Abstract
Lipopolysaccharide (LPS) and inflammatory cytokines cause activation of sphingomyelinases (SMases) and subsequent hydrolysis of sphingomyelin (SM) to produce a lipid messenger ceramide. The design of SMase inhibitors may offer new therapies for the treatment of LPS- and cytokine-related inflammatory bowel disease. We synthesized a series of difluoromethylene analogues of SM (SMAs). We report here the effects of the most potent SMase inhibitor, SMA-7, on the LPS-mediated release of tumour necrosis factor-α, interleukin-1β and interleukin-6 from THP-1 macrophages and the pathology of dextran sulphate sodium (DSS)-induced colitis in mice. SMA-7 suppressed the LPS-induced cytokine release and nuclear factor-κB activation. LPS stimulation caused a four-fold increase in acid SMase activation, but little increase in neutral SMase activity. The presence of 10 μm SMA-7 caused acid SMase to remain at the control levels and reduced the formation of ceramide. HT-29 cells had significantly decreased cell viability when incubated with media from LPS-stimulated THP-1 macrophages. However, incubating the colon cells in media from both SMA-7 and LPS-treated macrophages caused little decrease in viability, suggesting that ceramide has a role in the LPS-stimulated signalling that releases cytotoxic factors against colon cells. Oral administration of SMA-7 to mice with 2% DSS in the drinking water, for 10 or 21 consecutive days, reduced significantly the cytokine levels in the colon and the severity of colonic injury. These findings suggest a central role for acid SMase/ceramide signalling in the pathology of DSS-induced colitis in mice, indicating a possible preventive or therapeutic role for SMase inhibitor in inflammatory bowel disease.
Keywords: ceramide, inflammatory bowel disease, lipopolysaccharide, nuclear factor-κB, sphingomyelinase
Introduction
Ulcerative colitis and Crohn's disease are chronic inflammatory disorders of the bowel that fall in the category of inflammatory bowel disease (IBD). In the colons of patients with IBD, the number of macrophages is abnormally large and subpopulations of macrophages, not normally present in the lamina propria of the intestine, appear,1,2 indicating ongoing recruitment to the inflamed bowel.3,4 The macrophages may contribute to intestinal damage by releasing oxyradicals5 and by secreting inflammatory cytokines, such as interleukin-1 (IL-1), IL-2, IL-6, tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ).6 Therapies for IBD target one or more of these inflammatory mediators. Recent advances in drug development for IBD have involved the use of monoclonal antibodies to inhibit specific inflammatory cytokines such as ILs, IFNs and TNF-α.7,8 In particular, the anti-TNF-α antibodies, CDP571 and infliximab have been used clinically to treat Crohn's disease with some success.9,10
Lipopolysaccharide (LPS) is the major constituent of the outer membrane of Gram-negative bacteria; it plays a beneficial role in the course of bacterial infection because of its ability to stimulate the host immune response. LPS also induces the release of inflammatory cytokines such as IL-1β, IL-6, IL-8 and TNF-α from monocyte/macrophages11,12 via the mechanism of nuclear factor-κB (NF-κB) activation.13–15 LPS causes an increase in cellular ceramide by activating sphingomyelinase (SMase) in macrophages, similar to that seen upon IL-1β or TNF-α treatment.16,17 Ceramide triggers mitogen-activated protein kinase (MAPK), which leads to the expression of a variety of genes, resulting in inflammatory responses.18 Thus, the role of cellular ceramide in signal transduction may be important in treating Gram-negative bacteria-induced sepsis syndrome or IBD.
We wished to investigate new methods of inhibiting the inflammatory actions of these macrophage-produced cytokines on bowel tissues and to do so in an animal model of IBD. Blocking ceramide production by inhibiting SMase activity is expected to be an available method, because ceramide is the common signal transducer of at least LPS, IL-1β and TNF-α.16,17 Two SMases, neutral and acid SMases, are activated in response to many extracellular stimuli. Despite extensive studies, their precise cellular functions and mechanisms of regulation are not well understood. We have previously designed and synthesized difluoromethylene analogue of sphingomyelin (SMAs) which inhibit neutral SMase in bovine brain microsomes with 50% inhibitory concentrations (IC50) values of 3·3–377 μm.19,20 Our SMAs also showed the same inhibitory effect on acid SMase in the brain.19 The object of this study was to use the most potent SMA-7 with an IC50 value of 3·3 μm to evaluate the role of SMase in LPS-stimulated macrophages and the therapeutic effects of SMA-7 in intestinal inflammation. Here we show that SMA-7 suppresses acid SMase-catalysed ceramide production, NF-κB activation, and the inflammatory cytokine release from macrophages caused by LPS. The SMase inhibitor reduced dextran sulphate sodium (DSS)-induced intestinal inflammation in mice. To our knowledge this is the first demonstration that inhibition of acid SMase may have significant potential to treat patients with colitis.
Materials and methods
Reagents
The following reagents were obtained commercially: phorbol 12-myristate 13-acetate (PMA) from Wako Pure Chemicals, Osaka, Japan; RPMI-1640 from Nikken Bio Medical Laboratory, Tokyo, Japan; Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) from Gibco BRL (Grand Island, NY); Escherichia coli LPS (purified by ion-exchange chromatography: protein < 1%; RNA < 1%) from Sigma (St. Louis, MO); recombinant human TNF-α from Strathmann Biotec AG, Hanover, Germany; DSS (MW 40 000) from ICN Biomedicals, Inc., Aurora, OH; and lactacystin from CalBiochem, San Diego, CA.
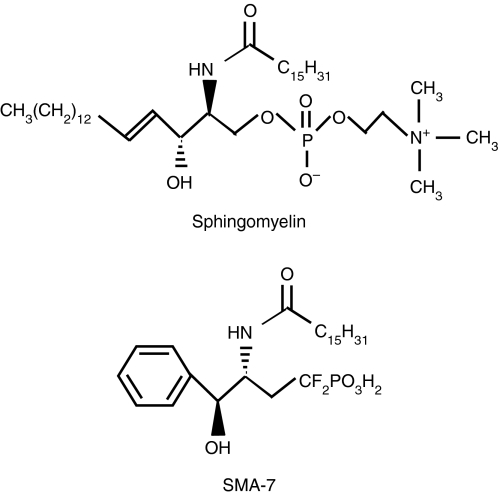
SMA-7 used in this study
We used the SM analogue SMA-7 in this study. Figure 1 gives the chemical structure. The long alkenyl chain and the phosphodiester moiety of SM are replaced by a phenyl and an isosteric difluoromethylenephosphonic acid, respectively.19 SMA-7 inhibits neutral SMase in bovine brain microsomes in a non-competitive manner: the IC50 value is 3·3 μm.20 While the compound has no effect on the activity of neutral SMase isolated from Bacillus cereus and ceramide synthase in bovine liver microsomes, it does inhibit acid SMase in bovine brain lysosomes with the same potency as the neutral SMase.19,20
Figure 1.
The chemical structures of SM and SMA-7.
Cell cultures and differentiation
The monocytic cell line THP-1 was purchased from Dainippon Pharmaceutical Co., Osaka, Japan. Stock cultures of THP-1 cells were maintained in 10 mm HEPES-buffered RPMI-1640, supplemented with 10% FBS, 50 U/ml penicillin and 50 μg/ml streptomycin at 37° in a humidified 5% CO2 atmosphere. Before experiments, the growing cells were seeded in six-well culture plates (3 × 106 cells/well) in the above medium supplemented with PMA (10 ng/ml) to induce differentiation into macrophage-like cells. After a 48-hr incubation, the macrophages were extensively washed with RPMI-1640 alone. Incubation followed for 12 hr in medium containing FBS.
The human peripheral blood mononuclear cells (HPBMC) line was obtained from BioWhittaker, Walkersville, MD. HPBMC were maintained in RPMI-1640, supplemented with 10% FBS, 50 U/ml penicillin, 50 μg/ml streptomycin and 0·05 mm 2-mercaptoethanol at 37° in a humidified 5% CO2 atmosphere. Before experiments, the cells were seeded in six-well culture plates (3 × 106 cells/well) in the medium.
The colon cell line HT-29 was obtained from Dainippon Pharmaceutical Co. HT-29 cells were maintained in DMEM supplemented with 10% FBS, 50 U/ml penicillin and 50 μg/ml streptomycin at 37° in a humidified 5% CO2 atmosphere. Before experiments, the cells were seeded in 96-well culture plates (5 × 103 cells/well). Incubation followed for 48 hr in medium containing FBS.
Measurement of cytokine release from macrophages
THP-1 macrophages in six-well plates were incubated in 10 mm HEPES-buffered RPMI-1640, supplemented with 10% FBS, 50 U/ml penicillin and 50 μg/ml streptomycin for 24 hr with 1 μg/ml of LPS alone or LPS plus 0·1–10 μm SMA-7 or 20 μm lactacystin. Levels of human TNF-α, IL-1β and IL-6 in the culture media were measured using enzyme-linked immunosorbent assay (ELISA) kits (Pierce-Endogen, Rockford, IL), according to the manufacturer's instructions.
Electrophoretic mobility shift assay
The DNA-protein binding assays were carried out using nuclear extracts from THP-1 macrophages treated with 1 μg/ml of LPS alone or LPS plus 10 μm SMA-7 or 20 μm lactacystin. Synthetic complementary oligonucleotides were 3′-biotinylated using a biotin 3′-end DNA labelling kit (Pierce, Rockford, IL) according to the manufacture's instructions and annealed for 1 hr at room temperature. The sequences of the oligonucleotides used were 5′-AGT TGA GGG GAC TTT CCC AGG C-3′ and 3′-TCA ACT CCC CTG AAA GGG TCC G-5′ containing a putative binding site for NF-κB. Binding reactions were carried out for 20 min at room temperature in the presence of 50 ng/μl poly (dI-dC) in 1 × binding buffer (LightShift chemiluminescent EMSA kit, Pierce) using 20 fmol of biotin end-labelled target DNA and 10 μl of the nuclear extract. The DNA-protein complexes were subjected to a 6% native polyacrylamide gel electrophoresis and transferred to a nylon membrane (Biodyne B membrane, Pierce). Transferred DNAs were cross-linked to the membrane for 10 min under a hand-held UV lamp with 254 nm bulbs and detected using horseradish peroxidase-conjugated streptavidin (LightShift chemiluminescent EMSA kit, Pierce) according to the manufacturer's instructions.
Measurement of ceramide
The amounts of ceramide in THP-1 macrophages (5 × 106 cells) were measured as the quantity of C16-ceramide, using the liquid chromatography/ion spray ionization mass spectrometry (LC/MS) procedure that we have previously developed.21 Briefly, the harvested cells were centrifuged and pelleted, and the resulting pellets were suspended in 250 μl RPMI-1640. The suspension was vigorously mixed with 4 ml chloroform/methanol (2 : 1, v/v) for 1 min. After the addition of 1 ml water, the sample was vortexed and centrifuged. The lower layer was collected, and the chloroform was allowed to evaporate. The residue was dissolved in a solvent and subjected to LC/MS analysis. High-performance liquid chromatography was performed using a Gulliver 1500 series system (JASCO, Tokyo, Japan) equipped with a Develosil ODS HG-5 reverse-phase column (35 mm × 2·0 mm inner diameter, 5 μm, Nomura Chemical, Nagoya, Japan). The mobile phases were as follows: A, 5 mm ammonium formate/methanol/tetrahydrofuran (5 : 2 : 3, v/v); B, 5 mm ammonium formate/methanol/tetrahydrofuran (1 : 2 : 7, v/v) containing 0·01% formic acid. Elution was performed at a flow rate of 0·2 ml/min with 70% of mobile phase A and 100% of mobile phase B in a linear gradient mode for 6·3 min. LC/MS analyses were performed with an electrospray ionization mass spectrometer (Finnigan-LCQ) and Navigator (Finnigan, Waltham, MA). Retention time (min) and mass charge ratio (m/z) of inner standards were as follows. for C8-ceramide (Sigma), 7·20 and 426·1; for C16-ceramide (Sigma), 10·35 and 538·2. For the C16-dihydroceramide (Sigma), they were 10·55 and 540·4.
Measurement of the activities of acid and neutral SMases
The activities of acid and neutral SMases were measured with an Amplex Red Sphingomyelinase Assay Kit (Molecular Probes Inc., Eugene, OR). Briefly, THP-1 macrophages in six-well plates (3 × 106 cells) were incubated at 37° for set periods ranging from 0 hr to 24 hr in 10 mm HEPES-buffered RPMI-1640 supplemented with 10% FBS, 50 U/ml penicillin and 50 μg/ml streptomycin in the presence or absence of LPS (1 μg/ml) or TNF-α (20 ng/ml). The cell layers were washed twice with ice-cold phosphate-buffered saline (PBS) and harvested. The cells were pelleted by centrifugation, and extraction followed for 60 min on ice in 1 ml of a lysis buffer, 20 mm Tris–HCl buffer (pH 7·4) containing 1% Triton X-100, 1 μg/ml aprotinin, 1 mm ethylenediaminetetraacetic acid, and 100 μg/ml phenylmethylsulphonyl fluoride, for neutral SMase or a lysis buffer for acid SMase, it was 50 mm sodium acetate buffer (pH 5·4) containing the above components. After centrifugation at 17 000 g for 10 min at 4°, the resulting supernatants and pellets were assayed, following the manufacturer's instructions. In this study, the supernatants (adjusted to pH 5·4) and the pellets (pH 7·4) were used for the assays of acid and neutral SMases, respectively. The assay mixture contained the following components in a total volume of 200 μl: 2 U/ml horseradish peroxidase, 0·2 U/ml choline oxidase, 8 U/ml alkaline phosphatase, 0·5 mm sphingomyelin (SM) and 100 µl of the enzyme source. Assay mixtures were incubated at 37° for 30 min, and the fluorescence was measured with a CytoFluor Series 4000 fluorophotometer (PerSeptive Biosystems, Framingham, MA) with excitation at 563 nm and emission at 587 nm.
Measurement of cell viability
HT-29 cells in 100 μl DMEM supplemented with 10% FBS, 50 U/ml penicillin and 50 μg/ml streptomycin were seeded in each well of 96-well plates (5 × 103 cells/well) and incubated at 37° for 48 hr. After being washed with DMEM, the cell layers were incubated for 24 hr in 100 μl of one of the media in which THP-1 macrophages had been incubated for 24 hr with 1 μg/ml LPS alone, 1 or 10 μm SMA-7 alone or LPS plus SMA-7. Cell viability was assayed with a Cell Counting Kit-8 (Dojindo, Kumamoto, Japan), following the manufacturer's instructions.
Fluorometric assay for caspase-3 activity
The activity of caspase-3 in HT-29 cells was measured by using a Caspase3/CPP32 Fluorometric Protease Assay kit (MBL, Inc., Nagoya, Japan). HT-29 cells (2 × 105 cells/well) were incubated at 37° for set periods ranging from 0 hr to 24 hr in the medium in which THP-1 macrophages in six-well plates (3 × 106 cells) were incubated at 37° for 24 hr with 1 μg/ml LPS alone or LPS plus 1 or 10 μm SMA-7. They were harvested and centrifuged. The cell pellets were washed with ice-cold PBS and suspended in 50 μl of the cell lysis buffer for 10 min on ice. The assay mixture contained the following components in a total volume of 105 μl: 50 μl cell lysate, 50 μl reaction buffer containing 10 mm dithiothreitol and 5 μl Asp-Glu-Val-Asp (DEVD)-AFC substrate. Incubation followed at 37° for 1 hr. The released 7-amino-4-trifluoromethyl coumarin (AFC) was measured by a fluorometric platereader with excitation at 400 nm and emission at 505 nm.
Animal experiments
Male BALB/c mice (5 weeks of age) were purchased from Crea Japan Inc., Tokyo, Japan. They were housed in a light-controlled room (light on 07.00–19.00 hr) at a room temperature of 24 ± 1° and a humidity of 60 ± 10% with food and water ad libitum. Animal treatment followed the animal care guidelines in Japanese Government Law no. 105 and Notification no. 6. Acute colitis was induced by giving 2% DSS orally in drinking water for 10 or 21 days ad libitum. SMA-7 was dissolved in water or vehicle and concomitantly administered to mice (100 mg/kg/day, orally). The daily dose was chosen by referring the administration dose (100 mg/kg) of scyphostatin,22 a neutral SMase inhibitor isolated from Trichopeziza mollissima, to carrageenin-induced paw oedema in rats. The animals were killed on day 10 or 21, the colons were removed and the distal parts were subjected to histochemical analysis and measurement of cytokines. The tissue samples were fixed in the Carnoy's solution. After fixation, these specimens were embedded in paraffin wax and cut into 5 mm sections by routine procedures. The sections were stained with haematoxylin & eosin (H&E) and examined under a light microscope to determine colonic mucosal damage.
Histology
Histological quantification was performed blinded using a scoring system as described previously.23 The three independent parameters measured were severity of inflammation (0–3: none, slight, moderate, severe), extent of injury (0–3: none, mucosal, mucosal and submucosal, transmural), crypt damage (0–4: none, basal one-third damaged, basal two-thirds damaged, only surface epithelium intact, entire crypt and epithelium lost). These changes were quantified by the percentage involvement by the disease process: (1) 1–25%; (2) 26–50%; (3) 51–75%; (4) 76–100%.
Measurement of cytokines in mouse colon tissues
The levels of TNF-α, IL-1β and IL-6 in mouse colon tissue homogenates were determined with a mouse TNF-α ELISA kit, a mouse IL-1β ELISA kit and a mouse IL-6 ELISA kit (Amersham Biosciences, Tokyo, Japan), respectively.
Statistical analysis
All values are expressed as the mean ± SD or the mean ± SE and the significance levels between groups were assessed by Student's t-test. P < 0·05 was considered statistically significant.
Results
The effect of SMA-7 on the LPS-induced release of TNF-α, IL-1β and IL-6 from macrophages and HPBMC
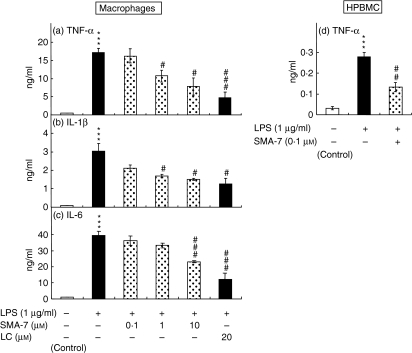
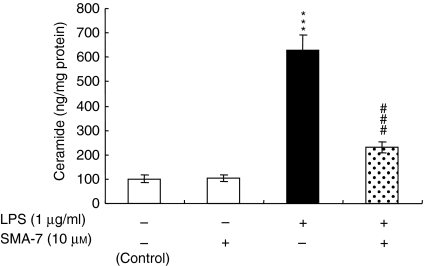
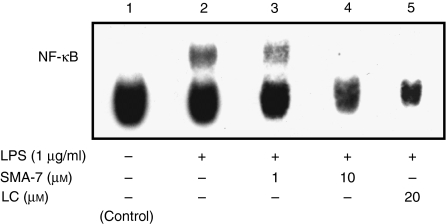
LPS induces the release of inflammatory cytokines, such as IL-1β, IL-6, IL-8 and TNF-α, from monocyte/macrophages11,12 and causes an increase in cellular ceramide in macrophages, similar to that seen with IL-1β or TNF-α treatment.16,17 These findings suggest that SM hydrolysis plays an important role in the LPS-induced release of these cytokines. To investigate the role of SMase in the release of inflammatory cytokines from THP-1 macrophages and HPBMC, both types of cells were treated for 24 hr with 1 μg/ml LPS in the presence or absence of 0·1–10 μm SMA-7. As shown in Fig. 2, SMA-7 suppressed the LPS-induced releases of TNF-α, IL-1β and IL-6 from the macrophages and that of TNF-α from HPBMC in a concentration-dependent manner. The result suggests that activation of SMase(s) is an underlying mechanism of the LPS-induced release of these cytokines from macrophages. The data also show that lactacystin, an NF-κB inhibitor, decreases the LPS-induced cytokine release. Figure 3 shows the ceramide content in the macrophages 3 hr after incubation with LPS (1 μg/ml) alone or LPS plus 10 μm SMA-7. LPS treatment caused a 6·4-fold increase in ceramide accumulation, compared to the control. However, the co-presence of 10 μm SMA-7 caused ceramide levels to remain low. LPS is known to activate NF-κB in macrophages.16,17,24 We next examined the effects of SMA-7 and lactacystin on the LPS-induced activation of NF-κB in macrophages. As shown in Fig. 4 (lane 2), incubating the cells with LPS (1 μg/ml) for 3 hr significantly increased the nuclear levels of NF-κB. However, lactacystin treatment reduced the NF-κB levels to that of the control (lane 5). Co-treatment of macrophages with 1 or 10 μm SMA-7 reduced the NF-κB levels in a concentration-dependent manner (lanes 3 and 4 in Fig. 4). Ceramide production following LPS-induced SM hydrolysis may trigger the activation of NF-κB in nuclei. Therefore, SMase inhibition by SMA-7 may reduce the cytokine secretion by reducing nuclear NF-κB levels.
Figure 2.
The effects of SMA-7 and lactacystin (LC) on the LPS-induced releases of TNF-α (a), IL-1β (b) and IL-6 (c) from macrophages and TNF-α (d) from HPBMC. Antigen levels in media were determined by ELISA. Each bar represents the mean ± SD of three independent experiments. ***P < 0·005, compared to the control. #P < 0·05; ##P < 0·01; and ###P < 0·005, compared to LPS alone.
Figure 3.
The effect of SMA-7 on the LPS-induced ceramide accumulation in macrophages. Cells were incubated for 3 hr with LPS alone or with LPS plus SMA-7. Total cellular C16-ceramide was measured by using the LC/MS procedure as described in the Materials and methods. ***P < 0·005, compared to the control. ###P < 0·005, compared to LPS alone.
Figure 4.
The effects of SMA-7 and lactacystin (LC) on the LPS-induced activation of NF-κB in macrophages. Cells were incubated for 3 hr with LPS alone or with LPS plus SMA-7 or LC. Nuclear extracts were prepared and subjected to the electrophoretic mobility shift assay of NF-κB described in the Materials and methods. Similar results were obtained in two independent experiments.
The effect of SMA-7 on the SMase activities in LPS-treated macrophages
To date, acid SMase and neutral SMase are candidates for those enzymes that respond to apoptotic and other stimulation, causing SM hydrolysis and subsequent ceramide generation.25–28 However, the precise cellular functions of the two SMases in inflammation are not well understood.
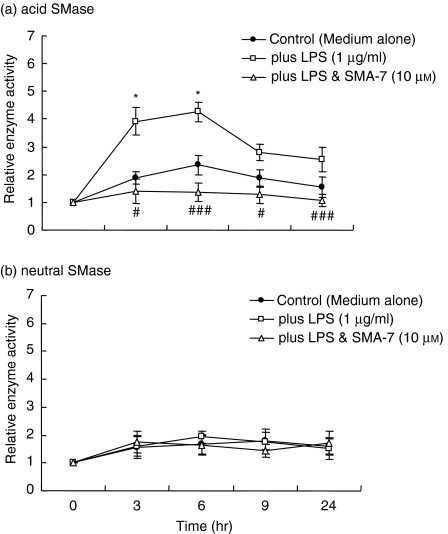
To confirm the origin of the accumulated ceramide in LPS-treated macrophages, we assessed the acid and neutral SMase activities in the cells and SMA's effects. As shown in Fig. 5(a), LPS treatment for 3 hr increased acid SMase activity approximately 4·0-fold over that in the control cells. The enzyme activity peaked at 6 hr (a 4·3-fold increase), and the activation of acid SMase was sustained up to 18 hr. The presence of 10 μm SMA-7 suppressed the increase in acid SMase activity. On the other hand, neutral SMase activity in the cells was unaffected by LPS treatment (Fig. 5b). These results suggest that acid SMase activation plays an important role in LPS inflammatory signalling in macrophages.
Figure 5.
The effect of SMA-7 on the acid (a) and neutral (b) SMase activities in macrophages treated with LPS. Cells were incubated at 37° for set periods ranging from 0 hr to 24 hr in serum-rich medium with LPS (1 μg/ml) alone or with LPS plus 10 μm SMA-7. Each bar represents the mean ± SD of three independent experiments. *P < 0·05, compared to the control. #P < 0·05; ###P < 0·005, compared to LPS alone.
The effect of SMA-7 on the SMase activities and IL-1β release in TNF-α-treated macrophages
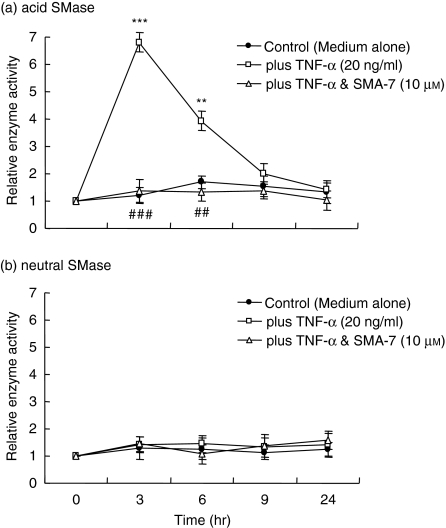
TNF-α plays a key role in many autoimmune and chronic inflammatory disorders, and seems to be a central mediator of the inflammatory process in IBD.29,30 As shown in Fig. 2, LPS induced the release of TNF-α from THP-1 macrophages. We examined the effect of SMA-7 on SMase activity in THP-1 macrophages treated with TNF-α (20 ng/ml). As shown in Fig. 6(a), the acid SMase activity in TNF-α-treated macrophages peaked at 3 hr (a 6·5-fold increase), and decreased to a level near the control cell level at 9 hr. The presence of 10 μm SMA-7 caused the enzyme activity to remain at the level seen in the control cells. The neutral SMase in macrophages was not activated by TNF-α (Fig. 6b) and was unaffected by LPS (Fig. 5b).
Figure 6.
The effect of SMA-7 on the acid (a) and neutral (b) SMase activities in macrophages treated with TNF-α. Cells were incubated at 37° for set periods ranging from 0 hr to 24 hr in serum-rich medium with TNF-α (20 ng/ml) alone or with TNF-α plus 10 μm SMA-7. Each bar represents the mean ± SD of three independent experiments. **P < 0·01; ***P < 0·005, compared to the control. ##P < 0·01; ###P < 0·005, compared to TNF-α alone.
We next investigated whether the released TNF-α acts on the macrophages as an autocrine mechanism. Incubation of macrophages with TNF-α (20 ng/ml) for 24 hr resulted in no enhancement of the release of other inflammatory cytokines such as IL-1β and IL-6 (data not shown). Additionally, treatment of the cells with TNF-α (20 ng/ml) for 3 hr did not activate NF-κB (data not shown). Thus, the LPS-released TNF-α may exert an additive effect on the LPS-induced activation of acid SMase and subsequent generation of ceramide in macrophages. However, TNF-α does not induce the release of other inflammatory cytokines via the mechanism of NF-κB activation.
The viability of HT-29 cells cultured in the media from LPS-treated macrophages
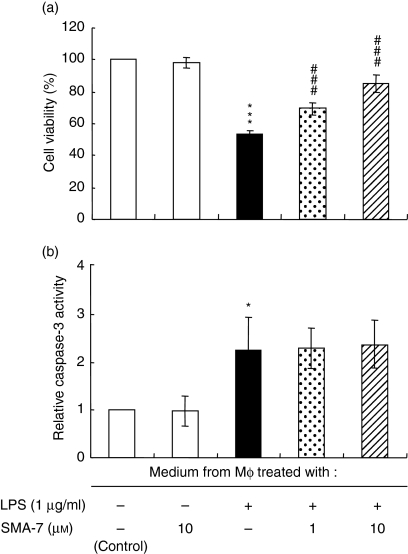
The intestinal epithelium serves as a barrier to numerous toxins and micro-organisms, and plays a major role in the immune system of the gut. In IBD, increased production of inflammatory cytokines causes the death of intestinal epithelial cells.31 Thus, we next examined the effect of humoral factors in the medium from LPS-treated macrophages on the viability of HT-29 intestinal epithelial cells. Before this experiment, THP-1 macrophages were incubated for 24 hr with LPS alone or LPS plus SMA-7, and media were collected. Figure 7(a) shows the viabilities of HT-29 cells cultured for 24 hr in the collected media. When compared to the control HT-29 cells, which were incubated in the medium from LPS-untreated macrophages, 57% of epithelial cells survived in the medium from LPS-treated macrophages. In the media in which macrophages had been cultured with LPS and SMA-7, cell viability increased in a concentration-dependent manner with SMA-7: 74 and 92% of cells survived at 1 and 10 μm, respectively. These data suggest that SMA-7 can suppress macrophage activation and the secretion of various cytotoxic factors via the inactivation of acid SMase. Figure 7(b) shows the effects of these media on the caspase-3 activity in HT-29 cells after 24 hr. The activity of caspase-3 in HT-29 cells incubated in the media from LPS-treated macrophages was approximately twice that in cells incubated in the media from LPS-untreated macrophages (control). The increased caspase-3 activity was unchanged in cells incubated in the media from both LPS-treated and SMA-7-treated macrophages. The result suggests that the cytotoxic factors present in the media from LPS-treated macrophages cause, in part, a caspase-3-dependent apoptosis, while other cytotoxic factors that induce caspase-3-independent cell death (necrosis) may be present in the media, and SMA-7 treatment can suppress their secretion.
Figure 7.
The effect of SMA-7 on the viability (a) and the caspase-3 activity (b) of HT-29 cells cultured in the media from LPS-treated macrophages. Macrophages were incubated for 24 hr with 1 μg/ml LPS alone or with LPS plus 1 or 10 μm SMA-7, and the media were collected. HT-29 cells were cultured for 24 hr in the collected media. Each bar represents the mean ± SD of three independent experiments. *P < 0·05; ***P < 0·005, compared to the control medium from LPS-untreated macrophages. ###P < 0·005, compared to the medium from LPS-treated macrophages.
The effect of orally administered SMA-7 on DSS-induced colitis in mice
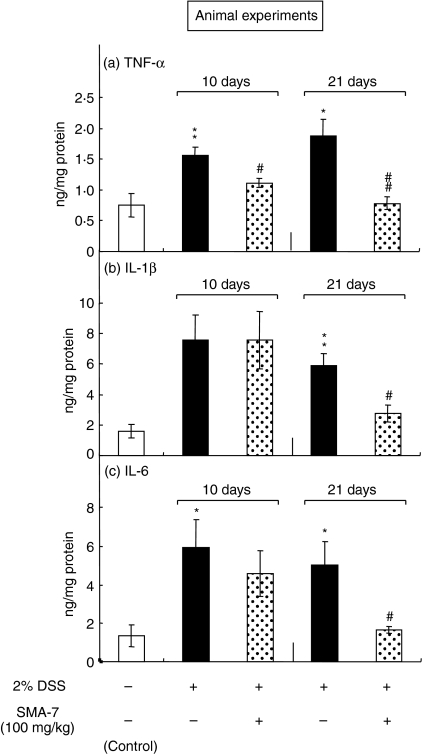
We next assessed the anti-inflammatory effects of SMA-7 in vivo by orally administering it into mice with DSS-induced colitis at a dose of 100 mg/kg per day. As shown in Fig. 8(a)–(c), the colon tissue levels of TNF-α, IL-1β and IL-6 were significantly increased in DSS-treated mice compared with the control animals. The mice treated with SMA-7 for 10 and 21 days had significantly lower tissue levels of TNF-α compared with the animals treated with DSS alone. The levels of IL-1β and IL-6 were not reduced significantly on day 10, but were reduced significantly on day 21.
Figure 8.
The effect of orally administered SMA-7 on the DSS-induced increase in TNF-α (a), IL-1β (b) and IL-6 (c) levels in mouse colon tissues. Antigen levels in colon tissue homogenates were determined by ELISA. Each bar represents the mean ± SE of three to five independent experiments. *P < 0·05 and **P < 0·01, compared to the vehicle alone; #P < 0·05 and ##P < 0·01, compared to DSS alone.
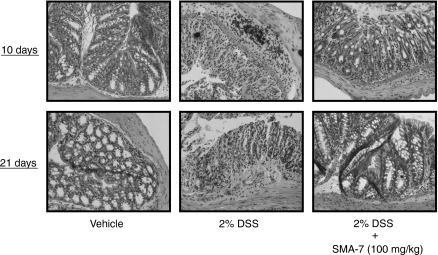
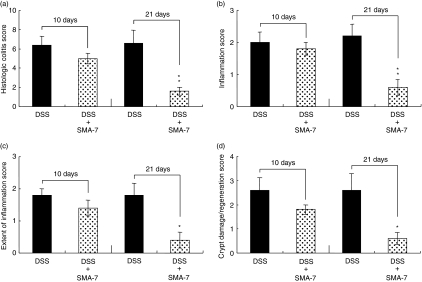
Histopathological evaluation of the colons in mice treated with DSS alone or with DSS plus SMA-7 is shown in Fig. 9. The left photographs (vehicle alone) show normal colonic architecture. Colon structure on day 21 (the middle photographs) after the beginning of 2% DSS administration was characterized by severe disintegration of tissue architecture, oedema and a massive, mixed immune cell infiltrate with ulcerations and large areas of complete epithelial denudation and muscular thickening. Conversely, in animals treated with SMA-7, alterations in mucosal architecture and damage to the colonic mucosal layer were minimal (the right-hand photographs). The histology scores indicate that administration of SMA-7 for 21 days in DSS-treated mice significantly reduces the colonic inflammation (Fig. 10). The present data are preliminary but suggest, for the first time, that inhibition of acid SMase in macrophages has a beneficial effect on intestinal inflammation damage.
Figure 9.
The effect of orally administered SMA-7 on the DSS-induced colonic damage in mice. The tissue slices were stained with haematoxylin & eosin. The data are the results from three to five individual mice in each group.
Figure 10.
Histology score of colitis in the DSS-treated mice. (a) Sum of the scores of (b), (c) and (d); (b) inflammation score; (c) extent of inflammation; (d) crypt damage/regeneration score. Each bar represents the mean ± SE of three to five independent experiments. *P < 0·05 and **P < 0·01, compared to DSS alone.
Discussion
IBD is multifactorial in origin and affects at least 1 in 1000 people in Western countries.32,33 Multiple lines of evidence suggest that macrophages have a critical role in the disease pathology of IBD. The macrophages produce various inflammatory cytokines such as TNF-α, IL-1β, and IL-6.6 These cytokines can stimulate the hydrolysis of SM, generating ceramide and phosphorylcholine.25–28 Ceramide production triggers MAPK activation, thereby leading to the expression of a variety of genes involved in inflammatory responses.18 Therefore, a drug that counteracts the action of ceramide would be expected to attenuate local inflammation. Resident intestinal macrophages are located close to the basal membrane of the intestinal epithelium and represent the first line of defence by immune cells. They do not normally express the LPS receptor CD14.34 However, macrophages in resected intestinal segments from patients with IBD express a unique phenotype with unusually high levels of CD1435 and are primed for the production of TNF-α, IL-1β and IL-6. These cytokines can directly or indirectly affect epithelial function, altering transport and barrier characteristics.36 The object of this study was to use our synthesized SMA-719,20 to evaluate the roles of the SMase-ceramide signalling pathway in activated macrophages and in an animal model of IBD.
We show that LPS stimulates the release of TNF-α, IL-1β and IL-6 from macrophages (THP-1 cells) and monocytes (HPBMC) via the mechanism of NF-κB activation. We found that LPS can specifically activate acid SMase in THP-1 macrophages. That an acid SMase inhibitor, SMA-7, can prevent inflammatory cytokine release, NF-κB activation, and rapid ceramide formation in activated macrophages is a new finding. In this study, incubation of macrophages with TNF-α (20 ng/ml) resulted in no enhancement of the release of other inflammatory cytokines, although TNF-α increases cellular ceramide, which can trigger the transcription of inflammatory genes and cytokine production. The concentration (20 ng/ml) of TNF-α used in this experiment corresponded to that of TNF-α released from LPS-treated macrophages. Higher concentrations of TNF-α may exhibit an autocrine mechanism for the cytokine release. We next demonstrated that the humoral factors released from LPS-treated macrophages cause the death of intestinal epithelial (HT-29) cells and that the release of the cytotoxic factors is suppressed by the treatment of macrophages with SMA-7. What the cytotoxic factors are and how they interfere with the function of epithelial cells remain unknown. However, these in vitro results suggest that therapeutic inhibition of acid SMase in activated macrophages may prove useful for treating IBD involving ulcerative colitis and Crohn's disease. Recently, anti-TNF-α therapy using the anti-TNF-α antibody, infliximab in Crohn's disease has been highlighted.7–10 In patients responding to this anticytokine therapy, rapid and thorough healing of the bowel has been demonstrated both endoscopically and histologically. However, the major drawbacks of the new protein therapy are the narrow spectrum of inflammatory mediator that it regulates, the high cost of production, in vivo instability, limited routes of administration and immunogenicity. TNF-α binding to the 55 000 MW receptor signals an apoptotic response.28 This receptor contains a C-terminal death domain which appears to be required for transmission of the apoptotic signal. The death domain is conserved in CD95 (Fas), a TNF receptor homologue that mediates apoptosis in lymphocytes.37 Activation of this domain system in the 55 000 MW TNF-α and CD95 receptors has been shown to be related to acid SMase.38,39 It is stherefore suggested that inhibition of acid SMase by SMA-7 exerts infliximab-like effects on downstream cellular apoptotic signalling.
The toll-like receptor 4 (TLR4), a transmembrane receptor for LPS, plays an important immunological role in the in vivo response of humans to inhaled LPS. LPS-induced signalling through TLR4 rapidly leads to NF-κB activation and cytokine expression in monocytes.40 In patients with IBD, TLR4 in monocyte/macrophages is strongly up-regulated and sensitive to LPS.41 Because monocyte/macrophages in IBD evoke epithelial dysfunction36 we investigated whether the influence of LPS-activated macrophages on intestinal epithelial cells can be altered by SMA-7 treatment of the macrophages. The result demonstrated that LPS-stimulated THP-1 macrophages release cytotoxic factors, which lead to HT-29 colonic cell death, and that SMA-7 treatment reduces the release of these humoral factors in a dose-dependent manner. Our data suggest the possibility that suppression of ceramide signalling in inflamed macrophages can contribute to the improvement of pathology in IBD. LPS-TLR4 signalling pathways include both MAPK and NF-κB activation pathways. The classical pathway leading to NF-κB activation after binding of LPS to TLR4 is not dependent on MAPK, but occurs via MYD88, IRAK, TRAF6, NIK and IKK complexes.42,43 However, it is reported that ceramide can induce activation of TAK1, a member of the MAPK family, in COS7 cells, which leads to IKK complex formation and subsequent activation of NF-κB.44 Therefore, it is tempting to speculate that ceramide induces NF-κB activation by acting on TAK1. Further studies are needed to clarify this possibility. Determining the effects of SMA-7 on these pathways may add important insight into the mechanisms by which SMase interacts with LPS-induced signalling pathways in this system.
To evaluate the in vivo effects of SMA-7 in intestinal inflammation, we administered the acid SMase inhibitor (100 mg/kg/day, orally) for 10 or 21 days in mice with DSS-induced colitis. DSS induces an acute colitis in BALB/c mice. In the colonic inflammation, NF-κB activity and its dependent cytokines, IL-1β, IL-6 and TNF-α, are up-regulated.45 Additionally, this acute colitis model increases the expression of TLR4.46,47 We demonstrated that SMA-7 can significantly suppress the NF-κB-dependent cytokine production. Histopathological evaluation of the colons in mice treated with DSS alone or plus SMA-7 showed that SMA-7 treatment clearly reduces DSS-induced alterations in mucosal architecture and colonic damage. Our results provide the first evidence that ceramide produced by the activation of acid SMase plays a critical role in the development of acute DSS-induced colitis and that acid SMase inhibitor may have a therapeutic value in IBD treatment, as well as affecting IBD targets such as TNF-α, IFNs and ILs. The dose of SMA-7 is based on that of scyphostatin in carrageenin-induced paw oedema in rats.22 Scyphostatin is a neutral SMase inhibitor that has a mild inhibitory effect on acid SMase, while SMA-7 has the ability to inhibit both acid and neutral SMases at the same level of effectiveness.19,20 Considering that acid SMase plays a key role in the carrageenin-induced paw oedema, reduction of the SMA-7 dose administered will be possible in our animal experiments. However, we have data showing that treatment of HT-29 colonic epithelial cells with LPS activates cellular neutral SMase but not acid SMase. Co-presence of SMA-7 reduced the activation of neutral SMase and the release of IL-8 (unpublished results). What is the role of each SMase in cell regulation? This should be the object of future studies.
Acknowledgments
This work was supported in part by funds from the Central Research Institute of Fukuoka University and from MEXT.HAITEKU (2002–06). Our thanks go to Mr Steven Sabotta for reading the manuscript.
Abbreviations
- DMEM
Dulbecco's modified Eagle's medium
- DSS
dextran sulphate sodium
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- HPBMC
human peripheral blood mononuclear cells
- IBD
inflammatory bowel disease
- IFN
interferon
- IL
interleukin
- LC/MS
liquid chromatography/ion spray ionization mass spectrometry
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- NF-κB
nuclear factor-κB
- PBS
phosphate-buffered saline
- PMA
phorbol 12-myristate 13-acetate
- SM
sphingomyelin
- SMA
difluoromethylene analogue of sphingomyelin
- SMase
sphingomyelinase
- TLR4
toll-like receptor 4
- TNF
tumour necrosis factor
References
- 1.Hume DA, Allan W, Hogan PG, Doe WF. Immunohistochemical characterisation of macrophages in human liver and gastrointestinal tract. Expression of CD4, HLA-DR, OKM1, and the mature macrophage marker 25F9 in normal and diseased tissue. J Leukoc Biol. 1987;42:474–84. doi: 10.1002/jlb.42.5.474. [DOI] [PubMed] [Google Scholar]
- 2.Oshitani N, Campbell A, Kitano A, Kobayashi K, Jewell DP. In situ comparison of phenotypical and functional activity of infiltrating cells in ulcerative colitis mucosa. J Pathol. 1996;178:95–9. doi: 10.1002/(SICI)1096-9896(199601)178:1<95::AID-PATH402>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 3.Rugtveit J, Brandtzaeg P, Halstensen TS, Fausa O, Scott H. Increased macrophage subset in inflammatory bowel disease: apparent recruitment from peripheral blood monocytes. Gut. 1994;35:669–74. doi: 10.1136/gut.35.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimm MC, Pullman WE, Bennett GM, Sullivan PJ, Pavli P, Doe WF. Direct evidence of monocyte recruitment to inflammatory bowel disease mucosa. J Gastroenterol Hepatol. 1995;10:387–95. doi: 10.1111/j.1440-1746.1995.tb01589.x. [DOI] [PubMed] [Google Scholar]
- 5.Britigan BE, Coffman TJ, Adelberg DR, Cohen MS. Mononuclear phagocytes have the potential for sustained hydroxyl radical production. Use of spin-trapping techniques to investigate mononuclear phagocyte free radical production. J Exp Med. 1988;168:2367–72. doi: 10.1084/jem.168.6.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogler G, Andus T. Cytokines in inflammatory bowel disease. World J Surg. 1998;22:382–9. doi: 10.1007/s002689900401. [DOI] [PubMed] [Google Scholar]
- 7.Atreya R, Mudter J, Finotto S, et al. Blockade of interleukin-6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn's disease and experimental colitis in vivo. Nat Med. 2000;6:583–8. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 8.Sandborn WJ, Targan SR. Biologic therapy of inflammatory bowel disease. Gastroenterology. 2002;122:1592–608. doi: 10.1053/gast.2002.33426. [DOI] [PubMed] [Google Scholar]
- 9.Stack WA, Mann SD, Roy AJ, et al. Randomised controlled trial of CDP571 antibody to tumour necrosis factor-α in Crohn's disease. Lancet. 1997;349:521–4. doi: 10.1016/s0140-6736(97)80083-9. [DOI] [PubMed] [Google Scholar]
- 10.Sandborn WJ, Feagan BG, Hanauer SB, et al. An engineered human antibody to TNF (CDP571) for active Crohn's disease: a randomized double-blind placebo-controlled trial. Gastroenterology. 2001;120:1330–8. doi: 10.1053/gast.2001.24042. [DOI] [PubMed] [Google Scholar]
- 11.Nathan CF. Secretory products of macrophages. J Clin Invest. 1987;79:319–26. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dentener MA, Bazil V, Von Asmuth EJ. Involvement of CD14 in lipopolysaccharide-induced tumor necrosis factor-α, IL-6 and IL-8 release by human monocytes and alveolar macrophages. J Immunol. 1993;150:2885–91. [PubMed] [Google Scholar]
- 13.Sweet MJ, Hume DA. Endotoxin signal transduction in macrophages. J Leukoc Biol. 1997;60:8–26. doi: 10.1002/jlb.60.1.8. [DOI] [PubMed] [Google Scholar]
- 14.Baeuerie PA, Baltimore D. NF-κB ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 15.Baeuerie PA. Pro-inflammatory signaling: last pieces in the NF-kappaB puzzle? Curr Biol. 1998;8:R19–R22. doi: 10.1016/s0960-9822(98)70010-7. [DOI] [PubMed] [Google Scholar]
- 16.MacKichan ML, DeFranco AL. Role of ceramide in lipopolysaccharide (LPS)-induced signaling: LPS increases ceramide rather than acting as a structural homolog. J Biol Chem. 1999;274:1767–75. doi: 10.1074/jbc.274.3.1767. [DOI] [PubMed] [Google Scholar]
- 17.Medvedev AE, Blanco JCG, Qureshi N, Vogel SN. Limited role of ceramide in lipopolysaccharide-mediated mitogen-activated protein kinase activation, transcription factor induction, and cytokine release. J Biol Chem. 1999;274:9342–50. doi: 10.1074/jbc.274.14.9342. [DOI] [PubMed] [Google Scholar]
- 18.Spiegel S, Foster D, Kolesnick R. Signal transduction through lipid second messengers. Curr Opin Cell Biol. 1996;8:159–67. doi: 10.1016/s0955-0674(96)80061-5. [DOI] [PubMed] [Google Scholar]
- 19.Yokomatsu T, Takechi H, Akiyama T, Shibuya S, Kominato T, Soeda S, Shimeno H. Synthesis and evaluation of a difluoromethylene analogues of sphingomyelin as an inhibitor of sphingomyelinase. Bioorg Med Chem Lett. 2001;11:1277–80. doi: 10.1016/s0960-894x(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 20.Yokomatsu T, Murano T, Akiyama T, Koizumi J, Shibuya S, Tsuji Y, Soeda S, Shimeno H. Synthesis of non-competitive inhibitors of sphingomyelinases with significant activity. Bioorg Med Chem Lett. 2003;13:229–36. doi: 10.1016/s0960-894x(02)00888-0. [DOI] [PubMed] [Google Scholar]
- 21.Soeda S, Iwata K, Hosoda Y, Shimeno H. Daunorubicin attenuates tumor necrosis factor-α-induced biosynthesis of plasminogen activator inhibitor-1 in human umbilical vein endothelial cells. Biochim Biophys Acta. 2001;1538:234–41. doi: 10.1016/s0167-4889(01)00073-8. [DOI] [PubMed] [Google Scholar]
- 22.Nara F, Tanaka M, Masuda-Inoue S, Yamasato Y, Doi-Yoshioka H, Suzuki-Konagai K, Kumakura S, Ogita T. Biological activities of scyphostatin, a neutral sphingomyelinase inhibitor from a discomycete, Trichopeziza mollissima. J Antibiot. 1999;52:531–5. doi: 10.7164/antibiotics.52.531. [DOI] [PubMed] [Google Scholar]
- 23.Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–91. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang FX, Kirschning CJ, Mancinelli R, et al. Bacterial lipopolysaccharide activates nuclear factor-κB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem. 1999;274:7611–14. doi: 10.1074/jbc.274.12.7611. [DOI] [PubMed] [Google Scholar]
- 25.Levade T, Jaffrezou JP. Signalling sphingomyelinases: which, where, how and why? Biochim Biophys Acta. 1999;1438:1–17. doi: 10.1016/s1388-1981(99)00038-4. [DOI] [PubMed] [Google Scholar]
- 26.Andrieu-Abadie N, Gouaze V, Salvayre R, Levade T. Ceramide in apoptosis signaling: relationship with oxidative stress. Free Radic Biol Med. 2001;31:717–28. doi: 10.1016/s0891-5849(01)00655-4. [DOI] [PubMed] [Google Scholar]
- 27.Goni FM, Alonso A. Sphingomyelinases: enzymology and membrane activity. FEBS Lett. 2002;531:38–46. doi: 10.1016/s0014-5793(02)03482-8. [DOI] [PubMed] [Google Scholar]
- 28.Marchesini N, Hannun YA. Acid and neutral sphingomyelinases. Roles and mechanisms of regulation. Biochem Cell Biol. 2004;82:27–44. doi: 10.1139/o03-091. [DOI] [PubMed] [Google Scholar]
- 29.Conroy CA, Cattell R. Infliximab treatment for Crohn's disease. Postgrad Med J. 2001;77:436–40. doi: 10.1136/pmj.77.909.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor α for Crohn's disease. N Engl J Med. 1997;337:1029–35. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 31.Schulzke J-D, Bojarski C, Zeissig S, Heller F, Gitter AH, Fromm M. Disrupted barrier function through epithelial cell apoptosis. Ann NY Acad Sci. 2006;1072:288–99. doi: 10.1196/annals.1326.027. [DOI] [PubMed] [Google Scholar]
- 32.Logan RF. Inflammatory bowel disease incidence: up, down or unchanged? Gut. 1998;42:309–11. doi: 10.1136/gut.42.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blumberg RS, Saubermann LJ, Strober W. Animal models of mucosal inflammation and their relation to human inflammatory bowel disease. Curr Opin Immunol. 1999;11:648–56. doi: 10.1016/s0952-7915(99)00032-1. [DOI] [PubMed] [Google Scholar]
- 34.Mahida YR, Patel S, Gionchetti P, Vaux D, Jewell DP. Macrophage subpopulations in lamina propria of normal and inflamed colon and terminal ileum. Gut. 1989;30:826–34. doi: 10.1136/gut.30.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimm MC, Pavli P, Van de Pol E, Doe WF. Evidence for a CD14+ population of monocytes in inflammatory bowel disease mucosa: implications for pathogenesis. Clin Exp Immunol. 1995;100:291–7. doi: 10.1111/j.1365-2249.1995.tb03667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zareie M, McKay DM, Kovarik GG, Perdue MH. Monocyte/macrophages evoke epithelial dysfunction: indirect role of tumor necrosis factor-α. Am J Physiol. 1998;275:C932–9. doi: 10.1152/ajpcell.1998.275.4.C932. [DOI] [PubMed] [Google Scholar]
- 37.Itoh N, Nagata S. A novel protein domain required for apoptosis. Mutational analysis of human Fas antigen. J Biol Chem. 1993;268:10932–7. [PubMed] [Google Scholar]
- 38.Wiegmann K, Schutze S, Machleidt T, Witte D, Kronke M. Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling. Cell. 1994;78:1005–15. doi: 10.1016/0092-8674(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 39.Cifone MG, Roncaioli P, De Maria R, Camarda G, Santoni A, Ruberti G, Testi R. Multiple pathways originate at the Fas/APO-1 (CD95) receptor. sequential involvement of phosphatidylcholine-specific phospholipase C and acidic sphingomyelinase in the propagation of the apoptotic signal. EMBO J. 1995;14:5859–68. doi: 10.1002/j.1460-2075.1995.tb00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–92. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 41.Zareie M, Singh PK, Irvine EJ, Sherman PM, McKay DM, Perdue MH. Monocyte/macrophage activation by normal bacteria and bacterial products: implications for altered epithelial function in Crohn's disease. Am J Pathol. 2001;158:1101–9. doi: 10.1016/S0002-9440(10)64057-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 43.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 44.Shirakabe K, Yamaguchi K, Shibuya H, et al. TAK1 mediates the ceramide signaling to stress-activated protein kinase/c-Jun N-terminal kinase. J Biol Chem. 1997;272:8141–4. doi: 10.1074/jbc.272.13.8141. [DOI] [PubMed] [Google Scholar]
- 45.Herfarth H, Brand K, Rath HC, Rogler G, Schölmerich J, Falk W. Nuclear factor-κB activity and intestinal inflammation in dextran sulphate sodium (DSS)-induced colitis in mice is suppressed by gliotoxin. Clin Exp Immunol. 2000;120:59–65. doi: 10.1046/j.1365-2249.2000.01184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ortega-Cava CF, Ishihara S, Rumi MA, et al. Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J Immunol. 2003;170:3977–85. doi: 10.4049/jimmunol.170.8.3977. [DOI] [PubMed] [Google Scholar]
- 47.Ohkawara T, Takeda H, Nishihira J, et al. Macrophage migration inhibitory factor contributes to the development of acute dextran sulphate sodium-induced colitis in Toll-like receptor 4 knockout mice. Clin Exp Immunol. 2005;141:412–21. doi: 10.1111/j.1365-2249.2005.02877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]