Abstract
The aim of this study is to evaluate the humoral autoimmune response in the experimental model of systemic sclerosis (SSc) induced by human type V collagen (huCol V). New Zealand rabbits were immunized with huCol V in Freund's complete adjuvant (FCA) and boosted twice with 15 days intervals with huCol V in Freund's incomplete adjuvant. Control groups included animals injected only with FCA or bovine serum albumin. Bleeding was done at days 0, 30, 75 and 120. Tissue specimens were obtained for histopathological investigation. Serological analysis included detection of antibodies against huCol V and anti-topoisomerase I (Anti-Scl70) by enzyme-linked immunosorbent assay, antinuclear antibodies (ANA) by indirect immunofluorescence, and rheumatoid factor (RF) by a latex agglutination test. Target antigens were characterized by immunoblot. Histological analysis revealed extracellular matrix remodeling with fibrosis and vasculitis. Anti-Scl70 and ANA were detected as early as 30 days in all huCol V animals. The universal ANA staining pattern was Golgi-like. This serum reactivity was not abolished by previous absorption with huCol V. Characterization of the target antigen by immunoblot revealed two major protein fractions of 175 000 and 220 000 MW. Similarly to ANA, there was a gradual increase of reactivity throughout the immunization and also it was not abolished by preincubation of serum samples with huCol V. RF testing was negative in hyperimmune sera. Conclusion: The production of autoantibodies, including anti-Scl70, a serological marker for SSc associated with histopathological alterations, validates huCol V induced-experimental model and brings out its potential for understanding the pathophysiology of SSc.
Keywords: scleroderma, systemic sclerosis, experimental model, Type V collagen, autoantibodies
Introduction
Experimental models for diseases affecting human beings are of utmost importance, because they allow studies on their pathogenesis and therapeutic approaches. In this group we can include this new animal model for an autoimmune disease, induced in rabbits by their immunization with human type Collagen V (huCol V), recently described by Teodoro et al.1 in which the histological alterations are very similar to those of human scleroderma.1–5
Scleroderma is a disease with an unknown etiology, characterized by vascular lesions and fibrosis in the skin and other organs because of the accumulation of proteins of the extracellular matrix.6–8 Immunological abnormalities in SSc include activation of B and T lymphocytes, imbalance in cytokine profile and the production of a myriad of circulating autoantibodies.8 Ninety to 95% of scleroderma patients present some autoantibody against intracellular antigens or extracellular matrix components like collagen.9
Antinuclear antibodies (ANA) found in scleroderma patients are varied, always involving a nuclear reactivity, with positive nucleoli in up to 41% of the cases.10 Some autoantibodies are specific to the scleroderma spectrum of diseases (SSD), such as the anti-RNA polymerase I, II and III, antitopoisomerase I (anti-Scl70) nucleoli antibodies and anticentriole antibodies, that appear in the diffuse form of the disease, and anticentromeric antibodies appearing in the chondrocalcinosis syndrome, Raynaud phenomenon, oesophageal dysmotility, sclerodactyly and telangiectasia (CREST).10–12 Unlike in systemic lupus erythematosus patients, scleroderma patients rarely present more than one autoantibody detectable in serum. This has been well documented regarding antibodies against Scl70 and centromere antigens, where the presence of one autoantibody appears to exclude the presence of others.9 On the other hand, the scleroderma cytoplasmic pattern is uncommon, appearing in just 3% of 735 patients classified according to the ANA pattern in HEp-2 cells.12
The aim of the present study is to extend knowledge on the experimental model of scleroderma by characterizing the autoantibody profile of the rabbits immunized with huCol V.
Materials and methods
New Zealand rabbits
All 26 animals used in the experiment were female adults with similar weights.
Immunization protocol
Ten animals received two subcutaneous injections (on days 0 and 30) of 1 mg of human type V collagen (huCol V) (Sigma Chemical Co, St Louis, MO) in Freund's complete adjuvant (FCA), and then two intramuscular boosters of 1 mg in Freund's incomplete adjuvant (FIA) 5 days apart. As control groups, animals were injected with FCA (n = 10) or bovine serum albumin (BSA) (n = 6), following the same immunization protocol. Blood samples were taken from all animals at day 0 and 30, 75, 120 days after primary immunization. Half the animals of each group (n = 13) were killed at 75 and 120 days to obtain tissue specimens of skin, kidney and lung for histopathological analysis.
Immunological assessments
A total of 91 sera were stored in aliquots at −70° until analysis. They were searched for detection of anti-huCol V and anti-Scl70 antibodies by enzyme-linked immunosorbent assay (ELISA); Antinuclear antibodies (ANA) were tested by indirect immunofluorescence (IIF) in human epithelial-2 (HEp-2) cells, and rheumatoid factor (RF) by latex agglutination. The target antigens were characterized by immunoblot (IB).
Detection of antibody to type V human collagen by ELISA
Briefly, wells of polystyrene microplates (Costar, San Diego, CA) were sensitized overnight with 50 µl of purified human collagen V (Sigma) (5 µg/ml) and then blocked with 100 µl of BSA 1% (Sigma) for 2 hr at room temperature. Serum samples 1 : 100 diluted were added to the wells and tested in duplicate. Plates were further incubated with alkaline phosphatase-conjugated goat anti-rabbit IgG (Sigma) and the reaction was developed with p-nitrophenyl phosphate (p-NPP; Sigma). The optical density (OD) was read at 405 ηm (ELISA Multiskan MS, Labsystems, Helsinki, Finland). To ensure consistency between assays, a hyperimmune rabbit serum for huCol V was systematically included in each assay and the reaction was stopped when its OD reached the value of 1·0. Positive results were defined as OD = 3 SD above the mean OD of 26 control serum samples obtained from rabbits before immunization (at day zero) and included in each assay.
Rheumatoid factor
Rabbit serum samples, at a 1 : 20 dilution, were tested for the presence of rheumatoid factor (RF) by a well-established latex agglutination test.13 RF titre was determined by testing serial dilutions of the positive samples. The results were expressed in International Units (IU), according to the manufacturer's guidelines (Laborclin, Parana, Brazil), which standardizes the initial dilution as 25 IU/ml.
Antinuclear antibodies
All sera were screened for the presence of ANA by indirect immunofluorescence on acetone-fixed preparations of HEp-2 onto microscope slides. Slides were incubated with 1 : 80 diluted serum samples followed by fluorescein isothiocyanate (FITC)-conjugated goat immunoglobulin G (IgG) fraction anti-rabbit IgG (Sigma). Cellular staining was examined under a fluorescence microscope (Olympus BX 550) and immunostaining pattern were categorized according to the II National Consensus on the Antinuclear Antibodies using HEp-2 as substrate.14
The specificity of ANA results was confirmed by preincubating three randomly selected high-titre anti-Golgi-like immunostaining-positive rabbit sera with huCol V (75 days after immunization) before the binding assessment. The sera were submitted to three successive overnight incubations at 4° with huCol V, immobilized in 12-well polystyrene plates (Costar) at a 50 µg/ml concentration. Similarly, a randomly selected FCA-injected rabbit serum was submitted to the same protocol. The absorption efficiency was checked by comparing anti-huCol V binding of preabsorbed serum samples with the original untreated paired samples in the same ELISA assay. The adsorbed sera were then re-tested for ANA on IIF and effective interference of absorption procedure was established if a twofold titre reduction in ANA titre was observed.
Immunoblot analysis
Total saline extracts and cytoplasmic enriched fraction of HEp-2 cells were prepared as described elsewhere.15 Samples of the cellular extracts were submitted to polyacrylamide gel electrophoresis (PAGE) on 7·5% gel under denaturing and reducing conditions.16 Fractionated proteins were further electrotransferred to nitrocellulose membrane, which was further cut in individual strips and probed with serum samples at a 1 : 100 dilution. Reactivity was revealed by incubating the strips with alkaline phosphatase-conjugated goat anti-rabbit IgG and then with developing salts. Human serum samples from patients with rheumatological diseases and presenting autoantibodies anti-Scl70 (100 000 MW native protein and 70 000 MW degradation product), anti-Golgi complex (97 000–376 000 MW) and anti-RNA polymerase I (12 500–210 000 MW) were systematically included in each assay as reference. Similar to the ANA findings, the specificity of immunoblot results was checked by analysing the reactivity pattern of three serum samples preabsorbed with huCol V as described above.
Purification of anti-175 000 MW antibodies
In order to delineate intracellular localization of antigens targeted by rabbit antibodies on immunoblot, an acid elution (100 mm glycine, pH 2·8) of antibodies bound to electrofractionated HEp-2 proteins was performed.17 We have arbitrarily selected antibodies bound to a 175 000 MW target antigen of the HEp-2 cells because was the common reactivity pattern of all serum samples obtained after immunization on immunoblot. Purified eluates were neutralized (1 m Tris buffer, pH 8·0) and tested on HEp-2 cells by immunofluorescence.
Detection of Anti-Scl70 by ELISA
Serum samples at 1 : 100 dilution were added to the wells of commercial available plates containing immobilized Scl70 antigen (ELISA Hemagen Diagnostics Inc., Columbia, NY) and tested in duplicate. Plates were further incubated with the immunoconjugate (alkaline phosphatase-conjugated goat anti-rabbit IgG (Sigma), and the reaction was developed with p-nitrophenyl phosphate (p-NPP) (Sigma). The optical density (OD) was read at 405 ηm (ELISA Multiskan MS, Labsystems). A human serum positive for anti-Scl70 were systematically included in each assay as control, and the reaction was stopped when its OD reached a 1·0-value. Positive results were defined as OD = 5 SD above the mean OD of 26 control serum samples obtained from rabbits before immunization (at day zero) and included in each assay.
Results
Humoral response testing of huCol V-immunized rabbits and of both control groups was always carried out along with the sera sampled at day 0 from each animal, because of reports on the occurrence of autoantibodies in healthy rabbits.18,19 The histological alterations compatible with scleroderma were restricted to the huCol V group (Fig. 1), and were in agreement with previously published histological results.1–5 The FCA (n = 10) and BSA (n = 6) control animals did not develop anticollagen, RF, ANA or anti-Scl70 antibodies.
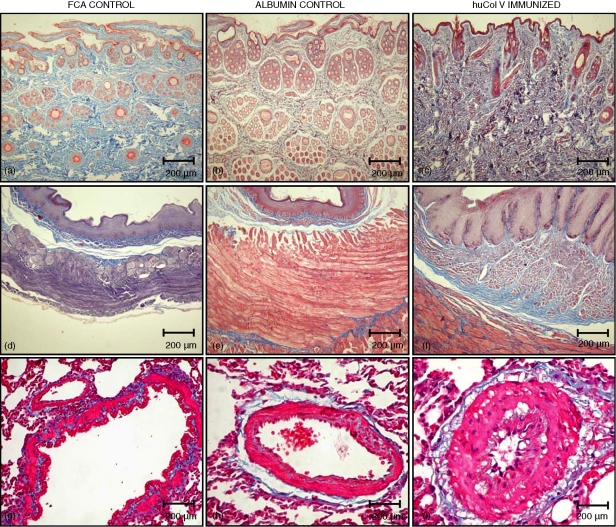
Figure 1.
Skin, oesophagus and lung vessel sections of controls (a, d, g), albumin control (b, e, h) and immunized rabbits (c, f, i) at 120 days after immunization with Masson's trichromic that stained collagen blue (original magnification ×100).
Anti-huCol V antibody
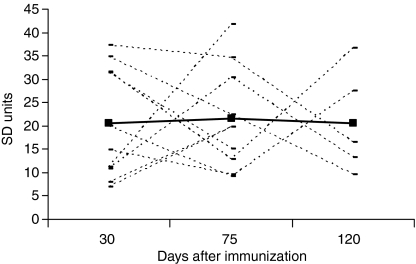
Reactivity to huCol V was found in 100% of the immunized animals from day 30, as shown in Fig. 2. The mean levels of anti-huCol V antibody throughout the immunization were above 20 times the standard deviation (SD) of basal levels (mean absorbance =0·034; SD = 0·016), showing a satisfactory immune response. The intensity of this reactivity decreased in 6/10 (60%) animals on day 75, with a recovery of antibody level in two out of the five animals that were killed on day 120.
Figure 2.
Levels of anti-huCol V antibody in rabbit sera on days 30, 75 and 120 after immunization (n = 10). Each line indicates antibody levels in individual rabbit sera. The continuous line indicates the mean level.
Rheumatoid factor
RF testing was negative in all huCol V hyperimmune sera from 30 to 120 days after immunization. Only one animal had a low titre of RF (50 IU/ml) at day 75. Animals from control groups were systematically negative for RF.
Antinuclear antibodies
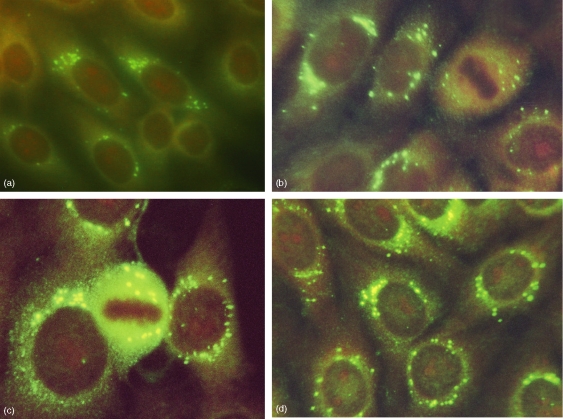
ANA revealed a cytoplasmic pattern with isolated, intensely coloured spots around the nuclear membrane, often in a polarized fashion, reminding the Golgi complex. These spots remained in the cytoplasm of the dividing cells with a negative metaphase, shown in Fig. 3. ANA reactivity was detected because 30 days after immunization, and antibody titre >1/320 was found in 100% of the animals after day 75.
Figure 3.
Representative immunostaining reactivity pattern of an anti-huCol V hyperimmune rabbit serum (day 75) on HEp-2 cells as detected by IIF showing a speckled Golgi-like cytoplasmic staining with negative metaphase plate on dividing cells (400×).
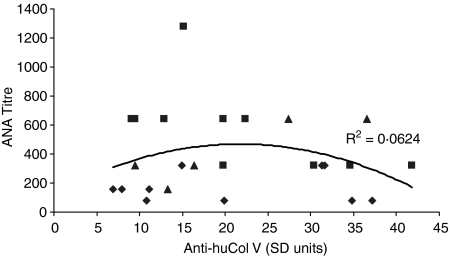
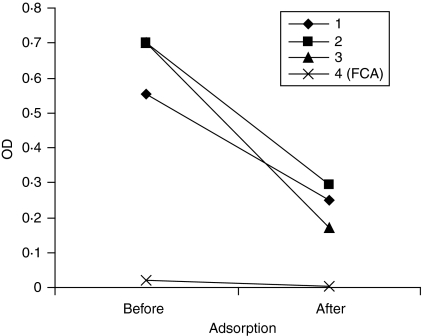
There was no significant correlation between titres of anti-Golgi-like antibody on IIF and antihuCol V as depicted in Fig. 4 (R2 = 0·06). Moreover, preadsorption of three huCol V hyperimmune sera with purified huCol V although effective in causing a marked reduction with the cognate antigen on ELISA (Fig. 5) did not interfere with ANA reactivity on IIF and immunoblot.
Figure 4.
Variation of titres of anti-Golgi-like antibody as a function of anti-huCol V serum levels. Each point represents one serum: (♦) = 30 days; (▪) = 75 days; (▴) = 120 days. (R2 = 0·0624).
Figure 5.
Efficiency of the preabsorption of 3 hyperimmune rabbit sera (75 days after primary immunization) with huCol V on antibody binding to cognate antigen as evaluated by ELISA. FCA control serum from Freund's complete adjuvant injected rabbit (75 days). OD: optical density.
Moreover, the acid eluates containing the specific antibodies against the 175 000 MW antigenic fraction, when tested on HEp-2 cell by IFI, reproduced the anti-Golgi-like staining pattern of the original paired sera.
Immunoblot results
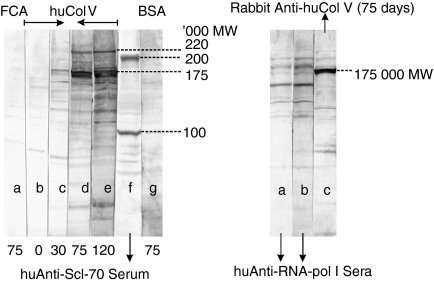
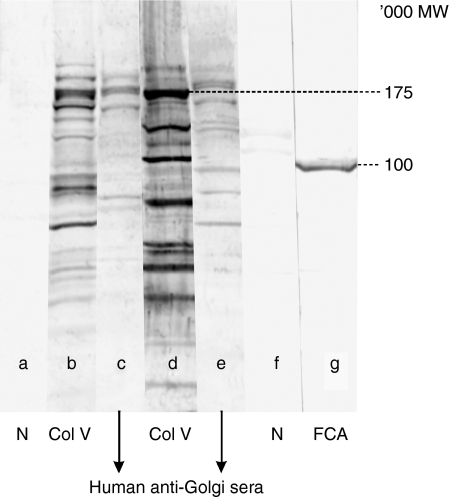
In the immunoblot tests, performed on HEp-2 extracts, it was quite evident a common reactivity to all anti-huCol V hyperimmune rabbit sera obtained from day 30 after immunization and on. Such reactivity was specific for high molecular weight protein fraction groups, being the 175 000 and 220 000 MW the most evident ones. Reactivity to these fractions gradually increased with time of immunization (Fig. 6a). In these tests, sera with specific human anti-Scl70 (100 000 and 200 000 MW), anti-RNA polymerase I (12 500–210 000 MW), and anti-Golgi (97 000–376 000 MW) antibodies were included (Fig. 6a, b). Figure 7 shows that the reactivity of the huCol V-immunized rabbits' sera was similar to that of the high molecular weight protein fractions found in two different human anti-Golgi sera.
Figure 6.
(a) Representative reactivity pattern of an anti-huCol V hyperimmune rabbit in HEp-2 cells, by immunoblot. Lane a: serum from a rabbit injected with Freund's complete adjuvant (FCA), at day 75; lanes b, c, d, e: sera from a hyperimmune rabbit obtained on days 0, 30, 75, and 120 after immunization; lane f: anti-Scl70 human serum; lane g: serum from a BSA-immunized rabbit (75 days). (b) Lanes a and b: Anti-RNA polymerase I human sera; lane c: anti-huCol V rabbit serum (75 days after immunization).
Figure 7.
Reactivity pattern of an anti-huCol V hyperimmune rabbit serum against cytoplasmic-enriched fraction of HEp-2 cells by immunoblot. Lanes a and f: normal rabbits; lanes b and d: huCol V rabbits' sera, obtained on day 75 after primary immunization; lanes c and e: two representative anti-Golgi human sera; lane g: control serum from rabbit injected with FCA (75 days).
Anti-Scl70
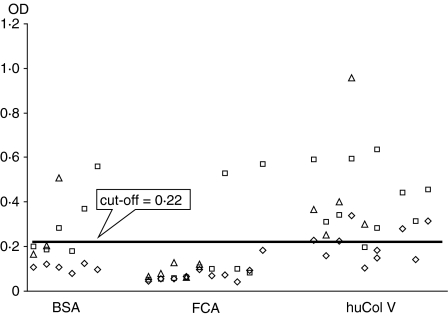
The ELISA tests for anti-Scl70, shown in Fig. 8, with the cut-off line at OD values 0,22 (mean absorbance = 0·094 and SD = 0·025), reveal a strong association between the huCol V hyperimmune rabbits' sera and the human antigen bound to the Hemagen plates, when compared to the BSA (χ2 after Yates' correction: 7·43; P < 0·01; N = 19/6/4/11) and FCA (χ2 after Yates' correction: 21·0; P < 0·01; N = 19/6/2/23) control groups. This reactivity appeared already in the first tests at day 30 after immunization, when compared to the FCA control (χ2 after Yates' correction: 4·27; P < 0·03; N = 5/5/0/10). When compared to the BSA group, the initial tests showed the same trend of positive association, but because of the reduced N it was not possible to obtain totally conclusive statistical data (χ2 after Yates' correction: 2·35; uncorrected χ2: 4·36; Fisher's exact P = 0·05; N = 5/5/0/6).
Figure 8.
Distribution of the reactivity of rabbit sera at days 30, 75 and 120 after immunization, by ELISA, for human Scl70. BSA = 6 bovine albumin control rabbits; FCA = 10 Freund's complete adjuvant control rabbits; huCol V = 10 rabbits immunized with huCol V. Each point represents one serum: (◊) = 30 days; (□) = 75 days; (▵) = 120 days. The cut-off line indicates optical density (OD) values = 3 SD above the mean OD of 26 control serum samples obtained from rabbits before immunization (at day zero).
Discussion
The animals immunized with huCol V and killed at days 75 and 120 from immunization reproduced the previously described histological alterations1–5 confirming the involvement of multiple organs: namely the skin, lungs, oesophagus, heart, synovia and kidneys, as in scleroderma. Animals injected with FCA or immunized with BSA did not show any histological complications. The microscopical analysis was not the main scope of this study, yet we needed to demonstrate that the experimental model can be reproduced. The study of humoral immunity, through the testing for autoantibodies in the hyperimmune sera, was important in order to give this original experimental model its credibility.
The presence of RF in this scleroderma experimental model was not significant. It was found in low titres, with just 5% of positive animals. In human scleroderma, this autoantibody has an incidence of 10 to 30%, regardless of the stage of illness and the graveness of the condition.20,21
The ANA testing in HEp-2 cells was characterized by a uniform, persistent response, restricted to the human Col V-immunized rabbits, with a Golgi-like cytoplasmic reactivity pattern that showed a reactivity dynamics different to that of the induced anti-huCol V, suggesting an autoimmune reaction against cytoplasmic antigens, apparently located at the Golgi complex. The anti-Golgi antibody (AGA) was first described in 1982 in a patient suffering from Sjögren syndrome (SS) and lymphoma.22 In the literature, it is regarded as a rare antibody, found after viral infections and other chronic rheumatic diseases, yet uncommon in lupus.22,23 Seelig et al.24 reported AGA in two patients, one with SS and the other with scleroderma. This autoantibody was initially called macrogolgin and then giantin. It is a resident protein of the Golgi complex, with the characteristics of a secretory protein, and it is incorporated into the endoplasmic reticulum membrane and transported to vesicles of the Golgi complex.25–27 Nozawa et al.28 analysed 80 AGA-containing human sera, defined by indirect immunofluorescence in HEp-2 cells and ELISA using recombinant autoantibodies and immunoprecipitation, in which 50% of the sera reacted with giantin. In the description of this reactivity pattern, the possible correlation of the found antibodies with the patients' pathology or clinical manifestations was not addressed.28 Other cytoplasmic antibodies were also described in scleroderma patients. Senécal et al.29 discovered a cytoplasmic antibody in two patients recently diagnosed with scleroderma, which reacted against microfibre proteins at anchorage sites (anti-MFAS) of the cell membrane, other than actin and vinculin, which are the two major proteins of the cytoskeleton. Tarsounas et al.30 demonstrated that sera from patients with the CREST syndrome contained an autoantibody that reacted with protein components of the Golgi complex and nuclear proteins of spermatocytes in mice. The autoantigens had a bovine and human homology, and were coded in the mouse chromosome 9.
The huCol V preadsorption study with the hyperimmune sera, performed in order to check for a cross-reaction between cytoplasmic organelles of the HEp-2 cell and this collagen, showed that the Golgi-like autoantibody found in the rabbits reacts independently from the anti-huCol V antibodies produced after the immunization. It is important to remember that the HEp-2 is of epithelial origin and does not produce collagens.
The immunoblotting tests identified two protein fractions of high molecular weight (220 000 and 175 000 MW), which intensity increased with immunization time and were close to those recognized by human AGA sera. The IFI reproduction of the Golgi-like pattern by the anti-175 000 MW eluate in HEp-2 cells also strengthens the hypothesis of an anti-Golgi autoantibody in this scleroderma animal model. The absence of an anti-Scl70 antibody in the immunoblotting assays and its presence in 100% of the huCol V animals (detected by ELISA in plates with purified human antigens) will be further studied, because we know that epitopes that generate autoimmunity may be conformational. In other words, these epitopes will be present only in the native molecules, which makes protein identification by immunoblotting harder once it is performed on denatured antigens.31
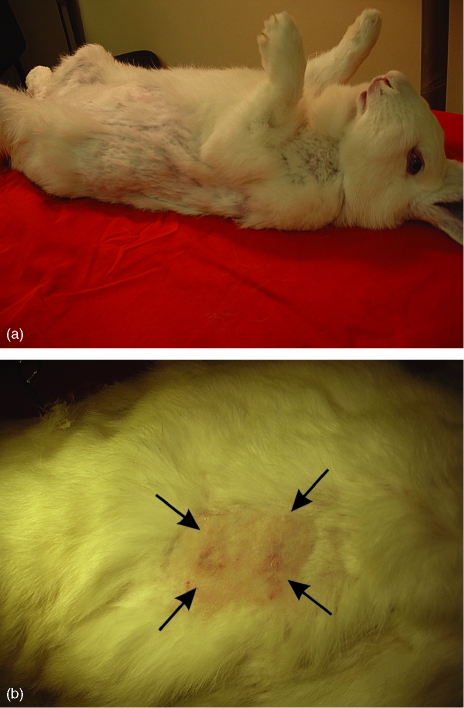
New experiments are being developed, aimed to consolidate this systemic sclerosis animal model. We should soon be able to answer some of the many questions raised by this line of research: What is the IgG subclass responsible for the ANA? What is its capacity to fix complement? Is the IgM anti-Golgi-like response significant? Does it fit into the behaviour of most IgM autoantibodies described in individuals with autoimmune diseases or healthy ones?32 Does the IgG response remain with the development of the disease in these animals? Will other antibodies appear? Is the huCol V antigenic stimulus excessive? Is it consuming the anti-huCol V antibodies, thus explaining the reduction of their plasmatic concentration by about 50% in the studied animals? Is the individual biological variation of each rabbit, regarding the formation of granulomas at the immunization sites, significant? Does it cause inflammatory reactions at different times that may explain the reduction or recovery of the anti-huCol V levels? Is the serum level of circulating immunocomplexes significant? Finally, the histological and immunological chemistry, electronic microscopy and anti-Golgi complex recombinant antibodies tests will point to the exact location of the proteins responsible for this autoantibody. However, their physiopathological role may remain just an epiphenomenon, like that of most autoantibodies found in human autoimmune diseases.32 Studies aimed to classify the population of mononuclear cells found in injured tissues will provide important information for the physiopathological understanding of this new systemic sclerosis animal model. In new tests with animals immunized at day 210 (Fig. 9), clinical alopecia was already diagnosed. At this initial stage, our data suggest that it is possible to reproduce the humoral immune response, with a production of antibodies similar to that seen in scleroderma, in normal rabbits immunized with huCol V. This animal model, proposed for the study of systemic scleroderma, is negative for the rheumatoid factor, positive for anti-Scl70, reactive for ANA with specificity for the Golgi complex, with its strongest expression against a 175 000 MW protein.
Figure 9.
(a) A huCol V immune animal at day 210, with abnormalities within its fur distribution. (b) Closer view of animal skin, showing a clinical alopecia region.
Acknowledgments
We tank José Rubens Costa Lima, MSc in Epidemiology of Fortaleza Health Secretary, for his assistance with the statistical data analysis; http://www.nftranslation.com for its translation services and Francisca Valtemar for her technical support. This study was supported by Fundação de Amparo à Pesquisa do Estado de Sao Paulo; grant 2003/02543-0.
References
- 1.Teodoro WR, Velosa APP, Witzel SS, Garipo AL, Farhat C, Parra ER, Sonohara S, Capelozzi VL, Yoshinari NH. Architectural remodeling in lungs of rabbits induced by type V collagen immunization: a preliminary morphologic model to study diffuse connective tissue diseases. Pathol Res Pract. 2004;200:681–91. doi: 10.1016/j.prp.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Teodoro WR, Miron BG, Ogido LTI, Velosa APP, Abatepaulo F, Capelozzi VL, Yoshinari NH. Synovial remodelling precess induced by type V collagen immunization in rabbits. Pathol Res Pract. 2003;199:605–12. doi: 10.1078/0344-0338-00468. [DOI] [PubMed] [Google Scholar]
- 3.Yoshinari NH, Teodoro WR, Ogido LTI, et al. Modelo experimental de doença difusa do tecido conjuntivo (DDTC) induzido por colágeno tipo V. Rev Bras Reumatol. 2002;42:295–305. [Google Scholar]
- 4.Bezerra MC, Teodoro WR, Oliveira CC, et al. Scleroderma-like remodeling induced by type V collagen. Arch Dermatol Res. 2006;298:51–7. doi: 10.1007/s00403-006-0645-5. [DOI] [PubMed] [Google Scholar]
- 5.Parra ER, Teodoro WR, Velosa APP, Oliveira CC, Yoshinari NH, Capelozzi VL. Interstitial and vascular type V collagen morphologic disorganization in usual interstitial pneumonia. J Histochem Cytochem. 2006;54:1315–25. doi: 10.1369/jhc.6A6969.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black CM. Scleroderma moves on. Curr Opin Rheumatol. 2001;13:493–4. [Google Scholar]
- 7.Ohtsuka T, Koibuchi N, Sakai H, Yamakage A, Yamakage S. Quantitative analisys of α1 (I) and α1 (III) procollagen mRNA expression in systemic sclerosis skin tissue – an in situ hybridization study. Arch Dermatol Res. 1990;291:575–82. doi: 10.1007/s004030050458. [DOI] [PubMed] [Google Scholar]
- 8.Tamby MC, Chanseaud Y, Guillevin L, Mouthon L. New insights into the pathogenesis of systemic eclerosis. Autoimmun Rev. 2003;2:152–7. doi: 10.1016/s1568-9972(03)00004-1. [DOI] [PubMed] [Google Scholar]
- 9.Tan EM. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:95–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- 10.Rothfield NF. Autoantibodies in scleroderma. Rheum Dis Clin North Am. 1992;18:483–98. [PubMed] [Google Scholar]
- 11.Bunn CC, Denton CP, Shi-Wen X, Knight C, Black CM. Anti-RNA polymerases and other autoantibody specificities in systemic sclerosis. Br J Rheumatol. 1998;37:15–20. doi: 10.1093/rheumatology/37.1.15. [DOI] [PubMed] [Google Scholar]
- 12.Okano Y, Medsger TA. In: Autoantibodies. Peter JB, Shoenfeld Y, editors. New York: Elsevier; 1996. pp. 727–34. [Google Scholar]
- 13.Singer JM. Standardization of the latex test for rheumatoid arthritis serology. Bull Rheum Dis. 1974;24:762–9. [Google Scholar]
- 14.Dellavance A, Gabriel AJ, Cintra AFU, et al. II. Consenso Brasileiro de Fator Antinuclear em Células HEp-2 Definições para a padronização da pesquisa de auto-anticorpos contra constituintes do núcleo (FAN HEp-2), nucléolo, citoplasma e aparelho mitótico e suas associações clínicas. Rev Bras Reumatol. 2003;43:29–40. [Google Scholar]
- 15.Storrie B, Madden EA. In: Guide to Protein Purification. Deutscher MP, editor. San Diego: Academic Press; 1990. pp. 203–5. [Google Scholar]
- 16.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Smith DE, Fisher PA. Identification, developmental regulation, and response to heat shock proteinof two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of a improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984;99:20. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connolly JA, Kalnins VI. Visualization of centriolos and basal bodies by fluorescent staining with nonimmune rabbit sera. J Cell Biol. 1978;79:526–32. doi: 10.1083/jcb.79.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turksen K, Aubin JE, Kalninsv I. Identification of a centriole – associated protein by antibodies present in normal rabbit sera. Nature. 1982;298:763–5. doi: 10.1038/298763a0. [DOI] [PubMed] [Google Scholar]
- 20.McGiven AR, Boer WGRM, Barnett AJ, Coventry DA. Autoantibodies in scleroderma. Med J Austr. 1968;2:533–6. doi: 10.5694/j.1326-5377.1968.tb83002.x. [DOI] [PubMed] [Google Scholar]
- 21.Cook L, Agnello V. In: Manual of Clinical Laboratory Immunology. Rose NR, Macario EC, Fahey JN, Friedman H, Penn GM, editors. Washington: American Society for Microbiology; 1992. pp. 762–4. [Google Scholar]
- 22.Renier G, Carrere F, Chevalier A. Les autoanticorps diriges contre láppareil de Golgi. Rev Med Interne. 1994;15:174–81. doi: 10.1016/s0248-8663(05)82144-7. [DOI] [PubMed] [Google Scholar]
- 23.Bradwell AR, Stokes RP, Johnson GD. Atlas of HEp-2 patterns. 1. San Diego: The Binding Site Inc; 1995. [Google Scholar]
- 24.Seelig HP, Schranz P, Schroter H, Wiemann C, Renz M. Macrogolgin – a new 376 kD Golgi complex outer membrane protein as target of antibodies in patients with rheumatic diseases and HIV infections. J Autoimmune. 1994;7:67–91. doi: 10.1006/jaut.1994.1006. [DOI] [PubMed] [Google Scholar]
- 25.Seelig HP, Schranz P, Schroter H, Wiemann C, Griffiths G, Renz M. Molecular genetic analyses of a 376 KDa Golgi complex membrane protein (giantin) Mol Cel Biol. 1994;14:2564–76. doi: 10.1128/mcb.14.4.2564. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Linstedt A D, Foguet M, Renz M, Seelig HP, Glick BS, Hauri HP. A C-terminally-anchored Golgi protein is inserted into the endoplasmatic reticulum and then transported to the Golgi apparatus. J Cell Biol. 1995(92):5102–5. doi: 10.1073/pnas.92.11.5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renier G, Fritzler JM, Chevalier A. Golgi apparatus autoantibodies. In: Peter JB, Shoenfeld Y, editors. Autoantibodies. New York: Elsevier; 1996. pp. 325–30. [Google Scholar]
- 28.Nozawa K, Fritzler MJ, Von Mühlen CA, Chan EK. Giantin is the major Golgi autoantigen in human anti-Golgi complex sera. Arthr Res Ther. 2004;6:95–102. doi: 10.1186/ar1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senécal JL, Fortin S, Roussin A, Joyal F. Anticytoskeletal autoantibody to microfilament anchorage sites recognizes novel focal contact proteins. J Clin Invest. 1987;80:778–85. doi: 10.1172/JCI113134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarsounas M, Heng HH, Ye CJ, Pearlman RE, Moens PB. Identification of the mouse beta′-COP Golgi component as a spermatocyte autoantigen in scleroderma and mapping of its gene Copb2 to mouse chromosome 9. Cytogenet Cell Genet. 1999;87:201–4. doi: 10.1159/000015467. [DOI] [PubMed] [Google Scholar]
- 31.Mackay IR, Rowley MJ. Autoimmune epitopes: autoepitopes. Autoimmun Rev. 2004;3:487–92. doi: 10.1016/j.autrev.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Rose NR. In: Autoantibodies. Peter JB, Shoenfeld Y, editors. New York: Elsevier; 1996. pp. xxvii–ix. [Google Scholar]