Abstract
Defective regulation of secondary immunoglobulin V(D)J gene rearrangement promotes the production of autoantibodies in systemic lupus erythematosus (SLE). It remains unclear, however, whether the regulation of the recombination-activating genes RAG1 and RAG2 is effective in SLE. RAG1 and RAG2 messenger RNA expression was analysed before and after in vitro activation of sorted CD19+ CD5– B cells with anti-immunoglobulin M antibodies, in 20 SLE patients and 17 healthy controls. The expression of CDK2 and p27Kip1 regulators of the RAG2 protein, were examined. The levels of interleukin-6 (IL-6) and its influence on RAG regulation were also evaluated in vitro. SLE patients had increased frequency of RAG-positive B cells. B-cell receptor (BCR) engagement induced a shift in the frequency of κ- and λ-positive cells, associated with a persistence of RAG messenger RNA and the maintenance of RAG2 protein within the nucleus. While expression of the RAG2-negative regulator CDK2 was normal, the positive regulator p27Kip1 was up-regulated and enhanced by BCR engagement. This effect was the result of the aberrant production of IL-6 by SLE B cells. Furthermore, IL-6 receptor blockade led to a reduction in p27Kip1 expression, and allowed the translocation of RAG2 from the nucleus to the cytoplasm. Our study indicates that aberrant production of IL-6 contributes to the inability of SLE B cells to terminate RAG protein production. Therefore, we hypothesize that because of constitutive IL-6 signalling in association with BCR engagement, SLE B cells would become prone to secondary immunoglobulin gene rearrangements and autoantibody production.
Keywords: B cells, cell activation, interleukin-6, recombination-activating gene, systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by abnormal B-cell activation leading to the production of autoantibodies that reflect a positive selection of autoantibody-secreting cells.1,2 Despite extensive studies to identify the molecular and cellular bases of their production, little is known about the regulatory mechanisms involved in the generation of these autoantibodies.
Recent studies have provided evidence for the existence of different checkpoints at which autoreactive B cells are regulated during development.3 Thus, the central tolerance checkpoint in the bone marrow is effective in most human patients with SLE.4 However, a defect in the second checkpoint in the periphery could account for the diversity of autoantibodies in mature B cells.4–6 Evidently, mechanisms that regulate tolerance in autoreactive B cells in the periphery are complex and undefined.7
In mice, secondary immunoglobulin gene rearrangements in mature peripheral B cells may act to maintain peripheral tolerance8 but they may also be instrumental in the development of disease, in the emergence of autoreactive B-cell clones,9 and so may contribute to SLE development.10 In a setting of sustained recombination-activating gene (RAG) gene expression and secondary immunoglobulin gene rearrangements, pathogenic autoantibodies could be generated. Consistent with this is the finding of a high frequency of RAG-positive B lymphocytes in the blood of SLE patients.11 Overexpression of RAG genes supports the notion that increased B-cell receptor (BCR) recombination occurs in SLE.12 The molecular basis for deregulated peripheral immunoglobulin gene recombination is unknown, although it is likely to involve RAG proteins. RAG2 accumulates in quiescent and dividing cells. Its cyclin-dependent kinase 2 (CDK2) phosphorylation seems to be a prerequisite to its translocation into the cytoplasm, in order for it to be degraded within the proteasome.13–15 Importantly, CDK2 is inhibited by p27Kip1,16 the level of which is raised in self-reactive CD4+ T lymphocytes in aged lupus-prone mice.17 Therefore, it is possible that exaggerated expression of p27Kip1 could influence RAG2 expression in SLE B lymphocytes. It has also been demonstrated that a BCR engagement turns RAG expression off in mature B cells.18,19 It is therefore possible to predict that the regulation of signalling pathways activated following antigen-cross-linked BCR could be defective in SLE B cells. The present study was designed to gain insight into the mechanisms resulting in elevated RAG expression in SLE patient B cells.
Materials and methods
Isolation of B cells
Peripheral blood mononuclear cells were isolated from 20 SLE patients fulfilling the 1982 criteria of the American College of Rheumatology for SLE,20 and from 17 healthy controls, using density gradient centrifugation on Ficoll–Hypaque. Cells were stained with anti-CD19 and anti-CD5 antibodies to sort the CD19+ CD5– B-cell subpopulation on an Epics Elite flow cytometer (Beckman Coulter, Villepinte, France).
Flow cytometry
Fluorescein isothiocyanate (FITC)-conjugated anti-CD19, phycoerythrin (PE) conjugated anti-CD5, PE-linked to cyanin 5-labelled anti-CD38 and anti-CD5, PE-linked to cyanin 7-labelled anti-CD19, and Enhanced Couple Dye anti-CD19 were obtained from Beckman Coulter (Villepinte, France). FITC-conjugated anti-immunoglobulin D (IgD) was obtained from BD Biosciences (Le Pont de Claix, France), PE-conjugated anti-λ, FITC-conjugated anti-κ and FITC-conjugated anti-IgM were purchased from Dako Cytomation (Trappes, France), anti-p27Kip1 and anti-CDK2 was supplied by Abcam (Cambridge, UK). Intracellular staining was performed after permeabilization of the cells with 70% methanol. Primary antibodies were revealed with biotinylated anti-rabbit antibodies followed by streptavidin–PE-linked to cyanin 5 (Beckman Coulter). Multi-colour analyses were performed on an Epics Elite flow cytometer.
Cell cultures
Sorted B cells, seeded at 2 × 105 cells/ml, were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum with 1 μg/ml of anti-IgM-coated beads for 24 hr, in the presence or not of 40 ng/ml of anti-interleukin-6 receptor (anti-IL-6R) antibody (R & D Systems, Lille, France) and recombinant IL-6 (ImmunoTools, Friesoythe, Germany).
RNA extraction and reverse transcription–polymerase chain reaction (RT-PCR)
RNA was extracted and reverse transcribed in 20 μl with SuperscriptTM II RNase H-reverse transcriptase according to the manufacturer's instructions (Invitrogen Corporation, Carlsbad, CA). For detection of RAG1 and RAG2 messenger RNA (mRNA), nested RT-PCR were performed using 1 μl cDNA with Taq DNA polymerase (Invitrogen) as previously described.21 For glyceraldehyde 3-phosphate dehydrogenase (GAPDH) RT-PCR, one round of PCR of 40 cycles was performed. Amplification products were identified on 2% agarose gels stained with ethidium bromide.
Single-cell PCR protocol
Individual B cells were sorted into PCR tubes containing 10 μl reverse transcriptase buffer [1 × first-strand buffer (Invitrogen), 5 μm random hexamer, 0·5 × reverse transcriptase buffer, 0·01 m dithiothreitol, 0·5 mm dNTP (all from Promega, Charbonnières, France)], using the flow cytometer outfitted with an Autoclone® single-cell deposition unit (Beckman Coulter). The mRNA conversion and RT-PCR for RAG1, RAG2 and GAPDH from a single cell were performed as described previously.21
Western blotting
Equal amounts of protein from cell lysates were separated on 12% sodium dodecyl sulphate–polyacrylamide gel electrophoresis in reducing conditions and transferred. Unoccupied sites were blocked by incubation in phosphate-buffered saline containing 0·5% Tween-20 and 5% non-fat milk for 1 hr, and the membranes were probed with rabbit anti-p27Kip1 or mouse anti-β-actin overnight at 4°. Bound antibodies were developed with biotinylated-secondary antibodies, revealed with streptavidin–horseradish peroxidase (Jackson Laboratories, West Grove, PA) and visualized using the enhanced chemiluminescence system. For quantification of band intensity, non-saturating exposures were scanned and densities were determined using a computing densitometer (Bio-Rad, Marnes-la-Coquette, France). The intensity of p27Kip1 was expressed relative to that of β-actin.
Enzyme-linked immunosorbent assay (ELISA)
Interleukin-6 was detected in the supernatants of cultured cells using ELISA kits according to the manufacturer's instructions (Beckman Coulter).
Quantitative RT-PCR
Quantitative RT-PCR was carried out in 20-μl mixtures containing 50 ng template cDNA, 1 × Sybr®Green PCR Master mix (Applied Biosystems, Foster City, CA), and 500 nm of each primer (p27Kip1 5′-CTGATCCGTCGGACAGCCAGAC-3′ with 5′-GACGTCTTCTGAGGCCAGGCTTC-3′, 18S 5′-GCCGCTAGAGGTGAAATTCTTG-3′ with 5′-CATTCTTGGCAAATGCTTTCG-3′). Amplifications consisted of one cycle at 50° for 2 min, one cycle at 95° for 10 min, followed by 40 cycles at 95° for 15 seconds and at 60° for 1 min. Comparison of cycle thresholds was carried out with the 2–ΔΔCt method using 18S as an internal control.
Statistical analysis
Results were expressed as mean ± SEM, and compared using the χ2 test, the paired Wilcoxon's test and the Mann–Whitney U-test for unpaired data.
Results
RAG mRNA expression and immunoglobulin gene rearrangement in blood B cells from patients with SLE
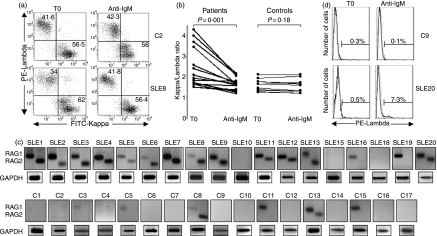
Transitional B cells have the capacity to express RAG mRNA in secondary lymphoid organs of mice.22,23 To ensure accurate determination of RAG expression in peripheral mature B cells, we sorted CD19+ CD5– lymphocytes and excluded CD19+ CD5+, since these latter contain the IgDlow CD38high transitional type 1 B cells (Fig. 1a) that have been detected in the circulation of some human patients with SLE.24,25 Messenger RNA levels for RAG genes in CD19+ CD5– B cells were determined by nested RT-PCR (Fig. 1b). Seventeen of the 20 SLE patients, and eight of the 17 healthy controls, expressed RAG1 (P < 0·02). RAG2 was found in 15 patients but only in two of the 17 healthy controls (P < 0·001). Up to 75% of the SLE patients (15/20) coexpressed RAG1 and RAG2 mRNA, whereas only 12% of the healthy controls (two of 17) did so (P < 0·001). For the V(D)J recombination machinery to operate, the RAG1 and RAG2 genes must be expressed at the same time.26 We therefore determined the level of both RAG1 and RAG2 transcripts in individual cells using single-cell RT-PCR (Fig. 1c). The data revealed that 18·1 ± 3·0% of the CD19+ CD5– peripheral B cells from three SLE patients tested coexpressed RAG1 and RAG2, compared with 4·2 ± 2·1% of those from the two healthy controls (P < 0·01) (Fig. 1d). These data suggest that SLE B cells may be prone to immunoglobulin gene rearrangements.
Figure 1.
RAG1 and RAG2 expression in peripheral blood B cells. (a) Peripheral cells from healthy controls (C) and systemic lupus erythematosus patients (SLE) were stained with anti-CD19 and anti-CD5 mAb, and expression of IgD and CD38 was assessed on CD19+ CD5+ and CD19+ CD5– B-cell subpopulations. A representative example is shown. IgDlow CD38high, including transitional type 1, B cells are indicated. (b) RAG1 and RAG2 mRNA were amplified by nested RT-PCR in fluorescence-activated sorted CD19+ CD5– B cells from SLE patients and healthy controls. (c) Individual B cells were sorted with the Autoclone® single-cell deposition unit of the flow cytometer. Among 40 cells sorted in each sample, only GAPDH mRNA-positive cells were analysed for expression of RAG1 and RAG2 mRNA by nested RT-PCR. A representative example of three SLE patients is shown (SLE2) where crosses indicate cells excluded from the RAG1 and RAG2 analyses because of the absence of cDNA or contamination with genomic DNA. (d) The frequencies of single RAG1-positive and/or RAG2-positive cells are shown. Mean ±SD of three SLE patients and the two healthy controls.
The recombination process was evaluated by determining the frequency of κ-positive and λ-positive B lymphocytes by flow cytometry following anti-IgM stimulation. Given that this activation leads to a high rate of apoptosis over 30 hr,27 the investigation was limited to the first 24 hr, during which 57·7 ± 13·1% and 61·1 ± 11·6% of SLE and control B cells were viable, respectively. Compared with controls, the data revealed a lower frequency of λ-positive SLE B cells before anti-IgM treatment which increases after BCR engagement (Fig. 2a). Thus, anti-IgM stimulation decreased the ratio of κ: λ, whereas it did not change in healthy control B cells (Fig. 2b, Table 1). Furthermore, secondary rearrangement was associated with the persistence of RAG1 and RAG2 mRNA in B cells from 13 of the 18 SLE patients examined, and in two of 17 controls (Fig. 2c). As expected,28 the ratio of κ:λ did not change in untreated B cells (not shown). To verify the defective termination of the RAG machinery following BCR engagement, κ-positive CD19+ CD5– B lymphocytes were sorted and stimulated with anti-IgM. Again, λ-expressing B cells were induced in the patients, but not in the controls (Fig. 2d, Table 1). These data confirm the aberrant expression of RAG1 and RAG2 mRNA in SLE B lymphocytes. Furthermore, they indicate that BCR-mediated regulation of the V(D)J recombination machinery in SLE is likely to be defective.
Figure 2.
Expression of κ and λ light chains, and RAG mRNA in IgM cross-linked B cells. The CD19+ CD5– B cells from patients with SLE and healthy controls were sorted and stimulated with anti-IgM-coated beads at 1 μg/ml for 24 hr. (a) FITC-anti-κ and PE-anti-λ antibodies were used to determine the proportion of κ- and λ-positive cells by flow cytometry. A representative example of 11 healthy controls and 14 SLE patients is shown (C2 and SLE8, respectively). (b) Ratios of κ+/λ+ B cells were determined before (T0) and after anti-IgM-stimulation (anti-IgM). (c) RAG1 and RAG2 mRNA expression after anti-IgM stimulation was analysed by nested RT-PCR and that of GAPDH by RT-PCR. (d) Sorted κ+ B cells were stimulated with anti-IgM antibody, and the expression of λ light chain was determined by flow cytometry. A representative example of three healthy controls and three SLE patients is shown (C9 and SLE20, respectively).
Table 1.
Variation of κ and λ light chain expression following anti-IgM stimulation
| Ratio κ+/λ+ from peripheral B cells | % of λ+ cells fro κ-positive sorted B cells | ||||
|---|---|---|---|---|---|
| Before anti-IgM | After anti-IgM | Before anti-IgM | After anti-IgM | ||
| Patients | 2·3 ± 0·21 (n = 12) | 1·6 ± 0·08 (n = 12) | P = 0·001 | 0·3 ± 0·12 (n = 3) | 7·4 ± 0·42 (n = 3) |
| Controls | 1·6 ± 0·08 (n = 8) | 1·4 ± 0·09 (n = 8) | P = 0·18 | 0·3 ± 0·08 (n = 3) | 0·2 ± 0·08 (n = 3) |
Increased expression of p27Kip1 in SLE B cells
To identify the underlying factors for defective regulation of RAG genes in SLE B lympocytes, we analysed CDK2 and p27Kip1 protein production in the peripheral CD19+ CD5– B cells. As depicted in Fig. 3(a,b), the frequency of CDK2-positive cells was comparable in SLE patients and controls (16·5 ± 5·1% and 13·5 ± 4·1%, P = 0·7), without variations in the mean fluorescence intensity (7·8 ± 1·7 and 7·9 ± 1·3, P > 0·99). In contrast, the proportion of p27Kip1-positive B lymphocytes was higher in the patients (58·9 ± 6·4%) compared with the controls (20·3 ± 6·2%, P = 0·034), associated with a slight elevated mean fluorescence intensity (10·5 ± 1·1 versus 7·3 ± 0·6, P = 0·051). Western blotting confirmed the increased level of p27Kip1 protein (Fig. 3c). There was a higher ratio of p27Kip1/β-actin in the SLE B cells compared with the controls (0·93 ± 0·19 versus 0·41 ± 0·04, P = 0·029). Quantitative RT-PCR studies revealed that the level of p27Kip1 mRNA (Fig. 3d) was not different between B lymphocytes from patients and controls (26·5 ± 8·7 versus 15·7 ± 3·3, P = 0·52). This suggests a post-transcriptional up-regulation of the p27Kip1 protein in SLE B cells. Therefore, the increased level of RAG2 gene expression in freshly isolated SLE B cells is probably the result of the overproduction of p27Kip1.
Figure 3.
CDK2 and p27Kip1 expression in peripheral blood B cells. (a) Fluorescence-activated sorted CD19+ CD5– B cells from patients with SLE and from healthy controls were stained intracellularly with anti-CDK2 or anti-p27Kip1 antibodies and analysed. A representative example of the healthy controls and SLE patients (C1 and SLE7, respectively) is shown. (b) Frequency of CDK2- and p27Kip1-positive B cells (filled symbols) and mean fluorescence intensity (MFI: open symbols) determined by flow cytometry. (c) The expression of p27Kip1 was analysed by Western blotting: four representative examples of the SLE patients and the healthy controls are shown. For all the samples tested, the level of the p27Kip1 protein was normalized relative to that of the β-actin by densitometry. (d) The expression of p27Kip1 mRNA was determined by quantitative RT-PCR and the level was established following normalization to that of the 18S. Medians are indicated.
To gain further insight into the nature of impaired BCR signalling in SLE B lymphocytes, p27Kip1 production was evaluated in cells stimulated with anti-IgM antibody (Fig. 4a). As expected,29 the levels of p27Kip1 protein decreased in control B cells following anti-IgM stimulation (0·41 ± 0·04 before compared with 0·26 ± 0·04 after stimulation, P = 0·04). Interestingly, the level of p27Kip1 protein was maintained, or even increased, in B cells from SLE patients following anti-IgM stimulation, with the exception of one patient (SLE 19). The mean ratio of p27Kip1/β-actin after stimulation was thus not significantly different from the mean level before stimulation (0·79 ± 0·08 versus 0·93 ± 0·19, P = 0·1).
Figure 4.
Variations in p27Kip1 protein and mRNA expression in anti-IgM-stimulated B cells. The CD19+ CD5– B cells from patients with SLE and from healthy controls were sorted and stimulated with anti-IgM-coated beads at 1 μg/ml for 24 hr. (a) Following Western blotting, the level of p27Kip1 protein was measured by densitometry analysis relative to that of the β-actin. Fold increases induced by anti-IgM stimulation for all the samples tested are shown. (b) The expression of p27Kip1 mRNA was determined by quantitative RT-PCR, and the level was normalized relative to that of the 18S. Fold increases induced by anti-IgM stimulation for all the samples tested is shown.
Moreover, these variations were associated with a striking increase in the p27Kip1 mRNA levels in SLE B cells (26·5 ± 8·7 before compared with 755·6 ± 6·4 after stimulation, P = 0·006) (Fig. 4b), while no changes were seen in the controls (15·7 ± 3·3 versus 19·4 ± 7·2, P = 0·95). These data suggest that the p27Kip1 protein is thus not degraded in SLE B cells following engagement of the BCR. Alternatively, synthesis of new proteins is triggered by up-regulated mRNA.
The role of IL-6 in p27Kip1 and RAG2 expression
Accumulation of p27Kip1 and consequent V(D)J recombination may be under the control of cytokines acting as growth inhibitory factors.13 In view of the observations that IL-6 induces G1 arrest by virtue of its ability to induce p27Kip1,30 and that its production is enhanced in SLE,31 we reasoned that it was a likely candidate responsible for RAG expression in SLE B lymphocytes. CD19+ CD5– B cells were sorted, stimulated with anti-IgM antibodies, and the amount of IL-6 released was measured (Fig. 5a). We observed that SLE B cells produced higher levels of IL-6 than the controls (410·9 ± 102·7 versus 157·8 ± 43, P = 0·012).
Figure 5.
IL-6-dependent regulation of p27Kip1 and RAG2. (a) The CD19+ CD5– B cells from patients with SLE and healthy controls were sorted and stimulated with anti-IgM-coated beads at 1 μg/ml for 24 hr; the concentration of IL-6 in the supernatant was then determined by ELISA and expressed as pg for 106 cultured B cells. (b) Some SLE B cells were costimulated with anti-IgM antibody at 1 μg/ml and anti-IL-6 receptor (IL-6R) antibody at 40 ng/ml for 24 hr. p27Kip1 mRNA level was estimated by quantitative RT-PCR following normalization relative to the 18S mRNA. (c) Following costimulation with anti-IgM and anti-IL-6R antibodies, p27Kip1 protein level was calculated by Western blotting and densitometry relative to that of β-actin. (d) Anti-IgM-stimulated SLE B cells were costimulated or not with anti-IL-6R antibody for 24 hr, permeabilized and stained with propidium iodide and anti-RAG2 antibody revealed by FITC-conjugated anti-rabbit IgG. The localization of RAG2 was determined by confocal microscopy. A representative example of three experiments is shown. (e) Healthy and SLE B cells were sorted, stimulated with anti-IgM antibody in the presence of a range of rIL-6 concentrations (0, 500 or 1000 pg/ml) with or without anti-IL-6R antibody for 24 hr, and RAG1 and RAG2 mRNA was amplified by nested RT-PCR. A representative example of three experiments is shown.
To demonstrate the role of IL-6 in p27Kip1 up-regulation, and furthermore in RAG expression, the experiments were repeated in the presence of anti-IL-6R antibody to inhibit the activity of IL-6. Anti-IgM-induced enhancement of the mRNA for p27Kip1 and protein product were eliminated (Fig. 5b,c). This effect indicates that IL-6 impedes engagement of the BCR from down-regulating p27Kip1 in SLE B cells. Moreover, confocal microscopy analyses showed that RAG2 was retained in the nucleus of SLE B cells under BCR-stimulated conditions (Fig. 5d). The enzyme was translocated into the cytoplasm when IL-6-induced signalling was blocked. These observations demonstrate that IL-6 is responsible for retaining RAG2 in the nucleus of SLE B cells once the BCR is engaged. To further confirm the role of IL-6, sorted B cells were stimulated with anti-IgM in the presence of recombinant IL-6. RAG expression was maintained in SLE B cells, but could not be induced on normal B cells whatever the concentration of IL-6 used (Fig. 5e). In the presence of anti-IL-6R antibdoy, however, maintenance of RAG expression was eliminated in SLE B cells. Overall, IL-6 seems to act upon the RAG2 mRNA overexpression in SLE B cells and the cellular localization of the RAG2 protein which is probably the result of its effect on p27Kip1 up-regulation.
Discussion
The production of pathogenic autoantibodies in SLE may be the result of a breakdown in B-cell tolerance, caused in part by immunoglobulin receptor editing in the bone marrow.3,4,10,12 Peripheral receptor revision may also be involved,8,11,12 although its precise driving force remains unknown.
In the present work, we confirmed that SLE patients have a higher frequency of RAG1- and RAG2-expressing B cells compared with controls.11 This raises a question about the nature of these cells. It has been demonstrated that CD5+ and CD5– human B cells display a near-identical response upon stimulation.32 Yet, a higher frequency of RAG-positive cells has been shown in the CD5+ B-cell population of patients with childhood SLE.33 Moreover, CD5 is expressed by transitional type 1B cells that are expanded in some SLE patients.24,25 Given that RAG-positive B cells in peripheral lymphoid organs could be immature transitional type 2 B cells,22,23 circulating RAG-positive B cells could be precursor cells that migrated recently from the bone marrow. However, we predict that this is unlikely in our study for the following reasons. (1) We have excluded the CD5-positive circulating IgDlow CD38high human transitional type 1 B cells from our analysis. (2) Though, we found that 1·4 ± 1·3% and 0·4 ± 0·2% of the CD19+ CD5– B cells were IgDlow CD38high in SLE and in controls, respectively (data not shown), human transitional B cells, circulating25 or in secondary lymphoid organs21, do not express RAG1 and RAG2 mRNA. (3) Although these transition-like B cells may be expressing RAG aberrantly in SLE patients, their frequency (1·4 ± 1·3%) is lower than that of the CD19+ CD5– B cells found to express RAG1 and RAG2 (18·1 ± 3·0%). (4) RAG-expressing B cells in SLE patients are pre-switch IgD+ cells that express CD69, CD38 and CD27 markers11, suggesting a mature, activated status rather than immature.
It has been recently demonstrated that the frequency of autoreactive mature B cells is higher in SLE4, indicating a defective tolerance checkpoint at the stage between new emigrant cells and the emergence of mature B cells. The increased frequency of RAG-expressing peripheral B cells reported herein may reflect this failure in peripheral tolerance. It can be noticed that the κ:λ ratio is higher in SLE B cells relative to controls before anti-IgM treatment. This suggests that SLE B cells might exit from the bone marrow before completing κ to λ editing because of a defect in the κ to λ rearrangement,34 though an intense receptor editing of Igκ chain may occur.35 Yet, we found a significant change in the proportion of κ-expressing and λ-expressing circulating B cells after anti-IgM stimulation. This suggests that the recombination machinery in peripheral SLE B lymphocytes continues to be active when the BCR is engaged. Our belief is that the BCR lacks the capacity to terminate RAG expression. Thus, uncontrolled ongoing secondary V(D)J rearrangement could account for excessive autoantibody production,35–38 and may be a characteristic of aberrant peripheral B cells in SLE, rather than of the expansion of a B-cell subset that normally do not express RAG.39 Furthermore, we did not observe any increase of κ- and λ-coexpressing B cells after anti-IgM stimulation. Though emergence of double-positive cells by receptor editing may be critical for the development of autoreactive B lymphocytes in mice,40 this might not be the case in SLE patients.
We found a greater proportion of p27Kip1-positive B cells associated with an increased protein level in the patients compared with the controls. Unexpectedly, however, the level of p27Kip1 mRNA was similar in both groups. This indicates that the regulation of p27Kip1 is altered, possibly, at the post-transcriptional level in SLE B cells. On assessing the effect of anti-IgM stimulation,41 we observed that it raised p27Kip1 in SLE B cells concurrent with mRNA up-regulation. Similar treatment led to a decrease in the level of the protein in B cells from the controls, as previously shown.29 Overall, our data reveal that the regulation of RAG2 expression in SLE B cells is likely to depend on p27Kip1, the expression of which is constitutively elevated but further enhanced by BCR cross-linking. In mice, anti-IgM stimulation of immature B cells inhibits the phosphatidyl inositol 3-kinase activity which up-regulates p27Kip1.42 Mice that are deficient in the phosphatidyl inositol 3-kinase catalytic subunit p110δ fail to suppress RAG expression and present ongoing light chain recombination.43 In SLE B cells, deficiency of BCR-induced down-regulation of p27Kip1 could be the result of an inhibition of phosphatidyl inositol 3-kinase activity. Such a default mechanism might sustain RAG2 expression, which would be retained in the nucleus,14 and contributes to ongoing recombination of the BCR.
Interleukin-6, which is spontaneously highly produced by SLE B lymphocytes,44 induces the accumulation of p27Kip1.45 Therefore, in the presence of constitutively expressed IL-6R,44,46 IL-6 probably contributes to the sustained excessive p27Kip1 protein in SLE B cells. We have shown that anti-IL-6R antibody prevented the anti-IgM-induced accumulation of p27Kip1 protein at transcriptional and translational levels. Further analysis by confocal microscopy revealed that RAG2 expression was maintained in the nucleus of anti-IgM-stimulated SLE B cells, but translocated into the cytoplasm when IL-6 actions were blocked. The following sequence can then be conceived: when IL-6 signals are neutralized, p27Kip1 level is down-regulated, which in turn allows RAG2 to be translocated into the cytoplasm where it would be ubiquitinated and degraded by the proteasome.13–15 Conversely, excessive IL-6 signalling raises the level of p27Kip1, which might maintain RAG2 in an un-phosphorylated state, and thus retains the protein in the nucleus.14 Furthermore, blockage of IL-6 action abrogated RAG mRNA expression, suggesting that IL-6 might also influence the maintenance of RAG expression at a transcriptional level. Overall, these experiments suggest that IL-6 is involved in secondary V(D)J rearrangements.
It is known that IL-6 is involved in the terminal differentiation of B lymphocytes,47,48 the production of autoantibodies49 and the development of the biological symptoms that characterize SLE.50 It is interesting in this respect that antinuclear autoantibodies are associated with the SLE susceptibility locus Sle1 in mice,7 which is linked to an aberrant activation of the STAT3 and ras-ERK signalling pathways. Such characteristics are associated with increased IL-6 production·51 Thus, treatment of mononuclear cells from Sle1ab mice with anti-IL-6 antibody abrogates the production of autoantibody.51 Intriguingly, IL-6 actively contributes to the production of autoantibodies by mature B cells in human SLE patients.41,45,52 A novel role for IL-6 relative to the regulation of RAG expression is highlighted by the current investigation. The IL-6-dependent autoantibody production is likely to be mediated by activation of RAG expression in peripheral B cells from SLE patients. Constitutive IL-6 signalling in these cells leads to the accumulation of p27Kip1 protein, which in turn keeps RAG2 in the nucleus and facilitates secondary V(D)J rearrangements. Concurrently, BCR cross-linking increases the level of IL-6 that contributes to sustained RAG expression and the ongoing V(D)J rearrangements. Both IL-6 and BCR signals may therefore act synergistically to recombine the BCR and probably induce the production of autoantibodies in SLE.
Acknowledgments
Thanks are due to Christelle Le Dantec for her technical help and to Simone Forest and Cindy Séné for their secretarial assistance. This work was supported by grants from the Ministère de l'Enseignement Supérieur et de la Recherche and from the Académie Nationale Française de Médecine.
References
- 1.Renaudineau Y, Pers JO, Bendaoud B, Jamin C, Youinou P. Dysfunctional B cells in systemic lupus erythematosus. Autoimmun Rev. 2004;3:516–23. doi: 10.1016/j.autrev.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 2.Ferry H, Jones M, Vaux DJ, Roberts IS, Cornall RJ. The cellular location of self-antigen determines the positive and negative selection of autoreactive B cells. J Exp Med. 2003;189:1415–25. doi: 10.1084/jem.20030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobi AM, Diamond B. Balancing diversity and tolerance: lessons from patients with systemic lupus erythematosus. J Exp Med. 2005;202:341–4. doi: 10.1084/jem.20050221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201:703–11. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pugh-Bernard AE, Silverman GJ, Cappione AJ, Villano ME, Ryan DH, Insel RA, Sanz I. Regulation of inherently autoreactive VH4-34 B cells in the maintenance of human B cell tolerance. J Clin Invest. 2001;108:1061–70. doi: 10.1172/JCI12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cappione A, Anolik JH, Pugh-Bernard A, Barnard J, Dutcher P, Silverman G, Sanz I. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest. 2005;115:3205–16. doi: 10.1172/JCI24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakeland EK, Liu K, Graham RR, Behrens TW. Delineating the genetic basis of systemic lupus erythematosus. Immunity. 2001;15:397–408. doi: 10.1016/s1074-7613(01)00201-1. [DOI] [PubMed] [Google Scholar]
- 8.Rice JS, Newman J, Wang C, Michael DJ, Diamond B. Receptor editing in peripheral B cell tolerance. Proc Natl Acad Sci USA. 2005;102:1608–13. doi: 10.1073/pnas.0409217102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekiguchi DR, Eisenberg RA, Weigert M. Secondary heavy chain rearrangement: a mechanism for generating anti-double-stranded DNA B cells. J Exp Med. 2003;197:27–39. doi: 10.1084/jem.20020737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edry E, Melamed D. Receptor editing in positive and negative selection of B lymphopoiesis. J Immunol. 2004;173:4265–71. doi: 10.4049/jimmunol.173.7.4265. [DOI] [PubMed] [Google Scholar]
- 11.Girschick HJ, Grammer AC, Nanki T, Vazquez E, Lipsky PE. Expression of recombination-activating genes 1 and 2 in peripheral B cells of patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:1255–63. doi: 10.1002/art.10264. [DOI] [PubMed] [Google Scholar]
- 12.Monestier M, Zouali M. Receptor revision and systemic lupus erythematosus. Scand J Immunol. 2002;55:425–31. doi: 10.1046/j.1365-3083.2002.01070.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Desiderio S. Cyclin A/CDK2 regulates V(D)J recombination by coordinating RAG-2 accumulation and DNA repair. Immunity. 1999;11:771–81. doi: 10.1016/s1074-7613(00)80151-x. [DOI] [PubMed] [Google Scholar]
- 14.Mizuta R, Mizuta M, Araki S, Kitamura D. RAG2 is down-regulated by cytoplasmic sequestration and ubiquitin-dependent degradation. J Biol Chem. 2002;277:41423–7. doi: 10.1074/jbc.M206605200. [DOI] [PubMed] [Google Scholar]
- 15.Jiang H, Chang FC, Ross AE, Lee J, Nakayama K, Desiderio S. Ubiquitylation of RAG-2 by Skp2-SCF links destruction of the V(D)J recombinase to the cell cycle. Mol Cell. 2005;18:699–709. doi: 10.1016/j.molcel.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 17.Sabzevari H, Propp S, Kono DH, Theofilopoulos AN. G1 arrest and high expression of cyclin kinase and apoptosis inhibitors in accumulated activated/memory phenotype CD4+ cells of older lupus mice. Eur J Immunol. 1997;27:1901–10. doi: 10.1002/eji.1830270813. [DOI] [PubMed] [Google Scholar]
- 18.Meffre E, Papavasiliou F, Cohen P, et al. Antigen receptor engagement turns off the V(D)J recombination machinery in human tonsil B cells. J Exp Med. 1998;188:765–72. doi: 10.1084/jem.188.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hertz M, Kouskoff V, Nakamura T, Nemazee D. V(D)J recombinase induction in splenic B lymphocytes is inhibited by antigen-receptor signalling. Nature. 1998;394:292–5. doi: 10.1038/28419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 21.Hillion S, Saraux A, Youinou P, Jamin C. Expression of RAGs in peripheral B cells outside germinal centers is associated with the expression of CD5. J Immunol. 2005;174:5553–61. doi: 10.4049/jimmunol.174.9.5553. [DOI] [PubMed] [Google Scholar]
- 22.Gartner F, Alt FW, Monroe RJ, Seidl KJ. Antigen-independent appearance of recombination activating gene (RAG)-positive bone marrow B cells in the spleens of immunized mice. J Exp Med. 2000;192:1745–54. doi: 10.1084/jem.192.12.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu W, Nagaoka H, Jankovic M, et al. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature. 1999;400:682–7. doi: 10.1038/23287. [DOI] [PubMed] [Google Scholar]
- 24.Wehr C, Eibel H, Masilamani M, Illges H, Schlesier M, Peter HH, Warnatz K. A new CD21low B cell population in the peripheral blood of patients with SLE. Clin Immunol. 2004;113:161–71. doi: 10.1016/j.clim.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood. 2005;105:4390–8. doi: 10.1182/blood-2004-11-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oettinger MA, Schatz DG, Gorka C, Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990;248:1517–23. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- 27.Pers JO, Jamin C, Le Corre R, Lydyard PM, Youinou P. Ligation of CD5 on resting B cells, but not on resting T cells, results in apoptosis. Eur J Immunol. 1998;28:4170–6. doi: 10.1002/(SICI)1521-4141(199812)28:12<4170::AID-IMMU4170>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 28.Ghia P, Gratwohl A, Signer E, Winkler TH, Melchers F, Rolink AG. Immature B cells from human and mouse bone marrow can change their surface light chain expression. Eur J Immunol. 1995;25:3108–14. doi: 10.1002/eji.1830251118. [DOI] [PubMed] [Google Scholar]
- 29.Solvason N, Wu WW, Kabra N, Wu X, Lees E, Howard MC. Induction of cell cycle regulatory proteins in anti-immunoglobulin-stimulated mature B lymphocytes. J Exp Med. 1996;184:407–17. doi: 10.1084/jem.184.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mori S, Murakami-Mori K, Bonavida B. Interleukin-6 induces G1 arrest through induction of p27Kip1, a cyclin-dependent kinase inhibitor, and neuron-like morphology in LNCaP prostate tumor cells. Biochem Biophys Res Commun. 1999;257:609–14. doi: 10.1006/bbrc.1999.0515. [DOI] [PubMed] [Google Scholar]
- 31.Robak E, Sysa-Jedrzejowska A, Stepien H, Robak T. Circulating interleukin-6 type cytokines in patients with systemic lupus erythematosus. Eur Cytokine Netw. 1997;8:281–6. [PubMed] [Google Scholar]
- 32.Gagro A, McCloskey N, Challa A, Holder M, Grafton G, Pound JD, Gordon J. CD5-positive and CD5-negative human B cells converge to an indistinguishable population on signalling through B-cell receptors and CD40. Immunology. 2000;101:201–9. doi: 10.1046/j.1365-2567.2000.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morbach H, Singh SK, Faber C, Lipsky PE, Girschick HJ. Analysis of RAG expression by peripheral blood CD5+ and CD5– B cells of patients with childhood systemic lupus erythematosus. Ann Rheum Dis. 2006;65:482–7. doi: 10.1136/ard.2005.040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki N, Harada T, Mihara S, Sakane T. Characterization of a germline Vk gene encoding cationic anti-DNA antibody and role of receptor editing for development of the autoantibody in patients with systemic lupus erythematosus. J Clin Invest. 1996;98:1843–50. doi: 10.1172/JCI118985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dorner T, Foster SJ, Farner NL, Lipsky PE. Immunoglobulin kappa chain receptor editing in systemic lupus erythematosus. J Clin Invest. 1998;102:688–94. doi: 10.1172/JCI3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dorner T, Farner NL, Lipsky PE. Ig lambda and heavy chain gene usage in early untreated systemic lupus erythematosus suggests intensive B cell stimulation. J Immunol. 1999;163:1027–36. [PubMed] [Google Scholar]
- 37.Hansen A, Dorner T, Lipsky PE. Use of immunoglobulin variable-region genes by normal subjects and patients with systemic lupus erythematosus. Int Arch Allergy Immunol. 2000;123:36–45. doi: 10.1159/000024422. [DOI] [PubMed] [Google Scholar]
- 38.Dorner T, Lipsky PE. Immunoglobulin variable-region gene usage in systemic autoimmune diseases. Arthritis Rheum. 2001;44:2715–27. doi: 10.1002/1529-0131(200112)44:12<2715::aid-art458>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 39.Potter KN, Mockridge CI, Rahman A. Disturbances in peripheral blood B cell subpopulations in autoimmune patients. Lupus. 2002;11:872–7. doi: 10.1191/0961203302lu309oa. [DOI] [PubMed] [Google Scholar]
- 40.Casellas R, Zhang Q, Zheng NY, Mathias MD, Smith K, Wilson PC. Igkappa allelic inclusion is a consequence of receptor editing. J Exp Med. 2007;204:153–60. doi: 10.1084/jem.20061918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frost V, Sinclair AJ. p27Kip1 is down-regulated by two different mechanisms in human lymphoid cells undergoing apoptosis. Oncogene. 2000;19:3115–20. doi: 10.1038/sj.onc.1203657. [DOI] [PubMed] [Google Scholar]
- 42.Banerji L, Glassford J, Lea NC, Thomas NS, Klaus GG, Lam EW. BCR signals target p27 (Kip1) and cyclin D2 via the PI3-K signalling pathway to mediate cell cycle arrest and apoptosis of WEHI 231 B cells. Oncogene. 2001;20:7352–67. doi: 10.1038/sj.onc.1204951. [DOI] [PubMed] [Google Scholar]
- 43.Llorian M, Stamataki Z, Hill S, Turner M, Martensson IL. The PI3K p110delta is required for down-regulation of RAG expression in immature B cells. J Immunol. 2007;178:1981–5. doi: 10.4049/jimmunol.178.4.1981. [DOI] [PubMed] [Google Scholar]
- 44.Kitani A, Hara M, Hirose T, et al. Autostimulatory effects of IL-6 on excessive B cell differentiation in patients with systemic lupus erythematosus: analysis of IL-6 production and IL-6R expression. Clin Exp Immunol. 1992;88:75–83. doi: 10.1111/j.1365-2249.1992.tb03042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klausen P, Pedersen L, Jurlander J, Baumann H. Oncostatin M and interleukin 6 inhibit cell cycle progression by prevention of p27Kip1 degradation in HepG2 cells. Oncogene. 2000;19:3675–83. doi: 10.1038/sj.onc.1203707. [DOI] [PubMed] [Google Scholar]
- 46.Nagafuchi H, Suzuki N, Mizushima Y, Sakane T. Constitutive expression of IL-6 receptors and their role in the excessive B cell function in patients with systemic lupus erythematosus. J Immunol. 1993;151:6525–34. [PubMed] [Google Scholar]
- 47.Muraguchi A, Hirano T, Tang B, Matsuda T, Horii Y, Nakajima K, Kishimoto T. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med. 1988;167:332–44. doi: 10.1084/jem.167.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kopf M, Baumann H, Freer G, et al. Impaired immune and acute-phase responses in IL-6-deficient mice. Nature. 1994;368:339–42. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 49.Sasai M, Saeki Y, Ohshima S, et al. Delayed onset and reduced severity of collagen-induced arthritis in interleukin-6-deficient mice. Arthritis Rheum. 1999;42:1635–43. doi: 10.1002/1529-0131(199908)42:8<1635::AID-ANR11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 50.Stuart RA, Littlewood AJ, Maddison PJ, Hall ND. Elevated serum interleukin-6 levels associated with active disease in systemic connective tissue disorders. Clin Exp Rheumatol. 1995;13:17–22. [PubMed] [Google Scholar]
- 51.Liu K, Liang C, Liang Z, Tus K, Wakeland EK. Sle1ab mediates the aberrant activation of STAT3 and Ras-ERK signaling pathways in B lymphocytes. J Immunol. 2005;174:1630–7. doi: 10.4049/jimmunol.174.3.1630. [DOI] [PubMed] [Google Scholar]
- 52.Llorente L, Zou W, Levy Y, et al. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med. 1995;181:839–44. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]