Abstract
Human leucocyte antigen (HLA)-B27 is strongly associated with spondyloarthropathies, including reactive arthritis. Several Gram-negative bacteria, such as Salmonella typhimurium, can trigger this disease. It has been suggested that peptides derived from bacterial proteins and presented by HLA-B27 to cytotoxic T lymphocytes might show molecular mimicry with autologous peptides, leading to T-cell cross-reaction and autoimmunity. Antigen presentation in Salmonella-infected cells could be modulated by changes in the composition of the proteasome, which is the major proteolytic system that generates major histocompatibility complex class I ligands. In this study we analysed whether the composition or activity of the 20S proteasome was altered upon infection of lymphoid cells by S. typhimurium. Two-dimensional gel electrophoresis failed to show any differences between the composition of 20S proteasomes from cells infected with S. typhimurium for 24 hr, relative to non-infected cells. In addition, digestions of oxidized insulin B-chain with purified 20S proteasomes from non-infected and infected cells generated the same products, indicating that the proteasomal cleavage specificity was not altered upon infection. These data indicate that infection of lymphoid cells by S. typhimurium fails to induce formation of immunoproteasomes or otherwise alter the proteolytic specificity of the 20S proteasome.
Keywords: antigens, arthritis, peptides, proteasome, Salmonella
Introduction
The onset of reactive arthritis (ReA) is caused by infection with several Gram-negative bacteria, including species of Yersinia, Campylobacter, Shigella, Chlamydia and Salmonella. The last of these is an intracellular bacterium that resides inside vacuoles. Salmonella typhimurium proliferates inside several cell types, such as macrophages and epithelial cells, but not in lymphoid cells.1,2 Spondyloarthropathies, including ReA, are strongly associated with the class I allotype human leucocyte antigen (HLA)-B27.3 The pathogenetic role of this molecule remains unknown, but several hypotheses have been proposed.4 Among them, the ‘arthritogenic peptide’ model claims that HLA-B27-restricted cytotoxic T lymphocytes (CTLs) activated against bacterial peptides might cross-react, through molecular mimicry, with endogenous peptides constitutively presented by HLA-B27, leading to autoimmunity.5
Proteasomes are the major proteolytic system generating peptide ligands of the major histocompatibility complex (MHC) class I molecules.6,7 They are located in the nucleus and cytosol and are involved in degradation of cytosolic and nuclear proteins, generally following ubiquitylation. Peptides are transported to the endoplasmic reticulum where they bind to nascent class I molecules. Some class I ligands can be directly generated by the proteasome8,9 but others require further processing.10–12
The 20S proteasome is the proteasomal catalytic core. It is a ring-barrel structure consisting of four heptameric rings. The external rings consist of seven structural subunits (α1 to α7). The internal rings consist of seven β subunits (β1 to β7) of which three are catalytic: β1, β2 and β5. These can be substituted for three interferon-γ (IFN-γ)-induced subunits: β1i, β2i and β5i, in an apparently cooperative process.13,14 Thus, there is a constitutive proteasome, containing the β1, β2 and β5 subunits, and an immunoproteasome, containing the corresponding IFN-γ-induced subunits.
It has been reported that infection of HeLa cells by S. typhimurium results in an increase of the inducible subunit β1i, and concomitant changes in the HLA-B27-bound peptide repertoire.15 Changes in the B27-bound peptide repertoire were also reported in L cells infected with Shigella flexneri.16 In contrast, in the lymphoid cell line C1R, the HLA-B27-bound peptide repertoires from S. typhimurium-infected and non-infected cells are very similar.1,17,18 Nevertheless, B27-restricted bacteria-specific CTLs isolated from synovial fluid of ReA patients killed Salmonella-infected HLA-B27-C1R targets,19 suggesting that small amounts of bacterial peptides were presented by HLA-B27 on these cells.
The question remained as to whether infection of lymphoid cells with S. typhimurium might also promote the induction of immunoproteasomes, leading to the modulation of antigen processing and HLA-B27-mediated peptide presentation. Thus, in the present work we analysed whether infection of C1R cells with S. typhimurium affects the subunit composition or activity of 20S proteasomes.
Materials and methods
Cell lines, bacteria and antibodies
HMy2.C1R (C1R) is a human lymphoid cell line with low expression of its endogenous class I molecules.20,21 The transfectant cell line expressing B*2705 has been previously described.22 Cells were cultured in Dulbecco's modified Eagles's medium (DMEM) supplemented with 7·5% heat-inactivated fetal calf serum (FCS) (both from Life Technologies, Paisley, UK). SL1344 is a virulent Salmonella serovar typhimurium strain.23 It was cultured overnight in Luria-Bertani (LB) medium without shaking. Human recombinant IFN-γ was obtained from Calbiochem (Darmstadt, Germany).
The following antibodies were used: the monoclonal antibody (mAb) W6/32 [immunoglobulin G2a (IgG2a), specific for a monomorphic HLA-A, -B, -C determinant],24 a polyclonal antiserum specific for the S. typhimurium lipopolysaccharide (LPS) (Difco, Detroit, MI), the polyclonal antibody 8016.2, which recognizes the proteasome subunit α6,25 the polyclonal antibody 8026.3, which recognizes the IFN-γ-induced subunit β1i, and cross-reacts with β5i, and the polyclonal antibody 8027.3, which recognizes the IFN-γ-induced subunit β5i. Both of these antibodies were produced by injection of the purified recombinant proteins into rabbits, as previously described.25 Antibodies PW.8840 and PW.8140, which recognize the proteasome subunits β1i and β1, respectively, were supplied by Affiniti (Mamhead, Exeter, UK). PW.8840 recognizes both the mature β1i subunit at 22 000 molecular weight (MW) and the 25 000 MW precursor form. The anti-β-actin mAb A.5316 was obtained from Sigma-Aldrich (St. Louis, MO).
Infection of B*2705-C1R cells with S. typhimurium
Large-scale infections were performed as previously described.1 Briefly, About 3 × 109 cells were grown in roller flasks, centrifuged for 10 min at 500 g and resuspended in 750 ml RPMI-1640 with 10% FCS. Bacteria (about 1011) were centrifuged and resuspended in 10 ml of the same medium, and added to the cells. Cells were mixed with bacteria and incubated at 37° for 2 hr. Then, cells were washed four times in phosphate-buffered saline (PBS) supplemented with 100 μg/ml gentamicin to eliminate extracellular bacteria, and incubated in DMEM with 5% FCS for 24 hr. Finally, cells were washed four times in PBS, and frozen at −80° until their use for proteasome purification. Alternatively, after washing, aliquots were taken to quantify the intracellular bacteria as colony-forming units (CFU) and to analyse the percentage of infected cells by immunofluorescence. The intracellular location of bacteria was confirmed by confocal and electron microscopy.1
Immunofluorescence of infected cells
Cover slips were overlaid with 1 mg/ml poly l-lysine (Sigma, St Louis, MO) for 30 min and washed three times with PBS. Infected cells were placed on cover slips, incubated for 30 min at room temperature and washed three times with PBS. Adhered cells were fixed with 3·5% paraformaldehyde (Merck, Darmstadt, Germany) in PBS for 20 min, and washed three times with PBS. Thirty-five microlitres 3% bovine serum albumin in PBS containing 0·1% saponin (both from Sigma) was added to cover slips, incubated for 5 min, and then incubated in 35 μl of a 1 : 200 dilution of rabbit anti-S. typhimurium LPS antiserum for 45 min. After this time, cells were blocked again with bovine serum albumin, and incubated with a 1 : 50 dilution of fluorescein-isothiocyanate-conjugated goat anti-rabbit IgG (Cooper Biomedical, Malvern, PA) for 45 min. After washing, cover slips were placed over Mowiol-treated slides and incubated for 1 hr at 37°.
Purification of 20S proteasomes
Proteasomes were purified from about 3 × 109 B*2705-C1R cells as previously described9,26,27 with modifications. Briefly, cells were potter-lysed in 50 mm Tris-HCl, 25 mm KCl, pH 8·0, centrifuged to 1500 g for 10 min and then ultracentrifuged for 1 hr at 100 000 g. The supernatant was loaded on a 35-ml diethylaminoethyl-cellulose (DE52) anion exchange column (Whatman, Maidstone, UK) equilibrated with buffer A (50 mm Tris-HCl, 25 mm KCl, pH 8·0). After washing the column with three volumes of equilibration buffer, elution was carried out with buffer B (50 mm Tris-HCl, 0·3 m KCl, pH 8). Protein-containing fractions were detected using the Bradford method, diluted three times in buffer A, concentrated in a 7-ml DE52 column equilibrated with the same buffer, washed with three volumes of the buffer, and eluted in buffer B. Protein-containing fractions were loaded onto a gradient of 10–30% glycerol in 50 mm Tris-HCl, 25 mm KCl, 1 m urea, pH 8·0, and centrifuged for 18 hr at 200 000 g. Five-drop fractions were taken, and analysed by a 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteasome-containing fractions were pooled, and subjected to anion-exchange chromatography in a monoQ SR5/5 column (Pharmacia, Uppsala, Sweden) at a flow rate of 0·5 ml/min, as follows: isocratic conditions with buffer A (50 mm Tris-HCl, 50 mm KCl, pH 8·0) for 10 min, followed by a linear gradient of 0–30% buffer B (50 mm Tris-HCl, 0·5 m KCl pH 8·0) for 5 min, and a linear gradient of 30–100% buffer B for another 30 min. Purity of fractions was assessed by SDS-PAGE and staining with Coomassie blue or silver. Aliquots were stored at −80°.
Western blot analysis
About 2 μg purified 20S proteasomes from non-infected and infected cells (24 hr postinfection time) were loaded in 12% SDS-PAGE gels. Proteins were transferred to polyvinylidene difluoride membranes. These were blocked for 30 min with 5% skimmed milk in 0·1% Tween-20 in PBS (T-PBS). β1i and β5i subunits were detected with the polyclonal antibodies 8026.3 and 8027.3, respectively. Second antibody was a horseradish peroxidase-labelled goat anti-rabbit. Detection was performed using ECLTM (both from Amersham).
In other experiments B*2705-C1R cells were grown in the presence or absence of IFN-γ (100 U/ml) for 24 hr. About 106 cells were boiled in SDS-PAGE loading buffer for 10 min and loaded in 12% SDS-PAGE gels. Proteins were transferred to nitrocellulose membranes. These were blocked for 60 min with 5% skimmed milk in T-PBS. β1i and β1 were detected with monoclonal antibodies PW.8840 and PW.8140, respectively, and with secondary antibody as above.
Two-dimensional gel electrophoresis of 20S proteasomes
Samples of purified 20S proteasomes from S. typhimurium-infected and control cells were loaded by hydration of immobilized pH gradient strips (IPG), non-linear pH 3–10, 18 cm in length (Pharmacia, Uppsala, Sweden), diluted previously up to a total volume of 350 μl in 6 m urea, 2 m thiourea, 2% CHAPS, ampholineTM pH 3–10, 1 mm tris-[2-carboxymethyl]-phosphine-HCl, and 0·1% bromophenol blue. In the first dimension, isoelectrofocusing (IEF) was performed in a IPGPhor (Pharmacia) under the following conditions: 30 V for 6 hr, 60 V for 6 hr, 500 V for 30 min, 1000 V for 30 min, a gradient of 1000–8000 V for 30 min, and 8000 V up to 32 000 Vh. After IEF, strips were equilibrated in 6 m urea, 30% glycerol, 2% SDS, and 0·1% bromophenol blue, twice for 20 min. Dithiothreitol (2%), and 4% iodoacetamide were added in the first and second equilibration steps, respectively. The second dimension was performed using 12·5% SDS-PAGE. Gels were stained with silver nitrate, scanned and analysed using the software ImageMaster (Pharmacia).
In-gel digestion of proteins
Protein spots were cut manually and processed automatically in a digestor InvestigatorTM ProGest (Genomic Solutions, Cambridgeshire, UK). Samples were washed with 25 mm ammonium bicarbonate and then with 100% acetonitrile, reduced with 10 mm dithiothreitol in 25 mm ammonium bicarbonate, alkylated with 100 mm iodoacetamide in 50 mm ammonium bicarbonate, washed in 50 mm ammonium bicarbonate and then with 100% acetonitrile, and dried with nitrogen. Modified pig trypsin (Promega, Madison, WI) was added to dried samples to a final concentration of 16 ng/μl in 25 mm ammonium bicarbonate and digested for 12 hr at 37°. Peptides were eluted with 100 μl 33% acetonitrile, 25 mm ammonium bicarbonate and 10% formic acid, and subjected to matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry (MS) fingerprinting analysis to identify each proteasome subunit.
Digestion of oxidized insulin B-chain
Ten micrograms of oxidized insulin B-chain (Sigma) was digested with 1 μg purified 20S proteasome for 24 hr at 37°. Digestion was stopped with trifluoroacetic acid 0·1% in water. Digestion products were fractionated as described elsewhere.8
Mass spectrometry
The MALDI-TOF MS analysis of proteasomal digestions was performed using a calibrated Reflex (Brucker Daltonics, Bremen, Germany) operating in positive ion reflectron mode as previously described.9 When necessary peptides were sequenced in an electrospray/ion trap mass spectrometer (Finnigan Thermoquest, San José, CA), as previously described.28,29
Results
C1R cells express a mixture of constitutive proteasome and immunoproteasome
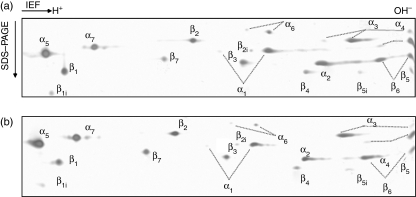
The subunit composition of 20S proteasome purified from B*2705-C1R cells was analysed by two-dimensional gel electrophoresis, and each subunit was identified by MS fingerprinting (Fig. 1a). In this analysis, both the constitutive proteasome subunits (β1, β2 and β5) and, in lower amounts, the corresponding inducible ones (β1i, β2i and β5i) were observed. Thus, C1R cells express a mixture of constitutive proteasome and immunoproteasome, but the former is more abundant.
Figure 1.
Two-dimensional gels of 20S proteasomes from non-infected (a) and S. typhimurium-infected (b) B27-C1R lymphoid cells. Proteasome purifications were performed as described in the Materials and methods. About 10 μg of proteasome were analysed by two-dimensional electrophoresis, and identification of spots were performed by mass spectrometry after trypsin digestion as described in the Materials and methods.
Infection with S. typhimurium does not change the composition of the 20S proteasome in lymphoid cells
To study the effects of Salmonella infection on the composition of 20S proteasomes, B*2705-C1R cells were infected with S. typhimurium with a postinfection time of 24 hr. C1R cells were used for two reasons: first, because this is a cell line that grows well in suspension, and it is possible to infect several billion cells, which are required for purification of the 20S proteasome. Second because the infection of C1R cells with Salmonella induces some changes in the HLA-B27-bound peptide repertoire that are recognized by T cells.19
We chose the SL1344 strain because it is perhaps the most extensively used serovar typhimurium strain for in vivo and in vitro infections. Although it is a His-negative strain, assays performed in epithelial and macrophage cell lines have shown that histidine auxotrophy does not affect proliferation of this particular strain inside these cells. Bacteria were grown in an overnight non-shaking culture to obtain highly motile and fully infective bacteria. Quantification of intracellular bacteria was performed measuring the CFU recovered after cell lysis with Triton X-100. The percentage of infected cells was estimated by immunofluorescence with an antiserum recognizing the Salmonella LPS. About 50% of the cells were infected, with a mean number of two intracellular bacteria per cell.
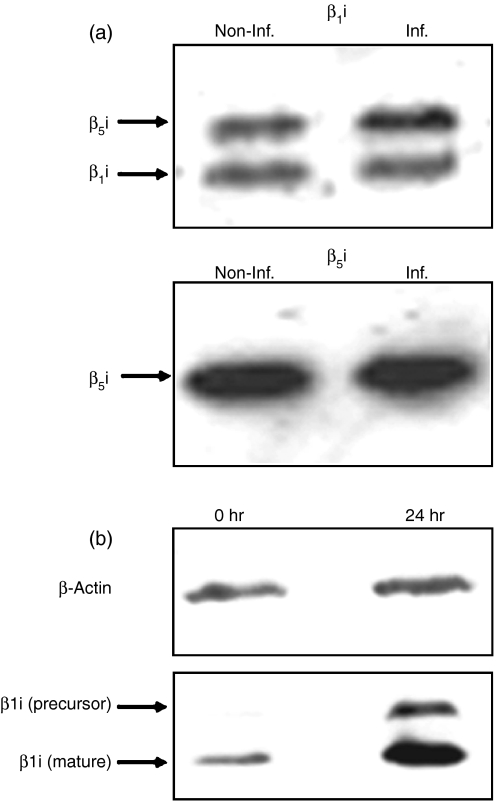
The 20S proteasomes from infected and non-infected B*2705-C1R cells were purified, and analysed by two-dimensional gel electrophoresis and MS fingerprinting. This analysis showed no significant difference in the 20S proteasome composition between non-infected and infected cells (Fig. 1). In addition, Western blot analysis using anti-β1i and anti-β5i antibodies did not show any change in the expression of these components between the proteasomes from non-infected and 24 hr-infected cells (Fig. 2a). The absence of immunoproteasome induction was not the result of an intrinsic inability of C1R cells to modulate the inducible subunits at this postinfection time, because the induction with IFN-γ for 24 hr produced an increase of β1i detectable by Western blot (Fig. 2b). These results indicate that S. typhimurium infection does not induce any significant changes in the proteasome/immunoproteasome ratio in the human lymphoid cells tested.
Figure 2.
Western blot analysis of proteasome subunits. (a) Western blot analysis of inducible β-subunits of the 20S proteasome from B27-C1R cells. About 2 μg purified 20S proteasomes from non-infected and infected (24 hr postinfection time) B27-C1R cells were subjected to 12% SDS-PAGE. Proteins were transferred to membranes, and analysed by Western blot. Upper panel: Western blot using the polyclonal antibody 8026.3, which recognizes the inducible subunit β1i, and cross-reacts with β5i. The intensity ratio of the β1i and the cross-reactive β5i bands between infected (Inf.) and non-infected (Non-Inf.) cells were 0·96 : 1 and 1·36 : 1, respectively. Lower panel: Western blot using the polyclonal antibody 8027.3, which recognizes the inducible subunit β5i. The intensity ratio of the bands corresponding to Inf. and Non-Inf. cells was 1·03 : 1. (b) Western blot analysis of constitutive (β1) and inducible (β1i) subunits in control and IFN-γ-induced C1R-05 cells. About 106 cells were lysed in SDS-PAGE loading buffer and subjected to 12% SDS-PAGE. Constitutive and induced subunits were detected as described in the Materials and methods. β-actin was used as a housekeeping protein. The intensity of the β1i bands, after IFN-γ stimulation was increased 2·84-fold, relative to non-stimulated cells, after normalizing to the intensity of the corresponding actin bands.
The chromatographic profiles of the digestion products of insulin B chain with 20S proteasome from Salmonella-infected and non-infected cells are indistinguishable
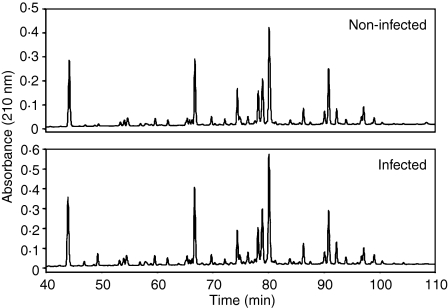
Digestions of oxidized insulin B chain have been previously used to establish the specificity differences between constitutive proteasomes and immunoproteasomes.30 Thus, 10 μg insulin B chain were digested with purified 20S proteasomes from non-infected and Salmonella-infected B*2705-C1R cells. These were infected and cultured for 24 hr after infection. About three bacteria per cell were counted, and the percentage of infected cells was about 50%.
Digestion products were fractionated by high-performance liquid chromatography (HPLC). The corresponding chromatographic profiles were very similar (Fig. 3). A small peak was found in the chromatogram of infected cells around a retention time of 49 min, which was not present in the control, but the corresponding HPLC fraction did not show any ion peak upon MALDI-TOF analysis (see below). Thus, the HPLC profiles of the digestion products failed to reveal any significant difference in the specificity of the 20S proteasomes following infection with S. typhimurium. Furthermore, no differences were found when a precursor peptide spanning residues 120/146 of the proteasome C5 subunit9 was digested with 20S proteasome from non-infected and infected cells (data not shown).
Figure 3.
HPLC chromatography of digestion products from oxidized insulin B chain with 20S proteasomes. About 10 μg substrate were digested with 1 μg purified 20S proteasomes from non-infected and S. typhimurium-infected B27-C1R cells for 24 hr at 37°. Digestion mixtures were fractionated by HPLC.
Insulin B chain digestion with 20S proteasome from Salmonella-infected and non-infected cells generates the same peptide products
Although the HPLC chromatograms of products obtained in digestions of insulin B chain with proteasomes from non-infected and infected C1R cells were very similar, this did not rule out the possibility that differentially produced peptides could be concealed in HPLC peaks containing multiple coeluting peptides. To test this possibility, a systematic analysis of the HPLC fractions corresponding to absorbance peaks was carried out by MALDI-TOF MS. When necessary, individual peptides were sequenced by quadrupole ion trap nanoelectrospray MS/MS. To quantify the yield of an individual peptide, the absorbance at 210 nm of the corresponding HPLC peak was considered, and was normalized to take into account peptidic length differences. When several peptides coeluted, the percentage of each peptide in the absorbance peak was estimated on the basis of their respective ion peak signal intensities in the MALDI-TOF spectra. This is only an approximation, because ion peak intensity does not necessarily correlate with peptide abundance. When a peptide eluted in more than one fraction, its total estimated abundance in all the fractions was considered.
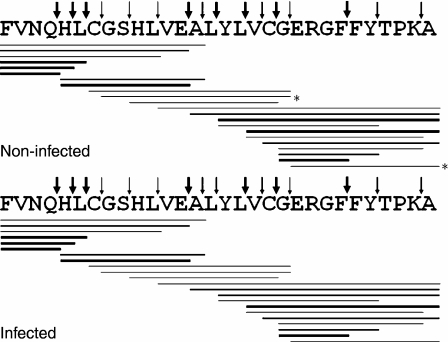
Many peptide bonds were hydrolysed at 24 hr digestion time. Figure 4 is a schematic representation of the peptides generated, and their estimated yields are shown in Table 1. The major cleavage sites were after Gln4, His5, Leu6, Glu13, Leu15, Leu17, Cys19 and Phe24. Various other bonds were cleaved less efficiently (Table 1). Cleavage efficiency at nearly all cleavage sites was very similar with 20S proteasome from infected or non-infected cells, except for a few of the peptide bonds cleaved with low efficiency (Table 1).
Figure 4.
Digestion pattern of oxidized insulin B chain by purified 20S proteasomes from non-infected and infected cells. About 10 μg substrate was digested with 1 μg proteasomes for 24 hr at 37°. Digestion mixtures were fractionated by HPLC, and absorbance peaks were analysed by MALDI-TOF or electrospray ion trap mass spectrometry. Thick, medium and thin lines correspond to peptides recovered at >4%, 1–4% and <1% yield of the total digest, respectively. Only peptides recovered with ≥0·2% yield are indicated. Thick, medium and thin arrows indicate cleavage sites that generated peptides with total yields >5%, 1–5% and <1% of the total digest, respectively. Peptides labelled with asterisks were found with an amount less than the 0·2% of the total of digest, but were included because in the counterpart they were recovered at ≥0·2%.
Table 1.
Proteasomal cleavage of insulin oxidized B chain1
| Cleavage after | Non-infected yield (%) | Infected yield (%) | Ratio non-infected : infected |
|---|---|---|---|
| Phe1 | Not observed | Not observed | – |
| Val2 | Not observed | Not observed | – |
| Asn3 | Not observed | Not observed | – |
| Gln4 | 31·3 | 24·2 | 1·3 |
| His5 | 7·0 | 10·0 | 0·7 |
| Leu6 | 11·2 | 11·0 | 1·0 |
| Cys7 | 0·1 | 0·2 | 0·5 |
| Gly8 | Not observed | Not observed | – |
| Ser9 | 0·2 | 0·3 | 0·7 |
| His10 | 0·1 | 0·1 | 1·0 |
| Leu11 | 0·8 | 0·9 | 0·9 |
| Val12 | Not observed | Not observed | – |
| Glu13 | 16·8 | 15·1 | 1·1 |
| Ala14 | 1·5 | 1·6 | 0·9 |
| Leu15 | 5·7 | 4·2 | 1·4 |
| Tyr16 | Not observed | Not observed | – |
| Leu17 | 10·7 | 9·9 | 1·1 |
| Val18 | 3·6 | 4·2 | 0·9 |
| Cys19 | 8·4 | 9·0 | 0·9 |
| Gly20 | 0·4 | 0·6 | 0·7 |
| Glu21 | 0·1 | 0·2 | 0·5 |
| Arg22 | Not observed | Not observed | – |
| Gly23 | 0·1 | Not observed | – |
| Phe24 | 5·4 | 6·2 | 0·9 |
| Phe25 | Not observed | 0·1 | – |
| Tyr26 | 2·8 | 2·4 | 1·2 |
| Thr27 | 0·1 | Not observed | – |
| Pro28 | 0·2 | 0·1 | 2 |
| Lys29 | 1·1 | 1·0 | 1·1 |
Cleavage yield at a peptide bond was estimated as the total percentage of peptides in the digestion mixture resulting from cleavage at that bond.
These results indicate that S. typhimurium infection of B*2705-C1R cells does not affect the specificity of the 20S proteasome after 24 hr infection. This is consistent with the lack of changes in subunit composition of the 20S proteasome observed at the same postinfection time.
Discussion
Although lymphoid cells are not the physiological target of S. typhimurium they were used in this study for two reasons. First, because the overwhelming majority of studies on HLA-B27-bound peptides have been carried out with these cells. Second, and more important, because lymphoid cells infected with S. typhimurium can be recognized by HLA-B27-restricted bacteria-specific CTLs as early as 4 hr after infection,19 implying that bacterial peptides have been processed and presented by HLA-B27 on the cell surface. The amount of bacterial antigen, although sufficient for CTL recognition, is presumably quite small because MS analysis of the HLA-B27-bound peptide repertoire failed to reveal virtually any peptide differentially expressed on infected cells.1,17,18 The nature of HLA-B27-restricted bacterial peptides that are relevant to the CTL response in patients with Salmonella-induced ReA remains unknown. Any putative alterations in the HLA class I antigen processing pathway following intracellular infection by S. typhimurium could influence the presentation of both the bacterial peptide antigens and of some of the endogenous HLA-B27 self-ligands. In this study we specifically asked whether Salmonella infection of lymphoid cells might influence the structure and/or the activity of the 20S proteasome at infection times sufficient for antigen presentation to occur. The C1R cell line used in our study contained a mixture of constitutive proteasome and immunoproteasome, in which the former was predominant. This pattern was not altered even at 24 hr after infection, as estimated by two-dimensional gel electrophoresis and Western blot analysis. These experiments do not rule out the possibility that a very small induction of immunoproteasome subunits might occur, but argue against any significant changes in the proteasome/immunoproteasome balance occurring upon infection.
These results are in contrast with a previous report15 in which infection of HeLa cells with S. typhimurium increased the reverse transcription-polymerase chain reaction values of the β1i, β2i and β5i subunits, and the amount of β1i protein as detected by Western blot analysis, although in that study the expected changes in the mature proteasomes purified from infected cells were not analysed. These differences might be attributed to the different cell lines used in both studies, and to the different behaviour of the bacteria in both cell types, because S. typhimurium proliferates inside HeLa cells, but not, or very little, inside C1R cells.
The cleavage specificity of the 20S proteasome, as assessed with a synthetic substrate previously used in proteasome activity studies,30–32 was not altered 24 hr after infection. These results suggest that bacterial antigen presentation in lymphoid cells occurs without any significant changes of the 20S proteasome following Salmonella infection. Absence of proteasomal changes also suggests that the proteasome-mediated processing of endogenous self-proteins should not be significantly altered. This is in agreement with previous reports indicating that intracellular Salmonella infection does not change the B27-bound peptide repertoires in lymphoid cells.1,17,18 It can be argued that a postinfection time of 24 hr might be too short to allow for a significant increase of immunoproteasome levels, but we were interested in knowing whether such changes can be detectable at times in which HLA-B27-restricted bacterial antigen presentation is known to take place. In C1R cells a postinfection time of 4 hr was enough to make infected cells a target for bacteria-specific CTLs.1 Furthermore, C1R cells were able to induce the immunoproteasome subunit β1i after incubation for 24 hr with IFN-γ, indicating the ability of this cell line to modulate the proteasome subunit composition at the postinfection times used in this study.
The class I antigen-processing pathway is complex, with various steps that together determine the presentation of any given ligand, including the generation of the ligand or N-terminally extended precursors of the proteasome, transport to the endoplasmic reticulum through transporter associated with antigen processing (TAP) and amino-peptidase-mediated trimming inside the lumen of endoplasmic reticulum.9 In this work we analysed the influence of Salmonella infection on the composition and activity of the 20S proteasome. In addition, although specific T-cell responses against Salmonella-infected C1R cells have been described even at a postinfection time of 4 hr,19 we increased the postinfection time to 24 hr. Even so, our data indicate that, although the effects produced by salmonella infection on B cells are enough for a specific T-cell response, the corresponding epitopes could not be detected using biochemical techniques. Together, our results indicate that infection of lymphoid cells with the arthritogenic bacterium S. typhimurium affects neither the composition nor the specificity of the 20S proteasome at infection times long enough for HLA class I-mediated antigen presentation, and suggest that changes in the generation of antigenic peptides based on modification of the proteasome are unlikely.
The percentage of infected cells was about 50% after a postinfection time of 24 hr. Thus, we cannot rule out that minor differences in the composition or activity of the 20S proteasome might be overlooked in our analysis. Nevertheless, our data suggest that Salmonella infection might induce minor changes in the B27-bound peptide repertoire but not a significant alteration of proteasome mediated processing.
The nature of the bacterial peptides presented by HLA-B27 after Salmonella infection and their processing is unknown. A limited number of bacterial proteins are injected into the cytosol through the type III secretion system33–35 and are degraded by the proteasome.36 Whether other bacterial proteins or peptides reach the class I processing-loading pathway is still unclear.
Our results concern only lymphoid cells and cannot be readily generalized to other cell types, such as macrophages or dendritic cells. However, the fact that CTL from Salmonella-induced ReA patients recognize infected C1R cells19 strongly suggests that the bacterial antigens eliciting the HLA-B27-restricted CTL response in vivo are the same as those presented by HLA-B27 on infected lymphoid cells.
Acknowledgments
This work was supported by grants SAF2002/00125, SAF2003/02213 and SAF2005-03188 from the Ministry of Science and Technology and 08.3/0005/2001.1 from the Comunidad Autónoma de Madrid to J.A.L.C. and SAF2002-00566 and CAM 08.5/0041/2002 to J.G.C. and EME2006-26 from the V.A.D. to P.A. We thank the Fundación Ramón Areces for an institutional grant to the Centro de Biología Molecular Severo Ochoa. We thank Anabel Marina (Centro de Biología Molecular Severo Ochoa), and Juan Antonio López and Francisco García del Portillo (Centro Nacional de Biotecnología) for assistance in MS, two-dimensional electrophoresis, and Salmonella infections, respectively.
Abbreviations
- C1R
HMy2.C1R
- CFU
colony-forming units
- CTL
cytotoxic T lymphocyte
- DMEM
Dulbecco's modified Eagle's medium
- FCS
fetal calf serum
- IEF
isoelectrofocusing
- IFN-γ
interferon-γ
- IPG
immobilized pH gradient
- LB
Luria–Bertani medium
- LPS
lipopolysaccharide
- mAb
monoclonal antibody
- MALDI-TOF
matrix-assisted laser desorption/ionization time of flight
- MHC
major histocompatibility complex
- MS
mass spectrometry
- PBS
phosphate-buffered saline
- ReA
reactive arthritis
References
- 1.Ramos M, Alvarez I, Garcia-del-Portillo F, Lopez de Castro JA. Minimal alterations in the HLA-B27-bound peptide repertoire induced upon infection of lymphoid cells with Salmonella typhimurium. Arthritis Rheum. 2001;44:1677–88. doi: 10.1002/1529-0131(200107)44:7<1677::AID-ART292>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 2.Verjans GM, Ringrose JH, van Alphen L, Feltkamp TE, Kusters JG. Entrance and survival of Salmonella typhimurium and Yersinia enterocolitica within human B- and T-cell lines. Infect Immun. 1994;62:2229–35. doi: 10.1128/iai.62.6.2229-2235.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brewerton DA, Hart FD, Nicholls A, Caffrey M, James DC, Sturrock RD. Ankylosing spondylitis and HL-A 27. Lancet. 1973;1(7809):904–7. doi: 10.1016/s0140-6736(73)91360-3. [DOI] [PubMed] [Google Scholar]
- 4.Kingsley G, Sieper J. Current perspectives in reactive arthritis. Immunol Today. 1993;14:387–91. doi: 10.1016/0167-5699(93)90139-C. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin R, Parham P. Guilt by association: HLA × B27 and ankylosing spondylitis. Immunol Today. 1990;11:137–42. doi: 10.1016/0167-5699(90)90051-a. [DOI] [PubMed] [Google Scholar]
- 6.Seemuller E, Lupas A, Stock D, Lowe J, Huber R, Baumeister W. Proteasome from Thermoplasma acidophilum: a threonine protease. Science. 1995;268(5210):579–82. doi: 10.1126/science.7725107. [DOI] [PubMed] [Google Scholar]
- 7.Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–71. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez I, Sesma L, Marcilla M, Ramos M, Marti M, Camafeita E, de Castro JA. Identification of novel HLA-B27 ligands derived from polymorphic regions of its own or other class I molecules based on direct generation by 20 S proteasome. J Biol Chem. 2001;276:32729–37. doi: 10.1074/jbc.M104663200. [DOI] [PubMed] [Google Scholar]
- 9.Paradela A, Alvarez I, Garcia-Peydro M, Sesma L, Ramos M, Vazquez J, Lopez De Castro JA. Limited diversity of peptides related to an alloreactive T cell epitope in the HLA-B27-bound peptide repertoire results from restrictions at multiple steps along the processing-loading pathway. J Immunol. 2000;164:329–37. doi: 10.4049/jimmunol.164.1.329. [DOI] [PubMed] [Google Scholar]
- 10.Craiu A, Akopian T, Goldberg A, Rock KL. Two distinct proteolytic processes in the generation of a major histocompatibility complex class I-presented peptide. Proc Natl Acad Sci USA. 1997;94:10850–5. doi: 10.1073/pnas.94.20.10850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoltze L, Dick TP, Deeg M, Pommerl B, Rammensee HG, Schild H. Generation of the vesicular stomatitis virus nucleoprotein cytotoxic T lymphocyte epitope requires proteasome-dependent and -independent proteolytic activities. Eur J Immunol. 1998;28:4029–36. doi: 10.1002/(SICI)1521-4141(199812)28:12<4029::AID-IMMU4029>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 12.Reits E, Neijssen J, Herberts C, Benckhuijsen W, Janssen L, Drijfhout JW, Neefjes J. A major role for TPPII in trimming proteasomal degradation products for MHC class I antigen presentation. Immunity. 2004;20:495–506. doi: 10.1016/s1074-7613(04)00074-3. [DOI] [PubMed] [Google Scholar]
- 13.Griffin TA, Nandi D, Cruz M, Fehling HJ, Kaer LV, Monaco JJ, Colbert RA. Immunoproteasome assembly: cooperative incorporation of interferon gamma (IFN-gamma)-inducible subunits. J Exp Med. 1998;187:97–104. doi: 10.1084/jem.187.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groettrup M, Standera S, Stohwasser R, Kloetzel PM. The subunits MECL-1 and LMP2 are mutually required for incorporation into the 20S proteasome. Proc Natl Acad Sci USA. 1997;94:8970–5. doi: 10.1073/pnas.94.17.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maksymowych WP, Ikawa T, Yamaguchi A, et al. Invasion by Salmonella typhimurium induces increased expression of the LMP, MECL, and PA28 proteasome genes and changes in the peptide repertoire of HLA-B27. Infect Immun. 1998;66:4624–32. doi: 10.1128/iai.66.10.4624-4632.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boisgerault F, Mounier J, Tieng V, et al. Alteration of HLA-B27 peptide presentation after infection of transfected murine L cells by Shigella flexneri. Infect Immun. 1998;66:4484–90. doi: 10.1128/iai.66.9.4484-4490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ringrose JH, Yard BA, Muijsers A, Boog CJ, Feltkamp TE. Comparison of peptides eluted from the groove of HLA-B27 from Salmonella infected and non-infected cells. Clin Rheumatol. 1996;15(Suppl. 1):74–8. doi: 10.1007/BF03342652. [DOI] [PubMed] [Google Scholar]
- 18.Ringrose JH, Meiring HD, Speijer D, Feltkamp TE, van Els CA, de Jong AP, Dankert J. Major histocompatibility complex class I peptide presentation after Salmonella enterica serovar typhimurium infection assessed via stable isotope tagging of the B27-presented peptide repertoire. Infect Immun. 2004;72:5097–105. doi: 10.1128/IAI.72.9.5097-5105.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermann EYuDT, Meyer zum Buschenfelde KH, Fleischer B. HLA-B27-restricted CD8 T cells derived from synovial fluids of patients with reactive arthritis and ankylosing spondylitis. Lancet. 1993;342(8872):646–50. doi: 10.1016/0140-6736(93)91760-j. [DOI] [PubMed] [Google Scholar]
- 20.Storkus WJ, Howell DN, Salter RD, Dawson JR, Cresswell P. NK susceptibility varies inversely with target cell class I HLA antigen expression. J Immunol. 1987;138:1657–9. [PubMed] [Google Scholar]
- 21.Zemmour J, Little AM, Schendel DJ, Parham P. The HLA-A,B ‘negative’ mutant cell line C1R expresses a novel HLA-B35 allele, which also has a point mutation in the translation initiation codon. J Immunol. 1992;148:1941–8. [PubMed] [Google Scholar]
- 22.Calvo V, Rojo S, Lopez D, Galocha B, Lopez de Castro JA. Structure and diversity of HLA-B27-specific T cell epitopes. Analysis with site-directed mutants mimicking HLA-B27 subtype polymorphism. J Immunol. 1990;144:4038–45. [PubMed] [Google Scholar]
- 23.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291(5812):238–9. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 24.Barnstable CJ, Bodmer WF, Brown G, Galfre G, Milstein C, Williams AF, Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978;14:9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- 25.Arribas J, Arizti P, Castano JG. Antibodies against the C2 COOH-terminal region discriminate the active and latent forms of the multicatalytic proteinase complex. J Biol Chem. 1994;269:12858–64. [PubMed] [Google Scholar]
- 26.Arribas J, Castano JG. Kinetic studies of the differential effect of detergents on the peptidase activities of the multicatalytic proteinase from rat liver. J Biol Chem. 1990;265:13969–73. [PubMed] [Google Scholar]
- 27.Ruiz de Mena I, Mahillo E, Arribas J, Castano JG. Kinetic mechanism of activation by cardiolipin (diphosphatidylglycerol) of the rat liver multicatalytic proteinase. Biochem J. 1993;296:93–7. doi: 10.1042/bj2960093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marina A, Garcia MA, Albar JP, Yague J, Lopez de Castro JA, Vazquez J. High-sensitivity analysis and sequencing of peptides and proteins by quadrupole ion trap mass spectrometry. J Mass Spectrom. 1999;34:17–27. doi: 10.1002/(SICI)1096-9888(199901)34:1<17::AID-JMS746>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Yague J, Vazquez J, Lopez de Castro JA. A single amino acid change makes the peptide specificity of B*3910 unrelated to B*3901 and closer to a group of HLA-B proteins including the malaria-protecting allotype HLA-B53. Tissue Antigens. 1998;52:416–21. doi: 10.1111/j.1399-0039.1998.tb03067.x. [DOI] [PubMed] [Google Scholar]
- 30.Ehring B, Meyer TH, Eckerskorn C, Lottspeich F, Tampe R. Effects of major-histocompatibility-complex-encoded subunits on the peptidase and proteolytic activities of human 20S proteasomes. Cleavage of proteins and antigenic peptides. Eur J Biochem. 1996;235:404–15. doi: 10.1111/j.1432-1033.1996.00404.x. [DOI] [PubMed] [Google Scholar]
- 31.Dick LR, Moomaw CR, DeMartino GN, Slaughter CA. Degradation of oxidized insulin B chain by the multiproteinase complex macropain (proteasome) Biochemistry. 1991;30:2725–34. doi: 10.1021/bi00224a022. [DOI] [PubMed] [Google Scholar]
- 32.Wenzel T, Eckerskorn C, Lottspeich F, Baumeister W. Existence of a molecular ruler in proteasomes suggested by analysis of degradation products. FEBS Lett. 1994;349:205–9. doi: 10.1016/0014-5793(94)00665-2. [DOI] [PubMed] [Google Scholar]
- 33.Kubori T, Matsushima Y, Nakamura D, Uralil J, Lara-Tejero M, Sukhan A, Galan JE, Aizawa SI. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science. 1998;280(5363):602–5. doi: 10.1126/science.280.5363.602. [DOI] [PubMed] [Google Scholar]
- 34.Galan JE, Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science. 1999;284(5418):1322–8. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- 35.Russmann H, Shams H, Poblete F, Fu Y, Galan JE, Donis RO. Delivery of epitopes by the Salmonella type III secretion system for vaccine development. Science. 1998;281(5376):565–8. doi: 10.1126/science.281.5376.565. [DOI] [PubMed] [Google Scholar]
- 36.Kubori T, Galan JE. Temporal regulation of salmonella virulence effector function by proteasome-dependent protein degradation. Cell. 2003;115:333–42. doi: 10.1016/s0092-8674(03)00849-3. [DOI] [PubMed] [Google Scholar]