony-forming units
- DMEM
Dulbecco's modified Eagle's minimum essential medium
- ELISA
enzyme-linked immunosorbent assay
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HBD
human β-defensin
- IL
interleukin
- IL-1Ra
interleukin-1 receptor antagonist
- MRSA
methicillin-resistant Staphylococcus aureus
- MYD88
myeloid differentiation antigen
- NEC
nasal epithelial cell
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- qPCR
quantitative PCR
- SA
Staphylococcus aureus
- SE
Staphylococcus epidermidis
- SEM
standard error of the mean
- TLR
Toll-like receptor
- TSA
trypticase soy agar
- TSB
tryptic soy broth
References
- 1.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med. 2001;344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 2.Kourbatova EV, Halvosa JS, King MD, Ray SM, White N, Blumberg HM. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA 300 clone as a cause of health care-associated infections among patients with prosthetic joint infections. Am J Infect Control. 2005;33:385–91. doi: 10.1016/j.ajic.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Rutar T, Zwick OM, Cockerham KP, Horton JC. Bilateral blindness from orbital cellulitis caused by community-acquired methicillin-resistant Staphylococcus aureus. Am J Ophthalmol. 2005;140:740–2. doi: 10.1016/j.ajo.2005.03.076. [DOI] [PubMed] [Google Scholar]
- 4.Pfeltz RF, Wilkinson BJ. The escalating challenge of vancomycin resistance in Staphylococcus aureus. Curr Drug Targets Infect Disord. 2004;4:273–94. doi: 10.2174/1568005043340470. [DOI] [PubMed] [Google Scholar]
- 5.Fritsche TR, Sader HS, Jones RN. Comparative activity and spectrum of broad-spectrum beta-lactams (cefepime, ceftazidime, ceftriaxone, piperacillin/tazobactam) tested against 12,295 staphylococci and streptococci: report from the SENTRY antimicrobial surveillance program (North America: 2001–02) Diagn Microbiol Infect Dis. 2003;47:435–40. doi: 10.1016/s0732-8893(03)00114-7. [DOI] [PubMed] [Google Scholar]
- 6.Cole AM, Tahk S, Oren A, Yoshioka D, Kim YH, Park A, Ganz T. Determinants of Staphylococcus aureus nasal carriage. Clin Diagn Laboratory Immunol. 2001;8:1064–9. doi: 10.1128/CDLI.8.6.1064-1069.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashimoto M, Tawaratsumida K, Kariya H, Aoyama K, Tamura T, Suda Y. Lipoprotein is a predominant Toll-like receptor 2 ligand in Staphylococcus aureus cell wall components. Int Immunol. 2006;18:355–62. doi: 10.1093/intimm/dxh374. [DOI] [PubMed] [Google Scholar]
- 8.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–71. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole AM, Liao HI, Stuchlik O, Tilan J, Pohl J, Ganz T. Cationic polypeptides are required for antibacterial activity of human airway fluid. J Immunol. 2002;169:6985–91. doi: 10.4049/jimmunol.169.12.6985. [DOI] [PubMed] [Google Scholar]
- 10.Bukharin OV, Kartashova OL, Kirgizova SB, Valysheva IV. Antilactoferrin activity of microorganisms. Zh Mikrobiol Epidemiol Immunobiol. 2005;6:7–10. [PubMed] [Google Scholar]
- 11.Meyer JE, Harder J, Gorogh T, Schroder JM, Maune S. hBD-2 gene expression in nasal mucosa. Laryngorhinootologie. 2000;79:400–3. doi: 10.1055/s-2000-4626. [DOI] [PubMed] [Google Scholar]
- 12.Birchler T, Seibl R, Buchner K, Loeliger S, Seger R, Hossle JP, Aguzzi A, Lauener RP. Human Toll-like receptor 2 mediates induction of the antimicrobial peptide human beta-defensin 2 in response to bacterial lipoprotein. Eur J Immunol. 2001;31:3131–7. doi: 10.1002/1521-4141(200111)31:11<3131::aid-immu3131>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 13.Hertz CJ, Wu Q, Porter EM, et al. Activation of Toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human beta defensin-2. J Immunol. 2003;171:6820–6. doi: 10.4049/jimmunol.171.12.6820. [DOI] [PubMed] [Google Scholar]
- 14.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–13. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 15.Midorikawa K, Ouhara K, Komatsuzawa H, et al. Staphylococcus aureus susceptibility to innate antimicrobial peptides, beta-defensins and CAP18, expressed by human keratinocytes. Infect Immun. 2003;71:3730–9. doi: 10.1128/IAI.71.7.3730-3739.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menzies BE, Kenoyer A. Signal transduction and nuclear responses in Staphylococcus aureus-induced expression of human β-defensin 3 in skin keratinocytes. Infect Immun. 2006;74:6847–54. doi: 10.1128/IAI.00389-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole AM, Dewan P, Ganz T. Innate antimicrobial activity of nasal secretions. Infect Immun. 1999;67:3267–75. doi: 10.1128/iai.67.7.3267-3275.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee IH, Cho Y, Lehrer RI. Effects of pH and salinity on the antimicrobial properties of clavanins. Infect Immun. 1997;65:2898–903. doi: 10.1128/iai.65.7.2898-2903.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 20.Zhang FX, Kirschning CJ, Mancinelli R, et al. Bacterial lipopolysaccharide activates nuclear factor-κB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem. 1999;274:7611–14. doi: 10.1074/jbc.274.12.7611. [DOI] [PubMed] [Google Scholar]
- 21.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucl Acids Res. 2001;29:2002–7. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Brien L, Kerrigan SW, Kaw G, et al. Multiple mechanisms for the activation of human platelet aggregation by Staphylococcus aureus: roles for the clumping factors ClfA and ClfB, the serine-aspartate repeat protein SdrE and protein A. Mol Microbiol. 2002;44:1033–44. doi: 10.1046/j.1365-2958.2002.02935.x. [DOI] [PubMed] [Google Scholar]
- 23.Walsh EJ, O'Brien LM, Liang X, Hook M, Foster TJ. Clumping factor B, a fibrinogen-binding MSCRAMM (microbial surface components recognizing adhesive matrix molecules) adhesin of Staphylococcus aureus, also binds to the tail region of type I cytokeratin 10. J Biol Chem. 2004;279(49):50691–9. doi: 10.1074/jbc.M408713200. [DOI] [PubMed] [Google Scholar]
- 24.Roche FM, Meehan M, Foster TJ. The Staphylococcus aureus surface protein SasG and its homologues promote bacterial adherence to human desquamated nasal epithelial cells. Microbiology. 2003;149:2759–67. doi: 10.1099/mic.0.26412-0. [DOI] [PubMed] [Google Scholar]
- 25.Mongodin E, Bajolet O, Cutrona J, Bonnet N, Dupuit F, Puchelle E, de Bentzmann S. Fibronectin-binding proteins of Staphylococcus aureus are involved in adherence to human airway epithelium. Infect Immun. 2002;70:620–30. doi: 10.1128/iai.70.2.620-630.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuroda M, Ohta T, Uchiyama I, et al. Whole genome sequencing of methicillin-resistant Staphylococcus aureus. Lancet. 2001;357(9264):1225–40. doi: 10.1016/s0140-6736(00)04403-2. [DOI] [PubMed] [Google Scholar]
- 27.Rao KV, Ramaswamy K. Cloning and expression of a gene encoding Sm16, an anti-inflammatory protein from Schistosoma mansoni. Mol Biochem Parasitol. 2000;108:101–8. doi: 10.1016/s0166-6851(00)00209-7. [DOI] [PubMed] [Google Scholar]
- 28.Miller LS, O'Connell RM, Gutierrez MA, et al. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Muzio M, Ni J, Feng P, Dixit VM. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278(5343):1612–15. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- 30.Sorensen OE, Thapa DR, Rosenthal A, Liu L, Roberts AA, Ganz T. Differential regulation of beta-defensin expression in human skin by microbial stimuli. J Immunol. 2005;174:4870–9. doi: 10.4049/jimmunol.174.8.4870. [DOI] [PubMed] [Google Scholar]