Abstract
We have previously shown that CD8+γδ T cells decrease late allergic airway responses, airway eosinophilia, T helper 2 cytokine expression and increase interferon-γ (IFN-γ) expression. We hypothesized that the effects of CD8+γδ T cells were IFN-γ mediated. Brown Norway rats were sensitized to ovalbumin on day 1. Cervical lymph node CD8+γδ T cells from sensitized animals were treated with antisense oligodeoxynucleotide (5 µmol/l) to inhibit IFN-γ synthesis or control oligodeoxynucleotide and 3·5 × 104 CD8+γδ T cells were injected intraperitoneally into sensitized recipients on day 13. Rats were challenged with aerosolized ovalbumin on day 15 and lung resistance was monitored over an 8 hr period, after which bronchoalveolar lavage was performed. Control oligodeoxynucleotide treated γδ T cells decreased late airway responses and eosinophilia in bronchoalveolar lavage. There was a complete recovery of late airway responses and a partial recovery of airway eosinophilia in recipients of antisense oligodeoxynucleotide treated cells. Macrophage ingestion of eosinophils was frequent in rats administered γδT cells but reduced in recipients of antisense oligodeoxynucleotide treated cells. These results indicate that CD8+γδ T cells inhibit late airway responses and airway eosinophilia through the secretion of IFN-γ. Defective or altered γδ T-cell function may account for some forms of allergic asthma.
Keywords: lung, allergy, inflammation, T lymphocytes, cytokines, asthma
Introduction
In human atopic asthma, the late airway response to allergen exposure appears to be relevant to chronic asthma because of its association with airway hyperresponsiveness and airway inflammation, two defining features of the disease.1 The late airway responses are T-cell dependent and their magnitudes are affected by activation of both CD4+ and CD8+ T cells in vivo. A mixed population of CD8+ T cells (αβ and γδ) inhibits allergen induced late airway responses and eosinophilia in rats.2–4 However, the subtype of CD8+ T cells has an important influence on the role they play. CD8+ T cells that bear the αβ T-cell receptor (TCR) are pro-inflammatory whereas CD8+ T cells bearing the γδ TCR are inhibitory.5,6
The γδ T cells that show tropism for mucosal epithelial cells, which is of potential importance to the epithelial repair process in diseases such as asthma7 are required for fully developed allergic responses in the mouse,8 but the CD8 subset of these cells in the rat is potently inhibitory.6 The mechanism by which the CD8+γδ T cells inhibit late airway responses is not clear but a candidate cytokine for the effect is interferon-γ (IFN-γ).9,10 Interestingly, respiratory syncytial virus infection, that is frequently associated with the development of childhood asthma, suppresses IFN-γ producing γδ T cells in the blood and the number of IFN-γ producing γδ T cells is lower in infants that developed recurrent wheezing.11 Although IFN-γ is a pro-inflammatory T helper 1 (Th1) cytokine, it has selective anti-inflammatory effects on allergic inflammation through suppression of the proliferation of Th2-type T cells12. Exogenous recombinant IFN-γ inhibits late airway responses in the rat13 and suppresses airway hyperresponsiveness and airway eosinophilia in mice.14,15 Deficient IFN-γ production causes a prolongation of eosinophilic inflammation after allergen challenge.16 Although IFN-γ is a plausible mediator of the suppressive effects of the CD8+γδ T cells, there is as yet no proof that this is the mechanism by which CD8+γδ T cells inhibit the late airway responses.
The aim of the current study was to test the hypothesis that IFN-γ mediates the inhibitory effects of the γδ T cells on the late airway responses and eosinophilia. We used a well-characterized rat model of allergic airway responses and employed the technique of adoptive transfer of T cells to explore the mechanisms of CD8+γδ T-cell suppressive effects. To do this CD8+γδ T cells were treated with antisense oligodeoxynucleotides (ODN)17 against IFN-γ prior to transfer. We hypothesized also that the inhibition of late airway responses by INF-γ and airway inflammation may involve the alveolar macrophage. Macrophages may serve as a source of cysteinyl leukotrienes that are the key mediators of late airway responses18 and the phagocytosis of eosinophils by alveolar macrophages is increased in rats administered CD8+γδ T cells.6 To test the potential role of the macrophages in mediating the suppressive effects of the CD8+γδ T cells through the release of IFN-γ we measured the effects of IFN-γ on the rate of macrophage phagocytosis of the yeast zymosan A and the synthesis of cysteinyl leukotrienes in vitro. Our results indicate that much of the inhibitory effect of CD8+γδ T cells on the late airway responses and airway inflammation are attributable to IFN-γ expression and are contributed to by alterations in macrophage function.
Materials and methods
Study protocol
All donor rats were sensitized to ovalbumin (OVA). Lymphocytes were harvested from the cervical lymph nodes of donors 12 days after the sensitization. CD8+γδ T cells were purified by immunomagnetic cell isolation. Recipient Brown Norway (BN) rats were given 3·5 × 104 of either antisense oligodeoxynucleotide (ODN)-treated CD8+γδ T cells (n = 6; interferon-γ (IFN-γ)-depleted CD8+γδ T cells group) or control ODN-treated CD8+γδ T cells (n = 8; CD8+γδ T cells group) intraperitoneally (i.p.) on day 13 whereas control rats were given phosphate-buffered saline (PBS; n = 9, PBS group). Like the donor rats, all recipient rats were sensitized to OVA on day 1 and were challenged with aerosolized OVA (5% in saline) two days after CD8+γδ T-cell transfer or PBS. Lung resistance was measured before aerosol challenge, at 5, 10, 15, 20, 30 min following aerosol challenge, and subsequently at 15-min. intervals for a total period of 8 hr. On completion of the lung function measurements, bronchoalveolar lavage was performed.
The study was approved by the University Animal Care Committee and the animals were treated according to the guidelines of the Canadian Council on Animal Care.
Animals and sensitization
BN rats (males; aged 7–9 weeks; 180–200 g) were purchased from Harlan Sprague Dawley UK (Blackthorn, UK). The animals were sensitized by a single subcutaneous injection of 200 µg of OVA (grade V, Sigma, St. Louis, MO) in 1·0 ml normal saline containing 8·5 mg of aluminium hydroxide gel (Anachemia Chemicals, Montreal, Canada) as an adjuvant. Simultaneously, 0·5 ml of Bordetella pertussis vaccine (Dr T. Issekutz, Dalhousie University, Halifax, NS, Canada) containing 1 × 109 heat-killed bacilli were injected i.p.
Treatment of CD8+γδ T cells with ODN and adoptive transfer
Cervical lymph node cells were harvested from donor rats 12 days after sensitization, pooled, and purified using negative selection with immunomagnetic cell sorting (MACS) and the following antibodies: anti-CD4, anti-B cell, anti-myeloid cell, anti-natural killer (anti-NK) cell, and anti-αβ TCR.6 The purity of the cells was measured using flow cytometry (FACScan, Becton Dickinson, Mountain View, CA).19 Phosphorothioate-ODN against IFN-γ and randomized ODN were designed and synthesized by Biognostik (Göttingen, Germany). Cells were incubated with ODN (5 µmol/l) and 30 nm of oligofectamine for 20 min to improve transfection efficiency. CD8+γδ T cells were incubated with the ODN/oligofectamine mix for a total of 6 hr in Dulbecco's modified Eagle's minimal essential medium (DMEM) and 1% fetal calf serum (added after 2 hr). 35 000 cells were transferred i.p. to OVA-sensitized recipients in groups CD8+γδ T cell and IFN-γ depleted CD8+γδ 2 days before challenge.
Antisense ODN and IFN-γ expression in lymphocytes
Mononuclear cells isolated using Ficoll-Hypaque from cells pooled from both the cervical lymph nodes and spleen of naïve rats were stimulated with anti-CD3/anti-CD28 antibodies (5 µg/ml) for 48 hr in medium with 10% fetal calf serum to evaluate the effects of antisense ODN on IFN-γ expression by T lymphocytes. After stimulation, cells were incubated with either 5 µmol/l IFN-γ antisense ODN/oligofectamine, control ODN/oligofectamine, or culture medium for 2 hr. Cells were re-stimulated with 10 ng/ml PMA and 0·5 µg/ml ionomycin in the presence of Golgistop (BD Biosciences, Mississauga, ON, Canada) and 1% fetal calf serum, and incubated for another 4 hr. The cells were analysed by means of flow cytometry (Becton Dickinson) with an fluoroscein isothiocyanate (FITC)-labelled anti-IFN-γ antibody and an FITC-labelled mouse immunoglobulin G1 as a control.
Measurement of airway responses to antigen challenge
Two days after the administration of CD8+γδ T cells or PBS, animals were anesthetized with urethane (1·95 g/kg administered i.p.), instrumented, and challenged for 5 min with aerosolized OVA (5% wt/vol). Lung resistance was measured for 8 hr and was calculated from airflow measurements and oesophageal pressure measurements using the technique of multiple linear regression.20
Bronchoalveolar lavage
Lavage was performed as previously described.20 Eight hrs after antigen challenge and total bronchoalveolar lavage cell counts were determined using a haemocytometer. Bronchoalveolar lavage cytospin slides were prepared using a Cytospin Model II (Shandon, Pittsburgh, PA). The slides were Wright-Giemsa stained and cellular differentials were assessed from a count of 300 cells.
Immunocytochemical evaluation of eosinophil-derived major basic protein expression
Bronchoalveolar lavage cytospin slides were fixed in 4% paraformaldehyde for 30 min, and were stained with mouse anti-human major basic protein monoclonal antibody (BMK-13) using the alkaline phosphatase–antialkaline phosphatase method. We had previously noted that eosinophils were underestimated by conventional staining.20
mRNA detection by in situ hybridization
Cytospin slides from bronchoalveolar lavage fluid were fixed with 4% paraformaldehyde. The detection of mRNA for interleukin (IL)-4, IL-5, and IFN-γ was performed according to previously described methodology.21 Antisense and sense riboprobes were prepared from cDNA coding for rat IL-4, IL-5, and IFN-γ mRNA. The probes for IL-4, IL-5, IL-13 and IFN-γ were gifts from Drs A. Neil Barclay (Oxford, UK), T. Blankenstein (Berlin, Germany), and P. H. van der Meide (Rijswijk, the Netherlands), respectively.
Phagocytosis and cysteinyl leukotriene synthesis by alveolar macrophages following stimulation by rrIFN-γ
Fluorescently labeled zymosan A (Molecular Probes, Eugene, OR) was mixed with opsonizing reagent (Molecular Probes) and naïve rat serum for 1 hr at 37°, and was re-suspended in complete DMEM with 1% antibiotics and 10% fetal bovine serum. Macrophages were obtained by bronchoalveolar lavage of naïve BN rats. Cells from two animals were pooled for each experiment. After centrifugation, red blood cell lysis was done. Cells were washed with PBS and re-suspended in 500 µl complete DMEM and then 0·25 × 106 cells/0·25 ml were placed in each well of low-adhesion plates (Ultra Low Attachment Plates; Costar/Corning, Acton, MA) with several concentrations (0, 0·2, 1·0 ng/ml) of rrIFN-γ (BD Biosciences, Mississauga, ON, Canada). The total volume was adjusted to 500 µl with complete DMEM. Macrophages were cultured for 18 hr at 37° and then opsonized zymosan A (0·25 × 106 particles) that was labelled with fluorescein or unlabelled control zymosan was cultured with the macrophages for a further 6 hr. Then supernatant from the cultures was removed and stored at −80° for subsequent assay of cysteinyl leukotrienes. Cells were re-suspended in cell dissociation buffer, centrifuged, re-suspended in 0·3–0·5 ml of 1% paraformaldehyde for analysis. Flow cytometry was used to count the number of macrophages that phagocytosed zymosan particles.
Measurement of cysteinyl leukotrienes
Enzyme immunoassay (Cayman Chemical, Ann Arbor, MI) was used to measure the levels of cysteinyl leukotrienes in bronchoalveolar lavage fluid and culture supernatant.18 The antiserum is reported to have cross-reactivity for leukotrienes C4 (100%), D4 (100%), and E4 (67%).
Statistical analysis
Unless otherwise indicated, data are presented as mean ± 1 standard error. Statistical comparisons were performed using the Kruskal–Wallis and the Mann–Whitney U-tests. P-values less than 0·05 were considered significant.
Results
CD8+γδ T-cell purification, antisense ODN treatment and transfer
The purity of CD8+γδ T cells harvested from the cervical lymph nodes, and isolated by negative selection, was assessed by flow cytometry. The ratio of CD8+γδ T/total cells was 88·1 ± 0·9% purity (n = 3). The viability of CD8+γδ T cells evaluated by Trypan Blue exclusion prior to their transfer into the animals was 95·2 ± 1·0% (n = 3) and 94·6 ± 1·4% (n = 3) for CD8+γδ and IFN-γ depleted CD8+γδ cells, respectively.
We confirmed the efficacy of antisense ODN treatment on IFN-γ expression by T cells by flow cytometry by comparing the effects of control and antisense ODN on intracellular IFN-γ expression in anti-CD3/anti-CD28 stimulated T-lymphocyte preparations. Approximately 25% of gated lymphocytes that were sense ODN treated expressed IFN-γ whereas approximately 10% of lymphocytes that were incubated with IFN-γ antisense ODN expressed IFN-γ.
Airway responses to OVA challenge in sensitized animals after transfer of CD8+γδ T cells
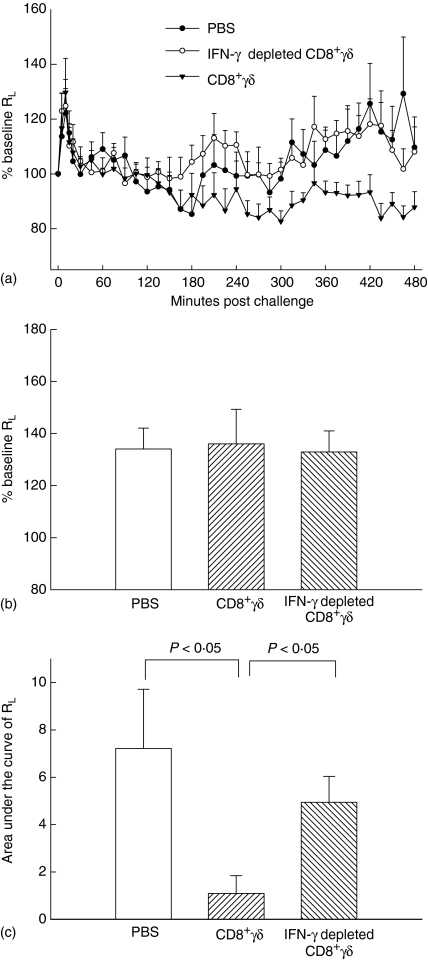
The transfer of CD8+γδ T cells did not alter mean baseline lung resistance among the three groups (Fig. 1a). The early airway responses were defined as the maximal value of lung resistance, expressed as a percentage of the baseline value, within the first 60 min after challenge, and there was no significant difference among groups (Fig. 1b).
Figure 1.
Transfer of CD8+γδ T cells abolishes the late response to antigen challenge. (a) Lung resistance measurements over 8 hr are depicted. Each data point represents the mean ± SE. The late airway response was greatly decreased in the CD8+γδ T-cell group and this effect was reversed in the IFN-γ depleted CD8+γδ T cell group. (b) Early airway responses were defined as the maximal value of lung resistance within the first 60 min of challenge and expressed as a percentage of baseline lung resistance. There was no significant difference in the group means. (c) The late airway response was calculated as the area under the curve of lung resistance against time from 3 to 8 hr, after correction for baseline lung resistance. The CD8+γδ T-cell group response was significantly lower compared to the PBS and to the IFN-γ depleted CD8+γδ T cell group: P < 0·05. There was no significant difference between the PBS and IFN-γ depleted CD8+γδ T-cell group.
The late airway responses were calculated as the area under the curve of lung resistance against time (in cmH2O/ml/s × min) from 3 to 8 hr following challenge and after correction of lung resistance for the baseline value. The transfer of control ODN treated CD8+γδ T cells (CD8+γδ T-cell group) significantly inhibited the late airway responses: surface areas: 1·09 ± 0·75 versus 7·21 ± 2·50 (PBS group; P < 0·05). Treatment of the CD8+γδ T cells with antisense ODN (IFN-γ depleted CD8+γδ T cell group) led to a complete restoration of the late airway responses surface area: 4·94 ± 1·06 (P < 0·05) (Fig. 1c).
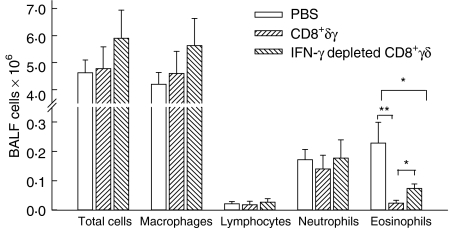
Effect of CD8+γδ T cells on the bronchoalveolar lavage leucocytes
There were no significant differences in the total cell counts among the groups (Fig. 2). The CD8+γδ T-cell group showed a non-significant trend for fewer neutrophils compared to the PBS and the IFN-γ depleted CD8+γδ T-cell groups. Both the CD8+γδ T-cell and the IFN-γ depleted CD8+γδ T-cell groups had significantly fewer eosinophils compared to the PBS group (P < 0·001 and P < 0·05, respectively). However, there was a significant rise in eosinophilia in the IFN-γ depleted CD8+γδ T-cell group compared to the CD8+γδ T-cell group (P < 0·05; Fig. 2).
Figure 2.
Effect of CD8+γδ T cells on the bronchoalveolar lavage leucocytes. There were no significant differences in the total cell counts, neutrophils or lymphocytes among the groups. The CD8+γδ T cell group and IFN-γ depleted CD8+γδ T cell group had significantly lower levels of eosinophils compared to the PBS group (**P < 0·001, *P < 0·05). However, there was a significant rise in eosinophilia in the IFN-γ depleted CD8+γδ T-cell group compared to the CD8+γδ T-cell group (P < 0·05).
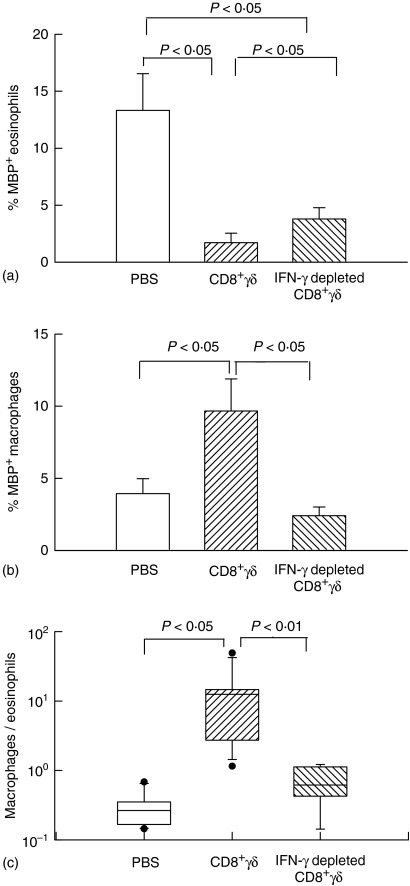
Eosinophil-derived major basic protein immunoreactive cells in the bronchoalveolar lavage
The percentage of major basic protein positive eosinophils in the bronchoalveolar lavage fluid is shown in Fig. 3. Major basic protein positive eosinophils were significantly fewer in the rats that were administered CD8+γδ T cells compared to the PBS control group. The inhibition of eosinophilia was partial in the IFN-γ depleted CD8+γδ T-cell group. The results of immunostaining for major basic protein were similar to the data from cell differentials evaluated by conventional staining as shown in Fig. 2.
Figure 3.
Effects of CD8+γδ T cells on eosinophilia and macrophages in the bronchoalveolar lavage. (a) The percentage of major basic protein (MBP) positive eosinophils in bronchoalveolar lavage fluid. Major basic protein positive eosinophils in CD8+γδ T-cell group were significantly lower compared to the PBS group. The eosinophilia was partially restored in the IFN-γ depleted CD8+γδ T cell group. (b) The number of major basic protein positive macrophages were more numerous in the CD8+γδ T cells group than in the PBS group. This increase was absent in the IFN-γ depleted CD8+γδ T cells group. (c) Ratio of major basic protein positive macrophages to eosinophils. The median, 25th, 75th, percentiles are shown. The ratio was higher in CD8+γδ T-cell group than in PBS group. In the IFN-γ depleted CD8+γδ T-cell group, the ratio was lower than in the CD8+γδ T-cell group.
There was evidence of major basic protein immunoreactivity in cells that were identified as macrophages by morphological criteria. We have previously identified ingested apoptotic eosinophils in macrophages following OVA challenge.6 Although the number of eosinophils was low in rats that received CD8+γδ T cells the number of major basic protein positive macrophages was greatly increased compared to the PBS group. This increase in major basic protein immunoreactive macrophages was not noted in the bronchoalveolar lavage fluid of the recipients of IFN-γ depleted CD8+γδ T cells.
The ratio of major basic protein positive macrophages to eosinophils in the bronchoalveolar lavage fluid was higher for the CD8+γδ T cells group than that of PBS group. In the IFN-γ depleted CD8+γδ T-cell group, the ratio was lower. These results are compatible with either an increase in the activity of the macrophages or a decrease in the survival of eosinophils in the CD8+γδ T-cell group.
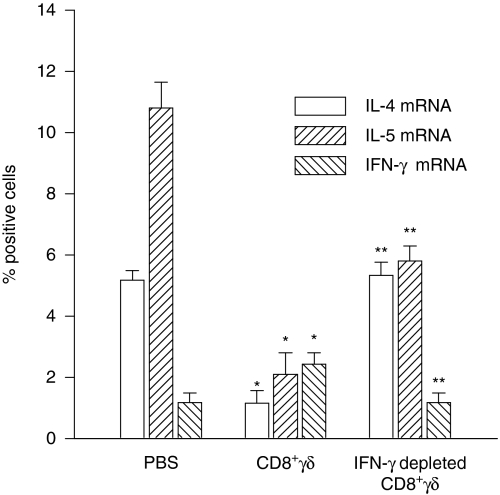
Cytokine mRNA expression in the bronchoalveolar lavage cells
IL-4 mRNA expressing cells (detected by in situ hybridization) were approximately 5% of the bronchoalveolar lavage fluid cells following OVA challenge of the PBS group (Fig. 4). Recipients of CD8+γδ T cells had fewer IL-4 expressing cells whereas in animals that received IFN-γ depleted CD8+γδ T cells IL-4 expression was restored. IL-5 showed a similar trend although its restoration was not complete in animals receiving IFN-γ depleted CD8+γδ T cells. A fraction of the IL-5 positive cells is eosinophils so that failure of complete restoration of the eosinophilia may account for the slightly lower number of IL-5 expressing cells (Fig. 4). In contrast, the number of cells expressing IFN-γ mRNA was increased after the CD8+γδ T-cell transfer, and was less in the IFN-γ depleted CD8+γδ T-cell transferred group, indicating the effectiveness of the antisense ODN treatment of the cells (Fig. 4).
Figure 4.
Effects of CD8+γδ T cells on cytokine mRNA expression. The percentage of IL-4 expressing cells (detected by in situ hybridization) was significantly lower (P < 0·05) in the CD8+γδ T-cell group but was restored to values comparable to the PBS group in the IFN-γ depleted CD8+γδ T-cell group. IL-5 expressing cells were reduced in CD8+γδ T-cell group compared to the PBS group (P < 0·005). Partial restoration of IL-5 positive cells occurred in the IFN-γ depleted CD8+γδ T-cell group. The number of IFN-γ expressing cells was greater in the CD8+γδ T-cell group (P < 0·05) but was reduced in the IFN-γ depleted CD8+γδ T-cell group (P < 0·05). *P < 0·05 compared to PBS group. **P < 0·05 compared to CD8+γδ T-cell group.
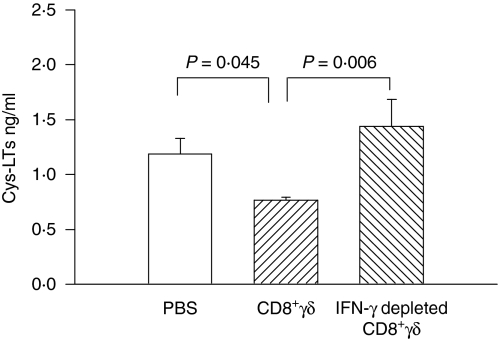
Cysteinyl leukotrienes in bronchoalveolar lavage fluid
The level of cysteinyl leukotrienes in the bronchoalveolar lavage fluid was assessed by specific enzyme immunoassay. Cysteinyl leukotrienes were elevated in bronchoalveolar lavage fluid following OVA challenge (PBS group in Fig. 5), compared to the values previously published for control animals.21 The level of cysteinyl leukotrienes was significantly lower in the CD8+γδ T-cell transferred group. However, when the CD8+γδ T cells were antisense ODN treated (IFN-γ depleted) the levels of cysteinyl leukotrienes were restored to values comparable to the OVA challenged control group.
Figure 5.
Cysteinyl leukotrienes in bronchoalveolar lavage fluid. Cysteinyl leukotrienes levels were reduced by CD8+γδ T-cell transfers and the levels restored in the IFN-γ depleted CD8+γδ T-cell group.
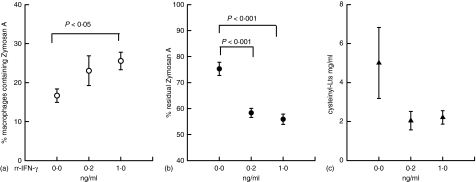
The assessment of phagocytosis and cysteinyl leukotrienes synthesis by alveolar macrophages following stimulation with rrIFN-γ
To mimic the effects of IFN-γ on macrophage functions in vivo we stimulated macrophages in culture with IFN-γ and examined the capacity of the macrophages for phagocytosis and for the synthesis of cysteinyl leukotrienes (Fig. 6). Zymosan A ingestion by macrophages was substantially increased in a concentration-dependent fashion following IFN-γ pretreatment (Fig. 6a and b). Macrophages showed a strong trend for decreased synthesis of cysteinyl leukotrienes following stimulation with IFN-γ, although variability in the control group was substantial and precluded statistical significance (Fig. 6c).
Figure 6.
Phagocytosis of zymosan A by macrophages analyzed by flow cytometry. (a) Phagocytosis of zymosan A by macrophages stimulated by rrIFN-γ. (b) Residual zymosan A remaining in supernatant. Phagocytosis was increased by IFN-γ. (c) Total cysteinyl leukotrienes in supernatant. Macrophage synthesis of cysteinyl leukotrienes was reduced by IFN-γ.
Discussion
To clarify the mechanisms by which CD8+γδ T cells may potentially modulate the late airway responses, we have used the technique of adoptive transfer in the BN rat model of allergic asthma.4 Recently, we have shown that administration of CD8+γδ T cells from naïve donors to rats undergoing sensitization resulted in a marked inhibition of the late airway response and of allergic inflammation evoked by aerosol challenge.6 The observed inhibition of the expression of the Th2 cytokines IL-4 and IL-5 in bronchoalveolar lavage fluid cells and the increase in cells expressing IFN-γ suggested that IFN-γ might provide the counter-regulatory inhibitory signal for Th2-type cytokine expression.12 The current study with IFN-γ depleted CD8+γδ T cells provides evidence that IFN-γ synthesis by CD8+γδ T cells is responsible for the inhibition of the allergen induced late airway responses and is, at least in part, also responsible for suppression of eosinophilic inflammation.
Both pro-inflammatory and anti-inflammatory roles have been proposed for γδ T cells in allergic inflammation.5,8 Fully developed inflammatory responses to allergen challenge require γδ T cells in the mouse.22 However, the CD8+γδ T-cell subset has been shown to mediate allergen tolerance in rodents and in mice undergoing repeated allergen exposures, through mechanisms involving the expression of IFN-γ.9 Even though we did not harvest the γδ cells from tolerized animals we reasoned nonetheless that the CD8 subset would be most likely to express the prototypic Th1 cytokine IFN-γ. There was complete inhibition of the late airway responses by the transferred CD8+γδ T cells incubated with the control ODN only. Previously we noted suppression of the late airway response when the CD8+γδ T cells were administered to rats prior to sensitization,6 whereas in the current experiments the suppression was evident even when the cells were administered 12 days after sensitization and only 2 days prior to challenge. In addition, there was marked suppression of IL-4 and IL-5 mRNA expressing cells, eosinophilia and cysteinyl leukotriene levels. Accompanying the suppression of Th2 inflammation there was an increase in IFN-γ expressing cells in the bronchoalveolar lavage fluid. A potential limitation of the study is the fact that the purity of the CD8+γδ T cells was about 90%. However, in a previous study6 we demonstrated that the contaminating cells were without effect on the responses of recipient rats to sensitization and challenge.
To test the role of IFN-γ in the observed inhibitory effects we targeted its synthesis using antisense ODN directed against IFN-γ. Efficacy of the antisense ODN treatment was confirmed using splenic T cells that were stimulated with anti-CD3/anti-CD28, phorbol 12-myristate 13-acetate and ionomycin and incubated with antisense ODN. This treatment produced about 60% inhibition of IFN-γ expression, as determined by flow cytometric assessment of intracellular cytokine expression. However, it seems likely that inhibition was even more complete in vivo given the potent biological effects of the antisense ODN treatment on the adoptively transferred cells. The successful restoration of the late airway responses by means of specific antisense ODN treatment was associated with a partial recovery of allergen induced eosinophilia in the bronchoalveolar lavage and a reduction in IFN-γ to control levels of expression. These findings are consistent with the reported inhibitory effects of IFN-γ on Th2-type cytokine expression by CD4+ T cells.23 The lack of complete recovery of eosinophilia may indicate that there are pathways other than IFN-γ for the inhibition of these cells. Alternatively the threshold for inhibition of eosinophilia by IFN-γ may be lower than for inhibition of the late response. The importance of IFN-γ expressed by γδ T cells in preventing airway hyperresponsiveness following repeated allergen challenge, in the absence of sensitization, has also been shown.21 In mice deficient in γδ T cells, airway hyperresponsiveness develops after three exposures to OVA; in mice that are both IFN-γ and γδ T-cell deficient airway hyperresponsiveness after OVA exposure is not further augmented by treatment with an anti-TCR Vγ4 antibody indicating, albeit indirectly, the importance of IFN-γ expressed by TCR Vγ4 in the phenomenon.24
The fact that IFN-γ antisense treatment of CD8+ T cells, not characterized as to their TCR (αβ or γδ), did not abrogate the suppressive effect of the cells on late airway responses in a previous study4 may be explained by the opposing effect of the two different CD8+ populations; CD8+αβ T cells seem to have pro-inflammatory effects25 whereas γδ T cells have been shown to suppress Th2-type cytokine production.6 In addition the efficacy of the antisense ODN treatment, at least as assessed in vitro, seems to have been greater in the present study.
CD8+γδ T cells expressing IFN-γ must have induced other lymphocytes in the bronchoalveolar lavage fluid to show a Th1-like phenotype whereas IFN-γ depleted CD8+γδ T cells did not affect the Th1 to Th2 balance compared with the PBS-injected control group. As the IFN-γ mRNA positive cells were 2·4% in bronchoalveolar lavage fluid after adoptive transfer of control ODN treated CD8+γδ T cells, these positive cells are in excess of the number of cells transferred and are likely to be CD4+ T cells. These results imply that CD8+γδ IFN-γ synthesis is involved in the propagation of the inhibitory process through other T cells that were not antigen specific. Such amplification is necessary to account for the effects exerted by the 35 000 cells that were transferred. This mechanism seems to be analogous to the collateral priming recently described involving IL-4.26 The γδ T cells are located in close proximity to dendritic cells and macrophages in the airways and this strategic location presumably accounts for their ability to modulate immune responses in the airway despite their relative paucity.27
Cysteinyl leukotrienes are the principal mediators of bronchoconstriction during the late airway response.18 The levels in bronchoalveolar lavage fluid following allergen challenge in the current study are comparable to those previously observed in this model19 and the degree of inhibition caused by CD8+γδ T cells brought the levels down to previously reported baseline values.19 Activation of the cysteinyl leukotriene receptor CysLT1 on smooth muscle cells28 can cause airway narrowing whereas diverse pro-inflammatory effects can result from its expression on CD34+ stem cells, mast cells, macrophages, basophils, and B cells.29 Cysteinyl leukotrienes are synthesized by mast cells and macrophages in rodents. The modulation of cysteinyl leukotriene synthesis by several cytokines has been reported.30–32 The macrophage is one of the major sources of cysteinyl leukotrienes33 and our data show that IFN-γ is inhibitory of their synthesis by macrophages, providing a mechanism by which this cytokine may exert its inhibitory effects on late airway responses in vivo. However, in addition to inhibiting cysteinyl leukotrienes synthesis IFN-γ stimulated phagocytosis by the alveolar macrophage. There are conflicting observations published concerning the role of alveolar macrophages in asthma.32,34 Macrophages stimulated by IFN-γ have increased antimicrobial properties, through the synthesis of tumour necrosis factor-α, nitric oxide, oxygen radicals, carbon monoxide and the costimulatory molecule B7.35,36 In the current study the enhancement of phagocytosis in vitro by IFN-γ was associated with the ingestion of larger numbers of eosinophils in bronchoalveolar lavage fluid in vivo. Thus the two actions of IFN-γ on the macrophage, to reduce cysteinyl leukotrienes synthesis and to enhance phagocytosis, are both anti-inflammatory in function and likely contribute to the reduction of airway inflammation and late responses by CD8+γδ T cells.
In conclusion, we have shown that CD8+γδ T cells transferred from sensitized donors to sensitized recipients are potently inhibitory of the late airway responses and allergic inflammation. Pre-treatment of these cells with antisense ODN against IFN-γ reverses the inhibitory effects of the CD8+γδ T cells on the late airway responses and partially restores airway eosinophilia. The results of our experiments demonstrate the critical role for IFN-γ in the immunomodulatory effects of CD8+γδ T cells on pulmonary allergic responses.
Acknowledgments
This work was supported by Canadian Institutes for Health Research Grant MT 10381 and JT Costello Memorial Fund. The authors wish to acknowledge the invaluable assistance of Dr Marie-Claire Michoud in the review of the manuscript.
Abbreviations:
- anti-NK
anti-natural killer cells
- BN
Brown Norway
- DMEM
Dulbecco's modified Eagle's minimal essential medium
- MACS
magnetic-activated cell sorting
- OVA
ovalbumin
- ODN
oligodeoxynucleotide
- PBS
phosphate-buffered saline
- TCR
T-cell receptor
References
- 1.O'Byrne PM, Dolovich J, Hargreave FE. Late asthmatic responses. Am Rev Respir Dis. 1987;136:740–51. doi: 10.1164/ajrccm/136.3.740. [DOI] [PubMed] [Google Scholar]
- 2.Olivenstein R, Renzi PM, Yang JP, Rossi P, Laberge S, Waserman S, Martin JG. Depletion of OX-8 lymphocytes from the blood and airways using monoclonal antibodies enhances the late airway response in rats. J Clin Invest. 1993;92:1477–82. doi: 10.1172/JCI116725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allakhverdi Z, Lamkhioued B, Olivenstein R, Hamid Q, Renzi PM. CD8 depletion-induced late airway response is characterized by eosinophilia, increased eotaxin, and decreased IFN-gamma expression in rats. Am J Respir Crit Care Med. 2000;162:1123–31. doi: 10.1164/ajrccm.162.3.9910001. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki M, Taha R, Ihaku D, Hamid Q, Martin JG. CD8+ T cells modulate late allergic airway responses in Brown Norway rats. J Immunol. 1999;163:5574–81. [PubMed] [Google Scholar]
- 5.Lahn M, Kanehiro A, Takeda K, et al. Negative regulation of airway responsiveness that is dependent on gammadelta T cells and independent of alphabeta T cells. Nat Med. 1999;5:1150–6. doi: 10.1038/13476. [DOI] [PubMed] [Google Scholar]
- 6.Isogai S, Rubin A, Maghni K, Ramos-Barbon D, Taha R, Yoshizawa Y, Hamid Q, Martin JG. The effects of CD8+ gammadelta T cells on late allergic airway responses and airway inflammation in rats. J Allergy Clin Immunol. 2003;112:547–55. doi: 10.1016/s0091-6749(03)01720-2. [DOI] [PubMed] [Google Scholar]
- 7.Wisnewski AV, Cain H, Magoski N, Wang H, Holm CT, Redlich CA. Human gamma/delta T-cell lines derived from airway biopsies. Am J Respir Cell Mol Biol. 2001;24:332–8. doi: 10.1165/ajrcmb.24.3.4325. [DOI] [PubMed] [Google Scholar]
- 8.Zuany-Amorim C, Ruffie C, Haile S, Vargaftig BB, Pereira P, Pretolani M. Requirement for gammadelta T cells in allergic airway inflammation. Science. 1998;280:1265–7. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]
- 9.McMenamin C, McKersey M, Kuhnlein P, Hunig T, Holt PG. Gamma delta T cells down-regulate primary IgE responses in rats to inhaled soluble protein antigens. J Immunol. 1995;154:4390–4. [PubMed] [Google Scholar]
- 10.Mueller R, Chanez P, Campbell AM, Bousquet J, Heusser C, Bullock GR. Different cytokine patterns in bronchial biopsies in asthma and chronic bronchitis. Respir Med. 1996;90:79–85. doi: 10.1016/s0954-6111(96)90202-4. [DOI] [PubMed] [Google Scholar]
- 11.Aoyagi M, Shimojo N, Sekine K, Nishimuta T, Kohno Y. Respiratory syncytial virus infection suppresses IFN-gamma production of gammadelta T cells. Clin Exp Immunol. 2003;131:312–7. doi: 10.1046/j.1365-2249.2003.02062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura T, Lee RK, Nam SY, Podack ER, Bottomly K, Flavell RA. Roles of IL-4 and IFN-gamma in stabilizing the T helper cell type 1 and 2 phenotype. J Immunol. 1997;158:2648–53. [PubMed] [Google Scholar]
- 13.Isogai S, Hamid Q, Minshall E, et al. Interferon-gamma increases IL-12 mRNA expression and attenuates allergic late-onset airway responses in the Brown Norway rat. Eur Respir J. 2000;16:22–9. doi: 10.1034/j.1399-3003.2000.16a05.x. [DOI] [PubMed] [Google Scholar]
- 14.Hofstra CL, Van A, Hofman G, Nijkamp FP, Jardieu PM, Van Oosterhout AJ. Differential effects of endogenous and exogenous interferon-gamma on immunoglobulin E, cellular infiltration, and airway responsiveness in a murine model of allergic asthma. Am J Respir Cell Mol Biol. 1998;19:826–35. doi: 10.1165/ajrcmb.19.5.3027. [DOI] [PubMed] [Google Scholar]
- 15.Iwamoto I, Nakajima H, Endo H, Yoshida S. Interferon gamma regulates antigen-induced eosinophil recruitment into the mouse airways by inhibiting the infiltration of CD4+ T cells. J Exp Med. 1993;177:573–6. doi: 10.1084/jem.177.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coyle AJ, Tsuyuki S, Bertrand C, Huang S, Aguet M, Alkan SS, Anderson GP. Mice lacking the IFN-gamma receptor have impaired ability to resolve a lung eosinophilic inflammatory response associated with a prolonged capacity of T cells to exhibit a Th2 cytokine profile. J Immunol. 1996;156:2680–5. [PubMed] [Google Scholar]
- 17.Wagner RW. Gene inhibition using antisense oligodeoxynucleotides. Nature. 1994;372:333–5. doi: 10.1038/372333a0. [DOI] [PubMed] [Google Scholar]
- 18.Abraham WM, Russi E, Wanner A, Delehunt JC, Yerger LD, Chapman GA. Production of early and late pulmonary responses with inhaled leukotriene D4 in allergic sheep. Prostaglandins. 1985;29:715–26. doi: 10.1016/0090-6980(85)90132-7. [DOI] [PubMed] [Google Scholar]
- 19.Eisenbarth SC, Zhadkevich A, Ranney P, Herrick CA, Bottomly K. IL-4-dependent Th2 collateral priming to inhaled antigens independent of Toll-like receptor 4 and myeloid differentiation factor 88. J Immunol. 2004;172:4527–34. doi: 10.4049/jimmunol.172.7.4527. [DOI] [PubMed] [Google Scholar]
- 20.Paulnock DM. Macrophage activation by T cells. Curr Opin Immunol. 1992;4:344–9. doi: 10.1016/0952-7915(92)90087-u. [DOI] [PubMed] [Google Scholar]
- 21.Martin JG, Suzuki M, Maghni K, et al. The immunomodulatory actions of prostaglandin E2 on allergic airway responses in the rat. J Immunol. 2002;169:3963–9. doi: 10.4049/jimmunol.169.7.3963. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe A, Mishima H, Renzi PM, Xu LJ, Hamid Q, Martin JG. Transfer of allergic airway responses with antigen-primed CD4+ but not CD8+ T cells in brown Norway rats. J Clin Invest. 1995;96:1303–10. doi: 10.1172/JCI118165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamid Q, Azzawi M, Ying S, et al. Expression of mRNA for interleukin-5 in mucosal bronchial biopsies from asthma. J Clin Invest. 1991;87:1541–6. doi: 10.1172/JCI115166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schramm CM, Puddington L, Yiamouyiannis CA, Lingenheld EG, Whiteley HE, Wolyniec WW, Noonan TC, Thrall RS. Proinflammatory roles of T-cell receptor (TCR) gammadelta and TCRalphabeta lymphocytes in a murine model of asthma. Am J Respir Cell Mol Biol. 2000;22:218–25. doi: 10.1165/ajrcmb.22.2.3620. [DOI] [PubMed] [Google Scholar]
- 25.Gajewski TF, Joyce J, Fitch FW. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol. 1989;143:15–22. [PubMed] [Google Scholar]
- 26.Lahn M, Kanehiro A, Takeda K, et al. MHC class I-dependent Vgamma4+ pulmonary T cells regulate alpha beta T cell-independent airway responsiveness. Proc Natl Acad Sci USA. 2002;99:8850–5. doi: 10.1073/pnas.132519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isogai S, Taha R, Tamaoka M, Yoshizawa Y, Hamid Q, Martin JG. CD8+ alphabeta T cells can mediate late airway responses and airway eosinophilia in rats. J Allergy Clin Immunol. 2004;114:1345–52. doi: 10.1016/j.jaci.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 28.Wands JM, Roark CL, Aydintug MK, et al. Distribution and leukocyte contacts of gammadelta T cells in the lung. J Leukoc Biol. 2005;78:1086–96. doi: 10.1189/jlb.0505244. [DOI] [PubMed] [Google Scholar]
- 29.Lynch KR, O'Neill GP, Liu Q, et al. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399:789–93. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- 30.Figueroa DJ, Breyer RM, Defoe SK, et al. Expression of the cysteinyl leukotriene 1 receptor in normal human lung and peripheral blood leukocytes. Am J Respir Crit Care Med. 2001;163:226–33. doi: 10.1164/ajrccm.163.1.2003101. [DOI] [PubMed] [Google Scholar]
- 31.Hsieh FH, Lam BK, Penrose JF, Austen KF, Boyce JA. T helper cell type 2 cytokines coordinately regulate immunoglobulin E-dependent cysteinyl leukotriene production by human cord blood-derived mast cells. Profound induction of leukotriene C (4) synthase expression by interleukin 4. J Exp Med. 2001;193:123–33. doi: 10.1084/jem.193.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cowburn AS, Holgate ST, Sampson AP. IL-5 increases expression of 5-lipoxygenase-activating protein and translocates 5-lipoxygenase to the nucleus in human blood eosinophils. J Immunol. 1999;163:456–65. [PubMed] [Google Scholar]
- 33.Steinhilber D, Radmark O, Samuelsson B. Transforming growth factor beta upregulates 5-lipoxygenase activity during myeloid cell maturation. Proc Natl Acad Sci USA. 1993;90:5984–8. doi: 10.1073/pnas.90.13.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei YF, Heghinian K, Bell RL, Jakschik BA. Contribution of macrophages to immediate hypersensitivity reaction. J Immunol. 1986;137:1993–2000. [PubMed] [Google Scholar]
- 35.Careau E, Bissonnette EY. Adoptive transfer of alveolar macrophages abrogates bronchial hyperresponsiveness. Am J Respir Cell Mol Biol. 2004;31:22–7. doi: 10.1165/rcmb.2003-0229OC. [DOI] [PubMed] [Google Scholar]
- 36.Nacy CA, Meierovics AI, Belosevic M, Green SJ. Tumor necrosis factor-alpha: central regulatory cytokine in the induction of macrophage antimicrobial activities. Pathobiology. 1991;59:182–4. doi: 10.1159/000163640. [DOI] [PubMed] [Google Scholar]