Abstract
Allergic asthma is a serious multifaceted disease characterized by eosinophil-rich airway inflammation, airway hyperreactivity and airway wall modifications known as remodelling. We previously demonstrated that the spores of two allergenic moulds, Alternaria alternata and Cladosporium herbarum, were potent inducers of immunoglobulin E (IgE) production. Moreover, mice sensitized by two intraperitoneal injections before intranasal challenge with A. alternata or C. herbarum spores developed an allergic lung inflammation and hyperreactivity. Here we report on the effect of chronic intranasal administration of C. herbarum spores or A. alternata extracts to unsensitized BALB/c mice. Our results demonstrate that this chronic treatment led to an increase of total serum IgE and the appearance of specific IgE and IgG1. Total cell number in bronchoalveolar lavage fluid from treated mice was highly increased compared to phosphate-buffered-saline-treated mice because of the accumulation of macrophages, neutrophils, lymphocytes and eosinophils. Airway hyperreactivity appeared after 3 weeks (extract) and 7 weeks (spores) and was maintained during the whole treatment. Increased interleukin-13 mRNA expression in the lungs and T helper type 2 cytokines (interleukin-4, -5, -6 and -13) and transforming growth factor-β secretion in bronchoalveolar lavage fluid were also observed. Lung hydroxyproline and fibronectin contents indicated increased fibrosis in mice treated with mould allergen. These observations were confirmed by histological analysis demonstrating airway wall remodelling and strong mucus production. These observations show that this model, using chronic intranasal administration of relevant particulate allergens, is an interesting tool for the study of mechanisms leading to allergic pulmonary diseases and lung remodelling.
Keywords: allergy, animal models/studies, mice/rats, eosinophils, lung disease, lung immunology, neutrophils
Introduction
Allergic asthma is a multifactorial chronic disease involving both genetic and environmental factors and characterized by a dysregulation of immunity. This dysregulation leads to a strongly polarized T helper type 2 (Th2) immune response and a chronic inflammation in the airways in response to innocuous antigens, e.g. airborne allergens.1 In asthmatic patients, the penetration of allergens into the lungs leads to airway inflammation consisting of a peribronchial infiltration of CD4+ T cells, macrophages, eosinophils and neutrophils and the presence of these cells in bronchoalveolar lavage fluid (BALF). Asthmatic patients also present a goblet cell metaplasia/hyperplasia and characteristic modifications of the airway wall including epithelial hyperplasia, thickening of the basement membrane, subepithelial fibrosis, increased airway smooth muscle mass and, finally, airway hyperreactivity (AHR) to specific and non-specific stimuli.2,3
So far, most experimental models of asthma have been developed in rodents and have used chicken egg ovalbumin (OVA) as a surrogate allergen. Although these OVA models have been valuable tools for the study of inflammatory mechanisms of asthma, OVA bears little relationship to the aeroallergens present in the day-to-day environment and the use of common aeroallergens involved in human asthma may be more relevant to a study of the pathophysiology of this chronic disease. Indeed, the administration of OVA through the airway is incapable of inducing a broad spectrum of allergic lung changes and in contrast induces a relative persistent tolerogenic state.4,5 However, OVA is readily capable of inducing goblet cell metaplasia, mucus oversecretion, airway eosinophilia, peribronchovascular inflammation and airway hyperresponsiveness if given systemically in a series of priming doses, typically with aluminium-based adjuvants before pulmonary challenge.6,7 These models, based upon sensitization by systemic administration of antigen (such as OVA) and subsequent short-term challenge by inhalation, are experimentally very convenient and have been widely used. However, these short-term exposure models are quite unlike the recurrent long-term exposure to allergens experienced by humans. Therefore chronic inhalational challenge models of asthma, in previously sensitized mice, using controlled exposure to nebulized OVA have also been developed,8,9 allowing the chronic inflammatory and epithelial changes that typify asthma to be studied.
Recently we described a new acute mouse model of lung allergy induced by the spores of two well-recognized human allergenic moulds, Alternaria alternata and Cladosporium herbarum.10 In contrast to Aspergillus, which is an opportunistic pathogen causing allergic and invasive diseases, spores from Alternaria and Cladosporium do not colonize the lungs and are rapidly cleared. Spores from these two moulds are important causes of both allergic rhinitis and asthma and exposure to airborne spores of A. alternata might trigger severe asthma and represents a risk factor for respiratory failure.11 We previously demonstrated that mice systemically sensitized with A. alternata and C. herbarum spores and then intranasally (i.n.) challenged developed a typical allergic airway inflammation and AHR.10
In this study we have analysed the effect of chronic i.n. instillations of mould spores and extracts in naive mice. We demonstrate that this treatment induces a typical Th2 immune response characterized by polyclonal immunoglobulin E (IgE) and specific IgE–IgG1 synthesis and lung inflammation containing numerous eosinophils. There is also the development of an AHR and important remodelling of the airway wall including epithelial hypertrophy, goblet cell hyperplasia/metaplasia and subepithelial fibrosis.
Materials and methods
Mould cultures, spore production and mould extract preparation
Alternaria alternata (strain 18586) was obtained from the BCCMTM/IHEM (Belgian Co-ordinated Collection of Micro-organisms, Institute of Public Health, Brussels, Belgium). Cladosporium herbarum (strain 19275) was obtained from the BCCMTM/MUCL (Belgian Co-ordinated Collection of Micro-organisms, Université Catholique de Louvain, Louvain, Belgium). These moulds were cultured at 27° on potato dextrose agar (Difco, Detroit, MI) plates for 1 week and then the spores were gently harvested with a cell scraper. Spores were diluted in phosphate-buffered saline (PBS) and counted with a haemocytometer.
Mould extracts were prepared as previously described12 with slight modifications. Mould cultures were grown for 3 weeks at 27° in flasks containing 250 ml Czapek's medium. Mould pellicles were harvested and homogenized in 0·4% NH4HCO3 + polyvinyl polypyrrolidone (Sigma, St. Louis, MO) using an Ultra-Thurax (IKA, Staufen, Germany). The homogenates were then agitated for 3 hr at 4°. Extracts were centrifuged twice for 30 min at 20 000 g, dialysed against PBS and stored at −20°.
Animals and immunizations
Female BALB/c mice were obtained from the Elevage Janvier (Le Genest Saint Isle, France) and maintained under standard laboratory conditions. To induce allergic lung inflammation, mice were administered i.n. with 2 × 105 spores or 5 μg extract (in 100 μl PBS), twice a week, every Monday and Friday for 10–12 weeks. Similar experiments were also performed with C. herbarum extracts and A. alternata spores (not shown). Results obtained after a chronic instillation of C. herbarum extract were very similar to those obtained with the A. alternata extract. Chronic instillation of 2 × 105A. alternata spores induced a cachexia, probably caused by a high production of tumour necrosis factor-α in response to this quantity of spores and therefore experiments were stopped after 5–7 weeks.
Control mice were treated with PBS. Mice were lightly anaesthetized with isofluran (Isoflo, Abbott Laboratories, North Chicago, IL). When the mice were unresponsive but breathing comfortably, 100 μl of the spore or extract solution was directly applied on the nostrils. The animals were allowed to slowly inhale the liquid and then to recover in a supine position.
For intraperitoneal (i.p.) immunizations mice were injected with 50 μg keyhole limpet haemocyanin (KLH, Pierce, Rockford, IL) in a final volume of 300 μl, emulsified in complete Freund's adjuvant (CFA, Pierce) and boosted 2 weeks later with 50 μg KLH emulsified in incomplete Freund's adjuvant (IFA, Pierce). Similarly, mice were immunized twice with KLH emulsified in aluminium hydroxide (Alum; Imject Alum, Pierce).
For all the experiments, data shown are representative of at least two independent experiments (except once for Fig. 3) Results were analysed using the paired Student's t-test. Significant P-values are indicated.
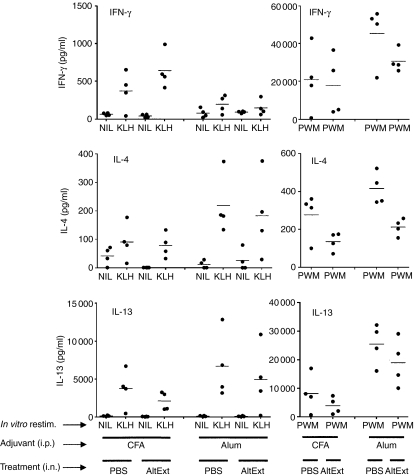
Figure 3.
Chronic i.n. instillations of Alternaria alternata extract do not influence the T-cell response induced by immunization with a model antigen. BALB/c mice were treated chronically twice a week i.n. with PBS or with 5 μg A. alternata extract during the entire experiment. Three weeks after the beginning of instillations, mice were immunized i.p. twice with 50 μg KLH emulsified in CFA or in alum. Mice were boosted 2 weeks later with 50 μg KLH emulsified in IFA or in alum, respectively. One week after the KLH boost, spleen cells were restimulated in vitro for 3 days with medium only or with medium supplemented with KLH or PWM. The concentrations of IFN-γ, IL-4 and IL-13 in supernatants were measured by ELISA.
Antibody detection
Mice were bled in the retro-orbital plexus 24 hr after the last i.n. instillation and antibodies in the sera were analysed by enzyme-linked immunosorbent asay (ELISA). Total serum IgE levels were determined using a sandwich ELISA. Plates were coated with a rat anti-mouse IgE monoclonal antibody (LO-ME-2, IMEX, UCL, Brussels, Belgium) and saturated with proteins from skimmed milk. Serial twofold dilutions of serum or purified monoclonal mouse IgE (LB-4, IMEX) were applied for 2 hr. Peroxidase-labelled rat anti-mouse IgE (LO-ME-3) was then added. Finally, plates were washed and developed by the addition of 100 μl 3,3′,5,5′-tetramethylbenzidine (TMB; Immunopure TMB substrate kit, Pierce Biotechnology, Rockford, IL). The reaction was stopped with 50 μl 1 m H2SO4and optical density was read at 450 nm (OD450) with an automatic Multiskan Reader MCC/340 (Titertek, Huntsville, AL). Serum titre was converted to IgE concentration by comparison with a purified LB-4 standard. Similar immunoglobulin concentrations for serum were calculated when serum and standard were titrated at any point on the linear part of the titration curve.
Mould-specific IgE and IgG1 levels were determined using an indirect ELISA. Plates were coated with mould extracts and saturated. Serial twofold dilutions of serum were applied for 16 hr. Peroxidase-labelled rat anti-mouse IgE (LO-ME-3) or anti-mouse IgG1 (LO-MG1-13) was then added during 3 hr. Finally, plates were washed and developed by the addition of 100 μl TMB.
In vitro spleen cell restimulation
Spleens were removed aseptically 1 week after the second KLH immunization. Spleen cells (5 × 105 cells in 200 μl) were cultured in triplicate in 96-well round-bottom microwell plates in RPMI-1640 medium supplemented with gentamycin, 2 mm l-glutamine, 5 × 10−5m 2-mercaptoethanol, 10% fetal calf serum with or without KLH (10 μg/ml) or pokeweed mitogen (PWM, 4 μg/ml, Sigma). Cultures were maintained in 5% CO2 at 37° for 3 days and then supernatants were collected and analysed by ELISA for the presence of interferon-γ (IFN-γ), interleukin-4 (IL-4) and IL-13.
Bronchoalveolar lavages
Mice were killed by cervical dislocation 24 hr after the last i.n. instillation. The trachea was exposed and incised. A needle (1·2 × 40 mm) was inserted into the trachea and BALF was harvested by rinsing the lungs twice with 1 ml PBS. Total cell counts were determined with a haemocytometer. Differential cell counts were obtained by examining at least 500 cells on cytospin slides stained with Diff-Quick (Dade Behring, Deerfield, IL).
Measurement of airway reactivity
The AHR was measured within 24 hr after the last instillation, in response to methacholine inhalation using a whole-body plethysmography system (EMKA, Paris, France). Mice were placed in the plethysmograph box and allowed to acclimatize for at least 5 min before analysis. Control measurements were obtained over a period of 5 min directly after a 3-min nebulization of PBS generated by an ultrasonic nebulizer (LS Syst'AM, Villeneuve sur Lot, France). Afterwards, increasing concentrations of methacholine (12·5, 25 and 50 mg/ml) in PBS were nebulized into the plethysmograph box for 3 min. Immediately after each nebulization the enhanced pause (Penh), a dimensionless index that reflects changes in the amplitude of the pressure waveform and expiratory time, was recorded and averaged for 5 min. Penh measurements have been validated by Hamelmann et al.13 with regard to identification of AHR because the heightened increase in Penh with methacholine challenge in OVA-sensitized/challenged mice was accompanied by a parallel enhancement of lung airway resistance (RL) responses to the methacholine with a high correlation between Penh and RL.
Total RNA extraction and IL-13 mRNA quantification
Total RNA was extracted from perfused lungs using Trizol (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. Residual DNA contamination was removed by treatment of the samples with DNA-free kit (Ambion, Austin, TX). One microgram RNA was reverse transcribed using SuperScript III reverse transcriptase (Invitrogen) with 350 pmol random hexamers (Eurogentec, Seraing, Belgium) in a final volume of 25 μl. The resulting cDNA was then diluted 10 times and used as the template in subsequent polymerase chain reactions (PCR).
Specific primer sets were as follows (Invitrogen): IL-13 sense AGACCAGACTCCCCTGTGCA, IL-13 antisense TGGGTCCTGTAGATGGCATTG, IL-13 probe (FAM-TAMRA) CGGGTTCTGTGTAGCCCTGGATTCC, β-actin sense AGAGGGAAATCGTGCGTGAC, β-actin antisense CAATAGTGATGACCTGGCCGT. For IL-13 and β-actin mRNA quantification, standards and samples (2·5 μl) were amplified with 300 nm of each primer and probe using, respectively, Taqman PCR Master Mix (Applied Biosystems, Foster City, CA) and SYBR Green PCR Master Mix (Applied Biosystems) in a total volume of 20 μl. PCR was performed on an ABI Prism 7000 Sequence Detector (Applied Biosystems) according to the following programme: 2 min at 50°, 10 min at 95° (15 s at 95°, 1 min at 60°) 40 times. Serial dilutions of a positive control sample of cDNA were used as standards for quantification. Results were calculated as a ratio of IL-13 expression to the expression of the reference gene, β-actin.
Cytokine detection
Cytokines were measured in BALF obtained 24 hr after the last i.n. inoculation. The levels of IL-4, IL-5, IL-6, IL-10, IL-13, IFN-γ and total transforming growth factor-β (TGF-β) were quantified by ELISA (sensitivities were 2 pg/ml, 7 pg/ml, 1·8 pg/ml, 4 pg/ml, 1·5 pg/ml, 2 pg/ml and 4·6 pg/ml, respectively) using Quantikine® kits according to the instructions provided by the manufacturers (R & D systems, Minneapolis, MN).
Quantification of fibrosis markers
Collagen deposition was estimated by measuring the hydroxyproline content of whole lung and soluble collagen in lung homogenates. For hydroxyproline assays, the lung was excised, homogenized and hydrolysed in 6 m HCl overnight at 110°. Hydroxyproline was assessed by high-performance liquid chromatography analysis14 and data were expressed as μg hydroxyproline/lung. Soluble collagen levels were estimated by Sircol collagen assay following the manufacturer's protocols (Biocolor, Westbury, NY). Fibronectin was measured in BALF by ELISA as previously described.15
Histological analysis
Tissues for histopathological examination were collected 24 hr after the last i.n. instillation. Mice were killed by cervical dislocation, the trachea was cannulated and the lungs were inflated with 4% buffered formalin. After fixation overnight, the lungs were embedded in paraffin. Tissues were sliced and 5-μm sections were stained with haematoxylin–eosin–safran for light microscopy examination of the lung inflammation, with periodic acid Schiff for the detection of mucus-producing cells and Masson trichrome for the detection of collagen.
Results
Chronic i.n. instillation of A. alternata extract or C. herbarum spores into naive BALB/c mice induces polyclonal IgE production and secretion of specific IgG1 and IgE antibodies
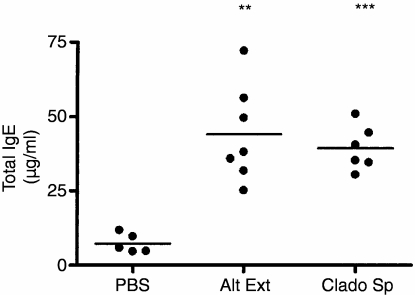
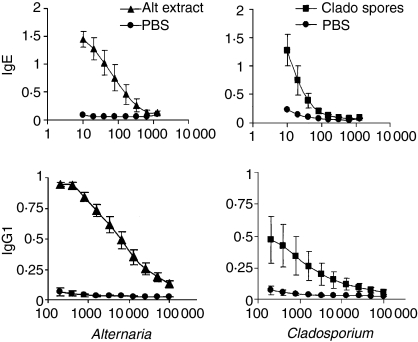
We previously demonstrated that the spores of A. alternata and C. herbarum are potent inducers of polyclonal IgE and specific IgG1 antibody synthesis when injected twice intravenously into BALB/c mice.10 To analyse the ability of mould spores and extracts to trigger a systemic Th2 response when introduced chronically into the airways, naive BALB/c mice were treated i.n. twice a week with A. alternata extracts (5 μg) or C. herbarum spores (2 × 105 spores) and 10 weeks later antibody synthesis and IgE serum levels were measured. As shown in Fig. 1, at the end of this treatment, total serum IgE levels were greatly increased in both groups compared to the PBS-treated control group and there was no significant difference between IgE levels of mice given the spores or the extract. The chronic i.n. administration of mould spores or extracts also led to the production of specific IgE and IgG1 antibodies in the serum (Fig. 2) although this synthesis was lower in mice given spores than those given extract. PBS-treated mice did not produce any specific IgE or IgG1 antibodies. We were unable to detect any secretion of specific IgG2a antibodies in mould spore-treated or extract-treated mice (not shown). These results clearly demonstrate that these antigens are able to induce a systemic type 2 response when introduced chronically into the airways. To analyse the consequences of this systemic type 2 activation on specific antigenic responses, mice were chronically administered A. alternata extract or PBS for 3 weeks. Mice were then immunized i.p. with KLH emulsified in CFA or Alum and boosted with KLH in IFA or Alum, respectively. One week after the boost the spleens were removed and the cells were restimulated in vitro with medium, KLH or PWM. Supernatants were collected and analysed for the presence of the prototypal Th1 cytokine IFN-γ and the Th2 cytokines IL-4 and IL-13. As shown in Fig. 3, spleen cells from mice immunized with KLH emulsified in CFA/IFA or with Alum and restimulated with PWM produced high levels of IFN-γ, IL-4 and IL-13. When restimulated with KLH, spleen cells from mice immunized with KLH emulsified in CFA/IFA secreted more IFN-γ while cells from mice immunized with KLH in Alum produced more IL-4 and IL-13. However, there was no difference in the secretion of these cytokines between mice given the mould extract and the control PBS-treated mice, indicating that the systemic Th2 activation induced by chronic mould exposure did not influence the T-cell response induced by i.p. injection of an unrelated antigen.
Figure 1.
Polyclonal IgE synthesis induced by chronic i.n. instillations of Alternaria alternata extract and Cladosporium herbarum spores. BALB/c mice were chronically administered i.n. with PBS, with 5 μg A. alternata extract (Alt Ext) or with 2 × 105 spores of C. herbarum (Clado Sp) for 10 weeks. Twenty-four hours after the last instillation mice were bled and total IgE levels were determined. Significant differences are shown between values from extract or spores treated mice versus PBS-treated mice; **P < 0·05, ***P < 0·01.
Figure 2.
Specific IgE and IgG1 antibody synthesis induced by chronic i.n. instillations of Alternaria alternata extract and Cladosporium herbarum spores. BALB/c mice were given PBS, 5 μg A. alternata extract or 2 × 105 spores of C. herbarum chronically i.n. for 10 weeks. Twenty-four hours after the last instillation mice were bled and specific IgE and IgG1 were determined. Data represent the mean ± SEM of the A450 values of five to seven mice per group.
Chronic i.n. instillation of A. alternata extracts or C. herbarum spores induces the appearance of inflammatory cells in BALF
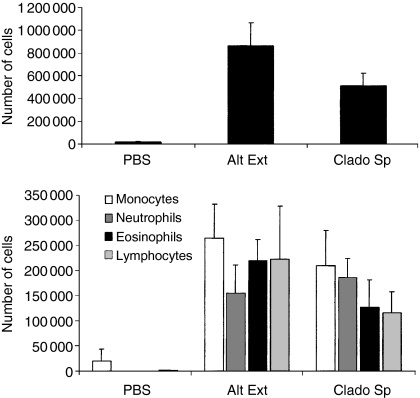
BALB/c mice sensitized by two i.p. injections of A. alternata or C. herbarum spores and then challenged i.n. develop a strong allergic inflammation in the airways consisting of an accumulation of macrophages, neutrophils, eosinophils and lymphocytes around the bronchioles and an accumulation of these cells in their BALF.10 To investigate the type of inflammation induced during chronic exposure to mould allergens, BALF cells were examined in naive BALB/c mice to which PBS, A. alternata extracts or C. herbarum spores were chronically administered i.n. for 10 weeks. The presence of inflammatory/immune cells was detected in BALF after 3 weeks (extract) to 5 weeks (spores) of treatment (not shown). After 10 weeks of treatment, the total numbers of cells in the BALF of mould spore-treated or extract-treated mice were highly increased as compared to the number of cells in BALF from the PBS-treated group (Fig. 4a). Analysis of the different cell subpopulations showed that i.n. spore or extract instillations led to the recruitment of a high number of macrophages, neutrophils, lymphocytes and eosinophils (Fig. 4b). This increase in the number of eosinophils in the airways has been considered as a hallmark of allergic asthma.3
Figure 4.
Inflammatory cell recruitment in the lungs of Alternaria alternata extract and Cladosporium herbarum spore-treated mice. BALB/c mice were chronically treated i.n. with PBS, with 5 μg A. alternata extract (Alt Ext) or 2 × 105 spores of C. herbarum (Clado Sp) for 10 weeks. (a) BALF was collected 24 hr after the last instillation and the total cell number was determined. (b) Differential cell count of the inflammatory subpopulation in BALF 24 hr after the last instillation. Data represent the mean ± SD of five or six individual mice per group.
Chronic i.n. instillation of A. alternata extracts or C. herbarum spores leads to a sustained AHR
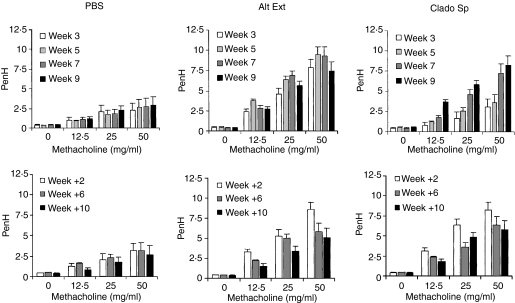
Whole body plethysmography was used to assess the development of AHR. The Penh values from BALB/c mice chronically administered A. alternata extracts or C. herbarum spores i.n. for 10 weeks were compared to those from PBS-treated mice. In PBS-treated mice, no AHR was observed in the course of the experiment. Mice chronically treated with A. alternata extracts did not show any AHR during the first 2 weeks of treatment. On week 3 AHR appeared and remained stable during the whole treatment (Fig. 5). In contrast Penh values for mice given C. herbarum spores were only slightly increased at weeks 3 and 5 but strong AHR was obtained at weeks 7 and 9. Two weeks after the end of the instillation AHR of the A. alternata extract-treated or C. herbarum spore-treated groups was not modified compared to the AHR observed after 9 weeks of i.n. instillation. Six and 10 weeks after the end of treatment, AHR decreased but was still above the baseline levels observed in the control PBS-treated group.
Figure 5.
Mice chronically treated with Alternaria alternata extract or Cladosporium herbarum spores develop a sustained AHR to inhaled methacholine. BALB/c mice were administered PBS, 5 μg A. alternata extract (Alt Ext) or 2 × 105 spores of C. herbarum (Clado Sp) chronically i.n. for 10 weeks. Penh were measured by whole body plethysmography after 3, 5, 7, 9 and 11 weeks. Results are expressed as means ± SEM of five to seven mice per group.
Chronic i.n. instillation of A. alternata extracts or C. herbarum spores increases Th2 cytokine lung production
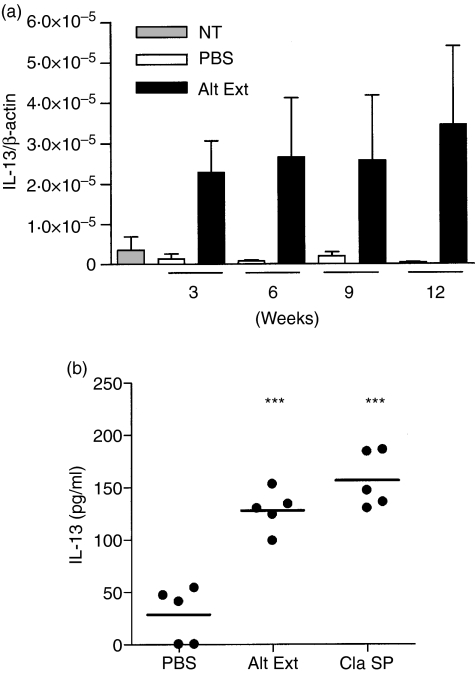
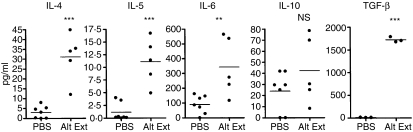
IL-4, IL-5 and IL-13 are Th2 cytokines playing key regulatory roles during allergic airway inflammation.16–19 Expression of IL-13 mRNA was measured by quantitative reverse transcription–PCR in the lungs of extract-treated mice compared to PBS-treated mice. Figure 6(a) shows that IL-13 mRNA was already strongly overexpressed in the lungs of mice given mould extract after 3 weeks and remained constant during the whole experiment while IL-13 expression was not increased in PBS-treated control mice as compared to untreated mice. The presence of IL-13 in BALF was confirmed by ELISA after 10 weeks of treatment. As shown in Fig. 6(b) a low concentration of IL-13 was found in BALF from control mice while IL-13 was significantly increased in BALF from mice chronically treated with either A. alternata extract or C. herbarum spores. The concentrations of IL-4, IL-5, IL-6 and IL-10 were also measured by ELISA in the BALF of A. alternata extract-treated mice. A significantly higher concentration of IL-4, IL-5 and IL-6 was observed in BALF from extract-treated mice compared to PBS-treated mice while IL-10 concentrations showed no significant variation. Interestingly, BALF levels of the pro-fibrotic mediator TGF-β were also increased in mice treated with A. alternata extracts (Fig. 7). Finally, IFN-γ was below the detection level in these BALF (not shown). These data clearly indicate that the mould extract and spores are strong and specific inducers of Th2 immunity.
Figure 6.
(a) Chronic instillations of Alternaria alternata extracts induce the production of IL-13 mRNA in the lungs. BALB/c mice were left untreated (NT), or were chronically treated i.n. with PBS or with 5 μg A. alternata extract (Alt Ext) for 10 weeks. At the indicated time-points (24 hr after the last instillations) total RNA was extracted from the lungs and transcriptional levels of IL-13 were analysed by quantitative RT-PCR. Data represent the mean ± SD of triplicate assays. (b) Chronic instillations of A. alternata extracts or C. herbarum spores induce an accumulation of IL-13 into the BALF. BALB/c mice were treated chronically i.n. with instillations of PBS, 5 μg Alternaria alternata extract (Alt Ext) or 2 × 105 spores of Cladosporium herbarum (Clad Sp) for 10 weeks. Twenty-four hours after the last instillation BALF were collected and IL-13 concentrations in BALF were analysed by ELISA. Significant differences between values from extract-treated or spore-treated mice versus PBS-treated mice are shown; ***P < 0·01. There was no significant difference between extract-treated versus spore-treated mice.
Figure 7.
Quantification of IL-4, IL-5, IL-6, IL-10 and TGF-β concentrations in the BALF of mice chronically treated with instillations of Alternaria alternata extract. BALB/c mice were treated chronically i.n. with PBS or with 5 μg A. alternata extract (Alt Ext) during 10 weeks. BALF were collected 24 hr after the last instillation and IL-4, IL-5, IL-6, IL-10 and TGF-β concentrations were determined by ELISA. Significant differences between values from extract-treated mice versus PBS-treated mice for IL-4, IL-5, IL-6 and TGF-β are shown; **P < 0·05, ***P < 0·01. Non-significant (NS) for IL-10.
Chronic i.n. instillation of A. alternata extracts or C. herbarum spores induce airway wall remodelling
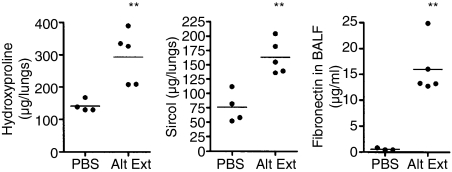
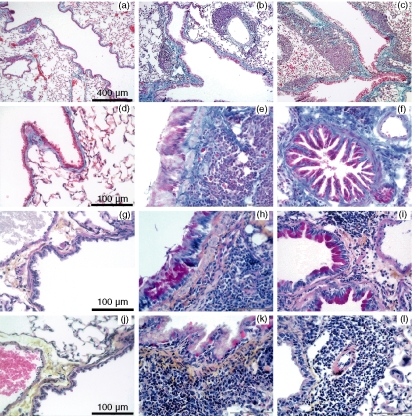
Chronic human asthma is characterized not only by chronic airway inflammation but also by modifications of the airway wall, called remodelling, such as subepithelial fibrosis, mucus production and epithelial proliferation. The amplitude of the pulmonary fibrosis induced by the chronic i.n. instillation of A. alternata extracts was determined after 10 weeks of treatment by measuring lung hydroxyproline, soluble collagen and fibronectin, as well as by histology. Compared to PBS-treated animals, mice exposed to A. alternata extracts presented an increase of hydroxyproline and soluble collagen and a high concentration of fibronectin in the BALF (Fig. 8). Histological examination of mice treated with A. alternata extract and C. herbarum spores confirmed the biochemical evidence of remodelling. Masson's trichrome staining of lung sections showed a pronounced thickening of the airway epithelium of mice treated with mould extract or spores compared to the PBS-treated mice and, more importantly, a strong accumulation of collagen beneath the airway epithelium of the extract and spore treated mice (Fig. 9a–f). No alveolar fibrosis was observed. In addition, periodic acid Schiff staining of lung sections from PBS-treated mice showed almost no goblet cells while numerous goblet cells were present in the airway epithelium of chronically treated mice (Fig. 9g–i). Finally, in accordance with the observations in BALF, intense inflammation was observed in both the perivascular and the peribronchial areas, containing numerous eosinophils, while no inflammation was detected in PBS-treated mice (Fig. 9j–l).
Figure 8.
Levels of hydroxyproline in lung homogenates, soluble collagen in supernatents of lung homogenates and fibronectin in BALF of BALB/c mice were administered instillations of PBS or Alternaria alternata extracts (Alt Ext) for 10 weeks. **P < 0·05.
Figure 9.
Histopathological changes in the lungs of mice chronically instilled with Alternaria alternata and Cladosporium herbarum. BALB/c mice were given i.n. instillations PBS (left column), A. alternata extracts (middle column) or C. herbarum (right colum) for 10 weeks. The first two rows show lung sections stained with Masson trichrome (a–f). The third row shows lung sections stained with periodic acid Schiff (g–i) and the last row shows lung sections stained with haematoxylin–eosin–safran (j–l).
Discussion
We previously demonstrated that mould spores from A. alternata and C. herbarum when injected i.p. into BALB/c mice induce the production of specific IgG1 antibodies and strongly increase IgE serum levels.10 Here we confirm and extend these results, showing that mice chronically exposed only through the airways with C. herbarum spores or A. alternata extract in the absence of adjuvant mount a specific IgG1 and IgE antibody response and present an increased serum IgE concentration. However, this strong non-specific systemic Th2 response does not influence specific T-cell responses and their production of IFN-γ, IL-4 and IL-13 induced by i.p. immunization with an unrelated antigen (KLH).
Shang et al.20 have elegantly shown that the non-specific polyclonal IgE production observed during a Th2 response was the result of enhanced production of IgE by already activated and committed B cells in the spleen and was dependent on IL-4. We believe that a similar mechanism might be responsible for the increase of IgE serum levels after inoculation of the mould spores or extracts.
Since IgE secretion is completely dependent on the activation of Th2 cells21 these results suggest that T cells secreting Th2 cytokines are also activated. This activation was confirmed by the up-regulation of IL-13 mRNA expression in the lungs and the presence of high levels of IL-4, IL-5 and IL-13 in the BALF of chronically treated mice.
The lung inflammation induced in mice chronically instilled with C. herbarum spores and A. alternata extract was characterized by the presence in the BALF of numerous macrophages, lymphocytes, eosinophils and neutrophils. Our previous study demonstrated the presence of neutrophils in BALF of naive mice inoculated with mould spores and a strong up-regulation of macrophage inflammatory protein-1α (MIP-1α), MIP-2 and eotaxin mRNA into the lungs while we noted that the accumulation of eosinophils into the lungs required a previous systemic sensitization.10 However, in the chronic settings of this study, neutrophils and eosinophils accumulated in the lungs without any previous systemic sensitization, as also observed in human asthma.22 Accumulation of eosinophils in the airways is a hallmark of allergic asthma3 and these cells have a critical role in allergic airway remodelling.23 Indeed, eosinophils are a main source of the pro-fibrotic factor TGF-β24 and eosinophil-derived cationic proteins can induce the production of remodelling factors by lung epithelial cells.25 A correlation has also been noticed between severity of asthma and number of neutrophils in the airway.26 In severe asthma the neutrophils are in an activated stage and therefore likely to contribute to tissue damage27 and neutrophils in the presence of IL-8 can increase the trans-basement-membrane migration of eosinophils.28 Moreover, in a mouse model of airway allergy induced by Aspergillus fumigatus depletion of neutrophils resulted in reduced AHR and remodelling whereas conditional transgenic overexpression of CXCL1 in airway walls, leading to increased accumulation of only neutrophils in the lungs, worsened the allergic responses.29 Activation of innate immunity and recruitment of neutrophils in response to Aspergillus fumigatus has been shown to be mediated by Dectin-1, an innate immune receptor expressed on alveolar macrophages and neutrophils. Dectin-1 specifically recognizes fungal β-glucans and triggers the inflammatory response and the release of MIP-1α, MIP-2 and tumour necrosis factor-α.30–32 Since spores and extracts from A. alternata and C. herbarum are rich in β-glucans, a similar mechanism involving the recognition of these pathogen-associated molecular patterns by Dectin-1 might explain the accumulation of neutrophils in the airways of chronically treated mice. Another explanation could be the presence of LPS in our spore and extract preparations but this seems not very likely because the concentration of LPS in our extract was very low (between 3 and 6 U/ml analysed with the QCL-1000 LAL, Biowhittaker, Walkersville, MD; corresponding to 0·15–0·3 U per instillation).
Mice chronically instilled with A. alternata extract developed an AHR 3 weeks after the beginning of the treatment that remained constant during the whole experiment and did not return to baseline levels even 10 weeks after ceasing the instillations. AHR appeared at week 7 in mice treated with C. herbarum spores, remained stable 2 weeks after termination of the experiments and then declined slowly. These differences concerning the evolution of AHR could be explained by intrinsic differences between the two moulds but it is more likely that the quantity of available allergens was higher in the extract than in the spore inoculum. Moreover, proteins instilled into the lungs are directly available to antigen-presenting cells whereas spores need to be engulfed and processed.
In this study we also observed that a chronic treatment with the C. herbarum spores and A. alternata extract led to an increase of lung hydroxyproline, soluble collagen and fibronectin levels, indicating that a remodelling process was occurring. This remodelling was confirmed by histological analysis showing a thickening of the airway epithelium, an accumulation of goblet cells and a strong subepithelial fibrosis. Subepithelial fibrosis is one of the predominant characteristics of airway remodelling observed in human asthma and is of great concern because several studies have linked the intensity of fibrosis to the severity of the disease in patients.33,34 Fibrogenesis is thought to result from chronic inflammation, mainly characterized by the recruitment of eosinophils and activated lymphocytes. Upon stimulation these cells, as well as resident epithelial cells, release inflammatory mediators and growth factors, leading to the activation and proliferation of fibroblasts and to the accumulation of the extracellular matrix proteins.35 Two cytokines detected in our model, IL-13 and TGF-β, are well known pro-fibrotic mediators.36 It is therefore likely that the remodelling process observed in our chronic model is linked to the production of both IL-13 and TGF-β while other undetected factors might also be contributing to this response.
One of the striking results of this study was the development of the allergic response after a chronic i.n. instillation of mould spores or extracts in the absence of any systemic sensitization. This immune activation is in contrast to the induction of tolerance generally observed after exposure of the airway mucosa to soluble antigens by i.n. administration.37 Indeed, i.n. exposure of mice to soluble OVA results in peripheral CD4+ T-cell unresponsiveness, which leads to prevention of AHR, lung inflammation and specific IgE production.38 However, the same i.n. OVA exposure leads to a lung allergic phenotype and an abrogation of tolerance if mice are presensitized with the use of adjuvant. In contrast to OVA, natural allergens, such as for instance Aspergillus fumigatus antigens or ragweed pollen extract, have been shown to elicit robust allergic lung inflammation when inoculated into the airways of naive mice and this response was dependent upon a proteolytic activity necessary to bypass the tolerogenic response to inhaled antigens.39 Indeed, natural allergens implicated in asthma are often either proteases or strongly associated with protease activity such as Der p I40 and Fel d I.41 The ability of Der p I to induce the release of proinflammatory cytokines from respiratory epithelial cells is known to be mediated by the activation of protease-activated receptor-2 (PAR-2) on these cells.40 Exogenous PAR-2 activation in parallel with OVA challenge enhances allergen-mediated AHR and airway inflammation.42 Moreover, PAR-2 activation also leads to the release of mediators such as eotaxin, granulocyte–macrophage colony-stimulating factor and metalloproteinases.43,44 Both A. alternata and C. herbarum extracts possess a high protease activity and are potent inducers of epithelial cell desquamation and production of pro-inflammatory cytokines (IL-6 and IL-8), which is strictly dependent on the protease activities of these extracts.45 Our data suggest that PAR-2 activation through protease activity from mould allergens might explain the inhibition of tolerance and the stimulation of lung inflammation by the allergens tested. Further experiments will aim to analyse the importance of the extract protease activity in this model.
In conclusion, we have described in this study a mouse model of lung allergy that does not require any sensitization, uses relevant particulate or proteic allergen from non-invasive moulds, and induces allergic inflammation, AHR and subepithelial fibrosis. This model could be an interesting tool to dissect the mechanisms leading to the activation of allergic immune responses in the lungs and remodelling and for the design of new therapeutics.
Acknowledgments
F.H. is a research associate with the Fonds National de la Recherche Scientifique (FNRS), Belgium. This work was partially supported by the ‘Fond National de la Recherche Scientifique’ (FNRS), grant no. 3 4616 04. We thank Youssof Yakoub for his excellent technical assistance.
References
- 1.Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat Immunol. 2002;3:715–20. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- 2.Arm JP, Lee TH. The pathobiology of bronchial asthma. Adv Immunol. 1992;51:323–82. doi: 10.1016/s0065-2776(08)60491-5. [DOI] [PubMed] [Google Scholar]
- 3.Busse WW, Lemanske RF. Asthma. N Engl J Med. 2001;344:350–62. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 4.Seymour BW, Gershwin LJ, Coffman RL. Aerosol-induced immunoglobulin (Ig) -E unresponsiveness to ovalbumin does not require CD8+ or T cell receptor (TCR) -γδ+ T cells or interferon (IFN)-γ. in a murine model of allergen sensitization. J Exp Med. 1998;187:721–31. doi: 10.1084/jem.187.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsitoura DC, DeKruyff RH, Lamb JR, Umetsu DT. Intranasal exposure to protein antigen induces immunological tolerance mediated by functionally disabled CD4+ T cells. J Immunol. 1999;163:2592–600. [PubMed] [Google Scholar]
- 6.Brusselle GG, Kips JC, Tavernier JH, van der Heyden JG, Cuvelier CA, Pauwels RA, Bluethmann H. Attenuation of allergic airway inflammation in IL-4 deficient mice. Clin Exp Allergy. 1994;24:73–80. doi: 10.1111/j.1365-2222.1994.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 7.Grunig G, Warnock M, Wakil AE, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–3. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMillan SJ, Lloyd CM. Prolonged allergen challenge in mice leads to persistent airway remodelling. Clin Exp Allergy. 2004;34:497–507. doi: 10.1111/j.1365-2222.2004.01895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Temelkovski J, Hogan SP, Shepherd DP, Foster PS, Kumar RK. An improved murine model of asthma: selective airway inflammation, epithelial lesions and increased methacholine responsiveness following chronic exposure to aerosolised allergen. Thorax. 1998;53:849–56. doi: 10.1136/thx.53.10.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Havaux X, Zeine A, Dits A, Denis O. A new mouse model of lung allergy induced by the spores of Alternaria alternata and Cladosporium herbarum molds. Clin Exp Immunol. 2005;139:179–88. doi: 10.1111/j.1365-2249.2004.02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black PN, Udy AA, Brodie SM. Sensitivity to fungal allergens is a risk factor for life-threatening asthma. Allergy. 2000;55:501–4. doi: 10.1034/j.1398-9995.2000.00293.x. [DOI] [PubMed] [Google Scholar]
- 12.Paris S, Fitting C, Latgé JP, Herman D, Guinnepain MT, David B. Comparison of conidial and mycelial allergens of Alternaria alternata. Int Arch Allergy Appl Immunol. 1990;92:1–8. doi: 10.1159/000235216. [DOI] [PubMed] [Google Scholar]
- 13.Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997;156:766–75. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- 14.Driscoll KE, Maurer JK, Poynter J, Higgins J, Asquith T, Miller NS. Stimulation of rat alveolar macrophage fibronectin release in a cadmium chloride model of lung injury and fibrosis. Toxicol Appl Pharmacol. 1992;116:30–7. doi: 10.1016/0041-008x(92)90141-e. [DOI] [PubMed] [Google Scholar]
- 15.Huaux F, Arras M, Tomasi D, et al. A profibrotic function of IL-12p40 in experimental pulmonary fibrosis. J Immunol. 2002;169:2653–61. doi: 10.4049/jimmunol.169.5.2653. [DOI] [PubMed] [Google Scholar]
- 16.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 17.Grunig G, Warnock M, Wakil AE, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–3. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emson CL, Bell SE, Jones A, Wisden W, McKenzie AN. Interleukin (IL)-4-independent induction of immunoglobulin (Ig) E, and perturbation of T cell development in transgenic mice expressing IL-13. J Exp Med. 1998;188:399–404. doi: 10.1084/jem.188.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riffo-Vasquez Y, Spina D. Role of cytokines and chemokines in bronchial hyperresponsiveness and airway inflammation. Pharmacol Ther. 2002;94:185–211. doi: 10.1016/s0163-7258(02)00217-6. [DOI] [PubMed] [Google Scholar]
- 20.Shang XZ, Armstrong J, Yang GY, Volk A, Li J, Grisworld DE, Emmell E, Li L. Regulation of antigen-specific versus by-stander IgE production after antigen sensitization. Cell Immunol. 2004;229:106–16. doi: 10.1016/j.cellimm.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Castigli E, Fuleihan R, Ramesh N, Tsitsikov E, Tsytsykova A, Geha RS. CD40 ligand/CD40 deficiency. Int Arch Allergy Immunol. 1995;107:37–9. doi: 10.1159/000236923. [DOI] [PubMed] [Google Scholar]
- 22.Kikuchi S, Nagata M, Kikuchi I, Hagiwara K, Kanazawa M. Association between neutrophilic and eosinophilic inflammation in patients with severe persistent asthma. Int Arch Allergy Immunol. 2005;137(Suppl. 1):7–11. doi: 10.1159/000085425. [DOI] [PubMed] [Google Scholar]
- 23.Humbles AA, Lloyd CM, McMillan SJ, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–9. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 24.Ohno I, Nitta Y, Yamauchi K, et al. Transforming growth factor beta 1 (TGF beta 1) gene expression by eosinophils in asthmatic airway inflammation. Am J Respir Cell Mol Biol. 1996;15:404–9. doi: 10.1165/ajrcmb.15.3.8810646. [DOI] [PubMed] [Google Scholar]
- 25.Pegorier S, Wagner LA, Gleich GJ, Pretolani M. Eosinophil-derived cationic proteins activate the synthesis of remodeling factors by airway epithelial cells. J Immunol. 2006;177:4861–9. doi: 10.4049/jimmunol.177.7.4861. [DOI] [PubMed] [Google Scholar]
- 26.Little SA, MacLeod KJ, Chalmers GW, Love JG, McSharry C, Thomson NC. Association of forced expiratory volume with disease duration and sputum neutrophils in chronic asthma. Am J Med. 2002;112:446–52. doi: 10.1016/s0002-9343(02)01047-1. [DOI] [PubMed] [Google Scholar]
- 27.Simpson JL, Scott RJ, Boyle MJ, Gibson PG. Differential proteolytic enzyme activity in eosinophilic and neutrophilic asthma. Am J Respir Crit Care Med. 2005;172:559–65. doi: 10.1164/rccm.200503-369OC. [DOI] [PubMed] [Google Scholar]
- 28.Kikuchi I, Kikuchi S, Kobayashi T, Hagiwara K, Sakamoto Y, Kanazawa M, Nagata M. Eosinophil trans-basement membrane migration induced by interleukin-8 and neutrophils. Am J Respir Cell Mol Biol. 2006;34:760–5. doi: 10.1165/rcmb.2005-0303OC. [DOI] [PubMed] [Google Scholar]
- 29.Park SJ, Wiekowski MT, Lira SA, Mehrad B. Neutrophils regulate airway responses in a model of fungal allergic airways disease. J Immunol. 2006;176:2538–45. doi: 10.4049/jimmunol.176.4.2538. [DOI] [PubMed] [Google Scholar]
- 30.Herre J, Willment JA, Gordon S, Brown GD. The role of Dectin-1 in antifungal immunity. Crit Rev Immunol. 2004;24:193–203. doi: 10.1615/critrevimmunol.v24.i3.30. [DOI] [PubMed] [Google Scholar]
- 31.Steele C, Rapaka RR, Metz A, Pop SM, Williams DL, Gordon S, Kolls JK, Brown GD. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 2005;1:323–34. doi: 10.1371/journal.ppat.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hohl TM, Van Epps HL, Rivera A, Morgan LA, Chen PL, Feldmesser M, Pamer EG. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. Plos Pathog. 2005;1:232–40. doi: 10.1371/journal.ppat.0010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chetta A, Foresi A, Del Donno M, Bertorelli G, Pesci A, Olivieri D. Airways remodeling is a distinctive feature of asthma and is related to severity of disease. Chest. 1997;111:852–7. doi: 10.1378/chest.111.4.852. [DOI] [PubMed] [Google Scholar]
- 34.Vignola AM, Mirabella F, Costanzo G, Di Giorgi R, Gjomarkaj M, Bellia V, Bonsignore G. Airway remodeling in asthma. Chest. 2003;123:417S–22S. doi: 10.1378/chest.123.3_suppl.417s. [DOI] [PubMed] [Google Scholar]
- 35.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–94. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee CG, Homer RJ, Zhu Z, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta1. J Exp Med. 2001;194:809–21. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMenamin C, Holt PG. The natural immune response to inhaled soluble protein antigens involves major histocompatibility complex (MHC) class I-restricted CD8+ T cell-mediated but MHC class II-restricted CD4+ T cell-dependent immune deviation resulting in selective suppression of immunoglobulin E production. J Exp Med. 1993;178:889–99. doi: 10.1084/jem.178.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsitoura DC, Blumentyhal RL, Berry G, Dekruyff RH, Umetsu DT. Mechanisms preventing allergen-induced airways hyperreactivity: role of tolerance and immune deviation. J Allergy Clin Immunol. 2000;106:239–46. doi: 10.1067/mai.2000.108429. [DOI] [PubMed] [Google Scholar]
- 39.Kheradmand F, Kiss A, Xu J, Lee SH, Kolattukudy PE, Corry DB. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol. 2002;169:5904–11. doi: 10.4049/jimmunol.169.10.5904. [DOI] [PubMed] [Google Scholar]
- 40.Asokananthan N, Graham PT, Stewart DJ, Bakker AJ, Eidne KA, Thompson PJ, Stewart GA. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells. The cysteine protease allergen, Der p I, activates protease-activated receptor (PAR) -2 and inactivates PAR-1. J Immunol. 2002;169:4572–8. doi: 10.4049/jimmunol.169.8.4572. [DOI] [PubMed] [Google Scholar]
- 41.Ring PC, Wan H, Schou C, Kroll Kristensen A, Roepstorff P, Robinson C. The 18-kDa form of cat allergen Felis domesticus 1 (Fel d I) is associated with gelatin- and fibronectin-degrading activity. Clin Exp Allergy. 2000;8:1085–96. doi: 10.1046/j.1365-2222.2000.00805.x. [DOI] [PubMed] [Google Scholar]
- 42.Ebeling C, Forsythe P, Ng J, Gordon JR, Hollenberg M, Vliagoftis H. Proteinase-activated receptor 2 activation in the airways enhances antigen-mediated airway inflammation and airway hyperresponsiveness through different pathways. J Allergy Clin Immunol. 2005;115:623–30. doi: 10.1016/j.jaci.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 43.Vliagoftis H, Befus AD, Hollenberg MD, Moqbel R. Airway epithelial cells release eosinophil survival-promoting factors (GM-CSF) after stimulation of proteinase-activated receptor 2. J Allergy Clin Immunol. 2001;107:679–85. doi: 10.1067/mai.2001.114245. [DOI] [PubMed] [Google Scholar]
- 44.Vliagoftis H, Schwingshackl A, Milne CD, Duszyk M, Hollenberg MD, Wallace JL, Befus AD, Moqbel R. Proteinase-activated receptor-2-mediated matrix metalloproteinase-9 release from airway epithelial cells. J Allergy Clin Immunol. 2000;106:537–45. doi: 10.1067/mai.2000.109058. [DOI] [PubMed] [Google Scholar]
- 45.Kauffman HF, Tomee JF, van de Riet MA, Timmerman AJ, Borger P. Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J Allergy Clin Immunol. 2000;105:1185–93. doi: 10.1067/mai.2000.106210. [DOI] [PubMed] [Google Scholar]