Abstract
Natural killer (NK) cells are activated early during inflammatory events and contribute to the shaping of the ensuing adaptive immune response. To further understand the role for NK cells in inflammation, we investigated the phenotype and function of synovial fluid (SF) NK cells from patients with chronic joint inflammation, as well as from patients with transient inflammation of the knee following trauma. We confirm that synovial NK cells are similar to the well-characterized CD56bright peripheral blood (PB) NK-cell subset present in healthy individuals. However, compared to this PB subset the synovial NK cells express a higher degree of activation markers including CD69 and NKp44, the latter being up-regulated also on CD56bright NK cells in the PB of patients. Activated synovial NK cells produced interferon-γ and tumour necrosis factor, and the production was further up-regulated by antibody masking of CD94/NKG2A, and down-regulated by target cells expressing human leucocyte antigen-E in complex with peptides known to engage CD94/NKG2A. We conclude that synovial NK cells have an activated phenotype and that CD94/NKG2A is a key regulator of synovial NK-cell cytokine synthesis.
Keywords: autoimmunity, cell surface molecules, cytokines, inflammation, natural killer cells
Introduction
Natural killer (NK) cells contribute to defence against infections and tumours, while their role in chronic inflammatory diseases is less clear. It is known that several cell types are involved in chronic joint inflammation. Among these, macrophages, fibroblasts, B cells and T cells all have well-documented roles in the pathophysiology of diseases such as rheumatoid arthritis.1–5 In addition, recent reports have shown that there is a significant fraction of NK cells in both the synovial fluid (SF) and the synovial membrane.6–8 However, their role in the pathogenesis of rheumatoid arthritis needs to be clarified.
Most studies characterizing human NK cells are performed on peripheral blood (PB) NK cells. Such NK cells are broadly divided into two major subgroups, the CD56bright and CD56dim subsets.9 Further phenotypical studies comparing these two NK-cell subsets have identified differences on the receptor repertoire for major histocompatibility complex (MHC) class I recognition. CD56bright NK cells express high levels of CD94/NKG2A but express no, or very low, levels of immunoglobulin domain-like receptors, such as killer cell immunoglobulin-like receptors (KIRs) and leucocyte immunoglobulin-like receptors (LIRs, also known as immunoglobulin-like transcripts or ILTs).10,11 On the other hand CD56dim NK cells express moderate levels of CD94/NKG2A, but express KIRs.
Moreover, CD16 expression is low/absent in the CD56bright subset but abundantly expressed on CD56dim NK cells, whereas CD25 is constitutively expressed on the CD56bright subset. Phenotypic studies also suggest that the CD56bright NK cells show distinct migratory properties.12 CD56bright NK cells have been reported to express lymph-node-homing molecules such as CD62 ligand and CCR7 and they have been detected in both inflamed and resting lymph nodes.13,14 In addition, CD56bright NK cells are enriched at different inflammatory sites.8,15,16
Successful triggering of NK-cell effector functions, however, depends on signals mediated through direct recognition by a number of activating cell surface receptors. These activating receptors are expressed on the cell surface in complex with adaptor molecules such as DAP12, DAP10, CD3ζ and FcRγ, which transduce signals leading to cytotoxicity and/or cytokine secretion. One important triggering receptor is the NKG2D homodimer, which is expressed at various levels on most NK cells and recognizes stress-induced human MHC class I related molecules (i.e. MHC class I related chain A and B (MICA/B) and UL16-binding protein (ULBPs)) leading to NK-cell activation through DAP10 signalling. Other more recently described activating NK-cell receptors, collectively known as natural cytotoxicity receptors (NCRs), include NKp46 and NKp30 which are widely expressed on NK cells and NKp44 which is expressed following NK-cell activation.17 These receptors have been implicated in cytotoxicity against tumours and in cytokine production. Finally, NK cells can also express activation molecules shared with other lymphocytes, e.g. CD25 and CD69 and certain chemokine receptors.
To understand the role of various NK-cell populations and their receptor usage in chronic arthritis, we aimed to characterize the phenotype of NK cells in the SF of arthritis patients, particularly regarding the above-mentioned NK-cell subpopulations. To this end we compared SF NK cells with PB NK cells from the same individual. To further differentially characterize the NK-cell subpopulation of SF in chronic arthritis, we made phenotypic comparisons between the PB NK-cell populations of the patients with chronic arthritis (the cell population from which the SF cells presumably are derived) and the PB of healthy controls (in whom SF sampling is not possible), as well as between SF and PB NK cells from patients with chronic arthritis and patients with post-traumatic knee joint exudates prompting non-acute arthroscopic examination. Second, we determined the activation status and functional capacity of SF NK cells from patients with chronic arthritis, including the capacity to secrete cytokines in response to stimulation, and analysis of the receptors involved in regulation of such responses.
Materials and methods
Patients, controls and cell separation
All patients with chronic joint inflammation suffered from arthritis of the knee, and had undergone therapeutic aspiration of SF at the time of analysis. The patient diagnoses included rheumatoid arthritis (n = 37), non-specific oligo/polyarthritis (n = 10), psoriatic arthritis (n = 5), ankylosing spondylitis (n = 5), juvenile arthritis (n = 4), Behçet's disease (n = 1), mixed connective tissue disease (n = 1) and reactive arthritis (n = 1). The median age of the whole patient group was 48·5 years (range 21–77). The median disease duration was 16 years (range 1 month to 41 years).
Trauma patients (n = 7) had had persistent symptoms for more than 12 weeks after the injury, specifically a swollen knee joint and pain with suspected intra-articular pathology requiring arthroscopy.
All SF samples were compared to PB from the same patient. For most patients SF was obtained from one knee joint. Where SF was available from both knees, each SF was processed and analysed as a distinct sample. For some analyses, healthy control PB was also used. All samples were collected into heparin tubes. Mononuclear cells were isolated within 2 hr of sample collection by density gradient centrifugation.
Monoclonal antibodies and flow cytometry
Anti-KIR antibodies DX9 (anti-KIR3DL1), DX27 (anti-KIR2DL2, anti-KIR2DL3 and anti-KIR2DS2), DX31 (anti-KIR3DL2), and DX22 (anti-CD94) were kindly provided by Drs Lewis L. Lanier and Joseph H. Phillips (UCSF, San Francisco and DNAX, Palo Alto, CA, respectively). Antibody to LIR-1/ILT2 (clone M405) was kindly provided by Amgen Inc (Thousand Oaks, CA). Phycoerythrin (PE)-conjugated antibodies specific for NKG2A, NKp46, NKp44 and NKp30 were purchased from Beckman Coulter (Fullerton, CA). Anti-CD3, anti-CD56, anti-CD25 and anti-CD16 antibodies were from BD Biosciences (Franklin Lakes, NJ) and anti-CD69 antibody was from Dako Cytomation (Glostrup, Denmark). Antibodies for NKG2C and NKG2D were purchased from R & D Systems (Minneapolis, MN). Antibodies against human interferon-γ (IFN-γ) and tumour necrosis factor (TNF) were from BD Biosciences. Second-step reagents were fluorescein isothiocyanate- and PE-conjugated goat anti-mouse immunoglobulin and isotype control antibodies (all from Dako Cytomation). NK cells were defined as CD56+ CD3– cells within the lymphocyte gate. Data in the text are expressed as median and range. Expression levels are given as geometric mean of fluorescence intensity. Patient samples with median nominal values were selected as representative individuals for illustration purposes.
HLA-E tetramers
The human leucocyte antigen (HLA)-E expression vector for tetramer production was kindly provided by Dr Veronique Braud (Oxford, UK). Tetrameric HLA-E complexes were generated essentially as previously described.12 Briefly, HLA-E heavy chain, fused with BirA substrate peptide (bsp) at the C terminus, and human β2-microglobulin (β2m) were over-expressed in Escherichia coli BL21 pLysS, purified from inclusion bodies and solubilized in an 8-m urea solution containing dithiothreitol. Complexes of HLA-E–bsp, human β2m and synthetic peptide (VMAPRTVLL) derived from the HLA-B*0701 leader sequence (Research Genetics Huntsville, AL) were produced as described in ref. 12. Tetramers were generated by mixing biotinylated HLA-E/β2m/peptide monomers with streptavidin–PE (Sigma, St Louis, MO) at a 4 : 1 molar ratio. Tetrameric mouse H-2Kb molecules conjugated to streptavidin–PE were used as control reagents.
Cells
Cell cultures were maintained in complete medium consisting of RPMI-1640 (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal calf serum, 2 mm l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma-Aldrich, St Louis, MO).
The K562-E*01033 transfected cell line was generated as previously described.12 Transfected cells were selected in complete medium supplemented with 1 mg/ml G418 (Bio-Rad, Hercules, CA). Stable transfected cells were isolated by flow cytometry on the basis of their cell surface HLA class I levels. HLA-E stabilization was performed in AIM-V (Invitrogen) serum-free medium at 26° in the presence of the indicated concentration of synthetic peptides (VMAPRTVLL, derived from the HLA-B*0701 leader sequence, QMRPVSRVL encoded within the heat-shock protein 60 leader sequence (hsp60sp) and RGPGRAFVTI an HIV-gp120 encoded peptide was used as control; all peptides obtained from Research Genetics, Huntsville, AL).
NK-cell stimulation
Peripheral blood mononuclear cells (PBMC) and synovial fluid mononuclear cells (SFMC) were diluted to 5 × 106/ml in complete medium and stimulated for 15 hr with interleukin-2 (IL-2; 10 ng/ml) or lipopolysaccharide (LPS; 10 μg/ml), followed by 4 hr in the presence of GolgiStopTM (BD Biosciences). Alternatively, PBMC and SFMC were grown in medium over night and stimulated next day with either K562 cells (1 : 1 ratio), monokines, namely IL-12 (10 ng/ml), IL-15 (10 ng/ml) and IL-18 (100 ng/ml), all from R & D Systems, or with 20 ng/ml phorbol 12-myristate 13-acetate and 0·5 μm ionomycin (Sigma) for 4 hr in the presence of GolgiStopTM (BD Biosciences). All stimulations were compared to cultures in complete medium alone. After stimulation, cells were first stained for CD3 and CD56 cell surface markers followed by fixation (CytoFix, BD Biosciences) and permeabilization (CytopermTM, BD Biosciences) and finally stained for intracellular cytokines (IFN-γ and TNF). The percentage of CD3– CD56+ NK cells producing the respective cytokine was assessed by flow cytometry.
For CD94 blocking experiments, cell cultures were stimulated in the presence of anti-CD94 [1 μg/ml, DX22, mouse immunoglobulin G1 (IgG1)]. Cultures stimulated in medium alone or in the presence of an anti-CD56 monoclonal antibody (1 μg/ml B159, mouse IgG1; BD Biosciences) were used as controls.
Statistics
Box plots depict the median as a line and the 25th and 75th percentiles limiting the box with the 10th and 90th percentiles indicated with bars. The Wilcoxon signed rank test was used to compare paired data of patient PB and SF, whereas the Mann–Whitney test was used for comparisons between data derived from PB of patients and healthy controls. Reported P-values were two tailed and considered significant when < 0·05.
Results
Phenotype of synovial and peripheral blood NK cells from patients with arthritis
The results presented below concern comparisons between SF NK cells from inflamed joints of patients with chronic, non-infectious arthritis with the circulating blood NK cells in each respective patient, and also comparisons with blood NK cells from healthy controls. As described in detail in the Materials and methods, the dominant diagnosis was rheumatoid arthritis but other rheumatic diseases were also represented. We sought distinguishing features associated with the different diagnoses, but found none. On the other hand, we found some consistent features of SF NK cells among the different arthritis patients that distinguished them from blood NK cells. We therefore report the results without the diagnostic classification for the chronic arthritis conditions.
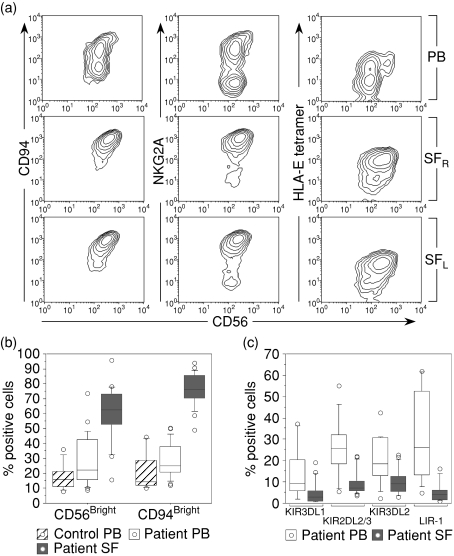
The total NK-cell frequencies did not differ between the SF and PB of patients. In PB, the NK-cell frequency ranged between 2·5 and 27·2% (median 9·4%) of total lymphocytes, whereas the range in SF was between 2·5 and 34·3% (median 10·6%). The percentages of CD56dim and CD56bright NK cells were determined according to the intensity of fluorescence staining for CD56. Whereas a minority of PB NK cells from patients and healthy controls belonged to the CD56bright NK-cell subset, the majority of SF NK cells expressed CD56 brightly (Fig. 1a–b). Similar to CD56, the expression of CD94 exhibits a bimodal distribution in the PB of healthy controls and patients, whereas a single CD94bright population was found in SF (Fig. 1a).
Figure 1.
Expression of NK-cell surface receptors on mononuclear cells isolated from peripheral blood (PB) and synovial fluid (SF) of patients with chronic arthritis. The cells were stained for CD3, CD56 and a panel of NK-cell-associated receptors. NK cells were defined by gating for CD3– CD56+ lymphocytes. (a) Expression profile of CD94/NKG2A, defined by staining with monoclonal antibodies as well as by tetramers of HLA-E in complex with the B7 leader encoded peptide. The data show one representative patient, with SF drawn from both knees denoted SFr for right knee and SFl for left knee. (b) Frequency of CD56bright and CD94bright NK cells (24 patients and eight healthy controls). (c) Frequency of NK cells expressing different KIR molecules and LIR-1 (Patient PB: n = 15 for KIR stainings and n = 12 for LIR-1, SF: n = 17 for KIR and n = 13 for LIR-1). For paired comparison between PB and SF NK cells: KIR3DL1 P < 0·001; KIR2DL2 P < 0·001; KIR3DL2 P < 0·001; LIR-1 P = 0·002).
The CD56bright NK-cell subset in PB was also characterized by negative/low expression of KIR and bright expression of CD94 and of NKG2A (data not shown), and similar to this subset most SF NK cells expressed NKG2A at bright levels and bound efficiently to HLA-E tetramers stabilized with an HLA-B7 encoded leader peptide (VMAPRTVLL) (Fig. 1a–b). The staining intensities, using antibodies against CD94 or NKG2A, as well as HLA-E tetramers, were similar in PB CD56bright NK cells and SF NK cells, suggesting that these two NK-cell subsets express similar levels of HLA-E receptors at the cell surface (not shown). NKG2A was expressed by 43·1% (range 28–81·5%) of patient PB NK cells and by 50·8% (range 31·0–75·0%) of healthy controls, whereas in SF it was expressed by 91·6% (range 52·4–96·7%) of the NK cells.
We also compared the expression of clonally distributed KIR molecules on patient PB and SF NK cells. KIR expression in PB did not differ between healthy controls and patients (data not shown). The proportion of KIR-expressing NK cells was, however, considerably lower in SF compared to PB NK cells isolated from the same patient (Fig. 1c). Furthermore, NK cells bearing LIR-1, a broadly reactive MHC class I inhibitory receptor, were also markedly under-represented among NK cells isolated from SF, compared to PB of both patients (Fig. 1c) and healthy controls (data not shown). Similar to SF NK cells, LIR-1 expression was absent on CD56bright PB NK cells from patients and controls (data not shown).
SF NK cells in patients with transient post-traumatic inflammation
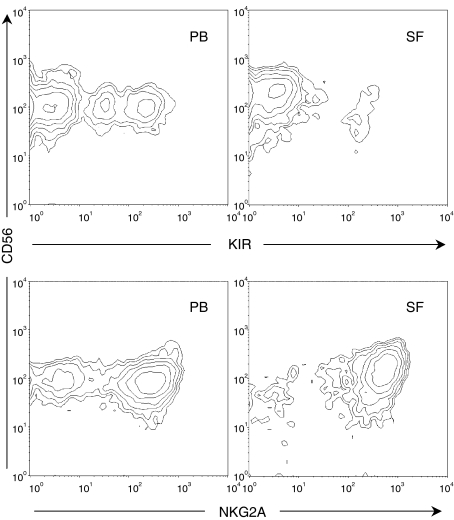
We further investigated whether the accumulation of a homogeneous NK-cell subset was a hallmark of chronic arthritis, or whether it is also observed in the joints of patients with knee inflammation associated with trauma. To avoid SF with massive blood contamination, we excluded acute trauma patients. We selected patients with persisting symptoms in terms of a swollen knee joint and pain for more than 12 weeks after the injury, who had suspected intra-articular pathology requiring arthroscopy. In general, these samples contained few cells, and we could only perform a satisfactory analysis of the SF in three out of seven patients. Similar to patients with chronic arthritis, these SF samples were dominated by CD56bright NKG2A+ NK cells (Fig. 2), and the frequency of KIR-expressing NK cells was lower in SF compared with paired PB (SF range 7·8–12%; PB range 30·6–43·5%).
Figure 2.
Expression patterns of KIRs (upper panels) using a cocktail of anti-KIR monoclonal antibodies (KIR3DL1, KIR2DL2/3 and KIR3DL2) and NKG2A (lower panels) on gated CD3– CD56+ NK cells derived from PB (left) and SF (right) of a patient with post-traumatic inflammation of the knee. Data are representative of three patients analysed.
Expression of activation-related receptors on SF and PB NK cells from patients with arthritis
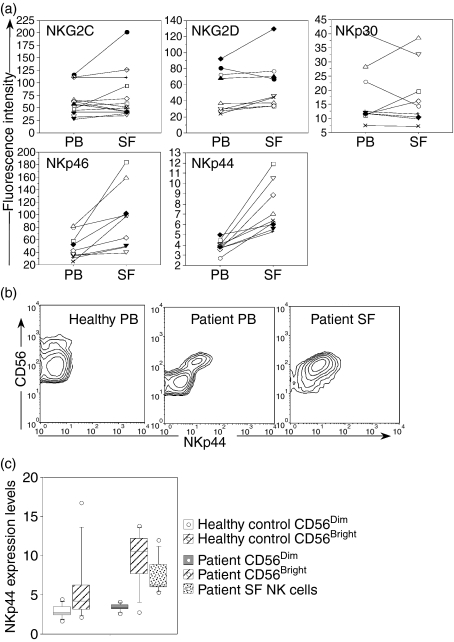
Apart from NKG2A, CD94 can also form functional heterodimers with other NKG2 chains, including NKG2C. CD94/NKG2C receptors signal through DAP12, and deliver an activating signal upon recognition of certain HLA-E ligands.18,19 NKG2C was expressed by 12·0% of PB NK cells (range 0·7–34·6%) and by 17·1% of the SF NK cells (range 1·1–42·6%). Furthermore, when comparing the expression level of NKG2C on positive cells there was no significant difference between PB and SF NK cells (Fig. 3a).
Figure 3.
(a) Expression levels of activating receptors on gated CD3– CD56+ NK cell from PB and SF samples of patients with chronic arthritis (n = 9). Fluorescence intensity is expressed as geometric mean (MFI). For NKG2C the fluorescence intensity was calculated on NKG2C-expressing NK cells. For NKG2D, NKp46, NKp44 and NKp30, the fluorescence intensity was calculated on all NK cells. Lines connect paired samples from each patient. Statistically significant differences on paired comparison between PB and SF NK cells; NKp46 P = 0·008, NKp44 P = 0·008. (b) Expression profile of NKp44/CD56 on gated CD3– CD56+ NK cells from one healthy donor PB and one patient PB and SF. (c) NKp44 expression levels portrayed as geometric mean of fluorescence intensity on PB NK-cell subsets (healthy donors n = 9, and arthritis patients n = 9) and on all SF NK cells (n = 9). CD56bright/dim subsets were defined according to the CD56 staining pattern on CD3– CD56+ lymphocytes in blood.
We next analysed the distribution and expression levels of other receptors known to be involved in NK-cell activation. Most PB NK cells (97·2%, range 85·8–99·3%) and SF NK cells (93·6%, range 89·2–98·8%) expressed NKG2D and displayed similar expression levels (evaluated as geometric mean fluorescence intensity, MFI) (Fig. 3a). In regard to the NCR group of receptors, NKp30 was detected among both PB NK cells (43·5%, range 12·5–96·6%) and SF NK cells (60·0%, range 6·4–96·9%) and MFI expression levels did not differ between compartments (Fig. 3a). Similar to NKp30, NKp46 was broadly expressed among both PB NK cells (59·9%, range 61·9–97·2%), and SF NK cells (96·0%, range 77·3−98·5%). However, in contrast to NKp30, the NKp46 MFI expression levels were elevated on SF NK cells compared to PB NK cells (Fig. 3a). NKp44 was expressed by a larger population of SF NK cells (13·6%, range 5·2–31·2%), compared to PB NK cells (2·5%, range 0·9–6·4%, P = 0·01). Similarly, the expression levels of NKp44 were elevated in SF NK cells (Fig. 3a).
When analysing the NCR staining profiles, we noted that in patient blood, the CD56bright NK cells displayed higher NKp44 expression levels compared to the CD56dim population (Fig. 3b). Since NKp44 is described as an inducible receptor expressed upon NK-cell activation, freshly isolated PB NK cells from healthy individuals are not expected to express this molecule on the cell surface.20 Indeed, in our analysis, NKp44 was absent from both PB NK-cell subsets in healthy individuals (n = 9), with one exception where NKp44 was detected on CD56bright NK cells (Fig. 3c). On the other hand, NKp44 was expressed by a considerable percentage of the CD56bright PB subset in all patients tested (Fig. 3c), suggesting a systemic activation of NK cells in patients with chronic arthritis. Similarly, NKp46 expression levels appeared elevated on CD56bright PB NK-cell subsets of healthy controls as well as of patients (data not shown). The overall expression of both NKp44 (Fig. 3a) and NKp46 was elevated on synovial NK cells compared to PB NK cells, indicating the presence of activated NK cells in the joint.
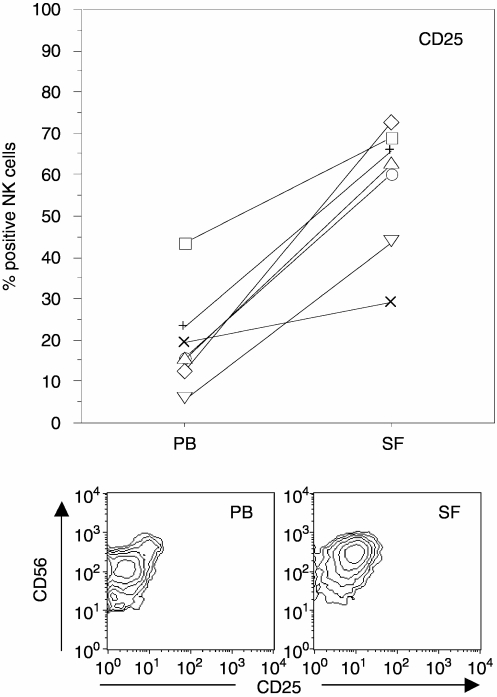
The activation marker CD25 (the high-affinity IL-2 receptor) was expressed by a minority of PB NK cells of both patients (Fig. 4) and healthy controls (data not shown), whereas in SF, most NK cells expressed CD25 (Fig. 4). Expression levels of this molecule were similar in PB NK cells from patients and healthy controls (data not shown). When analysing NK-cell subsets, the percentage of CD25+ NK cells in the PB CD56bright subset did not differ from the percentage of SF NK cells expressing this marker.
Figure 4.
Frequency of NK cells expressing CD25 in PBMC and SFMC from arthritis patients (n = 7). The lower panels illustrate CD25 and CD56 expression patterns from a representative patient. PB data are shown in the left panel and SF in the right panel. Paired comparison between PB and SF NK cells; P = 0·02. Lines connect paired samples from each patient.
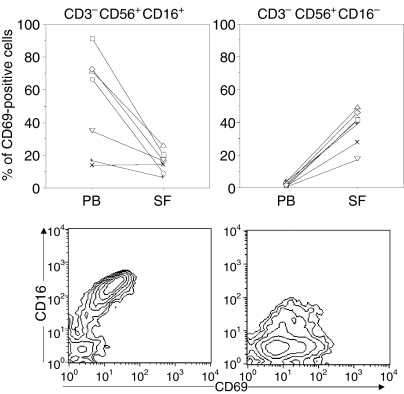
As previously described,6,21 the majority of SF NK cells lacked expression of CD16 (Fig. 5), another feature shared with circulating CD56bright NK cells from both patients and healthy individuals. CD69 expression on patient PB NK cells varied between individuals and was confined to the CD16+ PB NK-cell subset (Fig. 5). Of the few CD16+ NK cells present in the SF a minority expressed this marker (Fig. 5, left panel). Moreover, CD16– NK cells from the inflamed joint differ from CD16– PB NK cells, as PB CD16– NK cells lack CD69 expression (below 2%), whereas a considerable proportion of the CD16– SF NK cells expressed this marker (Fig. 5 right panel).
Figure 5.
CD69 expression on CD16+ and CD16– NK-cell subsets from patient PB and SF (n = 7). Lower panel shows CD16/CD69 staining patterns for one representative patient, where PB NK cells are depicted in the left panel and SF NK cells in the right panel. Paired comparison between PB and SF CD16+ NK cells; P = 0·02, and CD16– NK cells; P = 0·01. Lines connect paired samples from each patient.
In summary, the vast majority of NK cells in the SF display an activated phenotype and resemble, but are not phenotypically identical to, the previously described subset of NK cells in the blood expressing a CD56bright CD94bright NKG2D+ KIR– CD16– phenotype. The SF NK cells differ from this population by expressing higher levels of the activation marker CD69.
Cytokine production by SF NK cells
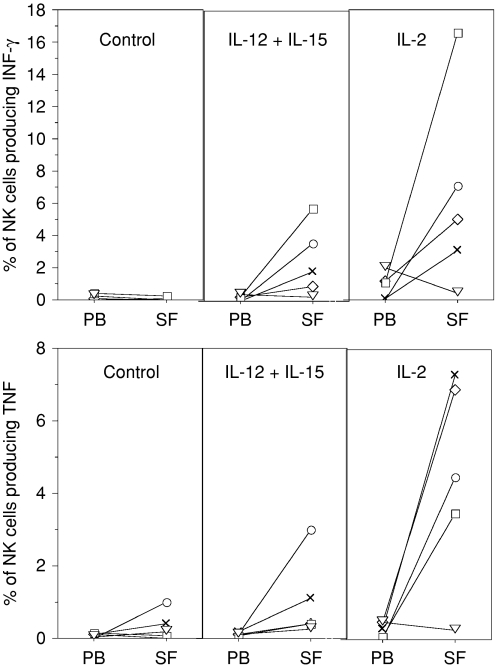
Previous reports have shown that NK cells with the CD56bright phenotype can produce high levels of cytokines, in particular IFN-γ.22 To further characterize SF NK cells from chronic arthritis patients, we stimulated PBMC and SFMC with different cytokines and estimated the frequency of IFN-γ-producing and TNF-producing NK cells. Freshly isolated NK cells from either SF or PB did not produce detectable levels of IFN-γ or TNF (data not shown). Likewise, NK cells from either SFMC or PBMC cultures in medium alone did not produce detectable levels of IFN-γ or TNF. Stimulation with IL-12, IL-15 or IL-18 alone resulted in sporadic increases of IFN-γ and TNF production by NK cells (data not shown). However, when the cultures were stimulated using a combination of IL-15 and IL-12 increased frequencies of IFN-γ-positive and TNF-positive NK cells were observed, particularly within SFMCs (Fig. 6).
Figure 6.
Frequency of IFN-γ-producing and TNF-producing NK cells in patient PBMC and SFMC following stimulation with IL-12 (10 ng/ml) in combination with IL-15 (10 ng/ml) or with IL-2 (10 ng/ml) for 4 hr (n = 5). Lines connect paired samples from each patient identified with the same marker in all panels.
As shown in Fig. 4, SF NK cells express higher levels of high-affinity IL-2 receptors and when stimulated by IL-2 a significant proportion of SF NK cells produced IFN-γ and TNF (Fig. 6). Combining IL-2 with either IL-12, IL-15 or IL-18 did not result in further increased frequencies of cytokine-positive NK cells compared to IL-2 alone (data not shown).
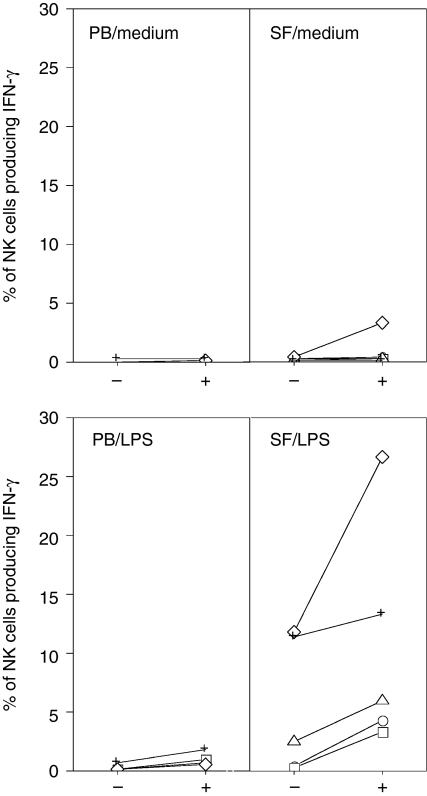
To test whether CD94/NKG2–HLA-E interactions regulate NK-cell cytokine synthesis, we stimulated PBMC and SFMC in the presence of an anti-CD94 monoclonal antibody to block the interaction of CD94 and HLA-E, and measured the proportion of NK cells producing IFN-γ. Blocking CD94 had no discernable effect on NK-cell cytokine production, in the absence of further stimulating factors (Fig. 7). In cultures stimulated with IL-12 and IL-15 there was a tendency towards an increased frequency of IFN-γ-producing cells in the presence of anti-CD94 blocking antibody, compared to control cultures, though not statistically significant (data not shown). We then reasoned that the effects of masking CD94 would become evident in a situation where NK-cell activation is dependent on direct cell contact with an activated target cell. Therefore we stimulated SFMC and PBMC with LPS, a well-described activator of myeloid cells, in the presence or absence of anti-CD94 monoclonal antibody. Indeed, an increased fraction of SF NK cells produced IFN-γ after stimulation with LPS, and the proportion of IFN-γ-producing NK cells was further increased upon blocking of CD94 (Fig. 7).
Figure 7.
Effect of CD94 blocking on the frequency of IFN-γ-producing NK cells. PBMC or SFMC of patients with arthritis (n = 5), were cultured in complete medium or stimulated with LPS (10 μg/ml) for 19 hr in the presence (+) or absence (–) of the CD94 blocking antibody DX22. Lines connect paired samples from each patient identified with the same marker in all panels.
HLA-E modulates cytokine production by activated synovial NK cells
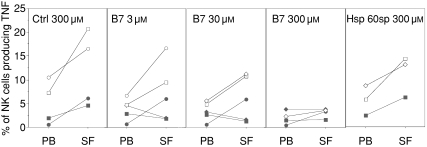
To further investigate the role of HLA-E on target cells during the regulation of SF NK-cell cytokine synthesis, we used HLA class I negative K562 cells stably transfected with the HLA-E*01033 allele (K562-E*01033) to stimulate PB-derived and SF-derived NK cells. Residual HLA-E expression could be detected on these cells, although it did not seem to contain any protective peptides because there was no obvious difference between untransfected and transfected cells concerning their sensitivity to NK cell-mediated lysis or their capacity to stimulate NK-cell cytokine synthesis (data not shown). To increase HLA-E cell surface expression, the K562-E*01033 cells were incubated with various synthetic HLA-E-binding peptides. HLA-E expression levels were assessed before stimulation. Stimulation of NK cells with K562-E*01033 cells in the absence of exogenous peptide (not shown) or incubation with a control peptide, resulted in production mainly of TNF by NK cells (Fig. 8) and marginal production of IFN-γ (data not shown). Stimulation of TNF synthesis was inhibited in a dose-dependent manner by incubating with an HLA-E binding protective peptide (i.e. B7sp), but not in the presence of an HLA-E binding non-protective peptide23 (hsp60sp) (Fig. 8). IFN-γ synthesis by the same NK cells was low and remained unaffected irrespective of the HLA-E/peptide combination (data not shown).
Figure 8.
Modulation of TNF production by HLA-E expression on target cells. PBMCs and SFMCs from arthritis patients were stimulated with K562 cells transfected with a cDNA plasmid encoding the HLA-E*01033 allele. HLA-E cell surface expression was stabilized by exogenous loading of the synthetic peptide VMAPRTVLL, encoded within the leader sequence of the HLA-B*0701(B7sp), or QMRPVSRVL encoded within the heat-shock protein 60 leader sequence (hsp 60sp). The frequency of NK cells (CD3– CD56+ lymphocytes) producing TNF was assessed by flow cytometry using standard intracellular cytokine staining protocol, after 4 hr of coculture (n = 3–5). Lines connect paired samples from each patient identified with the same marker in all panels.
Thus, an up-regulation of HLA-E complexes per se was not sufficient to decrease TNF production by SF NK cells when stimulated with K562-E*01033 cells. Rather, specific peptide-dependent recognition by CD94/NKG2A was required. This we conclude because B7 peptide, but not hsp60sp, affected NK-cell reactivity in the presence of similar levels of HLA-E expression (Fig. 8).
Discussion
The initial identification of two human NK-cell subsets (CD56bright and CD56dim) with different effector functions raised several questions as to where and when either of these subsets would be activated and how their actions could influence the outcome of an immune response. Differences in the expression pattern of chemokine receptors and adhesion molecules provided a first clue suggesting that CD56bright NK cells might preferentially circulate via lymph nodes. In fact, CD56bright are the dominant NK-cell population isolated from lymph nodes13,24 where they may interact with dendritic cells and T cells. CD56bright are also abundant at inflammatory sites.6,8,15,16 Thus, the anatomical location where CD56bright NK cells are found, together with their prominent ability to produce cytokines, suggests the possibility that they may have an important role in the co-ordination of inflammatory responses.
In the first part of this study, we show that synovial NK cells share some features with, but are not identical to, the CD56bright subset present in PB. Similar to this subset, SF NK cells express CD94/NKG2A but not KIR, and CD25 but not CD16. We found higher baseline levels of NKp44 in circulating CD56bright compared to CD56dim NK cells, and this difference was more pronounced in patient blood, indicating that disease-associated factors affect circulating NK cells, in particular the CD56bright subset. NKp44 expression was also increased among SF NK cells, which in combination with CD69 expression suggests a more activated phenotype. This indicates that the observed pattern of cell surface marker expression might not simply be a consequence of passive enrichment of CD56bright NK cells at the inflammatory site. It rather seems as though the latter cell population has been triggered to perform some function.
Our results on phenotype confirm the findings of previous studies of NK cells at inflammatory sites.6–8,16 For example, Dalbeth et al.8 described SF NK cells as CD56bright CD94+ KIR– CD69+. In addition, we provide several important extensions in the present study. It was not clear from previous observations whether the observed pattern was specific for chronic autoimmune arthritis or was a general feature of inflammation.6 In our material, we observed a dominance of the CD56 NKG2Abright KIR– phenotype in the SF of three out of three trauma patients with persisting inflammation and knee joint effusion 12 weeks after injury. These patients had major meniscal injury in combination with anterior cruciate ligament injury diagnosed at arthroscopy. These data indicate that the NK-cell pattern observed may be a characteristic of joint inflammation per se, rather than chronic autoimmune inflammation. Furthermore, we made a comparison of a number of markers between CD56bright cells in the blood and the SF, allowing us to demonstrate the activated phenotype of SF NK cells. The capacity of NK cells to secrete IFN-γ upon direct cell contact with macrophages has been demonstrated previously.8 We show that the SF NK cells can also be stimulated to secrete TNF, a key cytokine in the pathogenesis of RA, and that CD94/NKG2A, the main MHC class I recognizing inhibitory receptor expressed by SF NK cells, has a central role in regulating their expression of not only this cytokine but also IFN-γ.
The rapid responses of the cells performing non-adaptive immune functions presuppose the constitutive expression of activating molecules. In the present study we also analysed expression of activating receptors associated with NK cells for the first time. Expression of all three NCR was detected. NKp44 showed a markedly increased expression on SF NK cells. NKp46 levels were also elevated in SF NK cells, whereas NKp30 levels were unchanged.
Early studies noted that CD56bright NK cells express CD25, the high-affinity IL-2 receptor chain,25,26 and display a potent proliferative response to low levels of IL-2 in vitro. We show that, similarly to blood CD56bright NK cells, SF NK cells express high levels of CD25 and respond to IL-2 stimulation by producing both IFN-γ and TNF. The presence of this T-cell-derived cytokine in SF is disputed, but it could be a limiting factor for the regulation of NK-cell cytokine production. A recent study addressing this question27 found IL-2 in SF from patients with newly diagnosed synovitis that later progressed to rheumatoid arthritis, but not in fluids from patients with established disease. IL-2-dependent NK-cell activation could play a role in the early disease stages, whereas activation via macrophage-derived cytokines would be dominant in later stages. According to such a model, NK cells could participate in the perpetuation of inflammation throughout disease progression, but by different mechanisms in early compared to late disease.
As to the role of CD94/NKG2A, the inhibitory receptor recognizing HLA-E, we provide evidence that this receptor regulates synovial NK-cell effector functions, including IFN-γ and TNF synthesis. Antibody masking of CD94/NKG2A in coculture experiments with the NK-cell stimulating K562 tumour cell transfectants resulted in enhanced SF NK-cell cytokine production. Conversely, providing protective HLA-E binding peptides in coculture experiments to enhance CD94/NKG2A receptor ligation resulted in a decrease in SF NK-cell cytokine production, which was dependent on the peptide dose.
In the experiments demonstrating IFN-γ production by NK cells after LPS stimulation of the whole SF leucocyte population, there was also a markedly enhanced IFN-γ response by blockade of the CD94/NKG2A receptor. Since NK cells cannot respond to LPS directly, our interpretation is that LPS-stimulated macrophages or dendritic cells induce cytokine secretion by NK cells. The strong regulation by blockade of CD94/NKG2A on the NK cells indicates that the process is regulated through a cognate interaction, perhaps involving targeted release of cytokines to the NK cell. It is becoming increasingly evident that not only soluble factors but also cell-contact-mediated pathways between cytokine-activated lymphocytes and macrophages play important roles in the disease. For instance, enriched synovial T lymphocytes, especially when stimulated in vitro with IL-15, can enhance the synovial TNF synthesis.6 Similarly, freshly isolated synovial NK cells can effectively trigger TNF synthesis by macrophages in the absence of added cytokines. This response can be further amplified in the presence of monokines (IL-12, IL-15, and IL-18).8 Thus, both cell-contact-dependent and cell-contact-independent pathways may be involved in the activation of synovial NK cells in the inflamed synovium. It should be noted that freshly isolated SF NK cells do not contain detectable levels of intracellular IFN-γ or TNF, suggesting that cytokines present in the SF do not effectively induce cytokine responses by SF NK cells, or alternatively, that inhibitory cognate interactions or soluble factors in the fluid actively suppress cytokine production by synovial NK cells. It is also possible that levels induced in vivo are too low to detect.
Thus HLA-E molecules in the synovium or on SF cells may play an important role in regulating NK-cell functions, which may not necessarily depend on altered expression levels of this non-polymorphic MHC molecule. We have previously demonstrated that hsp60 signal peptides can bind to HLA-E, although these complexes do not allow binding to and inhibition via NKG2A on blood NK cells. We demonstrate the same specificity for synovial NK cells. The levels of hsp60 increase during cellular stress, which also redistributes the molecule from the mitochondria. The hsp60 is up-regulated in the synovial inflammation28 and if sufficient amounts of hsp60 signal peptides gain access to HLA-E molecules, one may speculate that an altered balance in the proportion of HLA-E molecules containing hsp60 signal peptides versus HLA signal peptides could influence NK-cell inhibition locally. Further research on this concept would require a direct demonstration of HLA-E expression in inflamed tissue and of whether a significant proportion of HLA-E molecules from cells in the synovium (or any other inflammatory site) actually contain hsp60 peptides.
Are there any implications of these findings for understanding and developing treatment modalities in arthritis? TNF is a major cytokine involved in the pathogenesis of rheumatoid arthritis. To develop novel effective therapies targeting pathways upstream of macrophage TNF synthesis, it is of critical importance to fully understand the mechanisms behind the inappropriate synthesis of this cytokine during chronic inflammation. It is known that the synthesis of TNF is dynamically influenced by multiple locally produced cytokines, of which IFN-γ and TNF itself, two major NK-cell-derived cytokines, are potent inducers of macrophage activation and TNF synthesis. We found that synovial NK-cell subsets are capable of producing TNF and IFN-γ when stimulated in vitro with exogenous monokines (IL-12, IL-15 and IL-18), known to be present in the synovium of RA patients. Therefore, NK-cell cytokine release may be involved in inducing or maintaining inflammation in the synovium. However, it should be stressed that our findings do no permit any conclusion in this respect; the SF NK cells could be disease-promoting, neutral bystanders, or may even protect against inflammation. What we can conclude is that if they have a role, it is likely that one may affect this by manipulation of the CD94/NKG2A receptor or its ligands.
Acknowledgments
We thank Professor Lars Klareskog and Rheumatology Unit at the Karolinska University hospital for continuous support and help with clinical samples. This work was supported by grants from the Swedish Medical Research Council (M.F.R.), Gustav V-80 års fond, Karolinska Institutet and the Swedish Cancer Society. C.T.M. is supported by a PhD fellowship from Fundação para a Ciência e a Tecnologia (PRAXIS XXI/BD/4047/94).
Abbreviations:
- β2m
β2-microglobulin
- HLA
human leucocyte antigen
- hsp
heat-shock protein
- IFN-γ
interferon-γ
- IgG
immunoglobulin G
- IL
interleukin
- ILT
immunoglobulin-like transcripts
- KIR
killer cell immunoglobulin-like receptors
- LIR
leucocyte immunoglobulin-like receptors
- LPS
lipopolysaccharide
- MFI
geometric mean fluorescence intensity
- MHC
major histocompatibility complex
- NCRs
natural cytotoxicity receptors
- NK cells
natural killer cells
- PB
peripheral blood
- PBMC
peripheral blood mononuclear cells
- PE
phycoerythrin
- SF
synovial fluid
- SFMC
synovial fluid mononuclear cells
- TNF
tumour necrosis factor
References
- 1.Firestein GS, Zvaifler NJ. How important are T cells in chronic rheumatoid synovitis? II. T cell-independent mechanisms from beginning to end. Arthritis Rheum. 2002;46:298–308. doi: 10.1002/art.502. [DOI] [PubMed] [Google Scholar]
- 2.Edwards JC, Leandro MJ, Cambridge G. B lymphocyte depletion therapy with rituximab in rheumatoid arthritis. Rheum Dis Clin North Am. 2004;30:393–403. doi: 10.1016/j.rdc.2004.01.006. viii. [DOI] [PubMed] [Google Scholar]
- 3.Malmstrom V, Trollmo C, Klareskog L. The additive role of innate and adaptive immunity in the development of arthritis. Am J Med Sci. 2004;327:196–201. doi: 10.1097/00000441-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 5.Muller-Ladner U, Gay RE, Gay S. Activation of synoviocytes. Curr Opin Rheumatol. 2000;12:186–94. doi: 10.1097/00002281-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Dalbeth N, Callan MF. A subset of natural killer cells is greatly expanded within inflamed joints. Arthritis Rheum. 2002;46:1763–72. doi: 10.1002/art.10410. [DOI] [PubMed] [Google Scholar]
- 7.Pridgeon C, Lennon GP, Pazmany L, Thompson RN, Christmas SE, Moots RJ. Natural killer cells in the synovial fluid of rheumatoid arthritis patients exhibit a CD56bright, CD94bright, CD158negative phenotype. Rheumatology (Oxford) 2003;42:870–8. doi: 10.1093/rheumatology/keg240. [DOI] [PubMed] [Google Scholar]
- 8.Dalbeth N, Gundle R, Davies RJ, Lee YC, McMichael AJ, Callan MF. CD56bright NK cells are enriched at inflammatory sites and can engage with monocytes in a reciprocal program of activation. J Immunol. 2004;173:6418–26. doi: 10.4049/jimmunol.173.10.6418. [DOI] [PubMed] [Google Scholar]
- 9.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs R, Hintzen G, Kemper A, Beul K, Kempf S, Behrens G, Sykora KW, Schmidt RE. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur J Immunol. 2001;31:3121–7. doi: 10.1002/1521-4141(2001010)31:10<3121::aid-immu3121>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Vitale M, Della Chiesa M, Carlomagno S, Romagnani C, Thiel A, Moretta L, Moretta A. The small subset of CD56bright CD16– natural killer cells is selectively responsible for both cell proliferation and interferon-gamma production upon interaction with dendritic cells. Eur J Immunol. 2004;34:1715–22. doi: 10.1002/eji.200425100. [DOI] [PubMed] [Google Scholar]
- 12.Kim CH, Pelus LM, Appelbaum E, Johanson K, Anzai N, Broxmeyer HE. CCR7 ligands, SLC/6Ckine/Exodus2/TCA4 and CKbeta-11/MIP-3beta/ELC, are chemoattractants for CD56(+) CD16(–) NK cells and late stage lymphoid progenitors. Cell Immunol. 1999;193:226–35. doi: 10.1006/cimm.1999.1483. [DOI] [PubMed] [Google Scholar]
- 13.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2. A potential new link between adaptive and innate immunity. Blood. 2003;101:3052–7. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 14.Ferlazzo G, Thomas D, Lin SL, Goodman K, Morandi B, Muller WA, Moretta A, Munz C. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol. 2004;172:1455–62. doi: 10.4049/jimmunol.172.3.1455. [DOI] [PubMed] [Google Scholar]
- 15.Schierloh P, Yokobori N, Aleman M, et al. Increased susceptibility to apoptosis of CD56dimCD16+ NK cells induces the enrichment of IFN-gamma-producing CD56bright cells in tuberculous pleurisy. J Immunol. 2005;175:6852–60. doi: 10.4049/jimmunol.175.10.6852. [DOI] [PubMed] [Google Scholar]
- 16.Katchar K, Soderstrom K, Wahlstrom J, Eklund A, Grunewald J. Characterisation of natural killer cells and CD56+ T-cells in sarcoidosis patients. Eur Respir J. 2005;26:77–85. doi: 10.1183/09031936.05.00030805. [DOI] [PubMed] [Google Scholar]
- 17.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 18.Braud VM, Allan DS, O'Callaghan CA, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391(6669):795–9. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 19.Lanier LL, Corliss B, Wu J, Phillips JH. Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity. 1998;8:693–701. doi: 10.1016/s1074-7613(00)80574-9. [DOI] [PubMed] [Google Scholar]
- 20.De Maria A, Fogli M, Costa P, Murdaca G, Puppo F, Mavilio D, Moretta A, Moretta L. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44) Eur J Immunol. 2003;33:2410–8. doi: 10.1002/eji.200324141. [DOI] [PubMed] [Google Scholar]
- 21.Hendrich C, Kuipers JG, Kolanus W, Hammer M, Schmidt RE. Activation of CD16+ effector cells by rheumatoid factor complex. Role of natural killer cells in rheumatoid arthritis. Arthritis Rheum. 1991;34:423–31. doi: 10.1002/art.1780340407. [DOI] [PubMed] [Google Scholar]
- 22.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells. A unique innate immunoregulatory role for the CD56 (bright) subset. Blood. 2001;97:3146–51. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 23.Michaelsson J, Teixeira de Matos C, Achour A, Lanier LL, Karre K, Soderstrom K. A signal peptide derived from hsp60 binds HLA-E and interferes with CD94/NKG2A recognition. J Exp Med. 2002;196:1403–14. doi: 10.1084/jem.20020797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferlazzo G, Pack M, Thomas D, et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci USA. 2004;101:16606–11. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagler A, Lanier LL, Cwirla S, Phillips JH. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989;143:3183–91. [PubMed] [Google Scholar]
- 26.Nagler A, Lanier LL, Phillips JH. Constitutive expression of high affinity interleukin 2 receptors on human CD16-natural killer cells in vivo. J Exp Med. 1990;171:1527–33. doi: 10.1084/jem.171.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raza K, Falciani F, Curnow SJ, et al. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res Ther. 2005;7:R784–95. doi: 10.1186/ar1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karlsson-Parra A, Soderstrom K, Ferm M, Ivanyi J, Kiessling R, Klareskog L. Presence of human 65 kD heat shock protein (hsp) in inflamed joints and subcutaneous nodules of RA patients. Scand J Immunol. 1990;31:283–8. doi: 10.1111/j.1365-3083.1990.tb02770.x. [DOI] [PubMed] [Google Scholar]