Abstract
Effective delivery systems are needed to design efficacious vaccines against the obligate intracellular bacterial pathogen, Chlamydia trachomatis. Potentially effective delivery vehicles should promote the induction of adequate levels of mucosal T-cell and antibody responses that mediate long-term protective immunity. Antigen targeting to the nasal-associated lymphoid tissue (NALT) is effective for inducing high levels of specific immune effectors in the genital mucosa, and therefore suitable for vaccine delivery against genital chlamydial infection. We tested the hypothesis that live attenuated influenza A viruses are effective viral vectors for intranasal delivery of subunit vaccines against genital chlamydial infection. Recombinant influenza A/PR8/34 (H1N1) viruses were generated by insertion of immunodominant T-cell epitopes from chlamydial major outer membrane protein into the stalk region of the neuraminidase gene. Intranasal immunization of mice with viral recombinants resulted in a strong T helper 1 (Th1) response against intact chlamydial elementary bodies. Also, immunized mice enjoyed a significant state of protective immunity (P > 0·002) by shedding less chlamydiae and rapidly clearing the infection. Furthermore, a high frequency of Chlamydia-specific Th1 was measured in the genital mucosal and systemic draining lymphoid tissues within 24 hr after challenge of vaccinated mice. Moreover, multiple epitope delivery provided a vaccine advantage over single recombinants. Besides, long-term protective immunity correlated with the preservation of a robustly high frequency of specific Th1 cells and elevated immunoglobulin G2a in genital secretions. Because live attenuated influenza virus vaccines are safe and acceptable for human use, they may provide a new and reliable approach to deliver efficacious vaccines against sexually transmitted diseases.
Keywords: Chlamydia trachomatis, vaccines, delivery systems, immunomodulation
Introduction
Chlamydia trachomatis genital infection poses a considerable public health challenge to many nations. According to the WHO, genital chlamydial infection is the most common bacterial sexually transmitted disease (STD) in several industrialized nations, accounting for more than 90 of the 500 million annual new STDs worldwide.1 Pelvic inflammatory disease (PID) and tubal factor infertility (TFI) are major complications of the genital infection, and constitute an enormous morbidity and socioeconomic burden.2 The USA spends over $2 billion annually on 4 million reported cases.3 While diagnosed cases can be treated with antibiotics, the rampant asymptomatic infections often result in clinical presentation of complications as the first evidence of an infection. Consequently, the current medical opinion is that an efficacious prophylactic vaccine would constitute the best approach to protect the human population from chlamydial infections.4 This opinion is reinforced by the findings that a significant proportion of treated infections may lead to persistence,5 casting doubt on the long-term value of certain chemotherapies. Furthermore, computer modelling has predicted that a partially protective chlamydial vaccine that prevents severe sequelae in a vaccination programme would constitute an acceptable short-term goal.6 The epidemiological data indicating increasing incidence of genital chlamydial infections among the youth emphasize the urgency for an efficacious vaccine.
Clinical studies in humans and experimentation in animal models have established that chlamydial immunity correlates with a strong T helper type 1 (Th1) response as well as a complementary antibody response that enhances immunity to reinfections.7–12 This finding has furnished important immunological correlates for vaccine testing and evaluation. The antichlamydial action of Th1 effectors is mediated principally via cytokine-induced antimicrobial mechanisms of CD4 T cells.7–9 These mechanisms include depletion of intracellular tryptophan by activation of indoleamine 2,3-dioxygenase, induction of elevated nitric oxide (NO) through inducible NO synthase, deprivation of iron (Fe), via down-regulation of transferrin receptors, and possibly the stimulation of phagolysosomal fusion or disruption of selective vesicular nutrient transport via p47/GTPase activation.7–9,13 Thus, chlamydial vaccines that induce these antimicrobial processes are potentially effective.
The possibility that the intact chlamydiae harbour pathogenic components,14 and the absence of genetic tools to modify and produce safe attenuated strains, make subunit vaccines the current research focus. Foremost among potential subunit vaccine candidates are: the 40 000, 60 000 and 15 000 MW outer membrane proteins (OMPs), which are encoded by the Omp-1 (omc A), Omp-2 (omp C) and Omp-3 (omp B) genes, respectively.7,15 Additional vaccine candidates are the polymorphic outer membrane proteins (POMP or pmp) and the conserved PorB family of membrane proteins,15,16 an ADP/ATP translocase,17 a clinically immunogenic plasmid protein (pgp3),18 the proteasome/protease-like activity factor (CPAF),19 a toxin mapped to the plasticity zone of several strains,20 and certain members of the type III secretory machinery.21 So far the efficacy of vaccines based on most of these candidates has been limited, caused partly by poor immunogenicity, and consequently producing only partial protective immunity.7 The lack of sterilizing immunity suggested that either single subunits are inadequate as vaccines, or the need for more effective delivery systems to optimize the effect of single subunit candidates. Thus, the immunogenicity and protection induced by a MOMP DNA vaccine were enhanced when delivered with an adjuvant carrier.22 Besides, a heterologous double subunit chlamydial vaccine delivered on the recombinant Vibrio cholerae ghost platform was superior in immunogenicity and protection to a single subunit construct.23 Therefore, effective delivery systems will likely enhance the efficacy of potential chlamydial subunit vaccines.
The vital role of mucosal immunity in protection against the oculogenital infections of C. trachomatis suggested that targeting vaccines to the specialized antigen-presenting cells (APCs) in certain mucosal inductive sites of the mucosa-associated lymphoid tissues (MALT) could lead to protective immunity. MALT includes the NALT, gut-associated lymphoid tissue (GALT), and bronchus-associated lymphoid tissue (BALT).24 Because the inductive and effector sites of the common mucosal immune system (CMIS) are compartmentalized, certain inductive and effector sites interact effectively to produce an optimal immune response. Hence, during vaccine delivery, it is important to select a route of immunization that favours an effective co-operation between a given mucosal inductive site and a targeted mucosal effector site of infection. In this respect, intranasal immunization with live chlamydiae or acellullar outer membrane complex induced protective immunity, which correlated with rapid elicitation of a genital mucosal Th1 response and the CMI-associated immunoglobulin G (IgG)2a and secretory IgA.25,26 Nasal immunization caused rapid generation of immune effectors detectable within days, and was superior to vaginal, gastric, peritoneal, or rectal immunization for inducing mucosal anti-human immunodeficiency virus (HIV) or anti-herpes simplex virus (HSV) responses,24,27 emphasizing the strong link between NALT and the genital mucosa. The cellular and molecular basis for this co-operation is still being studied but intranasal route is therefore a highly effective vaccine delivery route for the induction of protective immunity in the genital mucosa.
This study investigated whether the favourable co-operative immunological interaction between the inductive sites of NALT and immune effector site in the genital mucosa, and the proclivity of respiratory viruses to induce Th1 response, could be applied to design efficacious chlamydial vaccines. For instance, influenza viruses have been used successfully as vectors to deliver antigens targeted to NALT,28 and the live attenuated cold-adapted virus vaccine is an acceptable, FDA-approved vaccine for human use. We tested the hypothesis that intranasal delivery of recombinant influenza A virus expressing select immunodominant epitopes from C. trachomatis proteins29,30 is an effective vaccine delivery strategy against genital chlamydial infection. The results revealed that influenza viruses have potential as vaccine delivery vehicles for inducing protective immunity against genital chlamydial infection.
Materials and methods
Animals, stocks of C. trachomatis, influenza A viruses, cell lines, and antigens
Female C57BL/6 mice, 5–8 weeks old, were obtained from Taconic Farms (Germantown, NY). Animals were fed with food and water ad libitum, and maintained in Laminar flow racks under pathogen-free conditions of 12 hr light and 12 hr darkness. The animal use protocols described in this proposal have been approved by the CDC IACUC. Stocks of the human isolate of C. trachomatis serovars A, C, D, E, F, H, K, L2 and the agent of mouse pneumonitis (MoPn) were stored at −70°. Stocks of C. trachomatis were prepared by propagating elementary bodies (EBs) in McCoy or HeLa cells and titered in inclusion-forming units per millilitre (IFU/ml).31 Influenza A/PR8/34 virus (H1N1)(PR8), a mouse-adapted laboratory strain, was used as a backbone for deriving recombinant influenza viruses (rIVs) containing heterologous sequences. PR8 is non-pathogenic for humans and foreign fragment insertion results in attenuation that renders them non-pathogenic for mice although they induce protective immunity.32 Viral stocks were prepared by propagating the agents in Madin-Darby bovine kidney (MDCK), titred in plaque-forming units per millilitre (PFU/ml), and stored at −70°. Cell lines for growing agents include HeLa, McCoy, MDCK and 293T cells, and were maintained in minimal essential medium (MEM; Gibco-BRL, New York, NY) and Dulbecco's minimal essential medium (Gibco-BRL), respectively, containing 10% FCS and gentamicin. Chlamydial antigens were prepared by purifying EBs over renografin gradients, and inactivation under ultraviolet (UV) light for 3 hr. Virus antigens were prepared by inactivation of stocks under UV for 3 hr.
Plasmids for viral rescue and cloning chlamydial T cell epitopes and peptides
Plasmids encoding viral proteins required for encapsidation, transcription, and replication of the influenza virus genome (i.e. the three subunits of the viral RNA-dependent RNA polymerase complex, PB1, PB2 and PA, and the nucleoprotein (NP), along with others encoding the eight viral RNA segments of influenza virus were constructed for cotransfection into permissive cells to rescue the infectious virus. This plasmid-driven methodology allowed the genetic manipulation of viral genomes to generate live attenuated forms and vectors that express heterologous proteins or vaccines.33 Expression plasmids pCAGGS-PB2, pCAGGS-PB1, pCAGGS-PA, and pCAGGS-NP encode the PB2, PB1, PA, and NP proteins, respectively, of influenza A/WSN/33 (WSN) virus under the control of a chicken β-actin promoter. Expression plasmids for the viral RNAs of influenza A/PR/8/34 (PR8) virus (pPOLI-PB2/PR8, pPOLI-PB1/PR8, pPOLI-PA/PR8, pPOLI-HA/PR8, pPOLI-NP/PR8, pPOLI-NA/PR8, pPOLI-M/PR8, and pPOLI-NS/PR8) contain the corresponding cDNAs in a pUC18-based plasmid between a truncated human RNA polymerase (POL) I promoter and sequences of the hepatitis delta virus ribozyme. Plasmid pPOLI-NA/CT expresses a mutated neuraminidase (NA) protein from the PR8 virus in which a portion of the stalk domain of the NA were replaced by the sequence encoding T cell epitopes fragments from C. trachomatis serovar D, as described below.
Construction and characterization of recombinant influenza A viruses expressing C. trachomatis epitopes (rIV-CT)
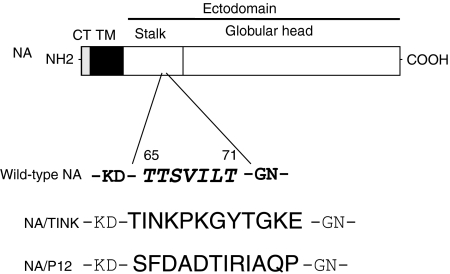
Recombinant viruses were generated by a modified transfection-based reverse genetics protocol for deriving mutant forms of influenza A virus.33 Briefly, viruses were rescued by transfecting a mixture of 293T and MDCK cells with a combination of 12 plasmids (0·5 µg of each) with Lipofectamine 2000 (2 µl/µg of plasmid; Invitrogen/Gibco-BRL) in OPTIMEM medium, as described previously.33 These plasmids were: four pCAGGS expression plasmids for viral polymerases PB1, PB2, and PA, and nucleoprotein NP; 7 pPolI-Rib plasmids for wild-type viral genomic segments PB1, PB2, PA, NP, HA, M and NS, and one plasmid containing an NA in which the sequences encoding amino acid residues 65–71 (TTSVILT) of the stalk domain were replaced by sequence encoding the immunodominant T-cell epitope defined by amino acids 232–243 (TINKPKGYVGKE) or another T-cell epitope corresponding to amino acids 293–304 (SFDADTIRIAQP) from C. trachomatis serovar D MOMP29,30,34 (http://chlamydia-http://www.berkeley.edu:4231/681.html#aa). These immunodominant T-cell epitopes form part of a longer sequence that was previously established to contain epitopes recognized by at least 80% of human T cell donors and murine T cells from several mouse strains,29,30,34,35 and are likely to be promiscuously bound and presented by several major histocompatibility complex (MHCs) although they elicit chlamydial-specific response. Rescued viruses are first grown at 37° in MDCK cells in MEM containing 3 µg/ml of trypsin (Sigma, St Louis, MO), 0·3% bovine serum albumin (Sigma), and penicillin-streptomycin, and subsequently amplified in embryonated chicken eggs. The identity of the rescued viruses was confirmed by reverse transcription–polymerase chain reaction (RT–PCR) and restriction sequence analysis of the mutated gene segments. Two recombinant influenza viruses expressing T-cell epitopes from C. trachomatis MOMP were characterized in this study: PR8-TINK (or TINK) which expresses the immunodominant T-cell epitope (TINKPKGYVGKE), and PR8-P12 (or P12) that expressed another T-cell epitope (SFDADTIRIAQP) (Fig. 1).
Figure 1.
Schematic representation of the procedure for inserting chlamydial epitopes into the neuraminidase (NA) of influenza A/PR8/34 virus (H1N1), as we previously described.28 The amino acid residues for the chlamydial epitopes TINK and P12 in the chimeric NA/TINK or NA/P12 protein replaced amino acid residues 65–71 in the wild-type NA stalk. CT/TM = cytoplasmic/transmembrane domains of NA.
Immunizations and immunogenicity evaluation
Groups of mice were anaesthetized and infected intranasally with 20 µl of phosphate-buffered saline (PBS) containing 105 PFU of the recombinant influenza A viruses (TINK or TINK + P12) or the parental PR8 virus. Animals were monitored daily. Animals received a secondary immunization of 105 PFU/mouse after 2 weeks of the primary. All experiments were conducted after the second immunization. The ability of the expressed peptide to induce antichlamydial T-cell response was measured by in vitro re-stimulation of T-cell enriched lymphocytes from the spleen, mandibular, anxillary, mesenteric and iliac lymph nodes with Chlamydia elementary bodies as antigen and splenic APCs (irradiated whole spleen cells), by the standard procedure.36 At the end of a 5-day incubation period, the supernatants were collected and assayed for interferon-γ (IFN-γ) content by a Luminex method (Bio-Rad Laboratories, Hercules, CA) according to the supplier's instructions. The concentration of the cytokine in each sample was obtained by extrapolation from a standard calibration curve generated simultaneously. Data were calculated as the mean values (± SD) of triplicate cultures for each experiment. Antigen-specific IFN-γ secretion by activated T cells is the measure of the Th1 response, as previously established.25
Genital challenge infection
Immunized mice were infected intravaginally with 105 IFU of C. trachomatis serovar D in a volume of 20 µL of PBS while under phenobarbitol anaesthesia.37 Also, in parallel experiments, nylon wool-purified immune T cells (>96% CD4/CD4) were adoptively transferred from vaccinated mice into naïve mice and the latter were challenged with 105 live chlamydiae after 24 hr.36 The course of the infection was monitored by periodic cervico-vaginal swabbing of individual animals and isolation of Chlamydia in McCoy cell culture according to standard procedures involving staining infected monolayers of cells with fluorescein isothiocyanate-labelled, genus-specific antichlamydial antibodies (Kallestad Diag., Chaska, MN).31 To assess long-term immunity, groups of animals were reinfected 97 days after the primary infection with 105 IFU of C. trachomatis serovar D per mouse and either swabbed for isolation of chlamydiae and/or killed to determine the development of hydrosalpinx. For assessment of hydrosalpinx, mice were surgically dissected to expose the entire reproductive system. The uterine and oviductal regions were inspected for visible evidence of classic hydrosalpinx in infected mice. Experiments were repeated three times.
Quantitation of cytokines, chlamydia-specific secretory and systemic antibodies
The Bio-Plex cytokine assay kits for simultaneously quantitating multiple cytokines were supplied by Bio-Rad (Hercules, CA). Serum was prepared from non-heparinized blood obtained by retro-orbital bleeding, and vaginal washes were performed with 200 µL PBS, as previously described.25 Samples were stored at −70° until assayed. The modified enzyme-linked immunosorbent assay (ELISA) procedure for measuring the levels of different immunoglobulin isotypes, including secretory IgA and IgG2a in vaginal washes has been described.25
Measurement of frequency of chlamydial-specific Th1 cells (Th1 frequency) after immunization or infection of mice
A modified procedure of the limiting dilution technique38 was used to measure the frequency of chlamydial-specific Th1 cells in immunized mice. Briefly, cultures were established by seeding T cells in a serial doubling dilution and stimulated with APCs (5 × 105 cells/well) and chlamydial antigen (10 µg/ml). Background cultures contained APCs and antigen. After a 5 day incubation, supernatants were assayed for IFN-γ by a sensitive ELISA.25 The mean and standard deviation (SD) of background cultures were calculated. Three times the value of the SD was added to the mean value and the sum was adopted as the baseline for positive experimental wells. The number of positive and negative wells per dilution of each T-cell population was calculated and the data were analysed by a limiting dilution computer program (LIDIA),38,39 which provided both the Th1 frequency and the conformity of input data with a single-hit Poisson model.36
Statistical analysis
The levels of IFN-γ in samples and IFUs from different experiments were analysed and compared by performing a one- or two-tailed t-test, and the relationship between different experimental groupings was assessed by analysis of variance (anova). Minimal statistical significance was judged at P < 0·05.
Results
Immunogenicity of chlamydial MOMP-derived T-cell epitopes delivered with recombinant influenza virus vectors
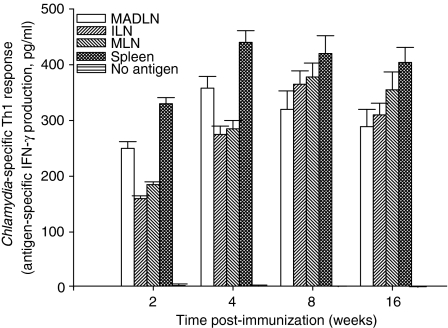
We tested the hypothesis that intranasal delivery of recombinant influenza viruses expressing immunodominant T-cell epitopes from C. trachomatis MOMP will induce specific T-cell response against the intact elementary bodies (EBs). A recombinant influenza virus, PR8-TINK (TINK) was derived from the parental A/PR8/34 (H1N1) virus by the insertion of the immunodominant T-cell epitope defined by amino acid sequences 232–243 of C. trachomatis serovar D MOMP29,34 into the stalk region of the NA (Fig. 1). When TINK was delivered intranasally to female mice and evaluated after 2, 4, 8, and 16 weeks later for T-cell response against EBs in several lymphoid tissues, a robust chlamydial-specific Th1 response was detected at all the time points and in all the lymphoid tissues (Fig. 2). In addition to serovar D, there was also a significant Th1 response to other serovars tested (E, G, and MoPn) above the control and background response (P > 0·021; data not shown), which confirms the conservation of TINK in these chlamydial strains. In addition, sera and genital washes from immunized mice during the first 2 weeks of immunization contained insignificant levels of antichlamydial antibodies (data not shown), which supported the fact that the immunogen was principally a T-cell epitope. The results supported the hypothesis that recombinant influenza viruses are effective vectors for inducing antichlamydial T-cell responses following intranasal delivery. However, the ability of such T-cell response to protect against chlamydial genital infection was uncertain.
Figure 2.
Chlamydia-specific Th1 response induced following intranasal administration of a recombinant influenza virus expressing an immunodominant T-cell epitope from C. trachomatis MOMP (PR8-TINK). Mice were immunized by intranasal administration of PR8-TINK and at the indicated time periods T-cell enriched cells were prepared from the spleen, mandibular, iliac and mesenteric lymph nodes (MADLN, ILN and MLN, respectively), according to standard procedures.25 Purified T cells (1 × 105 per well) were stimulated with splenic APCs plus chlamydial antigen (UV-inactivated EBs) in tissue culture plates for 5 days. At the end of the incubation period, the supernatants were collected and assayed for IFN-γ content by a quantitative sandwich ELISA, as previously described.25 The concentration of the cytokine in each sample (pg/ml) was obtained by extrapolation from a standard calibration curve generated simultaneously. Data were calculated as the mean values (± SD) of triplicate cultures for each experiment. The results were derived from at least three independent experiments. Control cultures containing T cells and APCs without chlamydial antigen showed extremely low measurable amounts of IFN-γ in the range of 0–5 pg/ml. Antigen-specific IFN-γ secretion by activated T cells is the measure of Th1 response, as previously established.25
Ability of nasally delivered recombinant influenza viral vectors expressing chlamydial epitopes to induce protective immunity against genital chlamydial infection
We investigated whether the immune effectors elicited by recombinant influenza viruses expressing immunodominant T-cell epitopes from MOMP were adequate and effective to confer protection against genital chlamydial infection. Recombinant influenza virus TINK was delivered intranasally to female mice or combined with another recombinant (P12) expressing a different immunogenic T-cell epitope from MOMP, although less potent than TINK.29,30,34 Following challenge, immunized mice showed a remarkably reduced infection burden measured by chlamydial shedding into the cervico-vaginal vault. Thus, the intensity of the infection in immunized mice was 4-log less IFUs than control mice that received the parental virus alone (Fig. 3). In addition, codelivery of TINK and P12 was superior to delivery of the immunodominant TINK alone, since the former resolved the infection earlier, suggesting that multiple epitope constructs will likely have a protective advantage.
Figure 3.
Protective immunity induced by recombinant influenza virus expressing immunodominant T-cell epitopes from C. trachomatis MOMP (PR8-TINK, and PR8-TINK + PR8-P12). Mice were immunized by intranasal administration of either PR8-TINK alone or PR8-TINK plus PR8-P12 as described in Materials and methods. Control mice received the parental PR8 alone. Two weeks after the last immunization, the three groups of mice were challenged intravaginally with live C. trachomatis serovar D and the course of the infection in each group was monitored by cervico-vaginal swabbing and isolation of chlamydiae in tissue culture according to standard procedure.31 The data are expressed as log of mean IFU from independent animal evaluation in each group. The experiments were repeated three times with six mice per experimental group.
Protection of immunized animals against genital chlamydial infection correlates with the rapid recruitment of high frequency of specific Th1 cells to the genital mucosa after infection
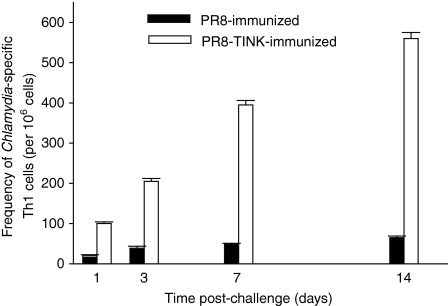
It was hypothesized that antichlamydial immune effectors elicited by recombinant influenza viruses in immune animals are recruited into the genital mucosa in a timely manner after challenge of animals to limit the productive growth of chlamydiae in the genital epithelium. To investigate this hypothesis, the frequency of chlamydial-specific Th1 cells in the genital mucosa was monitored with limiting dilution technique at different times after challenge of immunized animals. Figure 4 reveals that within 24 hr after challenge, a high frequency of Chlamydia-specific Th1 cells was induced in the genital mucosa of vaccinated mice that were challenged compared to recipients of the parental PR8 virus alone. The induction of Th1 cells increased progressively in immunized animals that rapidly cleared the infection and attained a peak response that was approximately 10-folds over the slowly developing response in control animals. The results indicated that the strong antichlamydial Th1 response induced by the recombinant virus resulted in the rapid recruitment of a high frequency of specific Th1 cells into the genital mucosa and this correlated with the clearance of the infection.
Figure 4.
Frequency of Chlamydia-specific Th1 cells after a challenge infection. A modified procedure of the limiting dilution technique38 was used to assess Th1 frequency in infected mice.36,38 T cells were isolated from the genital tract tissues of vaccinated and non-vaccinated mice that were challenged intravaginally with C. trachomatis, at the indicated times after infection. Limiting dilution cultures were established by seeding T cells in a serial doubling dilution into 96-well round bottom tissue culture plates, as described in Materials and methods. After incubation, the supernatants were assayed for IFN-γ by a sensitive ELISA, and frequency of chlamydial-specific Th1 cells were calculated as described in Materials and methods. T cells from naive mice have Th1 frequency of 15 (range: 9–21). The experiment was repeated twice with six mice per experimental group.
Immune correlates of long-term protective chlamydial immunity
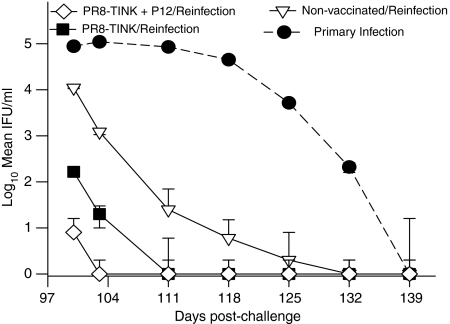
One of vital applications of protective experimental vaccine regimens is their utilization for defining the elements of protective immunity in a model system. Accordingly, we analysed long-term protective chlamydial immunity conferred by the recombinant influenza virus vaccine regimen and characterized the immune parameters that correlate with protection. Immunized and non-immunized animals that resolved their genital chlamydial infections were re-infected intravaginally after 97 days of the primary infection. The course of the infection was monitored at different time period (Fig. 5), and the frequency of chlamydial-specific Th1 cells and the levels of IgG1, IgG2a and IgA were measured in the genital washes on day 7 postreinfection (Table 1) when at least one group had resolved the infection. Figure 5 showed that animals that received the two recombinant viruses (TINK and P12) remained immune, since they resolved their infection within a week. Also, recipients of the recombinant virus harbouring the single immunodominant TINK epitope (TINK) were relatively immune, resolving their infection within 2 weeks. However, although non-vaccinated animals that were preinfected with chlamydiae were more immune than naïve mice with primary infection, the secondary infection in these mice was more remarkable than the vaccinated groups. The results indicated that the recombinant viruses induced a greater degree of protective immunity than the natural infection.
Figure 5.
Comparison of long-term protective immunity established after unvaccinated mice resolved a primary natural genital infection (•) and after mice immunized with PR8-TINK (▪) or PR8-TINK + PR8-P12 (◊) recombinant viruses cleared a challenge infection. Mice that cleared their infection (primary or challenge following vaccination with recombinants) were reinfected with C. trachomatis serovar D on day 97 after the cleared infection. The course of the infection in each group was monitored by cervico-vaginal swabbing and isolation of chlamydiae in tissue culture according to standard procedure.31 The data are expressed as log of mean IFU from independent animal evaluation in each of the three groups compared. The experiments were repeated twice with six mice per experimental group. The course of the primary infection in naïve mice (•) is transposed on the graph to illustrate the difference between a primary infection and reinfection.
Table 1.
Correlation of long-term protective immunity with levels of local Chlamydia-specific Th1 and IgG2a memory responsesa
| Group | Level of protection on day 7 of reinfection (%) | Frequency of specific Th1 cells/106 cells – (iliac node) | Genital IgA (µg/ml) | Genital IgG2a (µg/ml) | Genital IgG1 (µg/ml) |
|---|---|---|---|---|---|
| Immune-1° PR8-TINK Infection | 80 | 565 | 75 ± 5 | 27 ± 2+ | 45 ± 3 |
| Immune-PR8-TINK + PR8-P12 + 1° Infection | 95 | 1250 | 145 ± 6 | 28 ± 2 | 65 ± 2 |
| Immune-1° genital infection | 5 | 45 | 38 ± 3 | 30 ± 4 | 55 ± 3 |
| Naïve | 0 | 15 | 0 | 0 | 0 |
Immune animals were challenged at 97 days after the primary (1°) infection. The iliac lymph node (ILN) drains the genital tract in mice. All specimens were collected on Day 7 vaginal postreinfection.
Table 1 presents results that correlated the degree of protective immunity with specific immune parameters in each group of vaccinated and non-vaccinated animals on day 7 after reinfection. At least one group had resolved the infection during this time period after reinfection, which should correlate with certain measurable immune effectors, including specific T cells and antibodies. The results in Table 1 revealed that recipients of both recombinant viruses (TINK and P12) had 95% level of protective immunity (i.e. 19 of 20 mice were uninfected), which correlated with a robustly high frequency of chlamydial-specific Th1 cells (1250) in the iliac draining node and elevated titre of IgG2a (145 µg/ml) in the genital washes. The 80% protection yielded by group that received one recombinant also correlated with a relatively high frequency of Th1 cells and reasonably elevated titre of genital IgG2a. Non-vaccinated but preinfected animals that become susceptible to reinfection had only 5% protection since only one out of 20 animals had resolved the infection at this time after reinfection. Correspondingly, non-vaccinated animals that received a primary infection had a relatively low frequency of chlamydial-specific Th1 cells in their draining iliac lymph nodes and low specific IgG2a in their genital washes. The results support a strong correlation between protective immunity and a high frequency of specific Th1 and IgG2a in immune animals. Furthermore, these studies revealed that pre-exposure to the vector did not prevent a robust secondary antichlamydial immune response in long-term studies. This would indicate that vector elimination because of the presence of antivector antibodies in the host is unlikely to be an issue in the practical use of recombinant influenza viruses as vaccine vectors.
Discussion
Contemporary immunobiological paradigms guiding the design of chlamydial vaccine postulate that potentially effective delivery vehicles should have the capacity to target multiple vaccine subunits to specific immune inductive sites, and possess complementary immunomodulatory function to stimulate adequate costimulation and a favourable cytokine environment. These delivery conditions will foster the induction of appropriate levels of mucosal T cell and antibody responses that mediate long-term protective immunity against Chlamydia.7,8 The objective of this study was to design a delivery vehicle that integrates these requirements to induce protective immunity against genital chlamydial infection. First, the requirement for a delivery system to target vaccines to specific immune inductive site by vaccine administration via particular routes will ensure that immune effectors elicited are directed appropriately to the relevant mucosal effector site of chlamydial infection. In this respect, the functional relationship between the inductive sites of NALT and the genital mucosa24 may suggest that intranasal delivery of antigen will be an effective immunization approach to seed immune effectors to the genital tract and protect against genital chlamydial infection. Second, the need for a delivery vehicle that possesses effective immunomodulatory function to stimulate adequate costimulation and a favourable cytokine environment can be met by a vehicle comprising live attenuated influenza virus vectors, which are potent inducers of Th1 cytokines.40,41 We therefore tested the hypothesis that intranasal delivery of recombinant influenza viruses expressing immunodominant epitopes from a protective chlamydial antigen will induce protective immunity against genital chlamydial infection.
The results revealed that recombinant influenza A/PR8/34 (H1N1) viruses containing immunodominant T-cell epitopes from chlamydial MOMP in the stalk region of the NA gene were effectively immunogenic and induced a strong specific Th1 response against the intact chlamydiae following intranasal administration. The immune effectors induced by the recombinant TINK were rapidly recruited (within 24 hr) into the genital mucosa and draining lymph nodes following a genital chlamydial infection, and were protective against the infection. The remarkable immunogenicity of recombinant viruses appears to be due to the phenomenal ability of the constructs to induce a favourable cytokine and chemokine environment that supports a robust T-cell activation. In addition, intranasal administration was highly effective at inducing immune effectors that were recruited to the genital mucosa following a genital infection.
A major concern with genital chlamydial infection and the vaccine effort is that the natural infection does not lead to long-term protection against reinfections.42,43 Among other factors, rapid treatment of diagnosed cases that prevents the establishment of host immunity44 and the relatively low frequency of immune effectors induced during a natural infection have been advanced as reasons for the short-term immunity during chlamydial infection. Therefore, vaccine efforts have also focused attention on defining the immunological factors as well as the immune parameters that govern sterilizing long-term protective immunity. To our knowledge so far, only the interleukin-10 (IL-10)-deficient dendritic cell (DC)-based cellular vaccine has produced a sterilizing, long-term immunity in a mouse genital chlamydial infection model that correlated with the capacity to induce a high frequency of specific Th1 cells and elevated titers of the CMI-associated IgG2a and IgA antibodies.36 Chlamydial-pulsed IL-10-deficient DCs appear to possess the necessary antigenic, costimulatory and immunomodulatory machinery for inducing an optimal protective immunity. While the protective cellular vaccine approach is probably of limited practical application for a widespread infection as Chlamydia, it provides a benchmark for evaluating other potential vaccines, and designing more effective delivery vehicles. Besides, the phenomenal efficacy of the IL-10 deficient DC-based protection system indicates that given optimal conditions a protective chlamydial vaccine is possible, and given an effective delivery vehicle, inactivated chlamydial elementary bodies possess sufficient immunogenic epitopes to elicit a protective immunity. The challenge for vaccinology therefore is to develop a delivery system that will mimic the superior immunostimulatory properties of IL-10 deficient DCs to achieve an effective chlamydial vaccine. Incidentally, when long-term protective immunity was evaluated in mice vaccinated with recombinant influenza viruses expressing chlamydial T-cell epitopes, there was a strong correlation between protective immunity and a high frequency of specific Th1 and IgG2a in immune animals. This finding would suggest that the immunomodulatory action of influenza virus vectors was effective at inducing adequate immune effectors to support a significant level of long-term protective immunity against genital chlamydial infection.
Because live attenuated, cold-adapted influenza virus vaccines are safe and acceptable for human use, these studies may provide a new and reliable approach to deliver STD vaccines for optimal efficacy. In fact, using a malaria challenge model in mice, we previously found that recombinant cold-adapted influenza viruses were effective vectors for induction of immune responses against malarial epitopes.45
Results revealing a vaccine advantage in multiepitope delivery23 and the need for both T cells and antibodies for optimal chlamydial immunity7–12 would suggest that future studies will include the evaluation of recombinant viral constructs harbouring multiple chlamydial T- and B-cell epitopes. The objective is to enhance the simultaneous generation of both T cells and antibodies against Chlamydia during the immunization stage. While these results have provided the proof of principle that it is conceivable for a future Chlamydia vaccine regimen to be delivered with a live attenuated cold-adapted influenza virus,46,47 additional studies will be needed to define the multiepitope requirement in humans, which are unlikely to be identical in mice and humans. The continued progress in influenza virus genetics48 will likely facilitate the use of a suitable derivative of the licensed human vaccine strain as a vector for delivering vaccines against certain STD agents, including Chlamydia. A major concern with the use of common microbes as vaccine delivery vectors is vector elimination caused by the presence of host antibodies against them in the population of exposed individuals. However, our preliminary studies revealed that pre-exposure to the vector did not prevent a robust secondary antichlamydial immune response in long-term studies. In addition, vector elimination caused by frequent immunizations with influenza vaccines or natural infection could be prevented by using updated vaccine vectors based on circulating strains, which is also the basis for the effectiveness of the live attenuated vaccine after several immunizations.47 Although the emerging subtype of virus is unpredictable, rare viral subtypes can be used as delivery vectors as well. Besides, Bell's palsy diagnosed with intranasal administration of inactivated flu vaccine appears to be associated with the adjuvant used, not the route,49 and there is no indication of this undesired effect in people vaccinated with the live cold-adapted influenza virus vaccine, dispelling the concerns about this delivery approach.
Acknowledgments
This work was supported by PHS grants (AI41231, GM 08248 and RR03034) from the National Institutes of Health, and the Centers for Disease Control and Prevention (CDC). Research in the laboratories of AGS is supported by NIH and DOD grants. We appreciate the technical assistance of Kahaliah Joseph, Laurie Howard, Ila Churn and Richard Cadagan.
References
- 1.WHO. Global prevalence and incidence of selected curable sexually transmitted diseasesOverview and Estimates. Geneva: WHO; 1996. p. 1996. [Google Scholar]
- 2.Paavonen J, Wolner-Hanssen P. Chlamydia trachomatis: a major threat to reproduction. J HumReprod. 1989;4:111–24. doi: 10.1093/oxfordjournals.humrep.a136855. [DOI] [PubMed] [Google Scholar]
- 3.Johnson RE, Newhall WJ, Papp JR, et al. Screening tests to detect Chlamydia trachomatis and Neisseria gonorrhoeae infections – 2002. MMWR Morb Mortal Wkly Rep (Recomm Rep) 2002;51:1–40. [PubMed] [Google Scholar]
- 4.Mahdi OS, Byrne GI, Kalayoglu M. Emerging strategies in the diagnosis, prevention and treatment of chlamydial infections. Expert Opin Ther Patents. 2001;11:1253–65. [Google Scholar]
- 5.Bragina EY, Gomberg MA, Dmitriev GA. Electron microscopic evidence of persistent chlamydial infection following treatment. J Eur Acad Dermatol Venereol. 2001;15:405–9. doi: 10.1046/j.1468-3083.2001.00342.x. [DOI] [PubMed] [Google Scholar]
- 6.De la Maza MA, De la Maza LM. A new computer model for estimating the impact of vaccination protocols and its application to the study of Chlamydia trachomatis genital infections. Vaccine. 1995;13:119–27. doi: 10.1016/0264-410x(95)80022-6. [DOI] [PubMed] [Google Scholar]
- 7.Igietseme JU, Eko FO, He Q, Bandea C, Lubitz W, Garcia-Sastre A, Black C. Delivery of Chlamydia vaccines. Expert Opin Drug Deliv. 2005;2:549–62. doi: 10.1517/17425247.2.3.549. [DOI] [PubMed] [Google Scholar]
- 8.Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infect Immun. 2002;70:2741–51. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loomis PW, Starnbach MN. T cell responses to Chlamydia trachomatis. Curr Opin Microbiol. 2002;5:87–91. doi: 10.1016/s1369-5274(02)00291-6. [DOI] [PubMed] [Google Scholar]
- 10.Igietseme JU, Eko FO, He Q, Black CM. Antibody regulation of T-cell immunity: implications for vaccine strategies against intracellular pathogens. Expert Rev Vaccines. 2004;3:23–34. doi: 10.1586/14760584.3.1.23. [DOI] [PubMed] [Google Scholar]
- 11.Cohen CR, Koochesfahani KM, Meier AS, et al. Immunoepidemiologic profile of Chlamydia trachomatis infection: importance of heat-shock protein 60 and interferon-gamma. J Infect Dis. 2005;192:591–9. doi: 10.1086/432070. [DOI] [PubMed] [Google Scholar]
- 12.Morrison SG, Morrison RP. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J Immunol. 2005;175:7536–42. doi: 10.4049/jimmunol.175.11.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson DE, Virok DP, Heidi W, et al. Chlamydia IFN-γ immune evasion is linked to host infection tropism. Proc Natl Acad Sci USA. 2005;102:10658–63. doi: 10.1073/pnas.0504198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaVerda D, Kalayoglu MV, Byrne GI. Chlamydial heat shock proteins and disease pathology: new paradigms for old problems? Infect Dis Obstet Gynecol. 1999;7:64–71. doi: 10.1155/S1064744999000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephens RS. Chlamydial genomics and vaccine antigen discovery. J Infect Dis. 2000:S521–S23. doi: 10.1086/315631. [DOI] [PubMed] [Google Scholar]
- 16.Kawa DE, Stephens RS. Antigenic topology of chlamydial PorB protein and identification of targets for immune neutralization of infectivity. J Immunol. 2002;168:5184–91. doi: 10.4049/jimmunol.168.10.5184. [DOI] [PubMed] [Google Scholar]
- 17.Murdin AD, Dunn P, Sodoyer R, Wang J, Caterini J, Brunham RC, Aujame L, Oomen R. Use of a mouse lung challenge model to identify antigens protective against Chlamydia pneumoniae lung infection. J Infect Dis. 2000;181(Suppl 3):S544–51. doi: 10.1086/315605. [DOI] [PubMed] [Google Scholar]
- 18.Donati M, Sambri V, Comanducci M, Di Leo K, Storni E, Giacani L, Ratti G, Cevenini R. DNA immunzation with pgp3 gene of Chlamydia trachomatis inhibits the spread of chlamydial infection from the lower to the upper genital tract in C3H/HeN mice. Vaccine. 2003;21:1089–93. doi: 10.1016/s0264-410x(02)00631-x. [DOI] [PubMed] [Google Scholar]
- 19.Sharma J, Bosnic AM, Piper JM, Zhong G. Human antibody responses to a Chlamydia-secreted protease factor. Infect Immun. 2004;72:7164–71. doi: 10.1128/IAI.72.12.7164-7171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belland RJ, Scidmore MA, Crane DD, Hogan DM, Whitmire W, McClarty G, Caldwell HD. Chlamydia trachomatis cytotoxicity associated with complete and partial cytotoxin genes. Proc Natl Acad Sci USA. 2001;98:13984–9. doi: 10.1073/pnas.241377698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slepenkin A, de la Maza LM, Peterson EM. Interaction between components of the Type III secretion system of Chlamydiaceae. J Bacteriol. 2005;187:473–9. doi: 10.1128/JB.187.2.473-479.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong-Ji Z, Yang X, Shen C, Lu H, Murdin Am Brunham RC. Priming with Chlamydia trachomatis major outer membrane protein (MOMP) DNA followed by MOMP-ISCOM boosting enhances protection and is associated with increased immunoglobulin A and Th1 cellular immune responses. Infect Immun. 2000;68:3074–8. doi: 10.1128/iai.68.6.3074-3078.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eko FO, He Q, Brown T, et al. A novel recombinant multisubunit vaccine against Chlamydia. J Immunol. 2004;173:3375–82. doi: 10.4049/jimmunol.173.5.3375. [DOI] [PubMed] [Google Scholar]
- 24.Wu H-Y, Russell MW. Nasal lymphoid tissue, intranasal immunization, and compartmentalization of the common mucosal immune system. Immunol Res. 1997;16:187–201. doi: 10.1007/BF02786362. [DOI] [PubMed] [Google Scholar]
- 25.Igietseme JU, Uriri IM, Kumar SN, Ananaba GA, Ojior OO, Momodu IA, Candal DH, Black CM. Route of infection that induces a high intensity of gamma interferon-secreting T cells in the genital tract produces optimal protection against Chlamydia trachomatis infection in mice. Infect Immun. 1998;66:4030–5. doi: 10.1128/iai.66.9.4030-4035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pal S, Theodor I, Peterson EM, de la Maza LM. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein can elicit a protective immune respose against a genital challenge. Infect Immun. 2001;69:6240–7. doi: 10.1128/IAI.69.10.6240-6247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staats HF, Montgomery SP, Palker TJ. Intranasal immunization is superior to vaginal, gastric, or rectal immunization for induction of systemic and mucosal anti-HIV antibody responses. AIDS Res Hum Retroviruses. 1997;13:945–52. doi: 10.1089/aid.1997.13.945. [DOI] [PubMed] [Google Scholar]
- 28.Efferson CL, Schickli J, Ko BK, Kawano K, Mouzi S, Palese P, Garcia-Sastre A, Ioannides CG. Activation of tumor antigen-specific cytotoxic T lymphocytes (CTLs) by human dendritic cells infected with an attenuated influenza A virus expressing a CTL epitope derived from HER-2/neu proto-oncogene. J Virol. 2003;77:7411–24. doi: 10.1128/JVI.77.13.7411-7424.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knight SC, Iqball S, Woods C, Stagg A, Ward ME, Tuffrey M. A peptide of Chlamydia trachomatis shown to be a primary T-cell epitope in vitro induces cell-mediated immunity in vivo. Immunology. 1995;85:8–15. [PMC free article] [PubMed] [Google Scholar]
- 30.Stagg AJ, Elsley WAJ, Pickett MA, Ward ME, Knight SC. Primary human T-cell responses to the major outer membrane protein of Chlamydia trachomatis. Immunology. 1993;79:1–9. [PMC free article] [PubMed] [Google Scholar]
- 31.Ramsey KH, Soderberg LSF, Rank RG. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect Immun. 1988;56:1320–5. doi: 10.1128/iai.56.5.1320-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Sastre A. Transfectant influenza viruses as antigen delivery vectors. Adv Virus Res. 2000;55:579–97. doi: 10.1016/s0065-3527(00)55019-2. [DOI] [PubMed] [Google Scholar]
- 33.Fodor E, Devenish L, Engelhardt OG, Palese P, Brownlee GG, Garcia-Sastre A. Rescue of influenza A virus from recombinant DNA. J Virol. 1999;73:9679–82. doi: 10.1128/jvi.73.11.9679-9682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishizaki M, Allen JE, Beatty PR, Stephens RS. Immune specificity of murine T-cell lines to the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1992;60:3714–8. doi: 10.1128/iai.60.9.3714-3718.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen JE, Locksley RM, Stephens RS. A single peptide from the major outer membrane protein of Chlamydia trachomatis elicits T cell help for the production of antibodies to protective determinants. J Immunol. 1991;147:674–9. [PubMed] [Google Scholar]
- 36.Igietseme JU, Ananaba GA, Bolier J, et al. Suppression of endogenous IL-10 gene expression in dendritic cells enhances antigen presentation for enhanced specific Th1 induction: potential for cellular vaccine development. J Immunol. 2000;164:4212–9. doi: 10.4049/jimmunol.164.8.4212. [DOI] [PubMed] [Google Scholar]
- 37.Tuffrey M, Alexander F, Woods C, Taylor-Robinson D. Genetic susceptibility to chlamydial salpingitis and subsequent infertility in mice. J Reprod Fertil. 1992;95:31–8. doi: 10.1530/jrf.0.0950031. [DOI] [PubMed] [Google Scholar]
- 38.Kees U, Kynast G, Weber E, Krammer PH. A method for testing the specificity of influenz A virus-reactive memory cytotoxic T lymphocytes (CTL) clones in limiting dilution cultures. J Immunol Methods. 1984;69:215–27. doi: 10.1016/0022-1759(84)90320-x. [DOI] [PubMed] [Google Scholar]
- 39.Krammer PH, Dy M, Hultner L, et al. Production of lymphokines by murine T cells grown in limiting dilution and long-term cultures. In: Fathman CG, Fitch FW, editors. Isolation, Characterization, and Utilization of T Lymphocyte Clones. New York: Academic Press; 1982. pp. 252–62. [Google Scholar]
- 40.Julkunen I, Melen K, Nyqvist M, Pirhonen J, Sareneva T, Matikainen S. Inflammatory responses in influenza A virus infection. Vaccine. 2000;19(Suppl 1):S32–37. doi: 10.1016/s0264-410x(00)00275-9. [DOI] [PubMed] [Google Scholar]
- 41.Matsuo K, Iwasaki T, Asanuma H, Yoshikawa T, Chen Z, Tsujimoto H, Kurata T, Tamura SS. Cytokine mRNAs in the nasal-associated lymphoid tissue during influenza virus infection and nasal vaccination. Vaccine. 2000;18:1344–50. doi: 10.1016/s0264-410x(99)00401-6. [DOI] [PubMed] [Google Scholar]
- 42.Richey CM, Macaluso M, Hook EW. Determinants of reinfection with Chlamydia trachomatis. Sex Transm Dis. 1999;26:4–11. doi: 10.1097/00007435-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Veldhuijzen IK, Van Bergen JE, Gotz HM, Hoebe CJ, Morre SA, Richardus JH, Group PCS. Reinfections, persistent infections, and new infections after general population screening for Chlamydia trachomatis infection in the Netherlands. Sex Transm Dis. 2005;32:599–604. doi: 10.1097/01.olq.0000179887.01141.c3. [DOI] [PubMed] [Google Scholar]
- 44.Parks KS, Dixon PB, Richey CM, Hook E. Spontaneous clearance of Chlamydia trachomatis infection in untreated patients. Sex Transm Dis. 1997;24:229–35. doi: 10.1097/00007435-199704000-00008. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez-Aseguinolaza G, Nakaya Y, Molano A, et al. Induction of protective immunity against malaria by priming-boosting immunization with recombinant cold-adapted influenza and modified vaccinia Ankara viruses expressing a CD8+–T-cell epitope derived from the circumsporozoite protein of Plasmodium yoelii. J Virol. 2003;77:11859–66. doi: 10.1128/JVI.77.21.11859-11866.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Influenza virus vaccine live intranasal – MedImmune vaccines: CAIV-T, influenza vaccine live intranasal. Drugs R D. 2003;4:312–9. doi: 10.2165/00126839-200304050-00007. [DOI] [PubMed] [Google Scholar]
- 47.Abramson JS. Intranasal, cold-adapted, live, attenuated influenza vaccine. Pediatr Infect Dis J. 1999;18:1103–4. doi: 10.1097/00006454-199912000-00017. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe T, Watanabe S, Noda T, Fujii Y, Kawaoka Y. Exploitation of nucleic acid packaging signals to generate a novel influenza virus-based vector stably expressing two foreign genes. J Virol. 2003;77:10575–83. doi: 10.1128/JVI.77.19.10575-10583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Couch RB. Nasal vaccination, Escherichia coli enterotoxin, and Bell's palsy. N Engl J Med. 2004;350:860–1. doi: 10.1056/NEJMp048006. [DOI] [PubMed] [Google Scholar]