Abstract
Salmonella enterica serovar typhimurium (S. typhimurium) is an intracellular pathogen that causes macrophage cell death by at least two different mechanisms. Rapid cell death is dependent on the Salmonella pathogenicity island-1 protein SipB whereas delayed cell death is independent of SipB and occurs 18–24 hr post infection. Lipopolysaccharide (LPS) is essential for the delayed cell death. LPS is the main structural component of the outer membrane of Gram-negative bacteria and is recognized by Toll-like receptor 4, signalling via the adapter proteins Mal, MyD88, Tram and Trif. Here we show that S. typhimurium induces SipB-independent cell death through Toll-like receptor 4 signalling via the adapter proteins Tram and Trif. In contrast to wild type bone marrow derived macrophages (BMDM), Tram–/– and Trif–/– BMDM proliferate in response to Salmonella infection.
Keywords: monocyte/macrophages, bacterial infection, Toll-like receptor, cell death
Introduction
Salmonella can survive, grow and eventually trigger cell death in macrophages.1–4 At least two mechanisms of Salmonella-induced cell death have been identified depending on the growth phase of the bacterium when it infects the cell. When macrophages are infected with Salmonella grown to late-logarithmic phase, cell death occurs within 2 hr of infection. This rapid induction of cell death is dependent on the Salmonella Pathogenicity Island-1 (SPI-1)-encoded protein SipB1,4 and requires caspase-1 activity.5,6 SPI-1 mutants of S. typhimurium are attenuated in oral infection of mice, but not in systemic mouse typhoid infection.7 Caspase-1 is a serine protease that cleaves pro-interleukin (IL)-1β into active IL-1β. Although activation of caspase-1 is required for early macrophage cell death the underlying mechanisms are unclear. IL-1β does not kill cells and macrophages are not killed by classic apoptosis in response to Salmonella infection.8 Recent oral infection studies in caspase-1–/– mice show that these animals are more susceptible to infection with S. typhimurium9,10 and when infected by the intraperitoneal route show a higher organ bacterial load.9Salmonella can also kill macrophages independently of SipB 18–24 hr after infection.3,11,12 The mechanisms underlying the delayed cell death are unclear; however, caspase-1, the type III secretion system (TTSS) encoded by SPI2 (essential for intracellular bacterial survival) and pathogen-associated molecular pattern (PAMP) receptors (PRRs) may all play important roles.13–15
Salmonella has several PAMPs, such as lipopolysaccharide (LPS), lipoproteins and flagellin, which are recognised by the Toll-like receptors (TLRs) TLR4, TLR2 (in heterodimers with either TLR1 or TLR6) and TLR5, respectively.16 TLR4 signals through the recruitment of several adapter proteins (including MyD88, Mal, Tram and Trif) to the cytoplasmic TLR/IL-1 receptor (TIR) domains to activate signalling pathways within the cell. MyD88-dependent signalling through TLR2 and TLR4 specifically requires the association of the adapter protein, Mal, with MyD88.17 The adapter proteins Trif and Tram mediate MyD88-independent signalling, which leads to the activation of IRF3.17
Cell death can be triggered by TLR ligands such as lipoproteins, dsRNA and LPS.14,18–20 Many bacterial pathogens have developed strategies to exploit TLR signalling and interfere with the immune response of the host;21 hence TLR signalling could also play a role in Salmonella-induced killing of macrophages.22 Hsu et al.14 showed that Salmonella-induced SipB-independent cell death requires TLR4 stimulation of the dsRNA responsive protein kinase PKR and postulating that this may be Trif dependent. It is, however, unclear how TLRs influence Salmonella-induced delayed cell death and whether SipB contributes to stimulation of TLR-dependent activation of caspase-1. Here we show that TLR4, through activation of the Trif/Tram signalling pathway, is required for SipB-independent Salmonella-induced cell death. SipB-independent cell death is accompanied by a caspase-1 dependent, but largely TLR4-independent increase in IL-1β production at 24 hr. The SipB-independent production of IL-1β requires signalling through MyD88 and Mal, but is also critically dependent on Trif.
Materials and methods
Mice
Mice were bred under specific-pathogen free conditions. TLR4–/–,23 MyD88–/–,24 Mal–/–,25 Trif–/–26 and Tram–/–27 C57/BL6 mice are described elsewhere. TLR4–/–, MyD88–/– and their respective wild type mice were bred at University of Cambridge, while Mal–/–, Tram–/–, Trif–/– and their respective wild type mice were bred at The University of Massachusetts Medical School, Worcester, MA, USA. We routinely genotype our strains of knock-out mice.
Bacteria and preparation of LPS
S. typhimurium strain C528 and a congenic sipB mutant (gift from Dr A. Khan, University of Newcastle) were used for in vitro bacterial studies. S. typhimurium was prepared by diluting 1 : 10 an overnight culture in fresh Luria broth (LB) and incubating for a further 2 hr then washing the bacteria in LB broth and diluting as required in tissue culture medium.29
Cell culture and infection with S. typhimurium
Primary bone-marrow derived macrophages (BMDM) were isolated from the femurs and tibia of mice killed by cervical dislocation.29,30,31 The femurs and tibias from Mal–/–, Trif–/–, Tram–/– and their respective wild type mice were stored at 4° in tissue culture medium to allow transport from The University of Massachusetts Medical School to Cambridge (36–48 hr). Briefly, the bone marrow was flushed out with medium (RPMI + 10% fetal calf serum supplemented with 2 mm glutamine, 5% horse serum, 1 mm sodium pyruvate) and the macrophages were seeded into Petri dishes. For maintenance of the bone marrow macrophages in culture the RPMI medium was supplemented with 20% of supernatant taken from L929 cells (a murine granulocyte–macrophage colony-stimulating factor-producing cell line; BMDM medium). For experiments cells were plated onto six-well, or 96-well plates at a plating density of 2 × 106 or 2 × 105 per well, respectively.
S. typhimurium was added to the cells at the multiplicities of infection (MOI) of 10 or 30. Following a 2 hr incubation, the cells were incubated in BMDM medium containing 50 µg/ml gentamicin for 1 hr. Cells were then further incubated in BMDM medium containing 10 µg/ml gentamicin until 24 hr postinfection.
In experiments with the caspase-1 specific inhibitor Ac-YVAD-cmk (100 µm, Bachem, St Helens, UK), the inhibitor or the equivalent amount of vehicle control (dimethyl sulphoxide; DMSO, 1/500 diluted) was added to BMDM 1 hr prior to infection with S. typhimurium.
Measurement of IL-1β activity
To determine cumulative IL-1β production, supernatants were taken at 2 hr or 24 hr postinfection (cumulative 3–24 hr postinfection) and stored at −80° until analysed with the Duoset® enzyme-linked immunosorbent assay development system (R & D systems, Abingdon, UK). The materials were all diluted and stored according to the manufacturer's instructions. A seven-point standard curve of twofold dilutions from 1000 pg/ml to 15·625 pg/ml of recombinant mouse IL-1β was used. A volume of 100 µl of the standards and samples of the appropriate dilution were added to a 96-well microtitre plate.
Measurement of cell viability
Cell viability was determined at 2 hr and 24 hr postinfection using the Cytotox 96 Non-Radioactive Cytotoxicity Assay (Promega, Southampton, UK) according to the manufacturer's instructions.
Gentamicin protection assay
BMDM were infected with S. typhimurium at MOI = 10 or 30. After 2 hr incubation, the medium was changed to BMDM medium containing 50 µg/ml gentamicin for 1 hr. This was followed by two washes with warm phosphate-buffered saline (PBS) and lysis of cells using 0·5% (v/v) Triton-X-100/PBS for 20 min on ice. Serial dilutions were plated on LB agar.
Statistical analysis
For statistical analysis a two-way anova was performed using S-Plus software (Insight, Sheffield, UK) or Prism (GraphPad Inc, San Diego, CA). Data was considered significant when P < 0·05.
Results
SipB-dependent cell death and IL-1β production in response to S. typhimurium infection is caspase-1 dependent
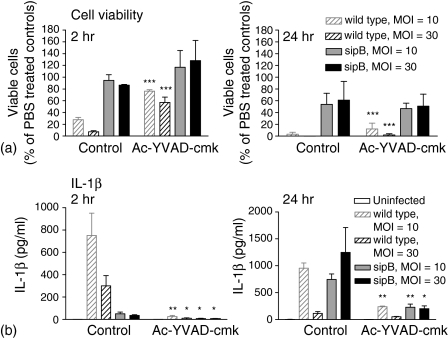
Infection of macrophages with Salmonella leads to cell death via at least two different mechanisms, a SipB-caspase-1-dependent early cell death and SipB-independent late cell death. For our experiments we used BMDM 14 days after isolation. BMDM culture extended beyond 14 days resulted in reduced bacterial uptake and hence less bacteria per macrophage were found (data not shown). Using the caspase-1 inhibitor Ac-YVAD-cmk macrophages were, as expected, protected against the early cell death induced by S. typhimurium, whilst in the absence of Ac-YVAD-cmk 75–85% of the macrophages were killed in an MOI-dependent manner (Fig. 1a, 2 hr). Inhibition of caspase-1 did not protect against the later, SipB-independent Salmonella induced cell death, where 40–50% cell death was seen in both the inhibitor treated and control group (Fig. 1a and 24 hr). There is a significant late SipB-dependent caspase-1-independent driven cell death at 24 hr post infection (Fig. 1a). IL-1β production in response to Salmonella infection is, as expected, largely caspase-1 dependent in the presence or absence of SipB (Fig. 1b).
Figure 1.
IL-1β production in response to S. typhimurium is caspase-1 dependent. Cell viability and IL-1β production after infection of macrophages from wild type mice with S. typhimurium. BMDM were preincubated with the caspase-1 specific inhibitor Ac-YVAD-cmk (100 µm) or the equivalent amount of vehicle control (DMSO) and were infected for 2 hr or 24 hr with S. typhimurium (MOI = 10 and 30) as described in the materials and methods section (n = the number of animals used to provide cells from each knock-out strain). (a) The remaining cells were lysed and assayed for cell viability (n = 4). (b) Media was removed and assayed for IL-1β production (n = 4). Mean values (± standard error of the mean) are shown, significant differences between inhibitor treated and vehicle control treated BMDM are indicated (*P < 0·05, **P < 0·01, ***P < 0·001).
Late SipB-independent cell death is TLR4 dependent via the Trif/Tram pathway
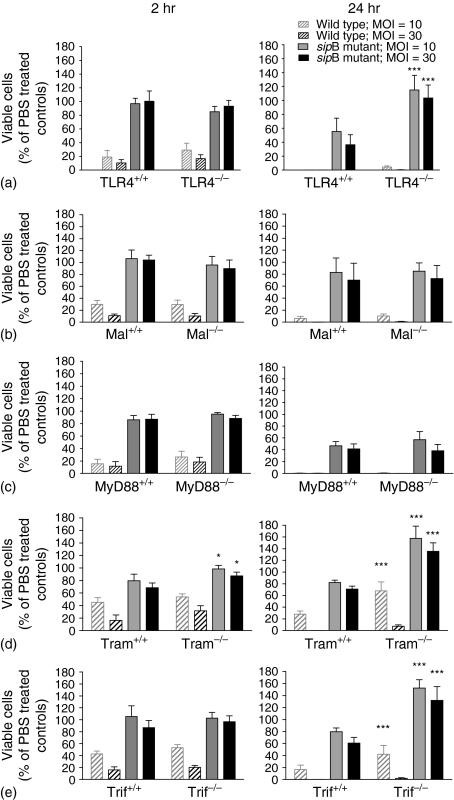
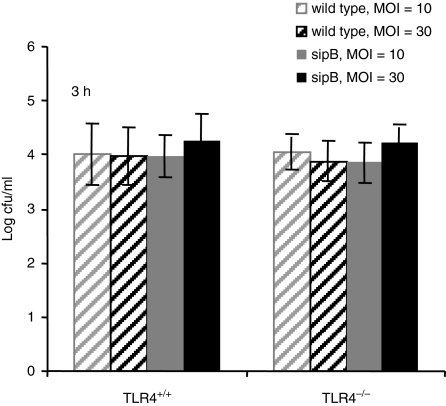
Early SipB-dependent, caspase-1-dependent Salmonella-induced cell death has been studied intensively1,4–6 but SipB-independent cell death is less well understood. LPS activation of TLR4 is critical for initiating a protective immune response to S. typhimurium.32,33 We therefore investigated whether TLR4 also plays a role in Salmonella induced cell death. To analyse this, we infected TLR4+/+ and TLR4–/– BMDM with S. typhimurium or its congenic sipB mutant. Salmonella induced cell death was similar at 2 hr post infection in both wild type and TLR4–/– macrophages. As expected, the early cell death was SipB dependent in all cell types. The sipB mutant strain of S. typhimurium did not kill BMDM at 2 hr post infection while the wild type Salmonella killed 80–90% of the cells in a MOI-dependent manner (Fig. 3a). At 24 hr postinfection, the wild type Salmonella strain killed more than 95% of both cell types. In contrast the sipB mutant strain killed 50–70% of the TLR4+/+ BMDM while little or no cell death was observed in the TLR4–/– BMDM (Fig. 3a). This difference in cell survival is not caused by differences in the initial uptake of Salmonella into the cells, because a gentamicin protection assay at 2 hr post infection showed similar numbers of intracellular Salmonella in TLR4+/+ and TLR4–/– cells (Fig. 2). These results demonstrate a role for TLR4 in SipB-independent Salmonella induced delayed cell death.
Figure 3.
Late Salmonella-induced cell death is TLR4 dependent via the Trif/Tram signalling pathway. Cell viability of TLR4–/–, Mal–/–, MyD88–/–, Tram–/–, Trif–/– and their respective wild type BMDM after infection with S. typhimurium for 2 hr or 24 hr. S. typhimurium C5 and the congenic sipB mutant were used at MOI = 10 and 30. The remaining cells were lysed and assayed for cell viability (n = the number of animals used to provide cells from each knock-out strain). (a) TLR4–/– BMDM, n = 4; (b) Mal–/– BMDM, n = 4; (c) MyD88–/– BMDM, n = 6; (d) Tram–/– BMDM, n = 3; (e) Trif–/– BMDM, n = 6. Cell viability is expressed as percentage viable cells compared to PBS treated control cells. Mean values (± standard error of the mean) are shown. Significant differences between BMDM from knock-out mice and their respective controls are indicated (*P < 0·05, ***P < 0·001).
Figure 2.
TLR4+/+ and TLR4–/– BMDM have similar bacterial counts in the early stages of infection. TLR4+/+ and TKR4–/– BMDMs were infected with S. typhimurium C5 wild-type and congenic sipB mutant at a MOI of 10 and 30. Bacterial numbers 3 hr post infection were detected using the gentamicin protection assay. Mean values (± standard error of the mean) of three experiments are shown.
Downstream of TLR4 the signalling pathway bifurcates at the level of the adapter proteins and is either Mal/MyD88 or Tram/Trif dependent. We have established that TLR4 is involved in SipB-independent Salmonella-induced cell death therefore we investigated which adapter proteins were required for this process. Mal–/– or MyD88–/– BMDM and their respective wild type BMDM were infected with S. typhimurium or the sipB mutant. At 2 hr and 24 hr there was no difference in cell survival in either MyD88–/– or Mal–/– BMDM compared to wild type macrophages (Fig. 3b, c). This shows that TLR4-dependent Salmonella induced cell death does not require signalling through Mal or MyD88. We next investigated whether Tram/Trif signalling is required for S. typhimurium-induced BMDM cell death. Tram–/– and Trif–/– BMDM were as susceptible as their respective wild type BMDM to early SipB-dependent cell death. Tram–/– and Trif–/– BMDM, in contrast, were protected against SipB-independent Salmonella-induced cell death (Fig. 3d, e). Tram+/+ BMDM showed cytotoxicity of 20% or 35% after infection with the S. typhimurium sipB mutant at an MOI = 10 or 30, respectively, whereas in Tram–/– BMDM no cell death was observed at either MOI (Fig. 3d). Similar results were observed in Trif–/– BMDM (no cytotoxicity) in comparison with Trif+/+ BMDM (20–40% cytotoxicity) after infection with the sipB mutant S. typhimurium (Fig. 3e). Both Tram–/– and Trif–/– BMDM also showed some resistance, in comparison to Tram+/+ and Trif+/+ BMDM, to delayed cell death induced by wild type S. typhimurium. Tram–/– and Trif–/– BMDM but not TLR4–/– BMDM were not only resistant to cell death, but proliferated during infection with S typhimurium SipB (Fig. 3d, e). In Tram–/– BMDM significantly higher numbers of viable cells were observed already at 2 hr post infection with Salmonella (Fig. 3d). These data show that SipB-independent Salmonella induced cell death is TLR4 dependent via the Tram/Trif pathway.
IL-1β production in response to S. typhimurium infection requires TLR4 adapter proteins
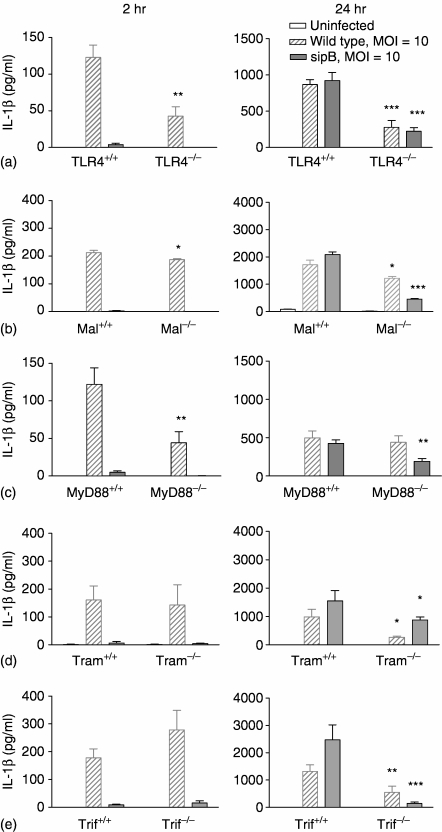
We assayed the production of IL-1β as a crude measure of caspase-1 activation in order to determine the effect of S. typhimurium on the activity of this protein. Different levels of IL-1β production were obtained from BMDM isolated from samples bred in The University of Massachusetts Medical School, and shipped for 36–48 hr (Mal–/–, Tram–/– and Trif–/–) in comparison to those from mice bred in the UK and the scale in the graphs were adjusted accordingly (Fig. 4b, d, e).
Figure 4.
IL-1β production in response to Salmonella infection requires TLR4 adapter proteins. IL-1β production from TLR4–/–, Mal–/–, MyD88–/–, Tram–/–, Trif–/– and their respective wild type BMDM after infection with S. typhimurium. S. typhimurium C5 and the congenic sipB mutant were used at MOI = 10 and 30. Media was removed at 2 hr and 24 hr and assayed for IL-1β production (n = the number of animals used to provide cells from each knock-out strain). (a) TLR4–/– BMDM, n = 4; (b) Mal–/– BMDM, n = 4; (c) MyD88–/– BMDM, n = 6; (d) Tram–/– BMDM, n = 3; (e) Trif–/– BMDM, n = 6. The mean IL-1β concentrations (value+-standard error of the mean) relative to the PBS treated control samples and are shown and significant differences between BMDM from knock-out mice and their respective controls are indicated (*P < 0·05, **P < 0·01, ***P < 0·001). Different levels of IL-1β production were obtained from BMDM isolated from samples bred in The University of Massachusetts Medical School and shipped for 36–48 hr and the scales in the graphs were adjusted accordingly (b, d, e).
At 2 hr post infection, IL-1β release and, hence, caspase-1 activation is largely, but not exclusively, SipB dependent in wild type and knock-out BMDM (Fig. 4). TLR4 contributes to early, SipB-dependent IL-1β release such that at an MOI of 10 TLR4–/– BMDM produced only 35% of the IL-1β compared to that produced by TLR4+/+ BMDM (Fig. 4a). The adapter proteins MyD88 and, to a lesser extent, Mal also have a role in early, SipB-dependent IL-1β release. At 2 hr postinfection, SipB-dependent IL-1β production in BMDM from MyD88–/– BMDM was reduced to 36% compared to MyD88+/+ BMDM (Fig. 4c). In contrast in BMDM from Mal–/– mice IL-1β production was only reduced by 10% (Fig. 4b), suggesting different roles for Mal and MyD88 in early, TLR4-dependent SipB-dependent IL-1β release. There is no difference in early SipB dependent IL-1β release in Tram–/– or Trif–/– BMDM compared to their respective wild type BMDM (Fig. 4d). This suggests that TLR4 contributes mainly via MyD88-dependent signalling pathway to the early SipB-dependent IL-1β release.
At 24 hr postinfection TLR4 and the adapter proteins Mal, Tram and Trif but not MyD88 contribute to SipB dependent IL-1β release (Fig. 4b, d, e). The SipB independent late IL-1β release at 24 hr postinfection is partially dependent on TLR4, being reduced to 24% of the wild type control BMDM IL-1β release (Fig. 4a). Both the Mal/MyD88 and the Tram/Trif pathways are involved. In the absence of either Mal, MyD88 or Tram SipB independent late IL-1β release is reduced to similar levels to that seen in TLR4–/– BMDM. In Trif–/– BMDM SipB-independent late IL-1β release is almost completely abolished (Fig. 4). This indicates a bifurcation in the Tram/Trif signalling pathway downstream of TLR4 in response to Salmonella infection.
Discussion
Here we show for the first time that signalling through TLR4 via the Tram/Trif pathway is important for SipB-independent Salmonella induced cell death in macrophages. Our data confirm that TLR4 is not necessary for early Salmonella induced SipB-dependent cell death. Our TLR4 dependent, SipB-independent Salmonella-induced cell death is consistent with the paper by Hsu et al.14 showing that in BMDM from C3H/HeJ mice (deficient in TLR4 activity) SipB-independent Salmonella-induced cell death was markedly reduced in comparison to BMDM from C3H/HeN mice (wild type TLR4). Weiss et al.22 in contrast, detected neither delayed Salmonella-induced cell death at 24 hr nor any differences in macrophage survival between TLR4+/+ and TLR4–/– BMDM. There are several experimental differences in our study to that of Weiss et al.22 including differences in Salmonella strains and bacterial growth protocols. Strain differences can alter the levels of LPS produced by the bacteria whilst the stage of bacterial growth can change the bacterial proteins that are expressed and is also known to influence the results of in vitro studies on S. typhimurium-induced cell death.34 Our data show that the delayed SipB-independent Salmonella-induced cell death is critically dependent on the Tram/Trif pathway. These data are also consistent with the observations of Hsu et al.14 where they show that bacterial-induced macrophage death is dependent on the protein kinase PKR, a kinase downstream of the TLR4/Tram/Trif signalling pathway. In conclusion, SipB-independent Salmonella induced cell death requires TLR4 signalling via the Tram/Trif pathway, probably via activation of PKR, while the MyD88/Mal signalling pathway is dispensable.
In this study we saw that BMDM without either Tram or Trif when infected with Salmonella sipB were not only resistant to cell death, but proliferated during the infection. Activation of TLRs has been linked to cellular proliferation.35–37 Recognition of the commensal microflora in the gut by TLRs is required for epithelial homeostasis through proliferation.37 Moreover, TLR signalling is involved in tissue repair after direct epithelial injury.37 TLR ligands, such as LPS, can induce cell cycle entry by overcoming p27 induced cell cycle arrest through a MyD88-dependent pathway36 but only if the action of type I interferon is blocked.36 TLR4 induces the production of interferon-β (IFN-β) through the recruitment of Tram and Trif therefore a possible explanation for the cellular proliferation seen here in Salmonella infected Tram–/– or Trif–/– macrophages is probably due to TLR4 activation without IFN-β production.
Macrophages undergoing Salmonella induced cell death exhibit heterogeneous morphological features indicative of both necrotic and apoptotic cell death.1,4,38,39 Caspase-1 plays an important role in Salmonella-induced cell death. Early, SipB-dependent cell death is largely but not completely caspase-1 dependent1,4 (Fig. 1a). The main biological function of caspase-1 is cytokine processing. The inactive precursors of IL-1β and IL-18 require splicing by caspase-1 to become active.40 Active caspase-1 is part of the inflammasome, an intracellular complex comprising several adapter proteins.41 The adapter protein apoptosis associated speck-like protein containing a CARD (ASC) directly binds caspase-1 and is essential for inflammasome function in response to Salmonella infection.42 Direct interaction of SipB with caspase-1 leads to the activation of caspase-1 as seen in its proteolytic maturation and the processing of its substrate IL-1β.5 Hernandez et al.43 propose that expression of Salmonella SipB leads to macrophage cell death by disrupting mitochondria, thereby inducing autophagy and cell death, although how this links to caspase-1 activity is unclear. Recently, it was shown that Trif, receptor-interacting protein-1 (RIP1) and reactive oxygen species (ROS) production are involved in LPS induced caspase-independent autophagy.44 The molecular pathways activated by caspase-1 that lead to Salmonella-induced cell death are unknown. Experiments using IL-1β–/– BMDM and anti-IL-18 neutralizing antibodies show that IL-1β and IL-18 are not required for rapid or delayed Salmonella-induced macrophage cell death.6 This suggests that caspase-1 has another direct role in response to Salmonella infection apart form cytokine activation, possibly by interacting with SipB or other Salmonella effector proteins.
Active caspase-1 is required for the maturation of the inactive precursor of IL-1β; we therefore used mature IL-1β release by macrophages as an indirect measure of caspase-1 activity. We show that in response to Salmonella infection, TLR4 and MyD88 but to a much lesser extent Mal, contribute to early, SipB-dependent IL-1 β release. While Trif is essential, Tram, Mal and MyD88 only contribute to late, SipB-independent, IL-1β release. Production of IL-1β at 24 hr is likely to be more complex, involving transcription of IL-1β, formation of precursor protein as well as potential autocrine inputs from other cytokines induced by Salmonella infection of BMDM. IL-1β precursor transcription is nuclear factor-κB dependent45 and is highly induced in response to LPS.46,47De novo synthesis of pro-IL-1β in response to LPS and hence also the release of IL-1β, is MyD88 dependent and partially requires Trif.48 Caspase-1 activation by LPS is ASC dependent but independent of the TLR associated MyD88 and Trif pathways.48,49 Caspase-1 also is important in the production of IL-18 in response to S. typhimurium infection and whilst IL-1 is important in enteric infection, it is the caspase-1-induced IL-18 production that probably explains the importance of caspase-1 murine salmonellosis.9
In conclusion we show that TLR4 signalling through Trif is required for Salmonella-induced SipB-independent macrophage cell death. Trif is also essential for SipB-independent IL-1β release, with the other adapter proteins (Mal/MyD88/Tram) contributing to the production of this cytokine. TLR4 signalling through the Tram/Trif signalling pathway is known to affect susceptibility to viral infections and LPS signalling50 but it is unclear how this will influence the host response to S. typhimurium infection. Our identification of Tram/Trif signalling in S. typhimurium-induced macrophage cell death suggest that signalling through Tram and Trif may well play a part in the immune response to salmonellosis.
Acknowledgments
This study was supported by the Biotechnology and Biological Sciences Research Council grant number BB/C005503/1. P.J.C. was supported by a BBSRC studentship. We would like to thank Rob Deardon for help with the statistical analysis.
References
- 1.Chen LM, Kaniga K, Galan JE. Salmonella spp. are cytotoxic for cultured macrophages. Mol Microbiol. 1996;21:1101–15. doi: 10.1046/j.1365-2958.1996.471410.x. [DOI] [PubMed] [Google Scholar]
- 2.Guilloteau LA, Wallis TS, Gautier AV, MacIntyre S, Platt DJ, Lax AJ. The Salmonella virulence plasmid enhances Salmonella-induced lysis of macrophages and influences inflammatory responses. Infect Immun. 1996;64:3385–93. doi: 10.1128/iai.64.8.3385-3393.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindgren SW, Stojiljkovic I, Heffron F. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:4197–201. doi: 10.1073/pnas.93.9.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monack DM, Raupach B, Hromockyj AE, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–8. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA. 1999;96:2396–401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monack DM, Navarre WW, Falkow S. Salmonella-induced macrophage death. the role of caspase-1 in death and inflammation. Microbes Infect. 2001;3:1201–12. doi: 10.1016/s1286-4579(01)01480-0. [DOI] [PubMed] [Google Scholar]
- 7.Baumler AJ, Tsolis RM, Valentine PJ, Ficht TA, Heffron F. Synergistic effect of mutations in invA and lpfC on the ability of Salmonella typhimurium to cause murine typhoid. Infect Immun. 1997;65:2254–9. doi: 10.1128/iai.65.6.2254-2259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guiney DG. The role of host cell death in Salmonella infections. Curr Top Microbiol Immunol. 2005;289:131–50. doi: 10.1007/3-540-27320-4_6. [DOI] [PubMed] [Google Scholar]
- 9.Raupach B, Peuschel SK, Monack DM, Zychlinsky A. Caspase-1-mediated activation of interleukin-1beta (IL-1beta) and IL-18 contributes to innate immune defenses against Salmonella enterica serovar Typhimurium infection. Infect Immun. 2006;74:4922–6. doi: 10.1128/IAI.00417-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lara-Tejero M, Sutterwala FS, Ogura Y, Grant EP, Bertin J, Coyle AJ, Flavell RA, Galan JE. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J Exp Med. 2006;203:1407–12. doi: 10.1084/jem.20060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santos RL, Tsolis RM, Baumler AJ, Smith R. 3rd, Adams LG. Salmonella enterica serovar typhimurium induces cell death in bovine monocyte-derived macrophages by early sipB-dependent and delayed sipB-independent mechanisms. Infect Immun. 2001;69:2293–301. doi: 10.1128/IAI.69.4.2293-2301.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Velden AW, Lindgren SW, Worley MJ, Heffron F. Salmonella pathogenicity island 1-independent induction of apoptosis in infected macrophages by Salmonella enterica serotype typhimurium. Infect Immun. 2000;68:5702–9. doi: 10.1128/iai.68.10.5702-5709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Browne SH, Lesnick ML, Guiney DG. Genetic requirements for salmonella-induced cytopathology in human monocyte-derived macrophages. Infect Immun. 2002;70:7126–35. doi: 10.1128/IAI.70.12.7126-7135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu LC, Park JM, Zhang K, et al. The protein kinase PKR is required for macrophage apoptosis after activation of Toll-like receptor 4. Nature. 2004;428:341–5. doi: 10.1038/nature02405. [DOI] [PubMed] [Google Scholar]
- 15.Libby SJ, Lesnick M, Hasegawa P, Weidenhammer E, Guiney DG. The Salmonella virulence plasmid spv genes are required for cytopathology in human monocyte-derived macrophages. Cell Microbiol. 2000;2:49–58. doi: 10.1046/j.1462-5822.2000.00030.x. [DOI] [PubMed] [Google Scholar]
- 16.Uematsu S, Akira S. Toll-like receptors and innate immunity. J Mol Med. 2006;84:712–25. doi: 10.1007/s00109-006-0084-y. [DOI] [PubMed] [Google Scholar]
- 17.O'Neill LA. How Toll-like receptors signal: what we know and what we don't know. Curr Opin Immunol. 2006;18:3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Aliprantis AO, Yang RB, Mark MR, et al. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–9. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 19.Aliprantis AO, Yang RB, Weiss DS, Godowski P, Zychlinsky A. The apoptotic signaling pathway activated by Toll-like receptor-2. EMBO J. 2000;19:3325–36. doi: 10.1093/emboj/19.13.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barber GN. Host defense, viruses and apoptosis. Cell Death Differ. 2001;8:113–26. doi: 10.1038/sj.cdd.4400823. [DOI] [PubMed] [Google Scholar]
- 21.Ruckdeschel K, Mannel O, Richter K, Jacobi CA, Trulzsch K, Rouot B, Heesemann J. Yersinia outer protein P of Yersinia enterocolitica simultaneously blocks the nuclear factor-kappa B pathway and exploits lipopolysaccharide signaling to trigger apoptosis in macrophages. J Immunol. 2001;166:1823–31. doi: 10.4049/jimmunol.166.3.1823. [DOI] [PubMed] [Google Scholar]
- 22.Weiss DS, Raupach B, Takeda K, Akira S, Zychlinsky A. Toll-like receptors are temporally involved in host defense. J Immunol. 2004;172:4463–9. doi: 10.4049/jimmunol.172.7.4463. [DOI] [PubMed] [Google Scholar]
- 23.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 24.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–22. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto M, Sato S, Hemmi H, et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–9. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto M, Sato S, Hemmi H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–3. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto M, Sato S, Hemmi H, et al. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol. 2003;4:1144–50. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- 28.Hormaeche CE. Natural resistance to Salmonella typhimurium in different inbred mouse strains. Immunology. 1979;37:311–8. [PMC free article] [PubMed] [Google Scholar]
- 29.Royle MC, Totemeyer S, Alldridge LC, Maskell DJ, Bryant CE. Stimulation of Toll-like receptor 4 by lipopolysaccharide during cellular invasion by live Salmonella typhimurium is a critical but not exclusive event leading to macrophage responses. J Immunol. 2003;170:5445–54. doi: 10.4049/jimmunol.170.11.5445. [DOI] [PubMed] [Google Scholar]
- 30.Totemeyer S, Kaiser P, Maskell DJ, Bryant CE. Sublethal infection of C57BL/6 mice with Salmonella enterica Serovar Typhimurium leads to an increase in levels of Toll-like receptor 1 (TLR1), TLR2, and TLR9 mRNA as well as a decrease in levels of TLR6 mRNA in infected organs. Infect Immun. 2005;73:1873–8. doi: 10.1128/IAI.73.3.1873-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yrlid U, Svensson M, Johansson C, Wick MJ. Salmonella infection of bone marrow-derived macrophages and dendritic cells. influence on antigen presentation and initiating an immune response. FEMS Immunol Med Microbiol. 2000;27:313–20. doi: 10.1111/j.1574-695X.2000.tb01445.x. [DOI] [PubMed] [Google Scholar]
- 32.Totemeyer S, Foster N, Kaiser P, Maskell DJ, Bryant CE. Toll-like receptor expression in C3H/HeN and C3H/HeJ mice during Salmonella enterica serovar Typhimurium infection. Infect Immun. 2003;71:6653–7. doi: 10.1128/IAI.71.11.6653-6657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinstein DL, Lissner CR, Swanson RN, O'Brien AD. Macrophage defect and inflammatory cell recruitment dysfunction in Salmonella susceptible C3H/HeJ mice. Cell Immunol. 1986;102:68–77. doi: 10.1016/0008-8749(86)90326-6. [DOI] [PubMed] [Google Scholar]
- 34.Hueffer K, Galan JE. Salmonella-induced macrophage death: multiple mechanisms, different outcomes. Cell Microbiol. 2004;6:1019–25. doi: 10.1111/j.1462-5822.2004.00451.x. [DOI] [PubMed] [Google Scholar]
- 35.Chow EK, O'Connell RM, Schilling S, Wang XF, Fu XY, Cheng G. TLR agonists regulate PDGF-B production and cell proliferation through TGF-beta/type I IFN crosstalk. EMBO J. 2005;24:4071–81. doi: 10.1038/sj.emboj.7600867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasan UA, Trinchieri G, Vlach J. Toll-like receptor signaling stimulates cell cycle entry and progression in fibroblasts. J Biol Chem. 2005;280:20620–7. doi: 10.1074/jbc.M500877200. [DOI] [PubMed] [Google Scholar]
- 37.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Boise LH, Collins CM. Salmonella-induced cell death: apoptosis, necrosis or programmed cell death? Trends Microbiol. 2001;9:64–7. doi: 10.1016/s0966-842x(00)01937-5. [DOI] [PubMed] [Google Scholar]
- 39.Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol. 2000;38:31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- 40.Fantuzzi G, Dinarello CA. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1) J Clin Immunol. 1999;19:1–11. doi: 10.1023/a:1020506300324. [DOI] [PubMed] [Google Scholar]
- 41.Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–74. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Mariathasan S, Newton K, Monack DM, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–8. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 43.Hernandez LD, Pypaert M, Flavell RA, Galan JE. A Salmonella protein causes macrophage cell death by inducing autophagy. J Cell Biol. 2003;163:1123–31. doi: 10.1083/jcb.200309161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu Y, Kim SO, Li Y, Han J. Autophagy contributes to caspase-independent macrophage cell death. J Biol Chem. 2006;281:19179–87. doi: 10.1074/jbc.M513377200. [DOI] [PubMed] [Google Scholar]
- 45.Cogswell JP, Godlevski MM, Wisely GB, Clay WC, Leesnitzer LM, Ways JP, Gray JG. NF-kappa B regulates IL-1 beta transcription through a consensus NF-kappa B binding site and a nonconsensus CRE-like site. J Immunol. 1994;153:712–23. [PubMed] [Google Scholar]
- 46.Fenton MJ, Clark BD, Collins KL, Webb AC, Rich A, Auron PE. Transcriptional regulation of the human prointerleukin 1 beta gene. J Immunol. 1987;138:3972–9. [PubMed] [Google Scholar]
- 47.Turner M, Chantry D, Buchan G, Barrett K, Feldmann M. Regulation of expression of human IL-1 alpha and IL-1 beta genes. J Immunol. 1989;143:3556–61. [PubMed] [Google Scholar]
- 48.Yamamoto M, Yaginuma K, Tsutsui H, et al. ASC is essential for LPS-induced activation of procaspase-1 independently of TLR-associated signal adaptor molecules. Genes Cells. 2004;9:1055–67. doi: 10.1111/j.1365-2443.2004.00789.x. [DOI] [PubMed] [Google Scholar]
- 49.Seki E, Tsutsui H, Nakano H, et al. Lipopolysaccharide-induced IL-18 secretion from murine Kupffer cells independently of myeloid differentiation factor 88 that is critically involved in induction of production of IL-12 and IL-1beta. J Immunol. 2001;166:2651–7. doi: 10.4049/jimmunol.166.4.2651. [DOI] [PubMed] [Google Scholar]
- 50.Hoebe KX, Georgel P, Janssen E, et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424:743–8. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]