Abstract
The ovarian tumour marker MUC16 (CA125) inhibits the cytotoxic responses of human natural killer (NK) cells and down-regulates CD16. Here we show that approximately 10% of the peripheral blood NK cells (PBNK) from the epithelial ovarian cancer (EOC) patients are CD16– CD56br whereas 40% of the peritoneal fluid NK (PFNK) carry this phenotype, which is usually associated with NK cells from the lymph nodes or human decidua. PBNK from healthy donors exposed to PF show a significant increase in the CD16– CD56br population. This shift in phenotype is not caused by increased apoptosis of the CD16+ CD56dim cells or selective proliferation of the CD16– CD56br NK cells. Thus, the terminal differentiation of the CD16– CD56br NK cells to CD16+ CD56dim subset that occurs during normal NK cell development may actually be a reversible step. A majority of the NK cell receptors (NKp46, NKp44, NKG2D, CD244, CD226, CD158a, CD158b, and CD158e) studied were down-regulated in the PFNK. MUC16 binds selectively to 30–40% of CD16+ CD56dim NK cells in EOC patients indicating that phenotypic alterations in these cells are mediated by tumour-derived soluble factors. Similar to EOC, MUC16 in early pregnancy also binds to NK cells suggesting shared mechanisms of NK cell suppression in feto-maternal tolerance and immune evasion by ovarian cancers.
Keywords: natural killer, ovarian cancer, MUC16, CA125, immune suppression
Introduction
Epithelial ovarian cancer (EOC) is the fifth leading cause of cancer related death in women.1 This disease is signified by vague clinical symptoms and no specific diagnostic molecular markers. Most patients are therefore diagnosed at an advanced stage, limiting therapeutic intervention. Restrictions in chemotherapeutic strategies make it necessary to explore other treatment options. Immune therapies against ovarian tumours are being investigated for use in primary or consolidation approaches.2
Natural killer (NK) cells are an important arm of the innate immune response against tumours and virally infected cells. The NK cells can directly cytolyse their targets and can also mediate antibody-dependent cellular cytotoxicity (ADCC) via the Fc receptor CD16. Only limited infiltration of the NK cells is observed within the primary ovarian tumour mass,3,4 however, 4–10% of the immune cells in the peritoneal exudates of EOC are NK cells.5 Paradoxically, increased numbers of peritoneal fluid NK (PFNK) cells have been associated with worse outcomes in EOC patients.6 Effector cells from PF mediate ADCC against target specific tumour cells albeit at significantly reduced levels compared to NK cells from PB of EOC or healthy donors (HD).7 In addition, NK cells associated with ovarian tumours exhibit reduced proliferation in response to interleukin-2 (IL-2).8 These observations suggest that a suppression of PFNK cell function could enhance ovarian tumour progression.
We have recently also demonstrated that MUC16, a mucin that carries the ovarian tumour marker CA125, is a potent inhibitor of NK cell function.9 Thus the ovarian tumours may employ several mechanisms to evade NK cell responses. Clarification of these immune evasion strategies requires studying the interplay between tumour-derived molecular factors and the NK cells.
Based on the expression of CD16 and CD56, two distinct subsets of mature NK cells have been identified in humans.10 The vast majority (>90%) of the NK cells in the human peripheral blood (PB) have a CD16+ CD56dim phenotype whereas NK cells in the secondary lymphoid tissue are CD16– CD56br. The CD16+ CD56dim NK cells have high amounts of intracellular cytolytic granules and are efficient mediators of cytotoxic responses.10 Conversely, the CD16– CD56br NK cells have low cytolytic potential but can express high amounts of cytokines.10
NK cells from ovarian tumour microenvironments show reduced expression of CD16.8 In in vitro experiments, exposure of NK cells derived from PB of HD to physiologically relevant concentrations of MUC16 induced down-regulation of CD16.9 Abundant amounts of MUC16 are present in the PF of EOC patients.11,12 Therefore MUC16 could actively play a role in altering the phenotype of NK cells in EOC patients. Such phenotypic changes in the NK cells of EOC patients may have a profound impact on the success of immunological therapies.
To date, an exhaustive phenotypic analysis of the NK cells from EOC patients has not been reported. Here, we address this issue by comparing the phenotype of the NK cells in the PF and the PB of individual EOC patients. We also contrast our results against the phenotype of NK cells from the PB of healthy individuals. We also provide data suggesting that MUC16 could play an important role in controlling NK cell phenotype and function during pregnancy.
Materials and methods
Sample processing
Blood and ascites samples were obtained from EOC patients (designated PT12, PT14, etc.) recruited at the time of their initial diagnosis. None of the EOC patients had been previously treated for this disease at the time of PB and PF sample collection. The CA125 (MUC16) levels in the blood and PF samples were determined by a clinical assay (Table S1). All patients and HD (designated HD1, HD2, etc.) signed an informed consent and the studies were approved by the Institutional Review Board of University of Wisconsin-Madison. Whole blood and bloody ascites were heparinized and the samples were immediately processed. Peripheral blood mononuclear cells (PBMC) from patients and HD were obtained and isolated under sterile conditions using Histopaque® (Sigma Aldrich, St Louis, MO). The PBMC layer was retained and washed twice with sterile phosphate-buffered saline (PBS) containing 1% bovine serum albumin (PBS-BSA) for 10 min at 300 g before being cryopreserved in 90% fetal bovine serum (FBS; Hyclone, Logan, UT) containing 10% dimethyl sulphoxide.
Cells from the PF were obtained by centrifugation at 500 g for 30 min. The supernatant was removed, and the cell pellet washed two times in PBS-BSA for 10 min at 300 g and then frozen as described above.
Treatment of HD PBMC with PF
Fresh PBMCs from HD were washed twice at 300 g for 10 min with RPMI-1640 (Mediatech, Herndon, VA) containing 10% FBS and 1% antibiotic/antimycotic (Mediatech) (RPMI-FBS). Cells were plated at a density of 1·7 × 106 cells/ml in a six-well plate and maintained either in RPMI-FBS (control) or in 90% sterile filtered PF and 10% FBS with antibiotic/antimycotic (test). After incubation 37° in 5% CO2 environment for 72 hr the cells were washed two times with PBS-BSA, and stained for flow cytometry.
Flow cytometry
For flow cytometric analysis, cryopreserved cells were briefly thawed and washed two times in PBS-BSA by centrifugation at 300 g for 10 min at 4°. Incubations with all the primary and secondary antibodies were performed for 30 min on ice. After incubation with each antibody the cells were washed with PBS-BSA at 300 g for 10 min at 4°. For each replicate, 5 × 105 cells/tube were used. For blocking purposes, cells were first incubated for 15 min with goat antibody before staining with the anti-MUC16 antibody VK8. After washing, fluoroscein isothiocyanate (FITC)-conjugated goat anti-mouse (GAM) IgG, Fc specific antibody (Table S2) at 1 : 100 dilution was then added to the cells. Following washing, the cells were incubated with mouse IgG for 15 min to bind any additional Fab sites on the GAM secondary. The cells were then incubated with a cocktail of directly conjugated antibodies (Table S2) to simultaneously stain for CD3, CD45, CD56, CD16, NKp46, and either, CD158a, CD158b, CD158e, CD226, CD244, NKG2A, NKG2D, or NKp44. After a final wash, cells were resuspended in ∼300 µl of PBS-BSA. Immediately before data acquisition on an LSRII (Beckton Dickinson, San Jose, CA) flow cytometer, the viability indicator DAPI (1 : 300; BD Biosciences, San Jose, CA) was added to each sample. Because of the complexity of compensation with multicolour flow cytometry, automatic compensation was applied. To compare the values of samples acquired on different days, SPHERO™ Rainbow Fluorescent Particles (BD Biosciences) were used to set instrument voltages.
FlowJo software (v. 4.6.1, TreeStar, Ashland, OR) was used for analysis of the raw flow cytometry data, and comparison of the data was done using GraphPad Prism software (v. 4, GraphPad Software, Inc., San Diego, CA). For statistical significance, GraphPad Prism was used to analyse samples using a non-parametric Wilcoxon Signed Rank test or a student t-test with two-sided P-values, where P < 0·05 indicated significance.
Apoptosis and proliferation assays
For apoptosis assays PBMC from three HD were incubated with either media only (control) or with PF (test) from two EOC patients as explained earlier. After incubation for 72 hr the cells were harvested and labelled for 30 min with anti-CD3, CD16, CD45, and CD56 antibodies conjugated to APC-Cy7, PE-Cy7, and PerCP-Cy5.5, and Alexa 700, respectively. Cells were then washed with 1 ml of 1X Annexin V binding buffer from the Annexin V–FITC Apoptosis Detection Kit (BD Pharmingen) for 10 min at 300 g. Cells were then labelled with Annexin V–FITC and DAPI; for 15 min before analysis by flow cytometry. The gating strategy depicted in Fig. S1 was used to monitor apoptosis in the NK cell subsets.
For proliferation assays the freshly isolated PBMC from two HD were initially labelled with 1·0 µm CellTrackerTM Green CMFDA (Molecular Probes, Eugene, OR) for 30 min in PBS at 37°. Labelled cells were washed twice in RPMI-FBS to remove excess dye and then incubated in media only (control) or in PF (test) of two EOC patients. The cells were harvested after culturing for 24, 48, and 72 hr. At each time point the cells were labelled with fluorophore conjugated anti-CD3, CD16, CD45, and CD56 antibodies. The cells were gated according to the strategy shown in Fig. S1. The CellTrackerTM Green labelled cells were analysed to determine distribution of the dye in the daughter cells as a indicator for proliferation.
Reverse transcription–polymerase chain reaction (RT–PCR) protocol
The RNeasy kit (Qiagen, Valencia, CA) was used to isolate mRNA and quantified using standard spectrophotometric protocols. Equal amounts of mRNA (0·5 µg/sample) were used to synthesize cDNA using the Oligo (dT) 15 primer (Promega, Madison, WI) and AMV reverse transcriptase (Promega) according to the manufacturer's protocol. Two primer sets (Set 1: forward, 5′-CGTTAAGGAAAAAAGCGGCCGCBTGTTCAAGAACACCAGTGT-3′; reverse, 5′-GCAACTAGATCTGTCCCTGAAGGACTCTCTC-3′; Set 2: forward, 5′-??GCCTCTACCTTAACGGTTACAATGAA-3′; reverse, 5′-GGTACCCCATGGCTGTTGTG-3′13) were used to monitor MUC16 expression. Both primers were obtained from Integrated DNA Technologies (Coralville, IA). RT–PCR reactions for the first primer set required 4 min at 94°, a pause to add GoTaq (Promega) enzyme, 1 min at 94°, 1 min at 65°, and 2 min at 72°. For the next nine cycles the annealing temperature was decreased 1°/cycle: 1 min at 94° (30 s at 65°−1°/cycle), and 2 min at 72°. For the next 20 cycles, the annealing temperature was kept constant: 1 min at 94°, 1 min at 55°, 2 min at 72°, and a final extension of 15 min at 72°. RT–PCR reactions for primer set 2 were performed as reported in an earlier study.13 Actin was chosen as the housekeeping gene control. The actin primer sequences (Integrated DNA Technologies) were as follows: forward, 5′-GGACTTCGAGCAAGAGATGG-3′; reverse, 5′-AGGAAGGAAGGCTGGAAGAG-3′. Reactions were performed at 4 min at 94°, a pause to add GoTaq enzyme, 1 min at 94°, 2 min at 51°, and 2 min at 72°. This was repeated for 30 cycles. The final extension was for 15 min at 72°. Ten μl of product was visualized on 2% agarose gels.
Results
CD16 and CD56 expression on NK cells from EOC patients
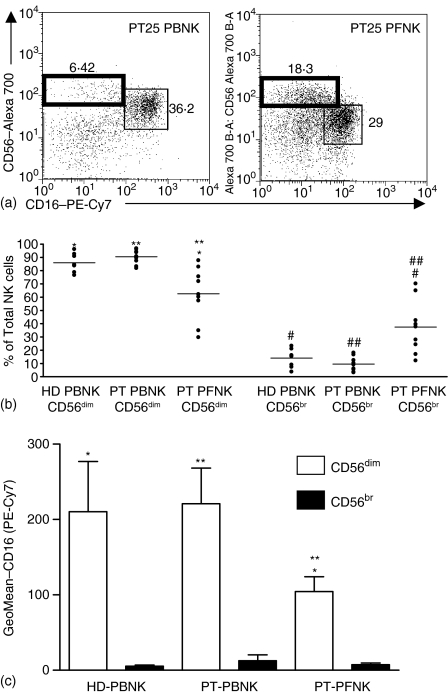
The NK cells constitute between 10 and 15% of the total PBMC in healthy individuals.14 A comparable number of NK cells could be identified in the PB and PF of nine advanced stage EOC patients participating in this study (data not shown). The NK cells in the PB and PF samples were identified among the CD45+ CD3– cells using antibodies directed against CD16 and CD56 (see Fig. S1 for gating strategy). All of our subsequent analysis was conducted on the CD16+ CD56dim and CD16– CD56br NK cell subsets (Fig. 1a). Initially we determined the distribution of these two NK cell subsets in the PB and PF samples of EOC patients and also in the PBMC of HD (Fig. 1b). For each EOC patient or HD the data was collected from eight individual flow cytometry measurements. The average values for all of the patients and HD samples were calculated. Approximately 90% of the PBNK in both the patients and HD were CD16+ CD56dim and 10% were CD16– CD56br. However, in the PF only 60% of the NK were CD16+ CD56dim and 40% were CD16– CD56br (Fig. 1b). The relative expression of CD16 on the CD56dim PFNK cells was significantly lower than the corresponding PBNK cells from EOC patients and HD (Fig. 1c). CD16 expression on the CD56dim subsets of PBNK from EOC patients and HD were comparable.
Figure 1.
Distribution of CD16+ CD56dim and CD16– CD56br NK subsets in the PB and PF of EOC patients. (a) The gating of the CD16– CD56br (bold box) and CD16+ CD56dim (thin box) for PT25 are shown. Similar gates were used for all samples in this study. (b) The numbers of CD16+ CD56dim and CD16– CD56br as a percentage of total NK cells are shown for all eight HD PBNK and nine EOC PB and PFNK samples. The data point for each HD or EOC sample represents a mean of eight independent measurements. (c) Relative expression of CD16 on the HD and EOC NK cells is shown. Statistical significance (P < 0·05, by Wilcoxon Sign Ranked test) is represented by matched symbols.
Activating and inhibitory receptor profile on PB- and PFNK of EOC patients
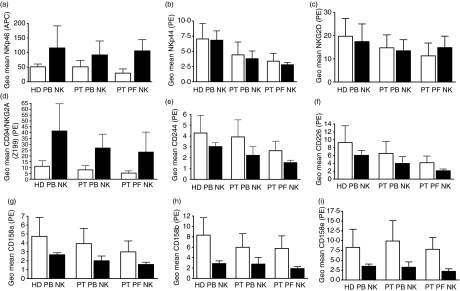
The shift in the CD16 and CD56 expression profile on the PBNK and PFNK prompted us to determine the expression pattern of prominent activating and inhibitory receptors (NKp46, NKp44, NKG2D, CD94/NKG2A, CD244, CD226, CD158a, CD158b, and CD158e) on these cells. The average expression levels for each one of these receptors was compared in the CD16+ CD56dim and the CD16– CD56br subsets of NK cells from the PB of HD and in the PB and PF of EOC patients (Fig. 2a–i). The phenotypic differences between these samples were determined by statistical analysis (Tables S3–S11). PFNK appear to express lower amounts of NK cell receptors than their PT or HD PBNK counterparts. For all receptors, except NKG2D, these differences were either statistically significant or demonstrated a downward trend in expression (Tables S3–S11). No major statistically significant differences in receptor expression were observed between PT and HD PBNK.
Figure 2.
Relative expression of NK cell receptors on the PB and PFNK cells of EOC patients. Expression of NKp46 (a), NKp44 (b), NKG2D (c), CD94/NKG2A (d), CD244 (e), CD226 (f), CD158a (g), CD158b (h), and CD158e (i) on the CD16+ CD56dim (open bars) and CD16– CD56br NK (dark bars) cell subsets are indicated. Each bar shown is an average reading obtained from eight HD or PB and PF samples from nine EOC patients. Complete statistical analysis is provided in Tables S3–+S11.
Phenotypic changes of NK cells are induced by the PF
The immune cells accumulating in the PF are exposed to high concentrations of soluble factors expressed by the ovarian tumours that could influence the expression levels of the various NK cell receptors. Alternatively, because the PF is formed because of the clogging of the lymphatic ducts15 the NK cells could originate from the lymphoid tissues and hence have an altered phenotype as compared to the PBNK. We therefore decided to determine the effect of PF on the expression of CD16, CD56, NKp46, on HD PBNK. NKp46 was especially chosen because it is down-regulated on the PFNK cells and is also known to be expressed primarily on the NK cells.16
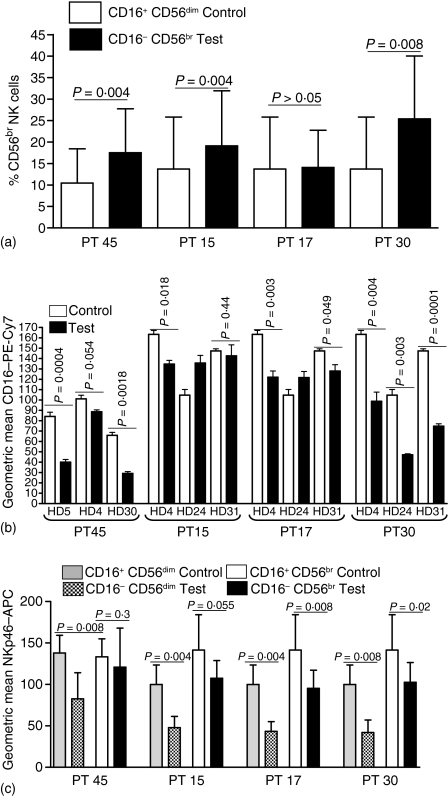
The HD derived PBMCs were incubated in PF from four EOC patients and cultured for 72 hr to at least partly mimic the long-term exposure scenario that the immune cells likely encounter in the peritoneal environment. In these experiments the effect of each PF sample was tested on the PBMC from three individual HD. Expression of CD56, CD16, and NKp46 on each control (cells incubated in media only) and test (cells incubated with PF) sample was measured by flow cytometry. Average measurements of PBMCs from three HD incubated in triplicate with PF from four EOC patients. Cells exposed to PF from PT45, PT15, and PT30 indicated a statistically significant increase in the percent of CD16– CD56br NK cells (Fig. 3a). Conversely, experiments with PF from PT17, demonstrated no difference in the numbers of CD16– CD56br NK cells. This was caused by the fact that cells from HD24 did not show increase in the CD16– CD56br population following treatment with PF from PT17. This was the only combination of samples where an increase in the CD16– CD56br population was not observed. In the same experiment, PBNK from HD4 and HD31 when treated with PF from PT17 did show a statistically significant increase in the CD16– CD56br NK cells. Furthermore, the percentage of CD16– CD56br cells increased when the PBNK for HD24 were treated with PF from PT30. These differences in the data may arise because of the heterogeneous nature of the PF and also because of the potential diversity in the responses of the HD derived PBMC. We have also noted that freshly obtained PF samples induce a more pronounced increase in the CD16– CD56br NK cells as compared to frozen PF samples that were stored in the laboratory for 3 months or more.
Figure 3.
Phenotypic changes in HD derived PBNK following treatment with EOC PF. (a) PBMC were treated with PF from EOC patients (test) or incubated in media only (control) for 72 h in triplicate. Effect of PF from PT45 was tested on PBMC from HD4, HD5, and HD30. PF from PT15, PT17, and PT30 was tested on PBMC from HD4, HD24, and HD31. Flow cytometry was performed and the numbers of CD16– CD56br cells as a percentage of total NK cells were determined. Each bar represents the average of the triplicate data obtained for the three HD PBMC samples (in effect, n = 9). (b) Relative expression of CD16 on the CD16+ CD56dim HD PBNK cells that were treated or untreated with PF is indicated. Each bar is a mean of three independent measurements. (c) Average expression of NKp46 on the CD16+ CD56dim and CD16– CD56br HD PBNK subsets following treatment with PF or media alone is show. The data represents the mean of triplicate measurements for each PF sample in three independent assays. APC, antigen-presenting cell.
As expected, the increase in the CD16– CD56br population was associated with a corresponding decrease in the CD16 expression on the CD16+ CD56dim NK cells (Fig. 3b). Again, only PBNK from HD24 incubated with PF from PT15 and PT17 showed an anomalous response. Finally, we also observed a significant decrease in the expression of NKp46 on both the CD16+ CD56dim and the CD16– CD56br HD PBNK subsets treated with PF (Fig. 3c). In this case the reduction in NKp46 was observed in all of the experiments conducted.
Exposure to PF does not lead to increased proliferation or apoptosis of any of the NK cell subsets
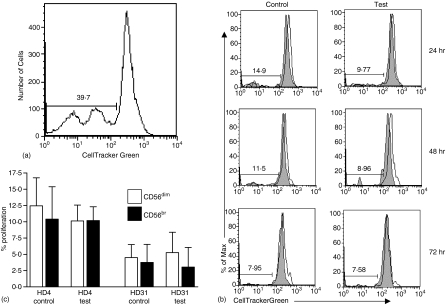
We conducted specific experiments to determine if the phenotypic changes following treatment with PF were caused by increased apoptosis of the CD16+ CD56dim cells or an increased proliferation in the CD16– CD56br cells. PBMC from two HD were freshly isolated and labelled with CellTrackerTM Green. The labelled cells from both donors were incubated in either media only (control) or in PF (test) obtained from three EOC patients. The cells were monitored for proliferation by flow cytometry at the 24, 48, and 72 hr time points. Similar to data shown in Fig. 3(a) we observed an increase in the percentage of the CD16– CD56br cells. Proliferation was detected in the entire population of PBMC treated with PF for 72 hr (Fig. 4a). However, no difference in the proliferation rate between the CD16+ CD56dim or the CD16– CD56br was observed at either the 24, 48, or 72 hr time points (Fig. 4b, c).
Figure 4.
Treatment with PF does not result in increased proliferation of NK cells. (a) Proliferation of entire population of HD4 derived PBMC incubated in PF of EOC PT45 for 72 hr is shown. Comparable results were obtained in all HD PBMC samples tested in this experiment. (b) PBMC from HD4 were treated for 24, 48, and 72 hr with media only (control) or PF (test) from EOC patient PT45. Proliferation of the CD16+ CD56dim (shaded histogram) and the CD16– CD56br (clear histogram) NK cell subsets was determined at the designated time intervals. (c) Combined proliferation at the 24, 48, and 72 hr time points from duplicate experiments of PBNK from HD4 and HD31 in EOC PT45 PF. Similar results were obtained when PBNK from HD4 and HD31 were incubated with PF from EOC PT30.
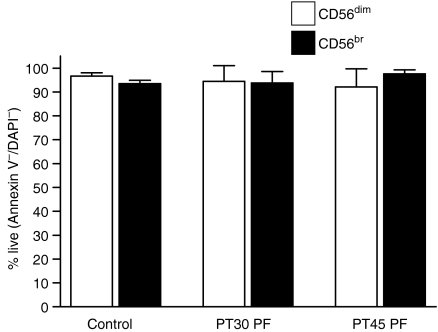
We also investigated the possibility that PF was inducing selective apoptosis in the CD16+ CD56dim NK cells. Again PBMC from two HD were cultured in media only (control) or PF (test) from two EOC patients for 72 hr. The cells were labelled with fluorophore conjugated Annexin V, and anti-CD3, -CD16, and -CD56 antibodies and the extent of apoptosis was determined. More than 90% of the control and test cells were viable according to this assay (Fig. S2). The extent of apoptosis in the NK cells was minimal following treatment with PF and was comparable in the CD16+ CD56dim and the CD16– CD56br NK cell subsets (Fig. 5).
Figure 5.
Treatment with PF does not result in increased apoptosis of the NK cells. PBMC from HD4, HD29, and HD31 were incubated with media only (control) or PF (test) from EOC patients PT30 and PT45 for 72 hr. The average of the percentage of viable cells for all three HD incubated with media or PF samples is shown.
EOC NK cells bind MUC16
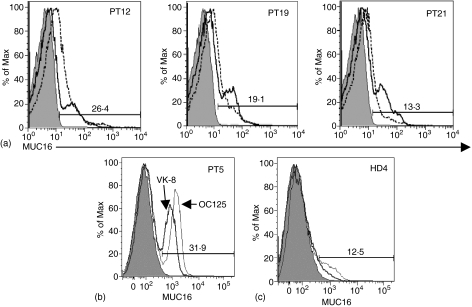
In previous studies we had shown that MUC16 could induce down-regulation of CD16 on HD PBNK cells.9 Because the PB and PF NK cells of EOC patients are continually exposed to MUC16, we tested the PBMC and PFMC for this mucin. Flow cytometry experiments using the anti-MUC16 antibody VK-8 indicated that this mucin was present on the cell surface of 10–35% of all lymphocytes present in the PB and PF of EOC patients (data not shown). The pattern of VK-8 binding to the lymphocytes in the PB (PBL) and the PF (PFL) was distinct and consistent for all patient samples tested (Fig. 6a). The patterns were similar in all of the nine patient samples tested. We have subsequently also tested five additional patient samples and have obtained comparable results (data not shown). To confirm that the binding of the VK-8 was not non-specific, we also tested the patient derived cells with OC125, the first antibody generated against MUC16 that is widely recognized for its specificity for this mucin. The binding of OC125 to the EOC PBL (shown in Fig. 6b) and PFL (data not shown) matched well with that of VK-8.
Figure 6.
MUC16 is present on PBL and PFL of EOC patients. (a) MUC16 on the PBL (solid line) and PFL (dotted line) of three EOC patients is shown. All nine patients studied showed similar flow cytometry patterns. The anti-MUC16 VK-8 antibody and a FITC-labelled goat anti-mouse secondary were used for detection. (b) MUC16 on the PBL was detected by using both the VK-8 and the OC125 antibodies as indicated. (c) The VK-8 (bold line) and OC125 (thin line) did not detect any appreciable amounts of MUC16 on PBL from HD. Data from HD4 are shown, although tests on PBL samples from an additional three donors gave identical results. The shaded histogram in all panels shows binding of isotype-matched control for both VK-8 and OC125.
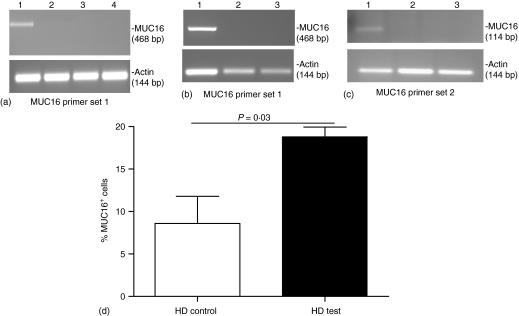
Both the VK-8 and OC125 did not bind to PBL of HD (Fig. 6c), suggesting that MUC16 is not normally found on the immune cells. MUC16 is also not endogenously produced by the PBMCs as indicated by RT–PCR experiments using two separate primer sets on mRNA isolated from healthy donors (Fig. 7a) or EOC PBMC (Fig. 7b). Because of potential contamination from MUC16+ ovarian tumour cells, RT–PCR on the EOC PFMC was not performed. PBMC from HD when incubated with EOC PF showed a significant increase in surface bound MUC16 (Fig. 7c), further suggesting that the immune cells were grabbing MUC16 from the PF.
Figure 7.
MUC16 is not endogenously expressed on PBMC but binds to the cells. (a) MUC16 expression in PBMC from HD4, HD22, and HD25, Lanes 2, 3, and 4, respectively, was determined by RT-PCR using primer set 1. RT–PCR product from OVCAR-3 mRNA was used as control, lane 1. (b) MUC16 expression on PBMC from PT6 and PT8, lanes 2 and 3, respectively, detected by using primer set 1. MUC16 expression in OVCAR-3 cells is in lane 1. (c) Primer set 2 was used to determine MUC16 expression in OVCAR-3 cells and in PB from PT22, and PT24, lanes 1–3, respectively. (d) PBMC from HD1, HD3, and HD4 treated with PF from PT36 for 72 hr were analysed by flow cytometry for MUC16. Control PBMC from the HD were incubated in media only. Average of the data from all three HD is shown. This data is representative of five separate experiments, each involving incubation of PBMC from three HD with PF from different EOC patients.
MUC16 binds to a restricted subset of NK cells
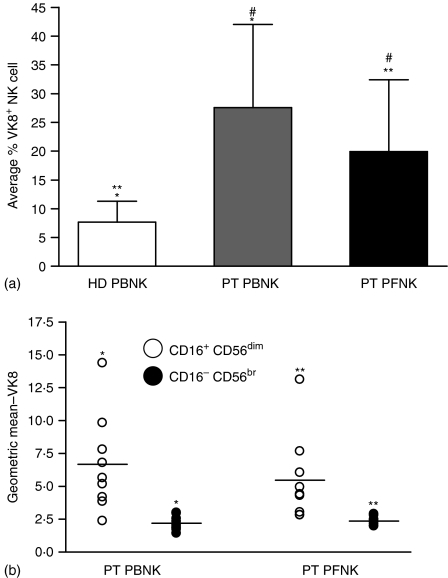
We determined that MUC16 was not present on the CD3+ PBL or PFL of EOC patients (data not shown). Attempts to characterize all of the immune cells that bind MUC16 are currently underway. However, we have determined that both the PBNK and PFNK of EOC patients carry MUC16 on their surface (Fig. 8a). On average, however, lower levels of MUC16 are present on the PFNK as compared to the PBNK even though the PF contains several fold higher amounts of MUC16. This discrepancy can likely be explained by the fact that MUC16 preferentially binds only to the CD16+ CD56dim NK cells from both the PB and PF of EOC patients (Fig. 8b). Very low amounts of MUC16 were detected on the CD16– CD56br subsets. The substantial increase in the CD16– CD56br subset in the PF likely explains the lower amounts of total MUC16 levels of the PFNK as compared to the EOC PBNK.
Figure 8.
Restricted binding of MUC16 to NK cell subsets. (a) MUC16 is present on a significant population of the PB and PF NK cells. Data shown is the average from seven HD and nine EOC patients. (b) Distribution of MUC16 on the CD16+ CD56dim and the CD16– CD56br NK cell subsets from the PB and PF of nine EOC patients is shown. Statistical significance (P < 0·05, by Wilcoxon Sign Ranked test) is represented by matched symbols.
MUC16 also binds to NK cells from pregnant women
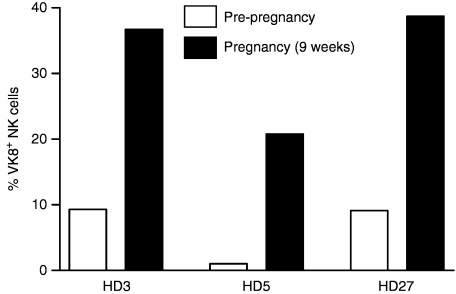
MUC16 is also expressed in the human decidua, and serum levels of this mucin (detected as CA125) are elevated in the serum of women in their first trimester of pregnancy.17,18 The PBNK of three women, HD3, HD5, and HD27, before they became pregnant showed no MUC16 on their cell surface. However, PB samples obtained from these three women when they were 9 weeks pregnant showed considerable binding of MUC16 (Fig. 9). Before pregnancy the serum CA125 levels in these three women were undetectable (<2 U/ml). In the ninth week of pregnancy the serum CA125 levels for these three women were 24 U/ml (HD3), 14 U/ml (HD5), and 40 U/ml (HD27), with a normal level defined as < 35 U/ml.19 The MUC16 bound NK cells were predominantly CD16+ CD56dim (data not shown).
Figure 9.
MUC16 is present on PBNK cells of pregnant women. PBMC samples from three HD were analysed for MUC16 before they conceived and then subsequently when they were 9 weeks into their pregnancy. The number of MUC16+ NK cells as a percent of total PBNK is shown.
Discussion
NK cells constitute an important arm of the innate immune response and are very useful in the generation of antiviral and antitumoral responses. Very early studies indicated that the cytolytic function of NK cells in the PF of ovarian cancer patients was suppressed.20 The suppression of PFNK cells was even more pronounced than that of the PBNK from EOC patients.21 Inhibition of the NK cell response was observed in direct cytolytic measurements and also for the ability of these PFNK in mediating ADCC. The decrease in ADCC responses could be tied to the lower expression of the Fc receptor CD16 on the PFNK cells as demonstrated in our study. This observation is also consistent with a previous report.8
In our studies we demonstrate that the CD16 expression on the PBNK from EOC patients is comparable to that of NK cells from healthy donors. Furthermore, the distribution of the CD16+ CD56dim and CD16– CD56br subsets in the PBNK is comparable in EOC patients and HD. Thus, the peritoneal presentation of the ovarian tumour does not appear to have any major impact on the phenotype and by inference on the cytolytic function of the PBNK.
The NK cells in the peritoneal environment are distinct from the PBNK of the same patients in many respects. The most obvious disparity is in the CD16– CD56br NK cells, which are found in large numbers in the PF. NK cells with this phenotype are normally found in the secondary lymphoid tissues.22 Because accumulation of the PF in EOC patients is in large part the result of the clogging of the peritoneal lymphatic circulation, selective retention of the NK cells with the CD16– CD56br phenotype may occur. Another reason could be the selective trafficking of NK cells with this phenotype in the peritoneal environment. The CD16– CD56br NK cells express CD62L (l-selectin) that likely helps in their localization to the lymph nodes.23 It remains to be demonstrated if the CD16– CD56br PFNK also express CD62L.
Finally, exposure of the NK cells to the tumour-derived factors may induce CD16+ CD56dim NK cells to acquire the CD16– CD56br phenotype. Partial support for this argument was provided by our previous observation that the ovarian tumour derived mucin MUC16 could induce a severe down-regulation of CD16 on HD derived PBNK.9 Further evidence is provided in our current study where HD derived PBNK, when treated with PF from EOC patients, exhibited an increase in the CD16– CD56br subset population. This phenotypic shift was associated with down-regulation of CD16 and the natural cytotoxicity receptor NKp46 (Fig. 3b, c). We have also demonstrated that the change in the phenotype is not caused by increased apoptosis of the CD16+ CD56dim cells (Fig. 5) or by selective proliferation of the CD16– CD56br NK cell subsets (Fig. 4) following exposure to PF of EOC patients.
This observation is important in the light of recent studies on the in vivo differentiation of human NK cells from haematopoietic progenitors.24,25 Development of the NK cells from the progenitors occurs in five distinct stages – defined by the relative expression of CD34, CD117, CD94 and CD56. CD34+ CD38– haematopoietic precursor cells, on their pathway towards terminal differentiation to NK cells, transition from pro-NK (stage 1; CD122– CD34+ CD117– CD94– CD16–) to pre-NK (stage 2; CD122+ CD34+ CD117+ CD94– CD16–) and to immature NK cells (stage 3; CD34– CD117+ CD94– CD16–)25,26. The immature NK cells initially differentiate into stage 4 where the phenotype is defined as CD34– CD117+/– CD94+ CD16– CD56br. Cells with this phenotype are primarily found in the secondary lymphoid tissues and only in minor numbers in the PB. Subsequent differentiation of the stage 4 cells into stage 5 cells results in the development of CD34– CD117– CD94+/– CD16+ CD56dim NK cells that are abundant in the PB.
At this time it is not clear if the CD56br population we have identified in the PFNK and in the HD PBNK exposed to PF are equivalent to the NK cells defined by the stage 4 phenotype. Our future studies on the expression of CD62L, CD117, perforin-containing granules and cytokines will help clarify this issue. However, if the CD56br cells identified in our study are equivalent to the stage 4 NK cells, it implies that the terminal differentiation (conversion from stage 4 to stage 5) of the NK cells may be a reversible step that can be influenced by the environment in which the NK cells reside.
The potential importance of the tumour generated soluble factors that may induce phenotypic alterations in the NK cells is highlighted by our observation that the ovarian tumour marker MUC16 binds to these and other immune cells of EOC patients. Although activated T cells can synthesize the mucin MUC1,27 our RT-PCR data indicates that the PBMCs do not express endogenous MUC16. Because incubation of healthy donor PBMC in ascites results in increased VK-8 binding to subsets of these cells and also to a large proportion of the NK cells, we believe that these cells grab the soluble MUC16 in the PB and the PF. High specificity for MUC16 was found within the total NK cell population because only the CD16+ CD56dim cells were positive for this mucin.
MUC16 was identified more than two decades ago as the antigen CA125.28 The majority of the research on this antigen has been focused on its diagnostic potential for EOC. From recent studies it is clear that MUC16 binds to galectin-1 and also to mesothelin.29,30 Binding to mesothelin, a glycoprotein expressed on the mesothelial cells that line the peritoneal cavity and organs31 helps the ovarian tumour cells to metastasize and form aggregates.12 MUC16 is also a potent inhibitor of NK cell responses in vitro.9 These observations together imply that the ovarian tumours may specifically up-regulate MUC16 expression to promote their metastasis and to evade immune responses.
The exact mechanism by which MUC16 attaches to the NK cells is not clear. Because MUC16 binds to galectin-1 it is possible that this mammalian lectin may be important in the attachment of the mucin to the NK cell surface. Another possibility is that in advanced stages, EOC patients may develop antibodies against MUC16 and these antibody–MUC16 complexes may bind via CD16 (FcγRIII) to the CD16+ CD56dim NK cells. Alternatively, MUC16 via its terminal sialic acid residues32 may be recognized by Siglec-9, an NK inhibitory receptor.33 Extensive studies are now being conducted in our laboratories to identify NK cell receptors that bind MUC16 and the subsequent cell signalling mechanisms that may be triggered by such an event.
The activity of the NK cells can be controlled at various levels. Major histocompatibility complex (MHC) class I ligand recognition by the inhibitory or activating KIR provides an important mechanism for the control of NK cell cytolytic responses. However, the cellular signals generated by the binding of the non-KIR receptors to their ligands also exert considerable influence on NK cell function. An appropriate example is the additive inhibitory effect generated by the binding of CD244 to its ligand CD48 on target cells.34,35 Target cells expressing MHC class I ligands and CD48 are even more protected when they interact with NK cells expressing inhibitory KIR and CD244.34 Thus the NK cell responses are layered and the final outcome is controlled by the type and relative signalling strength of receptors and ligands engaged.
There may also be another layer of NK cell activity control that is mediated following exposure of the cells to cytokine and non-cytokine factors expressed by the tumours or by pathogen infected target cells. This type of control may either supersede or work in conjunction with other NK cell regulatory mechanisms and may have its origins in the reproductive system where protection of the growing embryo/fetus from maternal immune responses is paramount.
Recent gene expression analysis of the human proliferative and secretory endometrium have indicated that MUC16 is expressed at sevenfold elevated levels in the proliferative phase just prior to the detection of NK cell specific genes in this tissue.36 The NK suppressive effect of MUC169 and glycodelin-A (PP14)37 a pregnancy-specific glycoprotein, imply that the human endometrium is programmed to express NK cell modulatory factors even before the mass scale recruitment of the NK cells and definitely prior to the implantation of the embryo. The infiltrating NK cells in the endometrium are likely primed beforehand to avoid immunological rejection of the fetus. We have proposed that such priming of the decidual NK cells may be mediated by pregnancy-specific glycoconjugates.38,39 Some proof for this hypothesis is provided by our observation that MUC16 is also bound to the PBNK cells of pregnant women (Fig. 9).
Maternal NK cells are recruited in large numbers to the site of implantation of the human embryo.40 A majority of these decidual NK cells are CD16– CD56br.40 It remains to be demonstrated if these decidual NK cells are homologous in phenotype and function to the CD16– CD56br NK cells we have detected in the PF of EOC patients. The decidual NK cells form immature synapses and are therefore compromised in their ability to lyse their targets.41 The decidual NK cells also play important supportive roles during pregnancy by modulating the migration of cytotrophoblasts42 and by expressing pro-angiogenic factors.43 It will be important to determine if the CD16– CD56br PFNK cells promote peritoneal tumour development by providing the angiogenic factors similar to the decidual NK cells.
Acknowledgments
We thank Drs. Robert Bast and Beatrice Yin for providing us the anti-MUC16 antibodies OC125 and VK-8, respectively. Funding for this research was provided by grants from the Department of Defense (#W81XWH-04-1-0102), Ovarian Cancer Research Fund (UW/UWM.05), charitable donation from Jean McKenzie, and start-up funds from the Department of Obstetrics and Gynecology to MSP and a T32 training grant from the National Institutes of Health to J.A.A.G. We are deeply grateful to Kathy Schell for her advice and help and acknowledge the support provided by the University of Wisconsin Comprehensive Cancer Centers Flow Cytometry facility which is supported by a core grant (CA14520) from the National Institutes of Health.
Glossary
Abbreviations
- ADCC
antibody-dependent cell-mediated cytotoxicity
- br
bright
- EOC
epithelial ovarian cancer
- FBS
fetal bovine serum
- GAM
goat anti-mouse antibody
- HD
healthy donor
- NK
natural killer
- PB
peripheral blood
- PBL
peripheral blood lymphocytes
- PBMC
peripheral blood mononuclear cells
- PBNK
peripheral blood NK cells
- PBS-BSA
phosphate-buffered saline containing bovine serum albumin
- PF
peritoneal fluid
- PFL
peritoneal fluid lymphocytes
- PFMC
peritoneal fluid mononuclear cells
- PFNK
peritoneal fluid NK cells
- PT
patient
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Reinartz S, Wagner U. Current approaches in ovarian cancer vaccines. Minerva Ginecol. 2004;56:515–27. [PubMed] [Google Scholar]
- 3.Santin AD, Hermonat PL, Ravaggi A, et al. Phenotypic and functional analysis of tumor-infiltrating lymphocytes compared with tumor-associated lymphocytes from ascitic fluid and peripheral blood lymphocytes in patients with advanced ovarian cancer. Gynecol Obstet Invest. 2001;51:254–61. doi: 10.1159/000058060. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 5.De Leonardis A, Casamassima A, Chiuri E, Addabbo L, De Frenza N, Falco G. [Lymphocytic subpopulations in malignant ascites of ovarian origin. Flow cytometric analysis] Minerva Ginecol. 1993;45:291–300. [PubMed] [Google Scholar]
- 6.Dong HP, Elstrand MB, Holth A, Silins I, Berner A, Trope CG, Davidson B, Risberg B. NK- and B-cell infiltration correlates with worse outcome in metastatic ovarian carcinoma. Am J Clin Pathol. 2006;125:451–8. [PubMed] [Google Scholar]
- 7.Connor JP, Felder M, Hank J, Harter J, Gan J, Gillies SD, Sondel P. Ex vivo evaluation of anti-EpCAM immunocytokine huKS-IL2 in ovarian cancer. J Immunother. 2004;27:211–9. doi: 10.1097/00002371-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Lai P, Rabinowich H, Crowley-Nowick PA, Bell MC, Mantovani G, Whiteside TL. Alterations in expression and function of signal-transducing proteins in tumor-associated T and natural killer cells in patients with ovarian carcinoma. Clin Cancer Res. 1996;2:161–73. [PubMed] [Google Scholar]
- 9.Patankar MS, Jing Y, Morrison JC, et al. Potent suppression of natural killer cell response mediated by the ovarian tumor marker CA125. Gynecol Oncol. 2005;99:704–13. doi: 10.1016/j.ygyno.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 10.Nagler A, Lanier LL, Cwirla S, Phillips JH. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989;143:3183–91. [PubMed] [Google Scholar]
- 11.Harlozinska A, Sedlaczek P, Van Dalen A, Rozdolski K, Einarsson R. TPS and CA 125 levels in serum, cyst fluid and ascites of patients with epithelial ovarian neoplasms. Anticancer Res. 1997;17:4473–8. [PubMed] [Google Scholar]
- 12.Gubbels JA, Belisle J, Onda M, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer. 2006;5:50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Argueso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest Ophthalmol Vis Sci. 2003;44:2487–95. doi: 10.1167/iovs.02-0862. [DOI] [PubMed] [Google Scholar]
- 14.Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Rev. 2006;20:123–37. doi: 10.1016/j.blre.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Feldman GB, Knapp RC, Order SE, Hellman S. The role of lymphatic obstruction in the formation of ascites in a murine ovarian carcinoma. Cancer Res. 1972;32:1663–6. [PubMed] [Google Scholar]
- 16.Pessino A, Sivori S, Bottino C, Malaspina A, Morelli L, Moretta L, Biassoni R, Moretta A. Molecular cloning of NKp46: a novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J Exp Med. 1998;188:953–60. doi: 10.1084/jem.188.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niloff JM, Knapp RC, Schaetzl E, Reynolds C, Bast RC., Jr CA125 antigen levels in obstetric and gynecologic patients. Obstet Gynecol. 1984;64:703–7. [PubMed] [Google Scholar]
- 18.Quirk JG, Jr, Brunson GL, Long CA, Bannon GA, Sanders MM, O'Brien TJ. CA 125 in tissues and amniotic fluid during pregnancy. Am J Obstet Gynecol. 1988;159:644–9. doi: 10.1016/s0002-9378(88)80026-7. [DOI] [PubMed] [Google Scholar]
- 19.Clement M, Bischof P, Gruffat C, et al. Clinical validation of the New ELSA-CA 125, II assay. Report of a European multicentre evaluation. Int J Cancer. 1995;60:199–203. doi: 10.1002/ijc.2910600212. [DOI] [PubMed] [Google Scholar]
- 20.Mantovani A, Allavena P, Sessa C, Bolis G, Mangioni C. Natural killer activity of lymphoid cells isolated from human ascitic ovarian tumors. Int J Cancer. 1980;25:573–82. doi: 10.1002/ijc.2910250505. [DOI] [PubMed] [Google Scholar]
- 21.Haskill S, Koren H, Becker S, Fowler W, Walton L. Mononuclear-cell infiltration in ovarian cancer. II. Immune function of tumour and ascites-derived inflammatory cells. Br J Cancer. 1982;45:737–46. doi: 10.1038/bjc.1982.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–7. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 23.Frey M, Packianathan NB, Fehniger TA, Ross ME, Wang WC, Stewart CC, Caligiuri MA, Evans SS. Differential expression and function of 1-selectin on CD56bright and CD56dim natural killer cell subsets. J Immunol. 1998;161:400–8. [PubMed] [Google Scholar]
- 24.Freud AG, Caligiuri MA. Human natural killer cell development. Immunol Rev. 2006;214:56–72. doi: 10.1111/j.1600-065X.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- 25.Freud AG, Yokohama A, Becknell B, Lee MT, Mao HC, Ferketich AK, Caligiuri MA. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med. 2006;203:1033–43. doi: 10.1084/jem.20052507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freud AG, Becknell B, Roychowdhury S, et al. A human CD34 (+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity. 2005;22:295–304. doi: 10.1016/j.immuni.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Agrawal B, Krantz MJ, Parker J, Longenecker BM. Expression of MUC1 mucin on activated human T cells: implications for a role of MUC1 in normal immune regulation. Cancer Res. 1998;58:4079–81. [PubMed] [Google Scholar]
- 28.Bast RC, Jr, Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981;68:1331–7. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seelenmeyer C, Wegehingel S, Lechner J, Nickel W. The cancer antigen CA125 represents a novel counter receptor for galectin-1. J Cell Sci. 2003;116:1305–18. doi: 10.1242/jcs.00312. [DOI] [PubMed] [Google Scholar]
- 30.Rump A, Morikawa Y, Tanaka M, Minami S, Umesaki N, Takeuchi M, Miyajima A. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004;279:9190–8. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]
- 31.Chang K, Pastan I, Willingham MC. Frequent expression of the tumor antigen CAK1 in squamous-cell carcinomas. Int J Cancer. 1992;51:548–54. doi: 10.1002/ijc.2910510408. [DOI] [PubMed] [Google Scholar]
- 32.Kui Wong N, Easton RL, Panico M, et al. Characterization of the oligosaccharides associated with the human ovarian tumor marker CA125. J Biol Chem. 2003;278:28619–34. doi: 10.1074/jbc.M302741200. [DOI] [PubMed] [Google Scholar]
- 33.Zhang JQ, Nicoll G, Jones C, Crocker PR. Siglec-9, a novel sialic acid binding member of the immunoglobulin superfamily expressed broadly on human blood leukocytes. J Biol Chem. 2000;275:22121–6. doi: 10.1074/jbc.M002788200. [DOI] [PubMed] [Google Scholar]
- 34.McNerney ME, Guzior D, Kumar V. 2B4 (CD244)–CD48 interactions provide a novel MHC class I-independent system for NK-cell self-tolerance in mice. Blood. 2005;106:1337–40. doi: 10.1182/blood-2005-01-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee KM, McNerney ME, Stepp SE, Mathew PA, Schatzle JD, Bennett M, Kumar V. 2B4 acts as a non-major histocompatibility complex binding inhibitory receptor on mouse natural killer cells. J Exp Med. 2004;199:1245–54. doi: 10.1084/jem.20031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Talbi S, Hamilton AE, Vo KC, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147:1097–121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- 37.Okamoto N, Uchida A, Takakura K, et al. Suppression by human placental protein 14 of natural killer cell activity. Am J Reprod Immunol. 1991;26:137–42. doi: 10.1111/j.1600-0897.1991.tb00713.x. [DOI] [PubMed] [Google Scholar]
- 38.Clark GF, Patankar MS. Opinion. Hu-FEDS. in search of ‘universal self’. Mol Hum Reprod. 1997;3:985–7. doi: 10.1093/molehr/3.11.985. [DOI] [PubMed] [Google Scholar]
- 39.Clark GF, Oehninger S, Patankar MS, Koistinen R, Dell A, Morris HR, Koistinen H, Seppala M. A role for glycoconjugates in human development: the human feto-embryonic defence system hypothesis. Hum Reprod. 1996;11:467–73. doi: 10.1093/humrep/11.3.467. [DOI] [PubMed] [Google Scholar]
- 40.Koopman LA, Kopcow HD, Rybalov B, et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–12. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kopcow HD, Allan DS, Chen X, Rybalov B, Andzelm MM, Ge B, Strominger JL. Human decidual NK cells form immature activating synapses and are not cytotoxic. Proc Natl Acad Sci USA. 2005;102:15563–8. doi: 10.1073/pnas.0507835102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu Y, Dutz JP, MacCalman CD, Yong P, Tan R, von Dadelszen P. Decidual NK cells alter in vitro first trimester extravillous cytotrophoblast migration: a role for IFN-gamma. J Immunol. 2006;177:8522–30. doi: 10.4049/jimmunol.177.12.8522. [DOI] [PubMed] [Google Scholar]
- 43.Hanna J, Goldman-Wohl D, Hamani Y, et al. Decidual NK cells regulate key developmental processes at the human fetal–maternal interface. Nat Med. 2006;12:1065–74. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]