Abstract
Allergen exclusion measures during pregnancy and lactation have been given consideration in studies of primary allergy prevention but complete avoidance of mother/neonatal allergen exposure has proven to be a difficult procedure. To evaluate a strategy to prevent allergen sensitization in early life in mice, we first established a neonatal model with ovalbumin sensitization through maternal allergen exposure during pregnancy or breastfeeding. The modulatory potential of preconception immunization was investigated on the neonatal development of subsequent allergic responses to maternal allergen exposure. Herein, we demonstrate that immunized mothers exposed to antigen during pregnancy or breastfeeding underwent intense vertical transmission of antibodies, including immunoglobulin G (IgG) in complex with ovalbumin and IgG1 antibody with anaphylactic function. It was further shown that maternal immunization efficiently decreased the passage of free antigens through breastfeeding and inhibited the enhanced IgE antibody response after postnatal antigen exposure. In addition, antenatal immunization decreased the antigen-specific proliferative response of immunized neonates, in parallel with profound downmodulatory effects on both the activation and differentiation of T and B cells after a non-specific stimulus and cytokine production. These findings showed that early life sensitization, subsequent to maternal allergen exposure during both the prenatal and postnatal periods, could be avoided by preventive vaccination of the mother.
Keywords: allergy prevention, breastfeeding, neonatal sensitization, pregnancy, T-cell anergy
Introduction
Allergen exposure in the gestational and perinatal periods has been associated with allergic disease and asthma.1 Generally, early postnatal exposure to high levels of allergen has been shown to enhance the risk for subsequent expression of allergic reactivity to that allergen in adult life.2 This suggests that allergen challenge during infancy predisposes the child toward the development of long-term T helper type 2 (Th2)-skewed allergen-specific immunological memory.3
Exposure to maternally derived dietary allergen via breast milk has long been known to provoke food-associated allergic symptoms.4 Therefore, dietary exclusion or allergen avoidance measures during pregnancy and lactation have been the focus of studies of primary allergy prevention.5 However, primary prevention to exclude maternal/neonatal allergen exposure is a difficult procedure to perform, reinforcing the need for preventive measures to control allergic sensitization in early infancy. The onset of allergic manifestation is usually during early childhood. Hence, the importance of primary preventive strategies focusing on early childhood, during the immune system maturation.
Paradoxically, allergen exposure in mothers could potentially inhibit the development of allergy. High levels of cord blood immunoglobulin G (IgG) antibodies to cat and birch were found to be associated with decreased atopy in children during the first 8 years of life.6 Indeed, children born to mothers who received rye-grass immunotherapy during pregnancy had fewer positive skin tests 3–12 years later compared to infants from mothers who stopped their treatment during pregnancy.7 Studies in mice have shown that allergen immunization of mothers represents a potential preventive measure for allergic responses; maternal IgG was found to suppress IgE responses in neonates.8 We observed that maternal immunization to allergens, before mating, transfers allergen-specific antibodies by transamniotic and transplacental routes as well as by breastfeeding, to inhibit IgE development efficiently in the offspring.9 This strategy did not induce allergen sensitization in the offspring, with long-lasting inhibitory effects on the allergen-specific IgE response until adulthood.10 Effectiveness of preconception immunization is linked with the time of allergen immunization; IgE suppression is stronger after maternal allergen exposure during early pregnancy than after exposure in late pregnancy.11 These investigations with rodent models have reported suppression of the IgE-specific response in offspring from both actively and passively immunized mothers. However, the prophylactic efficacy of maternal vaccination to control early allergen sensitization, when mothers are intensively exposed to allergen during fetal development or soon after birth, remains to be demonstrated. Also, the role of breastfeeding in neonatal allergen sensitization, subsequent to maternal allergen intake, has not been studied, and it is not known whether maternal antibodies would be effective in avoiding neonatal sensitization through this pathway. These remarks underscore the importance of investigating the impact of maternal vaccination on the first allergen sensitization of the offspring and on their antigen-specific or polyclonal cellular immune responsiveness, so as to improve the strategies of allergy prevention.
Several factors, during pregnancy and/or breastfeeding, can have a significant impact on the immunological repertoire of the offspring. A better understanding of the mechanism of maternal allergen immunization is essential for the safe modification of early immune development and for prevention of allergic disease. We sought to verify the efficacy of the murine model of preconception immunization to control early allergen sensitization of offspring. First, a neonatal model was established for ovalbumin sensitization through maternal allergen exposure during pregnancy or breastfeeding. The contribution of preconception immunization to the role of transamniotic/transplacental routes and breastfeeding in the transmission of antibodies was analysed; IgG1, which has anaphylactic function, and antigen transference to fetal circulation, either as free antigen or in complexes with IgG, were evaluated. We extended our studies to the repercussions of the preconception immunization on the allergen-specific and non-specific T-cell and B-cell responsiveness after neonatal immunization subsequent to prenatal and postnatal allergen exposure.
Materials and methods
Animals
Inbred female and male BALB/c mice (8–10 weeks old) were provided by the animal facilities of the São Paulo University Medical School. Wistar Furth rats, of both sexes, 3–4 months old and bred in our own laboratory's animal facilities, were used for passive cutaneous anaphylaxis reaction investigations. All experiments were approved by the Ethics Committee for Animal Research of the Institute of Biomedical Sciences.
Experimental protocols
Preconception immunization and ovalbumin exposure schedule
Female BALB/c mice were immunized subcutaneously with 200 μg ovalbumin (OVA, grade V, Sigma-Aldrich, St Louis, MO) in 6 mg Al(OH)3 and intraperitoneally (i.p.) boosted with 100 μg OVA without adjuvant, on the 10th and 20th days after immunization. One day later, females were mated with non-immunized male BALB/c mice. Immunized or non-immunized groups were given orally, using an oral feeding needle, five doses of 300 μg OVA in 0·5 ml saline solution during the pregnancy period, or three doses of 0·5 or 3·0 mg OVA in 0·5 ml saline solution during the postnatal period, corresponding to the 2nd, 3rd and 4th days of lactation.
Offspring immunization
Either 3-day-old or 25-day-old mice of both sexes were immunized i.p. with 10 or 200 μg OVA in 1·25 mg or 0·6 mg Al(OH)3, respectively. Ten days later, the offspring received an i.p. injection of 100 μg OVA in saline solution and were bled 7 days later.
Collection of amniotic fluid, fetal and maternal sera and milk
To evaluate the vertical transmission of antibodies or antigens, amniotic fluid and fetal sera were obtained by Caesarean section of full-term pregnant mice (21 days) and stored in a pool at −70° until use. Mothers were bled at parturition and 20 days postpartum. Milk samples were obtained as previously described.9
Passive transference of anti-OVA antibodies
On the day of neonatal immunization (3 days old), mice were injected i.p. with 200 μg rabbit anti-OVA IgG antibodies (Calbiochem, San Diego, CA) or rabbit IgG antibodies (Calbiochem). Mice were injected with the same dose of antibody after 6, 10, 13, 15 and 17 days of immunization, giving a total of 1·0 mg antibody. The other group of mice was injected with 200 μg antibodies twice, once on the day of the immunization and again 10 days after immunization.
Passive cutaneous anaphylaxis (PCA)
The anaphylactic activity of IgG1 was evaluated by PCA reaction in mice as described by Ovary.12 Mice were previously shaved and were given intradermal injections (50 μl) in each side of the dorsal skin of serial dilutions of sera that had been previously inactivated for 1 hr at 56°. After 2 hr, they were intravenously challenged with 0·25 mg OVA in 0·5 ml (0·25%) Evans Blue solution. All tests were conducted in triplicate. IgE antibodies were estimated by PCA in rats according to Mota and Wong.13 Serum dilutions were inoculated intradermally (100 μl) on the shaved backs of the rats. After 18 hr, the rats received an injection of 0·5 mg OVA in 1·0 ml (0·5%) Evans Blue solution through a tail vein. PCA titres were expressed as the reciprocal of the highest dilution that gave a spot bigger than 5 mm in diameter.
Assay for free OVA measurement and IgG–OVA complex detection
For measurement of OVA, 96-well microplates (A2, High binding, Costar, Cambridge, MA) were coated with goat anti-chicken egg albumin IgG antibody (ICN/Cappel, Aurora, OH), diluted in 0·1 m carbonate–bicarbonate buffer for 1 hr at 37° and overnight at 4°. After washing with Tris–HCl (50 mm), the plates were blocked with phosphate-buffered saline (PBS)/0·5% gel and samples and standard were applied for 1 hr at 37° and overnight at 4°, followed by rabbit anti-chicken egg albumin IgG antibody (Calbiochem) incubation for 1 hr at 37°. Detection was performed with biotinylated anti-rabbit IgG antibody (Calbiochem) and streptavidin–horseradish peroxidase (Sigma) and 3,3′,5,5′-tetramethyl-benzidine (TMB, Calbiochem). The reaction was stopped with 0·5 m sulphuric acid and read at 450 nm in an enzyme-linked immunosorbent assay (ELISA) microplate reader (Molecular Devices, Sunnyvale, CA) with the SOFT max PRO program. Optical density (OD) units were correlated to the protein concentration using OVA standard curves.
For the detection of OVA–IgG complexes, microplates (Costar) were coated with goat anti-chicken egg albumin IgG antibody (ICN) in PBS for 1 hr at 37° and overnight at 4°. After blocking the plates with PBS/1·0% bovine serum albumin (Sigma) for 1 hr at 37°, serial dilutions of samples were applied for 1 hr at 37° and overnight at 4°. Detection was performed with biotinylated anti-mouse chain antibodies (Southern Biotechnology Ass., Birmingham, AL), streptavidin-horseradish peroxidase and TMB. The reaction was stopped with sulphuric acid and plates were read at 450 nm in an ELISA assay microplate reader.
Determination of antibody levels
OVA-specific antibodies were measured by means of ELISA, as previously described [10]. The results were expressed as antibodies titres with reference to serial dilution of a titrated serum pool from immunized adult mice with high levels of specific antibodies.
Proliferation assay with [3H]thymidine
Spleens were aseptically collected from 20-day-old mice and pressed through a cell strainer (BD Bioscience, Bedford, MA) in RPMI-1640 supplemented with 10% fetal calf serum (Hyclone, Gibco BRL) and 10 mg/ml gentamicin. The red blood cells were lysed using ACK Lysing Buffer (Biosource, Rockville, MD) for 90 seconds. Splenic mononuclear cells at 2·0 × 105 cells/0·2 ml were cultured with OVA (Sigma, 200 μg/ml), anti-CD3 monoclonal antibody (mAb; PharMingen, 2 μg/ml) or lipopolysaccharide (LPS, Sigma, 50 μg/ml) at 37° in a humidified 5% CO2 incubator. Thymidine incorporation was measured on day 4 of culture 18 h after being pulsed with 1 μCi [3H]thymidine (GE Healthcare, Little Chalfont, UK).
In vitro cytokine production
Splenic mononuclear cells were isolated and cultured with anti-CD3 mAb (PharMingen, 2 μg/ml) or OVA (Sigma, 200 μg/ml) for 24 and 72 hr, respectively, as previously described,14 whereupon cell-free supernatants were stored at − 70° until the cytokines were measured by ELISA. Interleukin-4 (IL-4), IL-10 and interferon-γ (IFN-γ) (OptEIA™ Pharmingen, San Diego, CA) measurements were performed following the manufacturer's recommendations. Detection limits of IL-4, IL-10 and IFN-γ were estimated to be 7·8 pg/ml, 31·3 pg/ml and 31·3 pg/ml, respectively.
Statistical analysis
Values for all measurements are expressed as mean ± SD. Differences between groups were considered significant when P-values were < 0·05, using the Mann–Whitney test.
Results
Antibody transference by immune mothers after prenatal or postnatal ovalbumin exposure
First, we examined the transference of antibodies to fetuses by female mice, subcutaneously immunized with OVA 3 weeks before mating and given OVA orally five times during gestation, according to Fig. 1. Other immunized mothers received OVA during the postnatal period, three times, corresponding to the first week of breastfeeding. Immune mothers at parturition showed high antibody sera levels, mainly when they had received additional OVA during pregnancy (Fig. 2a). Maternal anti-OVA IgG antibody was transferred across the yolk sac during pregnancy and from milk postnatally, showing that both pathways contribute to antibody transference as verified in the circulation of 20-day-old offspring. Immune mothers exposed to antigen during the breastfeeding period did not modify IgG levels compared to the immune mothers that were not fed OVA (Fig. 2b). Antigen administration via the mucosal route per se barely induced antibody levels, irrespective of the maternal exposure period.
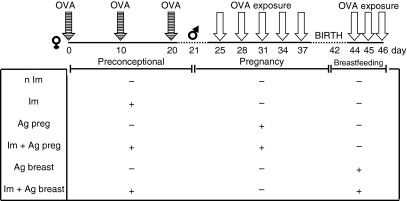
Figure 1.
Schematic representation of maternal immunization and exposure to OVA in prenatal or postnatal period. BALB/c mice were subcutaneously immunized with 200 μg OVA and i.p. boosted with 100 μg OVA after 10 and 20 days of immunization and mated one day after that. Immunized (Im) groups were orally administered with five 300 μg OVA doses during pregnancy (Im + Ag preg) or with three doses of 0·5 or 3·0 mg during the first week of breastfeeding (Im + Ag breast). Groups of non-immunized (n Im) pregnant mice exposed during pregnancy (Ag preg) or during the breastfeeding period (Ag breast) were also studied.
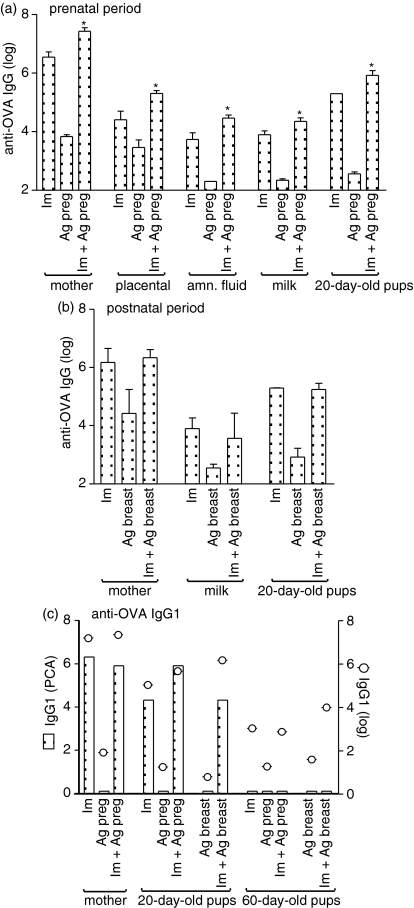
Figure 2.
Evaluation of antibody transmission by immune mother exposed to OVA in the prenatal or postnatal period. Female BALB/c mice immunized or not with OVA were mated and orally administered OVA during (a) gestation or (b) the breastfeeding period. Placental serum and amniotic fluid were obtained from Caesarean section from full-term pregnant mice. Breast-milk samples were obtained from the stomach of 5-day-old newborn and offspring serum at 20 and 60 days old. Maternal serum was collected at (a, c) parturition time or (b) 20 days postpartum. Results represent the means of anti-OVA antibody titres ± SD for five or six animals analysed by ELISA and pooled sera from four to six animals for the evaluation of anaphylactic IgG1 antibody by PCA reaction. *P ≤ 0·05 compared to pups from immune mothers.
One type of IgG1 has been described to have an anaphylactic activity in mice.15 We investigated whether preconception immunization followed by antigen exposure can transfer anaphylactic IgG1 antibody to the offspring. Figure 2(c) shows that full-term pregnant immune mothers produced IgG1 antibody with anaphylactic function, as measured by PCA, but the mothers who were only exposed to antigen during gestation did not. IgG1 anaphylactic antibodies were detected in the sera of offspring, with enhanced titres when the immune mothers were given antigen orally during pregnancy, which gradually dropped in those exposed during the postnatal period. In contrast, non-anaphylactic IgG1 antibody levels were transmitted at high levels to offspring, dependent on the maternal immunization schedule, mainly when they received antigen during the breastfeeding period. Interestingly, anaphylactic IgG1 antibody was undetectable in the sera of 60-day-old offspring, indicating a faster catabolism in the circulation than non-anaphylactic IgG1 antibody measured by ELISA.
Antenatal immunization decreased free antigen transference by breast milk of postnatally exposed mothers
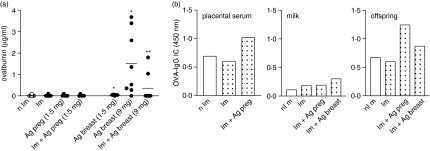
To assess whether antigen exposure during gestation or breastfeeding allows the transfer of antigen through the maternal–fetal barrier or by breast milk, OVA was measured by ELISA in placental sera (fetal sera), amniotic fluid and milk samples. The free form of OVA was undetectable in placental sera as well as in amniotic fluid obtained from mothers submitted to immunization or antigen exposure. Free antigen was measurable in a dose-dependent manner in the breast milk of mothers who had received OVA during the lactation period (Fig. 3a). According to the considerable amount of antigen present in breast milk after an intake of 9·0 mg OVA, we subsequently verified the effect of antenatal immunization in these females. Preconception immunization was able to reduce four-fold the amount of OVA in the breast milk samples, compared to only antigen-exposed mothers.
Figure 3.
Determination of antigen and OVA-IgG immune complex transference. Placental serum, milk and pup sera (20-day-old) from immunized or non-immunized mothers exposed to OVA in the prenatal or postnatal period were obtained as described in the Materials and methods. (a) Measurement of OVA in breast milk; (b) detection of OVA–IgG immune complex analysed by ELISA represent OD from a pool of five samples per group (1 : 200). *P ≤ 0·05 compared to non-immune mother; **P ≤ 0·05 compared to mother exposed to antigen postnatally.
Next, we examined whether antigen could be transferred in complex with IgG (OVA–IgG) in maternal fluids and offspring sera. Immune mothers that were antigen-exposed during pregnancy transferred OVA–IgG immune complex to fetuses mainly via the placental route, as also verified in the 20-day-old offspring sera (Fig. 3b). At the postnatal stage, although OVA–IgG complex was barely found in breast milk, it may have partially contributed to the transfer of antigen to offspring.
Influence of maternal immune status in the non-immunized offspring cellular responsiveness
Subsequently, we assessed whether prenatal or postnatal antigen exposure might influence the antigen-specific or non-specific cellular response of offspring. All non-immunized offspring showed a stimulation index less than 3 upon OVA stimulation, indicating a lack of relationship between the antigen cellular response and maternal antigen exposure (data not shown).
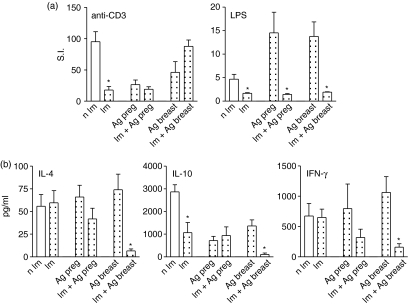
The offspring of immune mothers or those submitted to antigen exposure only at gestation showed an impaired T-cell response thorough T-cel receptor stimulation, compared to those from non-immunized mothers (Fig. 4a).
Figure 4.
Cellular responsiveness of non-immunized offspring. (a) Spleen cells from non-immunized offspring (20-day-old) from immune mothers and/or those exposed to OVA during gestation or breastfeeding periods were cultured with OVA, anti-CD3 mAb or LPS and [3H]thymidine uptake are represented by the stimulation index (SI); (b) cytokine measurement in supernatants of spleen cell culture after 24 hr of stimulation with anti-CD3 mAb by ELISA. Results represent mean ± SD from five to eight mice/per group assayed individually in three different experiments. *P ≤ 0·05 compared to control group.
The B-cell response to LPS was significantly increased by maternal antigen exposure, regardless of the time of antigen administration (Fig. 4a). The enhanced B-cell responsiveness was markedly inhibited by preconception immunization, decreasing 10-fold and 5·3-fold in offspring of immune mothers that were antigen-administered during pregnancy or the lactation period, respectively, compared to only exposed mothers. Although maternal antigen exposure did not induce an OVA-specific cellular response as well as cytokine production upon antigen stimulation, the offspring exhibited a marked alteration in the non-specific B- and T-cell responses.
Furthermore, pups from immunized mothers exposed to antigen postnatally showed a marked inhibition of IFN-γ, IL-4 and IL-10 after anti-CD3 mAb stimulus, despite their normal proliferative response (Fig. 4b). The impaired IL-10 production was consequent to the maternal immunization.
Preconception immunization inhibits the IgE response of neonates and weaned immunized offspring
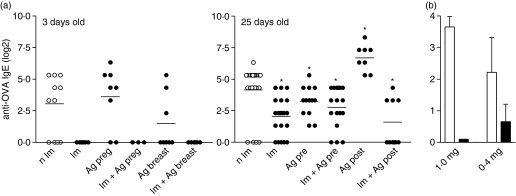
To investigate the effect of maternal immunization or antigen exposure on development of the IgE response, the offspring were immunized neonatally (3-day-old) or during weaning (25-day-old). Maternal immunization efficiently inhibited the development of anaphylactic IgE antibody production in the immunized neonates of mothers exposed in pregnancy or at the time of breastfeeding (Fig. 5a).
Figure 5.
Evaluation of IgE response in the neonatal and weaning immunization. (a) Three-day-old or 25-day-old offspring of immune mothers and/or those exposed to OVA at prenatal or postnatal period were immunized with OVA and IgE antibody evaluated by means of PCA reaction; (b) Neonatal pups (3-day-old) from non-immunized mothers were injected with 1·0 mg or 0·4 mg of rabbit anti-OVA IgG antibody or control antibody, immunized with OVA and sera were evaluated for IgE by PCA reaction. *P ≤ 0·05 compared to non-immunized mother.
To examine the IgE response later in life, offspring were immunized during weaning. Mice born to mothers exposed to antigen during pregnancy showed a decreased IgE response, while those exposed at the postnatal stage developed an enhancement of IgE antibody production in comparison to the offspring of non-immunized mothers (Fig. 5a). These data indicate that maternal antigen exposure very early in life, or in utero, may induce a peripheral tolerance instead of allergen sensitization. Regardless of the OVA priming of offspring as a result of postnatal antigen contact, preconception immunization significantly inhibited the IgE response of offspring.
To evaluate whether the inhibitory effect on the IgE response was mediated by neutralizing antibody, 3-day-old mice received injections of rabbit anti-OVA IgG antibody, simultaneously with the OVA immunization protocol. This treatment induced a marked inhibition of IgE production compared to immunized pups treated with control antibody (Fig. 5b).
Maternal immunization avoids antigen-specific cellular responsiveness in neonatal immunization
Considering the finding that preconception immunization decreases free antigen in breast milk as well as inhibiting the exacerbated IgE response induced by postnatal antigen exposure, we analysed the antigen-specific proliferative response and cytokine production after neonatal immunization.
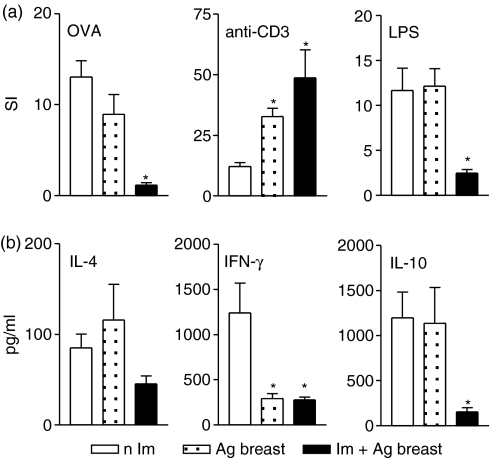
Figure 6(a) demonstrates that maternal immunization significantly reduces the antigen proliferative response as well as the B-cell response to LPS in offspring of mothers exposed postnatally. These pups exhibited an increased T-cell proliferation through T-cell receptor stimulation, with reduced ability to produce IFN-γ and IL-10 compared to offspring of non-immunized mothers (Fig. 6b).
Figure 6.
Effect of antenatal immunization with subsequent antigen exposure at postnatal period in the cellular responsiveness of immunized neonates. (a) Spleen cells of offspring immunized at the neonatal period born to immunized or non-immunized mothers exposed to OVA at postnatal period were cultured with OVA, anti-CD3 mAb or LPS and [3H]thymidine uptake are represented by the stimulation index (SI); (b) cytokine levels were evaluated in supernatants of spleen cell culture stimulated with anti-CD3 mAb for 24 hr by ELISA. Results represent mean ± SD from five to eight mice/per group assayed individually in three different experiments. *P ≤ 0·05 compared to pups of non-immunized mothers.
Discussion
There is accumulating evidence that allergen exposure during pregnancy or breastfeeding have the capacity to influence significantly a child's immune development and to alter the risk for allergic responses. This underscores the importance of defining the events in this early period of life to determine better strategies for allergy prevention. In this study, we aimed to evaluate the immunomodulatory potential of preconception immunization with OVA in the neonatal development of allergic responses under effect of antigen exposure at the prenatal or postnatal stages.
We demonstrated that antigen contact through mucosal sites in previously immunized mothers acted as an antigenic boost, leading to high IgG transference via the transamniotic/transplacental routes or through breast milk. The full-term pregnant immune mothers produced antibodies of IgG1 isotype, the main subclass transplacentally transferred to the fetus. For the IgG1 antibody, we observed diaplacental transmission of IgG1 antibodies with anaphylactic function in OVA immunized mice, which was further enhanced by antigen exposure in the pregnancy. Anaphylactic IgG1 antibody activity was undetectable within 2 months of offspring delivery, showing faster catabolism than non-anaphylactic IgG1 antibody, which still remained at high levels. In mice, both IgG1 and IgE antibodies can elicit anaphylactic reactions.13 IgG1 anaphylactic antibody is positively regulated by IL-415 and may enhance lung eosinophilic inflammation and induce airway hyperreactivity.16 It is not known whether anaphylactic IgG1 antibody transference contributes to the anaphylactic reaction during early life; however, other maternal IgG1 antibodies should be protective to prevent an allergy response. In fact, we showed that antenatal immunization with OVA exerted an inhibitory effect on the development of the type I hypersensitivity response after neonatal immunization. Moreover, we noticed the loss of inhibitory effect on the IgE response by maternal immunization with the dust mite Dermatophagoides pteronyssinus, when maternal IgG1 antibody levels dropped in the circulation of offspring.10
It is well recognized that postnatal environmental or maternal allergen exposure is central to the development of allergic responses,4 emphasizing the strategic studies to prevent allergen priming in early infancy. We observed for the first time that maternal vaccination could prevent postnatal allergen sensitization by significantly reducing the amounts of antigen in breast milk, indicating that this may be an important pathway for neonatal sensitization. Considering the difficulty of allergen diet exclusion during the lactation period, or even during pregnancy, maternal prophylactic immunization may be an efficient way to avoid allergy. The antigen/peptide clearance in breast milk might be a consequence of phagocytosis by activated cells from the GALT compartment, rather than maternal antibodies forming complexes with antigens, such as the immune complex, which was transferred mainly via the diaplacental route by immune mothers exposed during pregnancy.
Antigen loading through the immune complex form by transplacental transmission, fetal gut, or by swallowing in the amniotic compartment should be considered a possible route for neonatal priming. In humans, it has been suggested that peptides processed by the mother or inhalant allergen might access the fetal circulation, either as free antigens or in complexes with IgG.17–19 Detection of the major cat allergen with IgG, Fel d 1 IgG in cord blood correlated with a down-modulation of IFN-γ, probably representing a normal mechanism for inducing a primary immune response.20 Although maternal immunization generating an IgG–OVA complex can already provide the fetus with the initial trigger for the priming of the T-cell system in utero, we further noticed an intense immunomodulatory effect in the non-antigen-specific T-cell and B-cell responsiveness. It was observed that antenatal immunization was able to lead to an 87% reduction in the T-cell response of non-immunized offspring as well as to a 90% suppression of B-cell hyperresponsiveness followed by maternal antigen exposure. Otherwise, maternal immunization equilibrates T-cell activation and cell cycle, inducing a scarce Th1 and Th2 cytokine production, as a possible measure to prevent T-cell hyperresponsiveness after postnatal antigen exposure.
Prevention of IgE development by maternal immunization is a well-known effect8–11. However, how the maternal inhibitory antibodies prevent the offspring allergen sensitization in early life is yet to be determined; the efficacy of maternal vaccination while undergoing intense allergen exposure during pregnancy or breastfeeding remains to be evaluated. Here, we have demonstrated that maternal allergen intake during pregnancy may induce a tolerance state in the offspring. Conversely, maternal allergen intake postnatally triggered an enhancement in the offspring IgE antibody production. These results revealed that early antigen exposure in utero may induce fetal tolerance, whereas antigen contact soon after birth facilitates neonatal allergen sensitization. The finding that maternal immunization generates antibodies able to markedly reduce antigen delivery to the neonates, subsequent to antigen exposure during the postnatal period, may explain the efficacy of maternal vaccination to prevent IgE antibody exacerbation. One of the essential roles mediated by the maternal antibody is to form immune complexes that provide in vivo allergen neutralization to avoid offspring sensitization, which we have recorded as passive transference in anti-OVA antibody experiments. A complete suppression of early IgE production in the dependence on maternally derived, antigen-specific IgG1 antibodies directed against the same antigen has been shown to play a prominent role.21 However, despite a strong maternally mediated immunosuppression mediated by the antibodies, they have also been efficient at maintaining the balance in the effector functions of T and B cells. This was supported by the finding that maternal immunization, before antigen exposure during pregnancy, maintained the tolerance state in immunized offspring. The regulatory role of maternal immunization was also noted through the improvement of the strength of the T-cell responsiveness in immunized offspring, which were antigen-exposed postnatally. The re-equilibration of the T-cell response through T-cell receptor stimulation, already seen in non-immunized neonates, was enhanced with immunization in parallel with the reduction in cytokine production. These results showed that activation precedes the induction of T-cell anergy, probably induced by the maternal antibodies. Indeed, the inadequate costimulatory signal delivered by antigen-presenting cells from neonates and impaired cytokine production may contribute to avoiding full activation of T cells. A complex way of action, either by the inhibitory effect of immune complexes or by the inhibitory IgG receptor FcγRIIb, or by the modulation of the idiotype network, seems to be mediated by preconception immunization.
It is worth considering that the mechanism mediated for the maternal immunization through antigen-specific antibodies resembles the one applied in immunoglobulin replacement therapy. Human immunoglobulin prepared for intravenous administration (IVIG) has been increasingly used for the treatment of autoimmune and systemic inflammatory diseases and in the supportive therapy of immunodeficient patients.22 The mode of action of IVIG is complex, involving modulation of activation and effector functions of T and B cells and of antigen-presenting cells.23 Furthermore, IVIG suppresses proliferation and IgE production by human B cells stimulated with IL-4 and anti-CD40 antibodies, because of the inhibition of early events related to proliferation and progression in the cell cycle,24 which is not likely to be mediated through the inhibitory IgG receptor FcγRIIb.25 The complex mechanism mediated by the antigen-specific maternal antibodies altering the progeny immune repertoire needs further investigation.
Together, these findings showed that maternal immunization could avoid early antigen priming in the prenatal and postnatal period. The noticeable inhibitory effect of the mother's immunization should be considered as a potential prevention strategy in early life, which is susceptible to the Th2 response and allergen sensitization because of environmental or maternal allergen exposure. These findings establish the fundamental importance of maternal antibodies in regulation and functional cellular homeostasis, to prevent allergic response.
Acknowledgments
We thank Rachel Guedes Silva for the animal care and dedicated technical support. This research was supported by FAPESP n°02/11934–0 and LIM 56-HC/FMUSP.
Abbreviations
- ELISA
enzyme-linked immunosorbent assay
- IFN
interferon
- IgE
immunoglobulin E
- IgG
immunoglobulin G
- IL
interleukin
- i.p.
intraperitoneal
- IVIG
intravenous immunoglobulin
- LPS
lipopolysaccharide
- mAb
monoclonal antibody
- OD
optical density
- OVA
ovalbumin
- PBS
phosphate-buffered saline
- PCA
passive cutaneous anaphylaxis
- Th2
T helper type 2
- TMB
3,3′,5,5′-tetramethyl-benzidine
References
- 1.Prescott SL. Maternal allergen exposure as a risk factor for childhood asthma. Curr Allergy Asthma Report. 2006;6:75–80. doi: 10.1007/s11882-006-0014-7. [DOI] [PubMed] [Google Scholar]
- 2.Holt PG, McMenemin C, Nelson D. Primary sensitization to inhalant allergens during infancy. Pediatr Allergy Immunol. 1990;1:3–13. [Google Scholar]
- 3.Holt PG. Primary allergic sensitization to environmental antigens: perinatal T cell priming as a determinant of responder phenotype in adulthood. J Exp Med. 1996;183:1297–301. doi: 10.1084/jem.183.4.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saarinen KM, Juntunen-Backman K, Jarvenpaa AL, et al. Breast-feeding and the development of cows' milk protein allergy. Adv Exp Med Biol. 2000;478:121–30. doi: 10.1007/0-306-46830-1_10. [DOI] [PubMed] [Google Scholar]
- 5.Zeiger RS. Prevention of food allergy and atopic diseases. J R Soc Med. 1997;90(Suppl. 30):21–33. doi: 10.1177/0141076897090030s05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenmalm MC, Bjorksten B. Cord blood levels of immunoglobulin G subclass antibodies to food and inhalant allergens in relation to maternal atopy and the development of atopic disease during the first 8 years of life. Clin Exp Allergy. 2000;30:34–40. doi: 10.1046/j.1365-2222.2000.00771.x. [DOI] [PubMed] [Google Scholar]
- 7.Glovsky MM, Ghekiere L, Rejzek E. Effect of maternal immunotherapy on immediate skin test reactivity, specific rye I IgG and IgE antibody, and total IgE of the children. Ann Allergy. 1991;67:21–4. [PubMed] [Google Scholar]
- 8.Jarrett EEE, Hall E. IgE suppression by maternal IgG. Immunology. 1983;48:49–58. [PMC free article] [PubMed] [Google Scholar]
- 9.Fusaro AE, Maciel M, Victor JR, Oliveira CR, Duarte AJS, Sato MN. Influence of maternal murine immunization with Dermatophagoides pteronyssinus extract on the type I hypersensitivity response in offspring. Int Arch Allergy Immunol. 2002;127:208–16. doi: 10.1159/000053865. [DOI] [PubMed] [Google Scholar]
- 10.Victor JR, Fusaro AE, Duarte AJS, Sato MN. Preconceptional maternal immunization to dust mite inhibits the type I hypersensitivity response of offspring. J Allergy Clin Immunol. 2003;111:269–77. doi: 10.1067/mai.2003.39. [DOI] [PubMed] [Google Scholar]
- 11.Melkild I, Groeng EC, Leikvold RB, Granum B, Lovik M. Maternal allergen immunization during pregnancy in a mouse model reduces adult allergy-related antibody responses in the offspring. Clin Exp Allergy. 2002;32:1370–6. doi: 10.1046/j.1365-2745.2002.01458.x. [DOI] [PubMed] [Google Scholar]
- 12.Ovary Z. Passive cutaneous anaphylaxis in the mouse. J Immunol. 1958;81:325–8. [PubMed] [Google Scholar]
- 13.Mota I, Wong D. Homologous and heterologous passive cutaneous anaphylactic activity of mouse antisera during the course of immunization. Life Sci. 1969;8:813–20. doi: 10.1016/0024-3205(69)90099-x. [DOI] [PubMed] [Google Scholar]
- 14.Futata EA, Brito CA, Victor JR, Fusaro AE, Oliveira CR, Maciel M, Jr, da Silva Duarte AJ, Sato MN. Long-term anergy in orally tolerized mice is linked to decreased B7.2 expression on B cells. Immunobiology. 2006;211:157–66. doi: 10.1016/j.imbio.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Faquim-Mauro EL, Coffman RL, Abrahamsohn IA, Macedo MS. Cutting edge. Mouse IgG1 antibodies comprise two functionally distinct types that are differentially regulated by IL-4 and IL-12. J Immunol. 1999;163:3572–6. [PubMed] [Google Scholar]
- 16.Macedo-Soares MF, Itami DM, Lima C, Perini A, Faquim-Mauro EL, Martins MA, Macedo MS. Lung eosinophilic inflammation and airway hyperreactivity are enhanced by murine anaphylactic, but not nonanaphylactic, IgG1 antibodies. J Allergy Clin Immunol. 2004;114:97–104. doi: 10.1016/j.jaci.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 17.Brown MA, Halonen MJ, Martinez FD. Cutting the cord. Is birth already too late for primary prevention of allergy? Clin Exp Allergy. 1997;27:28–35. [PubMed] [Google Scholar]
- 18.Szépfalusi Z, Pichler J, Elsâsser S, van Duren K, Ebner C, Bernaschek G, Urbanek R. Transplacental priming of the human immune system with environmental allergens can occur early in gestation. J Allergy Clin Immunol. 2000;106:530–6. doi: 10.1067/mai.2000.108710. [DOI] [PubMed] [Google Scholar]
- 19.Casas R, Bjorksten B. Detection of Fel d 1-immunoglobulin G immune complexes in cord blood and sera from allergic and non-allergic mothers. Pediatr Allergy Immunol. 2001;12:59–64. doi: 10.1034/j.1399-3038.2001.012002059.x. [DOI] [PubMed] [Google Scholar]
- 20.Casas R, Jenmalmm C, Bjorksten B. Cat allergen-induced cytokine secretion and Fel d 1-immunoglobulin G immune complexes in cord blood. Clin Exp Allergy. 2004;34:591–6. doi: 10.1111/j.1365-2222.2004.1924.x. [DOI] [PubMed] [Google Scholar]
- 21.Uthoff H, Spencer A, Reckelkamm W, et al. Critical role of preconceptional immunization for protective and non-pathological specifc immunity in murine neonates. J Immunol. 2003;171:3485–92. doi: 10.4049/jimmunol.171.7.3485. [DOI] [PubMed] [Google Scholar]
- 22.Orange JS, Hossny EM, Weiler CR, et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2006;117:525–53. doi: 10.1016/j.jaci.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Bavry J, Lacroix-Desmazes S, Carbonneil C, et al. Inhibition of maturation and function of dendritic cells by intravenous immunoglobulin. Blood. 2003;101:758–65. doi: 10.1182/blood-2002-05-1447. [DOI] [PubMed] [Google Scholar]
- 24.Sigman K, Ghibu F, Sommerville W, Toledano BJ, Bastein Y, Cameron L, Hamid QA, Mazer B. Intravenous immunoglobulin inhibits IgE production in human B lymphocytes. J Allergy Clin Immunol. 1998;102:421–7. doi: 10.1016/s0091-6749(98)70130-7. [DOI] [PubMed] [Google Scholar]
- 25.Zhuang Q, Bisotto S, Fixman ED, Mazer B. Suppression of IL-4 and CD40-induced B-lymphocyte activation by intravenous immunoglobulin is not mediated through the inhibitory IgG receptor FcgammaRIIb. J Allergy Clin Immunol. 2002;110:480–3. doi: 10.1067/mai.2002.127284. [DOI] [PubMed] [Google Scholar]