Abstract
Glucocorticoids (GCs) are involved in the modulation of macrophage function and thereby control the host's immune responses to pathogens. However, neither the role of hormone concentration nor the differential contribution of the glucocorticoid (GR) and the mineralocorticoid receptors (MR) to these activities are known. Here we show that low levels of corticosterone enhance NO production as well as mRNA expression of pro-inflammatory cytokines, chemokines and enzymes required for mediator synthesis. In contrast, at high corticosterone concentrations macrophage function was strongly repressed. Importantly, inactivation of the GR by lentiviral delivery of siRNAs abrogated both the immunostimulatory and the immunosuppressive GC actions whereas inactivation of the MR had no effect. Furthermore, removal of endogenous GCs by adrenalectomy in vivo induced a preactivated state in macrophages that could be modulated by corticosterone. We conclude that GCs exert distinct effects on macrophage function dependent on their concentration, and that they primarily act through the GR despite concomitant expression of the MR.
Keywords: glucocorticoids, macrophage, nitric oxide, RNA interference, adrenalectomy
Introduction
Glucocorticoids (GCs) are important regulators of the immune system.1 While synthetic GCs are in widespread use to treat autoimmune and atopic inflammatory diseases, endogenous hormones are believed to protect the organism from the harmful effects of exaggerated immune responses. The major GC in rodents is corticosterone, which is synthesized in the adrenal gland in response to stress or systemic cytokine release. GCs either act via the glucocorticoid receptor (GR) or the mineralocorticoid receptor (MR), which reside in the cytoplasm sequestered in a heat-shock protein complex.2 Upon ligand binding they translocate into the nucleus and modulate transcription in a positive or negative manner. Whilst the GR is ubiquitously present in all cell-types, expression of the MR is more restricted.3 In addition, expression of the enzyme 11β-hydroxysteroid dehydrogenase type II (11β-HSD2) in epithelial organs such as the kidney prevents activation of the MR by corticosterone and thereby allows aldosterone to control its function.4 In contrast, 11β-HSD2 is not present in the hippocampus and therefore the GR and the MR are both activated by GCs. Because the affinity of the MR for corticosterone is much higher as compared to the GR it is assumed for the hippocampus that corticosterone at basal levels acts through the MR while at elevated concentrations it additionally binds to the GR.5 Interestingly, MR expression in leucocytes has not yet been reported with the exception of human CD34+ haematopoietic progenitor cells6 and rat microglial cells,7 a macrophage-related cell-type that resides in the central nervous system. In the latter case, it was suggested that the MR mediates immunostimulatory effects at low corticosterone concentrations whilst immunosuppressive GC effects at high doses are conferred through the GR. However, whether a similar phenomenon also exists for other types of immune cells is presently unknown.
The release of endogenous GCs is essential for preventing exaggerated immune responses and the management of sepsis.8 Consequently, adrenalectomy or treatment with the GR antagonist RU486 results in high mortality in various models of infection.9,10 In contrast, elevated GR expression is associated with increased resistance to endotoxic shock.11 GCs modulate the function of leucocytes at different levels including the control of gene expression, enzymatic activity and cellular migration.1 Adaptive immune responses are primarily modulated via their influence on T cells and dendritic cells whilst granulocytes and macrophages are primary targets in the context of innate immunity. In the case of macrophages, the expression of pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, IL-12 and tumour necrosis factor-α (TNF-α), chemokines such as CXCL-1, IL-8 and CXCL-10 and the enzymes inducible nitric oxide synthase (iNOS) and cyclo-oxygenase 2 (COX-2) are repressed by GCs.1 Consequently, production of nitric oxide (NO), oxygen radicals and prostaglandins is reduced and phagocytotic activity impaired. On the other hand, GCs are also known to exert immunostimulatory functions such as the induction of acute phase proteins, the enhancement of delayed-type hypersensitivity responses or the stimulation of T-cell mitogenesis.12–14 However, little is known about positive GC effects on macrophages so far. Based on the current knowledge we hypothesized that (i) corticosterone may distinctly modulate macrophage function dependent on its concentration, and that (ii) binding of GCs to different receptors represents the underlying principle. In agreement with our expectations we could demonstrate GR as well as MR expression in peritoneal exudate macrophages using PCR and immunofluoresence. Furthermore, we found immunostimulatory effects on various functions of such inflammatory macrophages at low corticosterone levels whilst high GC concentrations were purely immunosuppressive. However, both types of effects were abrogated by inactivation of the GR but not the MR using lentiviruses encoding specific small interfering RNA (siRNA) molecules. Furthermore, removal of endogenous GCs in vivo induced a preactivated state in macrophages. This suggests that the presence of GCs prevents premature activation of the immune system in the absence of stimulation. In summary, our findings reveal distinct concentration effects of GCs on innate immunity and show that despite the expression of the MR in macrophages, immunomodulatory activities of corticosterone are mediated through the GR.
Materials and methods
Animal experimentation
CD:Crl rats were purchased from Charles River (Sulzfeld, Germany) and kept in individually ventilated cages under specific pathogen-free conditions. To elicit peritoneal macrophages, the rats were injected intraperitoneally with 5 ml of 4% sterile thioglycollate medium (Sigma, Taufkirchen, Germany). Four days later the cells were harvested by peritoneal lavage with 20 ml balanced salt solution (BSS). For adrenalectomy (ADX), the rats were anaesthetized using Ketamin and Rompun. Subsequently the adrenals were quickly removed through two small dorsal incisions, the wounds sealed with tissue glue and the rats placed into their home cage. For sham-surgery the same procedure was performed, except that the adrenals were merely exposed instead of removed. To compensate for the disturbed salt homeostasis, the rats were offered ad libitum a 0·9% NaCl solution for drinking. Successful ADX was confirmed by measuring corticosterone serum levels 2 weeks after surgery by radioimmunoassay (RIA) as described previously.15 All animal experiments were approved by the Bavarian state authorities.
Isolation and culture of peritoneal macrophages
The peritoneal lavage was centrifuged at 450 gand 4° for 5 min and washed twice with cold phosphate-buffered saline (PBS). Subsequently, CD11b/c+ cells were magnetically purified using a biotinylated Ox42 monoclonal antibody in combination with streptavidin microbeads (Miltenyi Biotech, Bergisch-Gladbach, Germany). After washing with BSS containing 1% bovine serum albumin (BSA), the pellet was resuspended in RPMI-1640 medium complemented with 10% fetal calf serum (FCS) and penicillin/streptomycin. For macrophages from ADX rats charcoal/dextran treated FCS was used in all media (Hyclone, Logan, UT). The purified peritoneal macrophages were finally diluted to a final concentration of 1 × 106 cells/ml and plated either in 96-well plates for the analysis of nitric oxide production or 12-well plates for the analysis of mRNA expression. After allowing the macrophages to adhere for 4 hr at 37° and 5% CO2, they were washed three times to remove the non-adherent cells. Finally, the cultures were continued in fresh medium as described above. Routinely, the macrophages were stimulated with 100 ng/ml lipopolysaccharide (LPS; Sigma) and 100 units/ml recombinant rat interferon-γ (IFN-γ; R & D Systems, Wiesbaden, Germany) for 48 hr to determine NO production (in quadruplicate) or alternatively for 6 hr to analyse changes in gene expression (in duplicate). In parallel, corticosterone (Sigma) was added at various concentrations from a 1000× stock in 70% ethanol. In some cases, the macrophages were transduced with lentiviruses before stimulation was initiated.
Generation and transduction of siRNA expressing lentiviruses
The two lentiviral vectors pLL-siGR and pLL-siMR were cloned by inserting annealed oligonucleotides encoding shRNAs specific for the GR or the MR (sequences available upon request) into the lentiviral vector pLL-3.7.16 Virus particles were generated following published protocols, concentrated by ultracentrifugation and directly used to transduce peritoneal macrophages.17 After 48 hr the efficiency of virus infection was determined by flow cytometry on the basis of enhanced green fluorescent protein (eGFP) expression. Before treatment with LPS, IFN-γ and corticosterone the cells were washed twice to remove remaining free virus particles from the culture.
Nitric oxide (NO) release assay
The cell-free macrophage culture supernatants were individually collected from the 96-well plates and used for the nitric oxide release assay. To this end, 50 µl of supernatant was mixed with 50 µl of each of the two Griess reagents, 1% sulphanilamide and 0·1%N-naphthylethylene-diamine-dihydrochloride (both diluted in 2·5% phosphoric acid), and incubated for 5 min at RT for the colour to develop. The concentration of 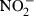 ions that are formed as a consequence of the reaction between NO produced by the macrophages and H2O was measured by spectrophotometry at 560 nm and compared to a NaNO2 standard curve ranging from 1 µm to 125 µm.
ions that are formed as a consequence of the reaction between NO produced by the macrophages and H2O was measured by spectrophotometry at 560 nm and compared to a NaNO2 standard curve ranging from 1 µm to 125 µm.
Quantitative polymerase chain reaction (PCR)
Total RNA was isolated using TriZol reagent according to standard procedures (Invitrogen, Karlsruhe, Germany). Potentially contaminating DNA was removed by DNaseI treatment followed by a purification step with the RNaesy mini Kit (Qiagen, Hilden, Germany). 1 µg of RNA was transcribed into cDNA with Superscript II Reverse Transcriptase (Invitrogen) by Oilgo-dT priming as previously described. Real-time PCR was performed on an iCycler instrument (Bio-Rad, München, Germany) using the SYBR mastermix from Abgene (Hamburg, Germany) according to the manufacturer's instruction. Primer sequences for the genes analysed as well as for β-actin used for normalization are available upon request. PCR conditions were as follows: 94° denaturation for 15 min followed by 50 cycles of 94° for 30 s, 64° for 30 s and 72° for 1 min.
Immunofluoresence analysis
Magnetically purified macrophages were attached on eight-well-chamber slides (LabTekII, Nunc, Wiesbaden, Germany) overnight at 37° at a density of 3 × 105 cells in 500 µl complete RPMI medium. After adding 4% paraformaldehyde (PFA) for 10 min, cells were fixed, permeabilized with 0·01% Triton-X-100 for 5 min, blocked with 5% BSA and incubated with an anti-GR antibody (M-20, Santa Cruz, Heidelberg, Germany) or an anti-MR antibody (a generous gift by Dr Wolfgang Schmid, Heidelberg, Germany18) diluted in 1% BSA overnight at 4°. After washing with 1% BSA the samples were incubated with an anti-rabbit Alexa488 secondary antibody (Invitrogen) for 45 min at room temperature followed by washing with PBS. Finally, the slides were mounted with Fluoromount-G mounting medium and analysed by confocal microscopy.
Results
GR and MR expression in peritoneal macrophages
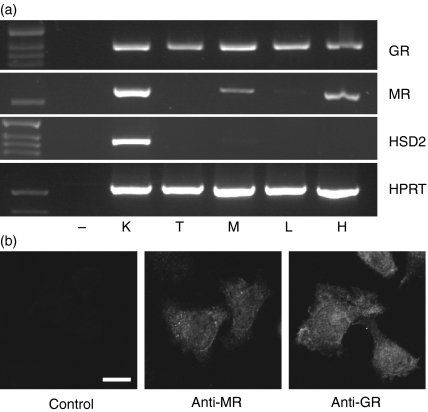
It was previously reported that the MR is expressed by microglial cells.7 Because these cells are functionally related to macrophages, we asked whether they may also express the MR. To this end, we studied mRNA expression of the GR and the MR in thymocytes, mature lymphocytes, peritoneal macrophages, kidney and hippocampus (Fig. 1a). While the GR was present in all cell-types, the MR was only expressed in kidney, hippocampus and macrophages. Lack of 11β-HSD2 expression in macrophages further suggests that both, the GR and the MR in these cells can be regulated by corticosterone (Fig. 1a). To confirm MR expression on the protein level we analysed peritoneal macrophages by immunofluoresence. Using confocal microscopy, we demonstrated the presence of both receptors in the cytosol as well as the nucleus (Fig. 1b). In summary, these data suggest that the MR is expressed in thioglycollate-elicited peritoneal macrophages and is able to respond to corticosterone.
Figure 1.
(a) Analysis of GR, MR and 11β-HSD2 mRNA expression in various cell types and tissues. HPRT expression served as a loading control. K = kidney, T = thymocytes, M = peritoneal macrophages, L = lymph-node cells, H = hippocampus. (b) Immunofluoresence analysis of peritoneal macrophages using GR and MR specific antibodies. A staining with the secondary antibody anti-rabbit-Alexa488 but lacking the primary antibody is shown for control. Bar = 10 µm.
Lentiviruses expressing siRNAs against the GR and the MR allow efficient gene inactivation in peritoneal macrophages
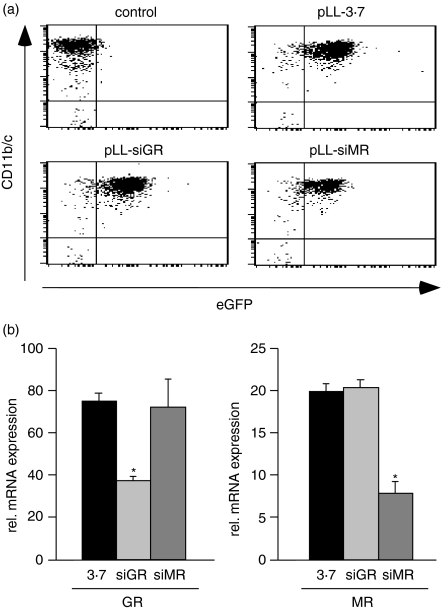
In order to selectively inactivate the GR and the MR in peritoneal macrophages we cloned specific short hairpin RNA (shRNA) sequences into the lentiviral vector pLL-3.7.16 The resulting constructs were designated pLL-siGR and pLL-siMR. Virus particles were concentrated by ultracentrifugation and used to transduce magnetically purified CD11b/c+ peritoneal macrophages. Transduction efficiency was confirmed by flow cytometric analysis of eGFP expression 2 days after infection (Fig. 2a). The level of gene inactivation was determined by quantitative PCR, which confirmed that the expression of the GR and the MR were both specifically reduced by 50–70% (Fig. 2b). Thus, our approach should allow assessment of the role of these receptors for the modulation of macrophage function by GCs.
Figure 2.
(a) Flow cytometric analyses of peritoneal macrophages transduced with the lentiviruses pLL-3·7, pLL-siGR and pLL-siMR for expression of CD11b/c and eGFP. Untransduced cells are shown for comparison. (b) GR and MR mRNA expression was determined by quantitative PCR in macrophages after lentiviral gene inactivation as in (a). Statistical analysis was performed by Student's t-test. The asterisk indicates P < 0·05.
Corticosterone modulates NO production by activated macrophages in a dose-dependent manner via the GR
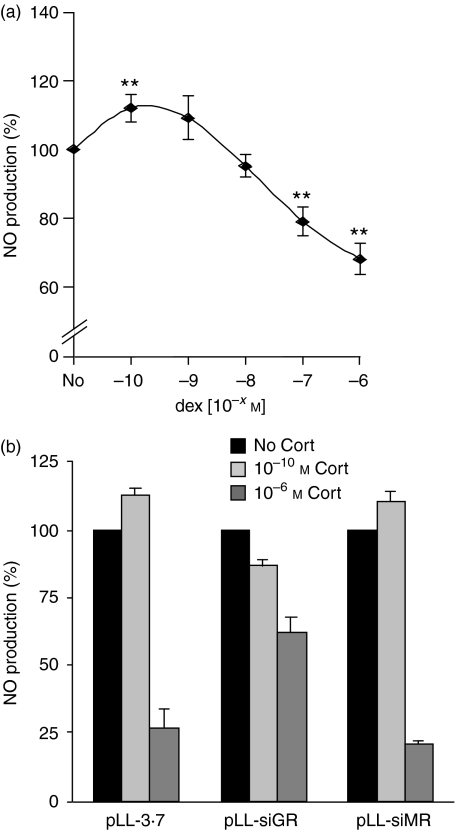
To analyse whether corticosterone exerts different effects on macrophage function dependent on its concentration, we first analysed the production of NO, an important antibacterial mediator. Thioglycollate-elicited peritoneal macrophages were activated by LPS and IFN-γ and concomitantly treated with increasing concentrations of corticosterone ranging from 10−10 m to 10−6 m. Forty-eight hr later we measured NO production in the supernatant using a photometric assay. As hypothesized, low concentrations of corticosterone potentiated the ability of macrophages to produce NO while high levels significantly repressed NO production (Fig. 3a). Importantly, GC treatment did not induce apoptosis of cultured macrophages. Whilst 89·5 ± 2·3% of the cells in control cultures were still alive after 2 days based on Annexin V-binding, this was the case for 88·2 ± 4·4% after treatment with 10−6 m corticosterone (n = 3). We conclude that GCs exerts opposing effects on macrophage function dependent on their concentration and that this effect is independent of apoptosis induction.
Figure 3.
(a) Dose–response curve of the immunomodulatory effect of GCs. Peritoneal macrophages were activated by LPS and IFN-γ in the absence (No) or presence of increasing amounts of corticosterone (10−10 m to 10−6 m). NO production was determined 48 hr later in the culture supernatant. NO production in the absence of corticosterone was set to 100%. (b) Impact of GR and MR inactivation on the ability of corticosterone to modulate NO production. Peritoneal macrophages were transduced with the lentiviruses pLL-3·7, pLL-siGR or pLL-siMR. Subsequently, they were activated with LPS and IFNγ in the absence or presence of corticosterone.
Next, we investigated the role that the GR and the MR play in the immunostimulatory and immunosuppressive activities of corticosterone. To this end, we transduced macrophages with the lentiviruses pLL-3.7, pLL-siGR or pLL-siMR for two days, activated them with LPS/IFN-γ and determined NO production as a measure for macrophage function. In addition, corticosterone was added either at a low (10−10 m) or a high (10−6 m) concentration. Similar to non-transduced cells, pLL-3.7-transduced macrophages produced high amounts of NO following activation, which could be further potentiated by low doses of corticosterone and repressed by high doses of corticosterone (Fig. 3b). Thus, lentiviral transduction does not interfere with macrophages function. Importantly, in cells transduced with pLL-siGR, a low concentration of corticosterone did no longer increase NO production and a high dose only inefficiently reduced it. In contrast, inactivation of the MR did not influence the ability of corticosterone to modulate NO production, neither at a low nor at a high concentration (Fig. 3b). Thus, the immunostimulatory and the immunosuppressive activities of GCs are both mediated via the GR and not the MR.
Corticosterone-dependent modulation of mRNA expression by activated peritoneal macrophages
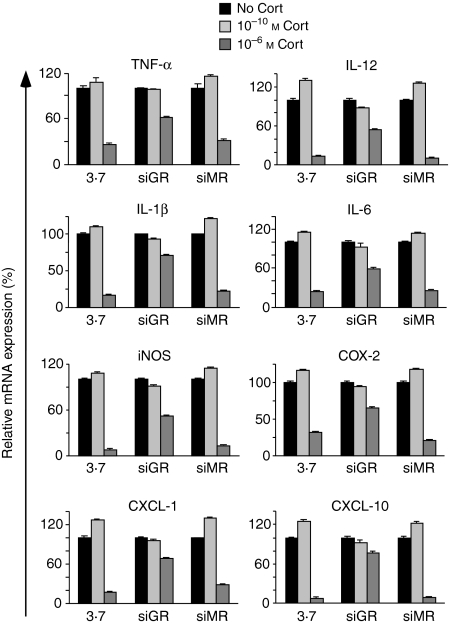
To extend our findings to other macrophage functions involved in the control of inflammation we studied mRNA expression of a variety of genes. As in the previous experiment, thioglycollate-elicited peritoneal macrophages were transduced with pLL-3.7, pLL-siGR or pLL-siMR followed by activation with LPS/IFN-γ in the presence or absence of a low or a high concentration of corticosterone. Subsequently, we analysed mRNA expression of cytokines (TNF-α, IL-1β, IL-6, IL-12), chemokines (CXCL-1, CXCL-10) and enzymes necessary for the production of mediators (iNOS, COX-2) by quantitative PCR. A low dose of corticosterone increased mRNA expression of all genes analysed whereas a high corticosterone concentration reduced it (Fig. 4). Similar to the effect on NO production, inactivation of the GR abrogated both effects while inactivation of the MR had no influence on the ability of corticosterone to modulate gene expression (Fig. 4). Taken together, corticosterone modulates macrophage function in a concentration-dependent manner by binding to the GR but not the MR.
Figure 4.
Peritoneal macrophages were transduced with pLL-3·7, pLL-siGR or pLL-siMR and subsequently activated with LPS and IFN-γ in the absence or presence of corticosterone for 6 hr. mRNA expression of pro-inflammatory genes was determined by quantitative PCR. The mRNA levels in the absence of corticosterone treatment were set at 100%.
Adrenalectomy induces macrophage priming
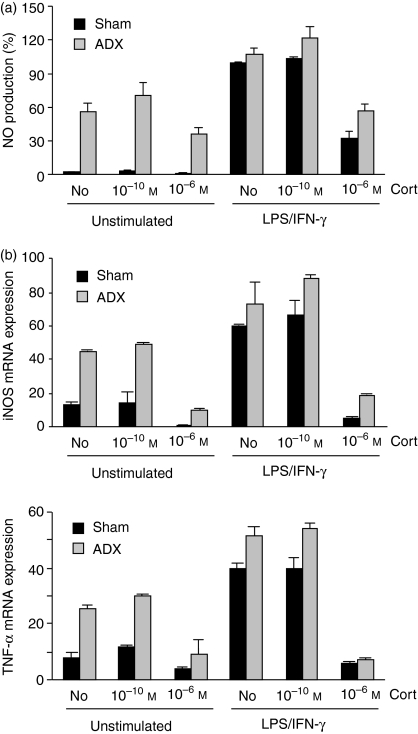
To investigate how the removal of endogenous GCs impacts on innate immunity, we isolated thioglycollate-elicited peritoneal macrophages from adrenalectomized (ADX) and sham-operated rats and took them in culture. As a control, macrophages from both types of animals were activated by LPS and IFN-γ. In addition, corticosterone was either added at a low or a high concentration. Most importantly, macrophages from ADX rats produced elevated levels of NO even in the absence of induction, which could only slightly be enhanced by LPS/IFN-γ treatment (Fig. 5a). Nevertheless, NO production by macrophages from ADX rats could still be positively and negatively modulated by corticosterone. Similarly, macrophages from ADX rats also expressed elevated levels of iNOS and TNF-α mRNA, indicating that these cells were generally preactivated and must have been primed by the lack of endogenous GCs (Fig. 5b). We conclude that the removal and the addition of increasing concentrations of corticosterone impact on macrophage function in a complex manner.
Figure 5.
Peritoneal macrophages were isolated from adrenalectomized (ADX) and sham-operated rats, either left unstimulated or activated with LPS and IFN-γ and cultured in the absence or presence of corticosterone. (a) NO production was determined after 48 hr by photometric assay. (b) mRNA expression of iNOS and TNF-α was measured by quantitative PCR 6 hr after activation.
Discussion
GCs modulate a plethora of physiological functions. While these activities are mostly mediated by the GR, the MR plays an equally important role in the hippocampus.2 In this case it is assumed that the MR mediates GC effects at basal levels while the role of the GR is largely refined to situations of elevated hormone concentrations, e.g. during stress.5 Guided by the observation that GCs are not purely immunosuppressive but also enhance certain immune functions, in particular at low levels13,14 we asked whether (i) concentration-dependent GC effects can also be found in peritoneal macrophages, and (ii) whether they are mediated by the GR or the MR.
First, our analysis provides clear evidence that thioglycollate-elicited peritoneal macrophages express both the GR and the MR. Furthermore, 11β-HSD2 is not present in macrophages, which is in line with previous data.19 Thus, corticosterone should be able to regulate these cells. Second, we found that macrophage function is enhanced at low corticosterone levels while high concentrations are immunosuppressive. Similar observations were previously made with regard to effects of GCs on a delayed-type hypersensitivity response, the stimulation of T-cell mitogenesis and NO production by microglial cells.7,13,14 Thus, opposing effects of GCs in dependence of their concentration appear to be a general characteristic of the immune system. Third, previous studies suggested that the enhancing activities of corticosterone are mediated by the MR while the repressive ones require the GR.7 Our data now clearly show that the MR is dispensable for both types of GC actions despite its expression in macrophages. However, the mechanism by which the opposing effects are achieved remains unknown.
The finding that corticosterone exerts dosage-dependent activities has important consequences for our understanding of immunoregulation by GCs. Whilst the repressive effects of high GC doses prevent exaggerated immune responses, we now found that macrophage functions are potentiated by low levels of corticosterone. We hypothesize that the release of limited amounts of GCs serves to alert the organism of potential threats thereby preparing it to respond to pathogens or wounding. One could imagine a model in which macrophage function is enhanced at an early stage of inflammation when GC levels are only mildly elevated, while leucocytes are held in check at a later stage when the massive release of endogenous GCs prevents a potentially deleterious host response. This would be in line with the model suggested by McEwen and colleagues arguing that acute stress situations are likely to prepare the organism for challenges.14
In the hippocampus, the differential ligand affinities of the GR and the MR underlie the opposing effects of low and high corticosterone concentrations. Pharmacological experiments performed on microglial cells suggested that a similar phenomenon may exist in the immune system.7 This was further supported by our finding that macrophages express MR mRNA and protein. However, selective inactivation of either the GR or the MR by lentiviral delivery of siRNAs to macrophages ex vivo revealed that the presence of the MR was dispensable for all the immunomodulatory functions of GCs tested. Thus, it remains unclear how two different concentrations of a single hormone are able to achieve opposing effects through one receptor. An explanation would be that different conformations of the GR are induced at low vs. high GC levels. For example, the GR binds as a homodimer to the enhancer region of the tyrosine-aminotransferase gene20 while concerted multimers are found in the case of the phenylethanolamine N-methyltransferase (PNMT) gene.21 Alternatively, cofactors could be differentially recruited to the GR dependent on receptor occupancy. Besides the possibility that opposing GC activities are mediated through genomic effects of the GR, non-genomic mechanisms also need to be considered.1 It is known that the GR is able to interact with cytosolic signalling molecules such as PI3K, and that GCs can act through a putative membrane receptor. Therefore it is tempting to speculate that genomic effects are seen at high GC concentrations while non-genomic mechanisms become effective at lower hormone levels. Taken together, it will be interesting in the future to further dissect the molecular basis of the opposing GC effects.
Another intriguing finding is the preactivation of macrophages following the removal of endogenous GCs by adrenalectomy, as it suggests that GCs serve to keep the immune system in an inactive stage in the absence of any stimulus. As a consequence, immune functions can be immediately modulated once GC levels rise in response to a challenge. In summary, our results revealed three different functions of GCs in controlling inflammatory macrophages that are all mediated through the GR. The presence of basal levels of GCs guarantees that the immune system is inactive in the absence of stimulation. Release of low amounts of corticosterone enhances immune functions and thereby prepares the organism to challenges. Finally, high concentrations of GCs repress macrophage function and thereby prevent exaggerated immune responses that may harm the organism. Thus, GCs exert complex modulatory activities in the control of the innate immune system.
Acknowledgments
We would like to thank Christian Bauer for expert technical help and Dr Jan Tuckermann for critical reading of the manuscript. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG 1631/1-3).
Abbreviations
- GCs
glucocorticoids
- GR
glucocorticoid receptor
- MR
mineralocorticoid receptor
- 11β-HSD2
11β-hydroxysteroid dehydrogenase type II
- iNOS
inducible nitric oxide synthase
- COX-2
cyclo-oxygenase 2
- NO
nitric oxide
- siRNA
small interfering RNA
- RIA
radioimmunoassay
- shRNA
short hairpin RNA
- eGFP
enhanced green fluorescent protein
- LPS
lipopolysaccharide
- IFN-γ
interferon-γ
- ADX
adrenalectomized
- PNMT
phenylethanolamine N-methyltransferase
References
- 1.Tuckermann JP, Kleiman A, McPherson KG, Reichardt HM. Molecular mechanisms of glucocorticoids in the control of inflammation and lymphocyte apoptosis. Crit Rev Clin Lab Sci. 2005;42:71–104. doi: 10.1080/10408360590888983. [DOI] [PubMed] [Google Scholar]
- 2.Beato M, Herrlich P, Schütz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–7. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 3.Funder JW. Mineralocorticoid receptors: distribution and activation. Heart Fail Rev. 2005;10:15–22. doi: 10.1007/s10741-005-2344-2. [DOI] [PubMed] [Google Scholar]
- 4.Holmes MC, Kotelevtsev Y, Mullins JJ, Seckl JR. Phenotypic analysis of mice bearing targeted deletions of 11beta-hydroxysteroid dehydrogenases 1 and 2 genes. Mol Cell Endocrinol. 2001;171:15–20. doi: 10.1016/s0303-7207(00)00386-5. [DOI] [PubMed] [Google Scholar]
- 5.Joels M. deKloet ER. Mineralocorticoid and glucocorticoid receptors in the brain. Implications for ion permeability and transmitter systems. Prog Neurobiol. 1994;43:1–36. doi: 10.1016/0301-0082(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 6.Grafte-Faure S, Leveque C, Vasse M, Soria C, Norris V, Vannier JP. Effects of glucocorticoids and mineralocorticoids on proliferation and maturation of human peripheral blood stem cells. Am J Hematol. 1999;62:65–73. doi: 10.1002/(sici)1096-8652(199910)62:2<65::aid-ajh1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka J, Fujita H, Matsuda S, Toku K, Sakanaka M, Maeda N. Glucocorticoid and mineralocorticoid receptors in microglial cells. the two receptors mediate differential effects of corticosteroids. Glia. 1997;20:23–37. [PubMed] [Google Scholar]
- 8.Yeager MP, Guyre PM, Munck AU. Glucocorticoid regulation of the inflammatory response to injury. Acta Anaesthesiol Scand. 2004;48:799–813. doi: 10.1111/j.1399-6576.2004.00434.x. [DOI] [PubMed] [Google Scholar]
- 9.Hawes AS, Rock CS, Keogh CV, Lowry SF, Calvano SE. In vivo effects of the antiglucocorticoid RU 486 on glucocorticoid and cytokine responses to Escherichia coli endotoxin. Infect Immun. 1992;60:2641–7. doi: 10.1128/iai.60.7.2641-2647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertini R, Bianchi M, Ghezzi P. Adrenalectomy sensitizes mice to the lethal effects of interleukin 1 and tumor necrosis factor. J Exp Med. 1988;167:1708–12. doi: 10.1084/jem.167.5.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reichardt HM, Umland T, Bauer A, Kretz O, Schütz G. Mice with an increased glucocorticoid receptor gene dosage show enhanced resistance to stress and endotoxic shock. Mol Cell Biol. 2000;20:9009–17. doi: 10.1128/mcb.20.23.9009-9017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reichardt HM. Immunomodulatory activities of glucocorticoids: insights from transgenesis and gene targeting. Curr Pharm Des. 2004;10:2797–805. doi: 10.2174/1381612043383575. [DOI] [PubMed] [Google Scholar]
- 13.Wiegers GJ, Croiset G, Reul JM, Holsboer F, de Kloet ER. Differential effects of corticosteroids on rat peripheral blood T-lymphocyte mitogenesis in vivo and in vitro. Am J Physiol. 1993;265:E825–30. doi: 10.1152/ajpendo.1993.265.6.E825. [DOI] [PubMed] [Google Scholar]
- 14.Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci USA. 1999;96:1059–64. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reichardt HM, Kaestner KH, Tuckermann J, et al. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–41. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- 16.Rubinson DA, Dillon CP, Kwiatkowski AV, et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003;33:401–6. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- 17.van den Brandt J, Wang D, Kwon SH, Heinkelein M, Reichardt HM. Lentivirally generated eGFP-transgenic rats allow efficient cell tracking in vivo. Genesis. 2004;39:94–9. doi: 10.1002/gene.20037. [DOI] [PubMed] [Google Scholar]
- 18.Berger S, Wolfer DP, Selbach O, et al. Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity. Proc Natl Acad Sci USA. 2006;103:195–200. doi: 10.1073/pnas.0503878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilmour JS, Coutinho AE, Cailhier JF, et al. Local amplification of glucocorticoids by 11 beta-hydroxysteroid dehydrogenase type 1 promotes macrophage phagocytosis of apoptotic leukocytes. J Immunol. 2006;176:7605–11. doi: 10.4049/jimmunol.176.12.7605. [DOI] [PubMed] [Google Scholar]
- 20.Schmid W, Strähle U, Schütz G, Schmitt J, Stunnenberg H. Glucocorticoid receptor binds cooperatively to adjacent recognition sequences. EMBO J. 1989;8:2257–63. doi: 10.1002/j.1460-2075.1989.tb08350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams M, Meijer OC, Wang J, Bhargava A, Pearce D. Homodimerization of the glucocorticoid receptor is not essential for response element binding: activation of the phenylethanolamine N-methyltransferase gene by dimerization-defective mutants. Mol Endocrinol. 2003;17:2583–92. doi: 10.1210/me.2002-0305. [DOI] [PubMed] [Google Scholar]