Abstract
Microbial adjuvants are essential for the development of T-cell-dependent antibody production, recall T-cell proliferation and interferon-γ production following immunization with protein antigens. Using an adoptive transfer approach, we showed that the adjuvant lipopolysaccharide enhanced the frequency of cells producing interleukin-2, enhanced clonal expansion by antigen-specific CD4 T cells and increased CD86 and interleukin-1α production by antigen-presenting cells. All of these effects were dependent on Toll-like receptor-4 (TLR4) expression by cells other than the antigen-specific CD4 T cells. The ability of lipopolysaccharides to increase the number of antigen-specific CD4 T cells that survive after immunization probably explains the previous finding that antigen-specific proliferation by T cells from normal mice depends on previous exposure to antigen and adjuvant.
Keywords: adjuvants, cellular proliferation, costimulation, lipopolysaccharide, T lymphocytes
Introduction
The pioneering work of Freund1 and Dresser2 established that efficient adaptive immune responses to purified foreign protein antigens only occur when microbial products, known as adjuvants, are present. Recently it has become clear that certain adjuvant molecules are sensed by cells of the innate immune system via Toll-like receptors (TLR).3 For example, TLR4 plays a role, along with CD14 and MD2, in the sensing of bacterial lipopolysaccharides (LPS).3 TLR signalling culminates in the activation of nuclear factor κB transcription factors,3 which drive the expression of inflammatory cytokines and costimulatory ligands such as interleukin-1 (IL-1), CD80 and CD86 in antigen-presenting cells (APC).3 Since these molecules have been shown to enhance the capacity of APC to activate naive T cells,4 it is possible that their induction contributes to the effects of adjuvants on adaptive immunity. This possibility is supported by the finding that recall antigen-specific T-cell proliferation and interferon-γ (IFN-γ) production in normal mice immunized with foreign protein antigen in complete Freund's adjuvant (CFA), are absent in myeloid differentiation primary-response protein 88 (MyD88)-deficient mice in which TLR signalling is defective.5
Previously we showed that naive CD4 T cells produce more IL-2 and accumulate in greater numbers in the draining lymph nodes following subcutaneous injection of antigen and LPS or IL-1 than in response to antigen alone.6,7 It is therefore possible that defective clonal expansion explains the poor recall T-cell proliferation observed in MyD88-deficient mice after immunization with antigen and CFA. Here we tested this possibility by tracking naive antigen-specific CD4 T cells from T-cell receptor (TCR) transgenic mice after adoptive transfer into TLR4-deficient recipients, which were then injected with antigen and LPS.
Materials and methods
Mice
Male and female C57BL/10 (B10), C57BL/6 (B6) or C57BL/10ScCr mice (TLR4-deficient) were purchased from The Jackson Laboratory (Bar Harbor, ME). All of the B6-derived or B10-derived strains express H-2b major histocompatibility complex (MHC) molecules. SM1 RAG-2-deficient B6 mice expressing CD90.1 and a transgenic T-cell receptor (TCR) specific for Salmonella typhimurium FliC peptide 427–441:I-Ab complexes, were produced in our laboratory.8 OT-II B6 mice expressing CD90.1 and a transgenic TCR specific for ovalbumin (OVA) peptide 323–339:I-Ab complexes were originally provided by Leo Lefrancois.9 All TCR transgenic strains and C57BL/10ScCr mice were bred under specific pathogen-free conditions in our facility.
Antigens
FliC peptide 427–441 was solubilized to a concentration of 15 mg/ml in dimethyl sulphoxide. OVA peptide 323–339 was solubilized in phosphate-buffered saline (PBS) to a concentration of 5 mg/ml. Escherichia coli J5 LPS (List Biologicals Laboratories, Campbell, CA) was re-extracted through an additional phenol/water precipitation step to remove contaminating lipopeptides as previously described.10 After re-extraction, the LPS concentration was established using a Limulus assay (Associates of Cape Cod, Falmouth, MA). Ten to 100 μg peptide with or without 25–50 μg LPS was injected intravenously (i.v.) into each recipient in 0·25 ml PBS. Mice were also injected in the tail vein with a dead non-virulent S. typhimurium strain SL3261. Bacteria were grown overnight at 37° in Luria-Bertani (LB) broth without shaking, diluted in PBS for estimation of bacterial concentration using a spectrophotometer and heat-killed at 65° for 1 hr at 1 × 109 colony-forming units/ml. Each recipient mouse was inoculated with 1 × 108 heat-killed bacteria in 0·25–0·5 ml 2 days after SM1 T-cell transfer. The negative control Salmonella strain BC490 did not express the FliC protein.
Staining methods and reagents
Spleens were collected on ice 6 hr after mice were pulsed with i.v. antigen with or without LPS. To prevent loss of cytokines during processing, single-cell suspensions in complete Eagle–Hank's amino acid (EHAA) medium (Invitrogen, Carlsbad, CA) were mixed 1 : 1 (v/v) with 4% formaldehyde in PBS for 20 min at room temperature. Cells were then washed with PBS, Fc-blocked with anti-FcR monoclonal antibody (mAb) 2.4G2 for 20 min and permeabilized with a buffer containing 0·3% saponin and 25% fetal bovine serum (FBS). Surface and intracellular staining were performed simultaneously for 30 min using phycoerythrin-labelled anticytokine or anti-CD86 mAbs and the appropriate combination of mAbs against surface markers to detect CD11c+ CD11b– dendritic cells (DCs), CD11c– CD11b+ macrophages (Mφ), CD4+ T cells from the recipient or CD90.1+ CD4+ T cells from the donor mouse. Cells were then sequentially washed with PBS; then with a buffer containing 0·5% saponin and 2% FBS; with PBS again; and finally with staining buffer (PBS, 2% FBS, 0·02% sodium azide). IL-2 production by individual cells was then measured by flow cytometry, collecting at least 1000 CD4+ CD90.1+ events (as well as an equal number of CD4+ CD90.1 events for endogenous CD4 T cells) for each sample on a FACScan flow cytometer. The frequency of antigen-specific T cells was determined by acquiring 20 000 events in the forward and side scatter lymphocyte gate as previously described.6,7 Single cell data were analysed with CellQuest software (BD Bioscience, San Jose, CA). Negative controls were the recipient's CD4+ T cells and SM1 or OT-II CD4+ T cells from mice primed with either PBS or LPS in PBS that were stained in parallel. IL-1α production in Mφ was assessed as described above by gating on CD11c– CD11b+ cells 6 hr after i.v. activation with 50 μg LPS. Surface CD86 expression on myeloid DC was assessed by gating on CD11c+CD8– cells. The following antibodies and reagents were purchased from BD PharMingen (San Diego, CA): fluorescein isothiocyanate-labelled anti-CD11b mAb; phycoerythrin-labeled anti-CD90.1, anti-CD86, anti-IL-1α, anti-IL-2 mAb; Peridinin chlorophyll protein-labelled anti-CD8 and streptavidin; CyChrome-labelled anti-CD4 and anti-CD8 mAb; and allophycocyanin-labelled anti-CD11c and CD11b mAb.
Adoptive transfer of congenic antigen-specific CD4 T cells
Spleen, mesenteric and other peripheral lymph nodes were harvested from TCR transgenic mice. T cells were enriched by negative selection using the SpinSep buoyant density method (StemCell Technologies, Vancouver, BC). To detect antigen-specific T-cell proliferation TCR transgenic T cells were labelled with the dye 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) at 5–7·5 μm final concentration before transfer as previously described.11,12 A sample of the cells was stained using antibodies specific for CD4 and the marker to be used to track the T cells in normal recipients, for example, CD90.1 to determine the frequency of TCR transgenic CD4 T cells. TCR transgenic CD4 T cells (2 × 106 to 5 × 106 per recipient) were then injected into the tail veins of non-TCR transgenic, MHC-compatible recipient mice. SM1 recombination activating gene2 (RAG2)-deficient B6 (CD90.1) cells were transferred into B6, C57BL/10, or C57BL/10ScCr mice (TLR4-deficient). Although the TCR transgenic T cells, C57BL/10 and C57BL/10ScCr mice are from different genetic backgrounds, the TCR transgenic T cells survived for the same amount of time in B6, C57BL/10 and C57BL/10ScCr recipients (data not shown). This finding was probably explained by the fact that these closely related strains differ by only a few weak minor histocompatibility antigens.
Clonal expansion of antigen-specific T cells 3 days after priming was calculated by multiplying the percentage of cells in the CD4+ CD90.1+ gate by the total number of leucocyte cells in the spleen calculated on a haemocytometer. Calculation of the mean fluorescence intensity (MFI) in the FL1 channel of CD4+, CD90.1+ T cells labelled with CFSE corroborates clonal expansion calculations. The total number of antigen-specific T cells and the percentage of IL-2+ cells in TLR4-deficient and normal C57BL mice in paired treatment groups were compared with Student's t-test.
Measurement of IL-2 production and CD86 expression in vivo in response to peptide antigen stimulation using flow cytometry
Recipient C57BL/10 or C57BL/6 and C57BL/10ScCr mice were injected in the tail vein with 2 × 106 to 5 × 106 SM1 RAG-2-deficient CD90.1+ CD4+ T cells or OT-II CD90.1+ CD4+ T cells. Transferred cells were parked for 1 or 2 days before priming with antigens. Mice were injected i.v. with antigens with or without LPS and killed 0–12 hr after. Instead of FliC, OVA peptide 323–339 was used for to stimulate transferred OT-II cells. Spleens were rapidly removed, mashed to a single cell suspension and fixed with formaldehyde and permeabilized with saponin (as described above). For detection of intracellular IL-2 in transferred T cells, the spleen cells were stained with fluorochrome-labelled anti-CD4 mAb, a mAb specific for the congenic marker expressed by the transferred T cells (CD90.1) and anti-IL-2 mAb in a permeabilization buffer containing 0·3% saponin and 25% FBS. The percentage of antigen-specific IL-2-producing cells was defined as the percentage of cytokine-positive cells within the CD4+ CD90.1+ gate (Fig. 1a) in both the SM1 and OT-II transferred cells when compared to PBS control. For detection of APC activation, fixed and permeabilized spleen cells were stained with fluorochrome-labelled anti-CD11c or anti-CD11b mAb and anti-CD86 or anti-IL-1α mAb in the identical permeabilization and staining protocol. Data from three experiments were pooled and paired groups were compared with Student's t-test.
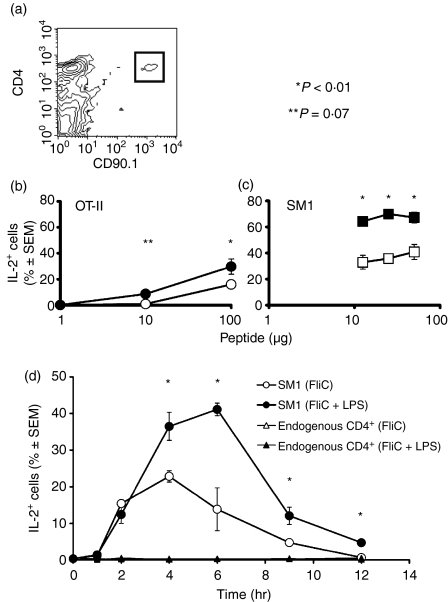
Figure 1.
LPS enhances antigen-stimulated IL-2 production. (a) SM1 cells were identified in the spleens of B10 recipients as CD4+ CD90.1+ cells (gated population), 1 day after adoptive transfer. CD4+ CD90.1+ events were not detected in B10 mice that did not receive SM1 cells (data not shown). The percentages ± SEM of transferred OT-II (b) or SM1 (c) CD4 T cells (identified as shown in a) that contained intracellular IL-2, 6 hr after i.v. injection of the indicated amounts of OVA peptide (b) with (•) or without (○) 25 μg LPS, or FliC peptide (c) with (▪) or without (□) 50 μg LPS are shown. (d) Percentages ± SEM of transferred SM1 T cells that contained intracellular IL-2 at the indicated times after i.v. injection of 25 μg FliC peptide with (filled symbols) or without (open symbols) 50 μg LPS. *P < 0·001; **P = 0·07.
Results
Antigen dose–response and kinetics of the LPS effect on IL-2 production by naive CD4 T cells
Since the endogenous naive CD4 T cells specific for a given peptide–MHC complex are below the limit of detection in flow cytometry, the effects of LPS were studied by tracking naive CD4 T cells from TCR transgenic mice after adoptive transfer into normal histocompatible mice.13 As shown in Fig. 1(a), adoptive transfer produced an easily identifiable population of naive TCR transgenic CD4 T cells in the spleens of the recipient mice, accounting for ∼ 0·1–0·2% of the cells in this organ (Fig. 1a). As predicted from our earlier results,6,7,14 LPS enhanced the fraction of adoptively transferred naive OT-II cells that produced IL-2 in the spleen after i.v. injection of 1, 10 or 100 μg OVA peptide (Fig. 1b). Intravenous injection was chosen over intraperitoneal delivery to ensure synchronous delivery of the peptide antigen to APC in the spleen. In a comparable system that recognizes the OVA peptide 323–339 on I-Ad we have shown that 100 μg peptide induces TCR-dependent c-Jun phosphorylation in 100% of antigen specific T cells in vivo just 60 min after i.v injection.15 This indicates that 100 μg petide is a saturating dose and suggests that the additional effect of LPS on the frequency of cells producing IL-2 is independent of a saturating dose of antigen.
Although this result clearly showed the beneficial effect of LPS on this response at the antigen dose we tested, the effect may depend on the affinity of the transgenic TCR of OT-II mice for the OVA peptide 323–339:I-Ab complex. To confirm that the effect of LPS was reproducible even in T cells expressing a TCR of different affinity, the experiment was repeated using CD4 T cells from the SM1 TCR transgenic line, which responds to much lower doses of the relevant FliC peptide. As shown in Fig. 1(c), LPS increased the fraction of adoptively transferred naive SM1 cells that produced IL-2 in the spleen 6 hr after i.v. injection of all three doses of FliC antigen tested.
A time–course experiment was then performed to assess the importance of LPS at earlier times after antigen injection. In the absence of LPS, SM1 T cells in the spleen began to produce IL-2 2 hr after i.v. injection FliC peptide, with a maximum of 20% of the T cells producing IL-2 at 4 hr (Fig. 1d). The frequency of T cells producing IL-2 then declined to an undetectable level by 12 hr. Two hours after i.v. injection of FliC peptide plus LPS, about the same fraction of SM1 cells contained IL-2 as was observed in the absence of LPS. However, at 4 and 6 hr, the presence of LPS increased the fraction of IL-2-producing SM1 cells by two- to three-fold. Similar results were obtained with OT-II cells after i.v. injection of OVA peptide with or without LPS (data not shown). Therefore, the effect of LPS on enhancing the number of naive CD4 T cells producing IL-2 was apparent 4–6 hr after i.v. injection of antigen, but not earlier.
Role of TLR4 in the LPS effects on CD4 T-cell activation
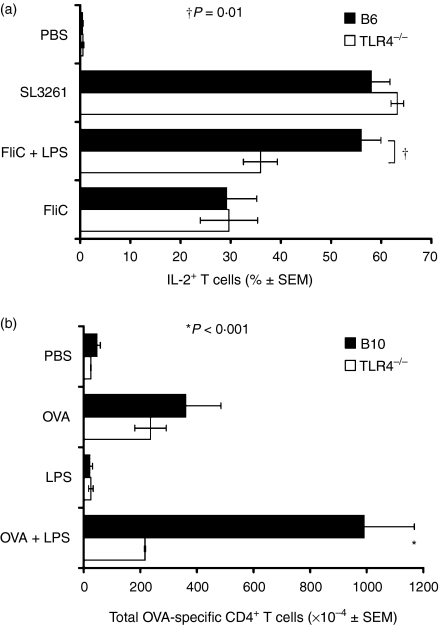
Since TLR4 is one of the primary LPS-sensing molecules16,17 we tested whether the adjuvant effects of LPS on naive CD4 T cells were dependent on TLR4. Naive SM1 T cells were transferred into normal C57BL/6 mice or C57BL/10 ScCr mice, which do not express TLR4 because of a natural mutation.16 The LPS used in these experiments was subjected to a purification process designed to remove the potentially confounding effects of other TLR ligands that contaminate many LPS preparations.10 This purified LPS enhanced the production of IL-2 by SM1 T cells in response to FliC peptide in C57BL/6 mice, but not TLR4-deficient C57BL/10ScCr mice (Fig. 2a), demonstrating that TLR4 expression by the recipient was essential for the adjuvant effect of LPS on the percentage of IL-2-producing SM1 T cells. More SM1 cells produced IL-2 after i.v. injection of 108 heat-killed S. typhimurium than after injection of FliC peptide alone in both C57BL/6 mice and C57BL/10ScCr mice (Fig. 2a), showing that TLR4-deficient mice were capable of sensing a stimulus containing adjuvants in addition to LPS. We observed similar results when unpurified LPS was used (data not shown), probably as the result of lipopetide contamination interacting with TLR2 receptors in TLR4-deficient mice.
Figure 2.
Effects of LPS on CD4 T-cell activation are dependent on TLR4. (a) Percentages ± SEM of transferred SM1 T cells that contained intracellular IL-2 6 hr after i.v. injection of 12·5–25 μg FliC alone, or with 50 μg purified LPS or 108S. typhimurium organisms into C57BL/6 (filled bars) or TLR4-deficient C57BL/10ScCr (open bars) mice. The data are the mean values of at least three experiments per antigen/adjuvant used. (b) Mean number ± SEM of OT-II T cells present in the spleens of C57BL/10 (filled bars) or TLR4-deficient C57BL/10ScCr (open bars) mice, 3 days after i.v. injection of 100 μg OVA with or without 50 μg LPS. †P < 0·01; *P < 0·001.
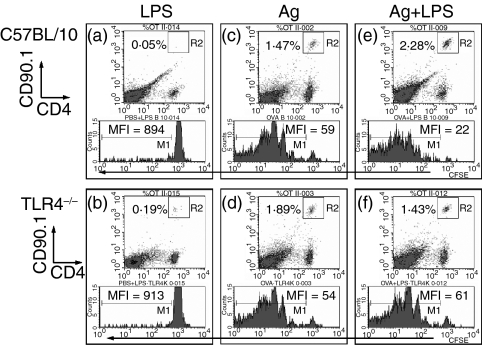
A requirement for TLR4 expression in the proliferation and accumulation of antigen-stimulated CD4 T cells that follows IL-2 production was also tested (Fig. 3 density plots).7 OT-II cells proliferated in the spleens of C57BL/10 recipients 3 days after i.v. injection of OVA peptide alone to a level several fold above the baseline, and to an even greater level after injection of OVA peptide plus LPS (P < 0·001) (Fig. 2b). Analysis of CFSE profiles demonstrated that more than 70–80% of the total antigen-specific T cells was attributable to proliferation and not to increased survival of naive T cells 3 days after priming (Fig. 3 histograms). The percentage of dividing/divided cells (i.e. CFSE low) was proportional to the increase in the total number of antigen-specific CD4+ T cells when identified by the congenic marker CD90.1 (Fig. 3 density plots). OT-II cells proliferated to the same level in TLR4-deficient C57BL/10ScCr mice as they did in C57BL/10 mice after injection of OVA peptide alone (Fig. 2b, 3c,d). However, OT-II cells did not proliferate to a higher level in TLR4-deficient C57BL/10ScCr mice after injection of OVA peptide plus LPS (P > 0·05). Lower proliferation in TLR4-deficient mice is reflected in higher CFSE MFI values and lower frequency of CD4+ CD90.1+ T cells (Fig. 3f). Therefore, TLR4 expression by the recipient mice was required for OT-II cells to undergo enhanced clonal expansion in response to antigen plus LPS.
Figure 3.
Effects of antigen plus LPS on proliferation of CD4 T-cell activation are dependent on TLR4. (a–f) Average frequency of CD4+ CD90.1+ OT-II T cells in the lymphocyte gate and relative CFSE dilution profile in C57BL/10 (a,c,e) or TLR4-deficient C57BL/10ScCr mice (b,d,f) 3 days after PBS alone (data not shown), 50 μg LPS in PBS (a,b; negative controls), 100 μg OVA peptide in PBS (c,d) or 100 μg OVA peptide + 50 μg LPS in PBS (e,f). MFI of CFSE dilution profile shows absence of significant proliferation in (a) and (b), similar proliferation in (c) and (d) and higher proliferation in (e) than in (f). These data are computed and partly presented as total OVA-specific T cells in Figure 2(b).
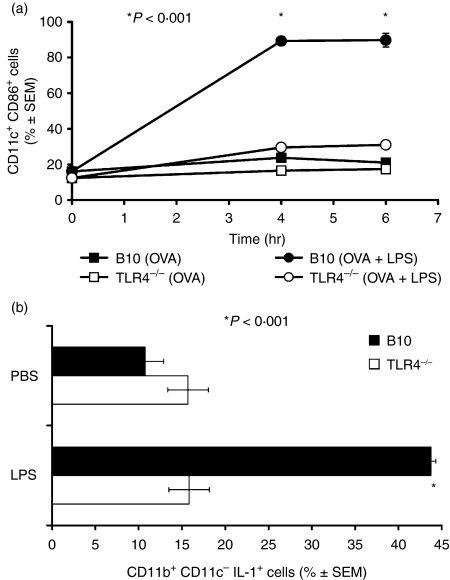
The finding that a lack of TLR4 expression in the recipient resulted in an inability of the CD4 T cells to benefit from the presence of LPS, suggested that the TLR4-dependent effects of LPS might act through APC. Previously we demonstrated that the adjuvant effects of LPS depend on CD28 expression by the CD4 T cells7 and can be mimicked with the APC-derived cytokine IL-1.6 Therefore, it was of interest to test the role of TLR4 in the ability of LPS to stimulate CD28 ligand expression and IL-1 production by APC. As shown in Fig. 4 i.v. injection of purified LPS induced CD86 on CD11c+ dendritic cells (Fig. 4a) and IL-1β production by CD11b+ CD11c– macrophages (Fig. 4b) in the spleens of normal C57BL/10 mice but not TLR4-deficient C57BL/10ScCr mice. Therefore, the effects of LPS on CD4 T cells correlated with TLR4-dependent effects on APC activation.
Figure 4.
Effects of LPS on CD86 and IL-1α production. (a) Percentage ± SEM of CD11c+ CD8– myeloid dendritic cells that expressed higher than basal amounts of CD86 in the spleens of C57BL/10 (▪, •) or TLR4-deficient C57BL/10ScCr (□, ○) mice at the indicated times after i.v. injection of OVA peptide alone (▪, □) or OVA peptide plus 50 μg purified LPS (•, ○). *P < 0·001 between filled symbols at 4 and 6 hr. (b) Percentage ± SEM of CD11b+, CD11c– macrophages that expressed higher than basal amounts of intracellular IL-1α in the spleens of C57BL/10 (filled bars) or TLR4-deficient C57BL/10ScCr (open bars) mice, 6 hr after i.v. injection of PBS or 50 μg purified LPS. *P < 0·001.
Discussion
Recent work has shown that TLR signalling plays an important role in the capacity of microbial molecules such as LPS to act as adjuvants for T-cell responses. MyD88, an important signal transducer downstream of many TLRs3 is critical for the development of recall T-cell proliferation and IFN-γ production following immunization with antigen in CFA.5 In addition, TLR4 is required for the development of antigen-specific T helper type 2 (Th2) cells following inhalation of antigen and LPS.18,19 Since TLR4 appears to be required for LPS to drive Th1 and Th2 differentiation, it is possible that it acts at an earlier step in the T-cell activation process. Indeed our results show that LPS has a TLR4-dependent effect on the frequency of cells producing IL-2 and consequently on the clonal expansion of antigen-specific CD4 T cells. Thus, the major effect of LPS may be to increase the number of antigen-specific precursors that are then candidates for Th1/Th2 differentiation.
Our conclusion that the effects of LPS on the frequency of T cells producing IL-2 and their clonal expansion are TLR4-dependent rests on the assumption that a lack of TLR4 is the important difference between the C57BL/10ScCr strain and wild-type C57BL/10 mice. Although C57BL/10ScCr mice also possess a defect in the IL-12 R pathway,20 several pieces of evidence indicate that this cannot explain the failure of LPS to act as an adjuvant for CD4 T cells in this strain. First, IL-12 does not mimic the effects of LPS on IL-2 production and T-cell expansion.6 Second, S. typhimurium, or unpurified LPS extracts which contain other inflammatory stimuli including TLR2 ligands, had adjuvant effects on CD4 T cells in C57BL/10ScCr. Since IL-12 would be expected to be produced in response to all TLR signals, the fact that the adjuvant defect in C57BL/10ScCr mice was limited to a purified LPS strongly implicates TLR4.
Because activation of wild-type CD4 T cells in response to antigen plus LPS was defective in TLR4-deficient recipients, LPS must act on a TLR4-expressing cell other than the naive CD4 T cells. One possibility is that the critical TLR4-expressing cell is the APC. This possibility is supported by the finding that LPS failed to stimulate CD28 ligand expression and IL-1 production in TLR4-deficient mice, both of which have been implicated in adjuvant effects of LPS in TLR4-sufficient mice.6 IL-1α and IL-1β are important pro-inflammatory cytokines that regulate the expression of costimulatory and MHC class II molecules. In vivo, IL-1α induces persistent clonal expansion of CD4+ T cells by either sustaining proliferation or inhibiting post-stimulation apoptosis6 even when CD28 is absent (A. Khoruts, personal communication). Using LPS we showed that TLR4 is essential for the production of IL-1α in DCs and macrophages (Fig. 4a,b). Lack of IL-1 is particularly detrimental to the activation of APCs because it reduces the positive feedback obtained through IL-1-receptor signalling. In addition, the finding that LPS enhanced IL-2 production at 4 and 6 hr, but not 2 hr is consistent with the known timing of CD28 ligand induction by LPS.21 Alternatively, it has recently been shown that TLR signalling prevents CD4+ CD25+ regulatory T cells from exerting suppressive effects on other T cells.22 Further experiments will be required to assess the relative roles of these two mechanisms of TLR4 action on IL-2 production and T-cell clonal expansion.
Acknowledgments
This work was supported by Public Health Service grants DE014371 (M. Costalonga) from the National Institute of Dental and Craniofacial Research and AI27998, AI35296, and AI39614 (M.K. Jenkins) from the National Institute of Allergy and Infectious Diseases. We thank Dr Mark C. Herzberg and Dr Marc Jenkins for their support and their insightful discussions and Jennifer Walter for technical assistance.
Abbreviations
- CFSE
5-(and-6)-carboxyfluorescein diacetate succinimidyl ester
- EHAA
Eagle–Hank's amino acid medium
- FBS
fetal bovine serum
- IFN
interferon
- IL
interleukin
- OVA
ovalbumin
- TLR
Toll-like receptors
References
- 1.Freund J, Casals J, Hosmer EP. Sensitization and antibody formation after injection of tubercle bacilli and paraffin oil. Proc Soc Exp Biol Medical. 1937;37:509–13. [Google Scholar]
- 2.Dresser DW. Effectiveness of lipid and lipidophilic substances as adjuvants. Nature. 1961;191:1169–71. doi: 10.1038/1911169a0. [DOI] [PubMed] [Google Scholar]
- 3.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, Itano A, Pape KA. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- 5.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–50. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 6.Pape KA, Khoruts A, Mondino A, Jenkins MK. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J Immunol. 1997;159:591–8. [PubMed] [Google Scholar]
- 7.Khoruts A, Mondino A, Pape KA, Reiner SL, Jenkins MK. A natural immunological adjuvant enhances T cell clonal expansion through a CD28-dependent, interleukin (IL)-2-independent mechanism. J Exp Med. 1998;187:225–36. doi: 10.1084/jem.187.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McSorley SJ, Asch S, Costalonga M, Reinhardt RL, Jenkins MK. Tracking Salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity. 2002;16:71–83. doi: 10.1016/s1074-7613(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 9.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 10.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165:618–22. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 11.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Meth. 1994;171:131–7. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 12.Merica R, Khoruts A, Pape KA, Reinhardt RL, Jenkins MK. Antigen-experienced CD4 T cells display a reduced capacity for clonal expansion in vivo that is imposed by factors present in the immune host. J Immunol. 2000;164:4551–7. doi: 10.4049/jimmunol.164.9.4551. [DOI] [PubMed] [Google Scholar]
- 13.Pape KA, Kearney ER, Khoruts A, et al. Use of adoptive transfer of T-cell-antigen-receptor-transgenic T cell for the study of T-cell activation in vivo. Immunol Rev. 1997;156:67–78. doi: 10.1111/j.1600-065x.1997.tb00959.x. [DOI] [PubMed] [Google Scholar]
- 14.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410(6824):101–5. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 15.Zell T, Khoruts A, Ingulli E, Bonnevier JL, Mueller DL, Jenkins MK. Single-cell analysis of signal transduction in CD4 T cells stimulated by antigen in vivo. Proc Natl Acad Sci USA. 2001;98:10805–10. doi: 10.1073/pnas.191567898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282(5396):2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 17.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 18.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–51. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dabbagh K, Dahl ME, Stepick-Biek P, Lewis DB. Toll-like receptor 4 is required for optimal development of Th2 immune responses: role of dendritic cells. J Immunol. 2002;168:4524–30. doi: 10.4049/jimmunol.168.9.4524. [DOI] [PubMed] [Google Scholar]
- 20.Merlin T, Sing A, Nielsen PJ, Galanos C, Freudenberg MA. Inherited IL-12 unresponsiveness contributes to the high LPS resistance of the Lps(d) C57BL/10ScCr mouse. J Immunol. 2001;166:566–73. doi: 10.4049/jimmunol.166.1.566. [DOI] [PubMed] [Google Scholar]
- 21.DeSmedt T, Pajak B, Muraille E, et al. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med. 1996;184:1413–24. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299(5609):1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]