Abstract
Natural regulatory CD4+ CD25+ T cells play an important role in preventing autoimmunity by maintaining self-tolerance. They express CD25 constitutively and are produced in the thymus as a functionally mature T-cell population. Changes in the potential of these cells to regulate the activity of conventional effector lymphocytes may contribute to an increased susceptibility to infection, cancer and age-associated autoimmune diseases. In this study we demonstrated that the thymi of aged mice are populated by a higher percentage of CD4+ CD25+ thymocytes than in young animals. The expression of several surface markers (CD69, CD5, CD28, CTLA-4, CD122, FOXP3), usually used to characterize the phenotype of CD4+ CD25+ T regulatory cells, was compared between young and aged mice. We also examined the ability of sorted thymus-deriving regulatory T cells of young and aged BALB/c mice to inhibit the proliferation of lymph node lymphocytes activated in vitro. Natural regulatory T cells isolated from the thymi of young mice suppress the proliferation of responder lymph node cells. We demonstrated that thymus-deriving CD4+ CD25+ T cells of old mice maintain their potential to suppress the proliferation of activated responder lymphocytes of young mice. However, their potential to inhibit the proliferation of old responder T cells is abrogated. Differences in the occurrence and activity of CD4+ CD25+ thymocytes between young and old animals are discussed in relation to the expression of these surface markers.
Keywords: ageing, CD4+ CD25+ regulatory T cells, natural regulatory T cells, thymus-deriving regulatory T cells
Introduction
Natural regulatory CD4+ CD25+ T cells (nTreg cells), initially described by Sakaguchi and co-workers,1 are the best characterized subset of regulatory CD4+ T cells. This population was defined primarily from its constitutive expression of CD25, the interleukin-2 receptor α-chain. CD4+ CD25+ cells play a crucial role in suppressing autoimmune and inflammatory reactions, in tolerance induction, and in maintaining the peripheral T-cell homeostasis.2–6 The population of CD4+ CD25+ T cells present in the peripheral lymphoid organs is a potent inhibitor of polyclonal T-cell activation in vitro. Suppression is mediated by a cell–cell contact-dependent mechanism that requires activation of the suppressor cells by the T-cell receptor (TCR).7,8 These cells are positively selected for self antigens and acquire the suppressive phenotype in the thymus; they represent a constant population in the peripheral blood and lymphoid organs of naive mice.5,8,9 Deficiency in the nTreg-cell population results in multiorgan autoimmune diseases.2,4,6 The phenotypic characterization of naturally arising regulatory T cells is still not fully established. CD4+ CD25+ Treg cells are characterized by their constitutive expression of several activation-related or maturation-related markers: CD69, CD5, CD62 ligand, CD28, CD103, CD152, CD134, GITR, FOXP3.8,10–13 The significance of these molecules in the mechanism of action of nTreg cells is still far from being elucidated. While the human peripheral CD4+ CD25+ T-cell population contains both Treg and T effector populations, murine CD4+ CD25+ T cells are rather homogeneous and are highly enriched in Treg cells. The potential of CD4+ CD25+ T cells to inhibit the proliferation of activated CD4+ CD25– T cells is still the best functional marker of Treg cells.14 T-cell-dependent immunosenescence results from the thymic involution and from changes in T-cell development.15–17 Aging did not affect the developmental potential of T-cell progenitors, but their maturation is blocked by age-related changes in the thymic and extra-thymic environment.18,19 The accumulation of CD44+ CD25– cells (DN1 stage in thymocyte development) in the thymi of old mice is the result of age-related changes in the thymic environment that prevent their maturation. Thus, ageing could result in the selective loss of thymocyte populations. We may expect that changes in T-cell maturation also refer to the development of natural CD4+ CD25+ Treg cells. Since thymic involution with ageing leads to the decrease of the population of naive T cells, increased susceptibility to infection, cancer and age-associated autoimmune diseases, we postulate that at least a part of these age-related changes can result from impaired generation of CD4+ CD25+ nTreg cells or decreased activity of these cells. In this study we investigated thymus-deriving natural regulatory cells in young and old mice. We compared their distribution in a single-positive (SP) CD4+ thymocyte population, phenotype characteristics and the potential to suppress the proliferation of activated lymph node lymphocytes. We demonstrated the increase in the percentage of CD4+ CD25+ T cells in the thymi of old mice. Moreover, the suppressing function of the thymus-deriving nTreg CD4+ CD25+ cells of old mice is not impaired against T responder cells from young individuals. However, the potential of nTreg cells of old mice to inhibit the proliferation of activated responder T cells of old animals is abrogated. The inhibiting activity of thymic nTreg cells is discussed in relation to their increased percentage in the thymus and changes in the expression of several surface markers.
Materials and methods
Mice
BALB/c female mice bred in our animal facility were used at 8–12 weeks old and 15–17 months old (young and aged animals, respectively). The animals were maintained at 22–24° under a 10/14-hr light/dark cycle, with food and water ad libitum. The experiments on animals were performed with the permission of the local authorities: permission number 449/2005 of the Local Ethical Commission, 3 Pasteur Street, 02–093 Warsaw, Poland.
Isolation of thymocytes
Freshly isolated thymi were placed in cold phosphate-buffered saline (PBS), and immediately homogenized. The cells were filtered through cotton gauze to remove tissue debris. Thymocyte suspensions for surface marker analysis were prepared from separate thymi.
Flow cytometry analysis of surface markers
Freshly isolated thymocytes were stained with monoclonal antibodies to CD4, CD8, CD25, CD5, CD69, CD28, CTLA-4 and CD122 labelled with different fluorochromes, at 1 μg/ml final concentration, all from BD PharMingen (San Diego, CA). A phycoerythrin anti-mouse/rat/human FOXP3 Flow Kit (BioLegend, San Diego, CA) was used to determine the intracellular expression of the transcription factor FOXP3. Four-colour analysis was performed on a fluorescence-activated cell sorter (FACSCalibur; Becton-Dickinson, San Diego, CA). The percentage of positively stained cells and the level of surface-marker expression estimated by the fluorescence intensity were analysed using the CellQuest program.
Sorting of CD4+ CD25+ from the thymus
A two-step procedure for the isolation of CD4+ CD25+ SP T cells from the thymus was performed. A magnetic cell sorter was used to deplete the whole cell suspension of pooled thymic cells of CD8+ CD4– and CD4+ CD8+ thymocytes. Thymocytes were suspended in an appropriate volume of sorting buffer and incubated with microbeads conjugated with monoclonal anti-mouse CD8 antibodies. After incubation the cells were layered on the magnetic antibody cell sorting (MACS) column. The procedure was conducted according to the Miltenyi Biotec (Bergisch Gladbach, Germany) protocol. Thymocytes eluted from the column (CD4+ CD8– and CD4– CD8–) were stained with anti-CD4/PerCP and anti-CD25/phycoerythrin (BD PharMingen) monoclonal antibodies and sorted by FACSCalibur for isolation of CD4+ CD25+ T cells. The viability of the cells was estimated by the trypan blue exclusion method. The purity of CD4+ CD25+ population exceeded 98%.
Proliferation assay
The suppressive function of sorted CD4+ CD25+ thymocytes was estimated by the inhibition of proliferation of lymph node lymphocytes as responder cells. Pooled lymph node cell (from three to five mice per experiment) suspensions were used for the proliferation assay. In our model, lymph node lymphocyte proliferation was induced by soluble anti-CD3 and anti-CD28 monoclonal antibodies (BD PharMingen) at concentrations of 0·5 and 2·5 μg/ml, respectively. The concentrations of anti-CD3 and anti-CD28 antibodies were determined by their ability to induce the proliferation of responder lymphocytes. Sorted CD4+ CD25+ thymocytes did not proliferate in the presence of anti-CD3 nor anti-CD28 antibodies used separately or in combination. Non-activated cultures were used as controls. Cells were cultured in RPMI-1640 medium with GlutaMAX I supplemented with 20 mm HEPES, 1 mm sodium pyruvate, 50 μm 2-mercaptoethanol, 5% inactivated fetal calf serum and 5 μg/ml gentamicin (all reagents from Gibco invitrogen™, Paisley, UK). The CFSE (5,6-carboxyfluorescein diacetate succinimidyl ester) technique was used to track proliferation.20 The suspension of lymph node lymphocytes at a concentration of 2 × 107/ml in 0·1% bovine serum albumin (BSA) in PBS was prepared for CFSE loading. CFSE in the form of a 5-mm stock solution in dimethylsulphoxide was diluted in 0·1% BSA in PBS to give a final concentration of 20 μm. The two equal volumes of cell suspension and CFSE solution were mixed to initiate labeling at 37° for 10 min. The labelling process was quenched by adding 10 × volumes of 0·1% BSA in PBS. After 1 min, the CFSE-labelled cells were washed twice, and adjusted to a concentration of 2 × 106/ml in culture medium. Responder CFSE-labelled lymph node cells were cocultured with sorted CD4+ CD25+ thymocytes at a ratio of 10 : 1, in 96-well plates at 37°, in a humidified atmosphere of 7% CO2 for 72 hr. The proliferation was measured by flow cytometry. The progressive loss of CFSE fluorescence was a hallmark of the cell proliferation. The proliferation was measured by flow cytometry. Each cell division reduces the CFSE fluorescent intensity of the daughter cells. Cells from each well were acquired at a fixed speed (high setting) for 1 min to measure an equal volume from each sample. Progressive loss of CFSE fluorescence, indicating the cell proliferation, is presented in histograms.
Statistical analysis
Analysis of variance (anova) was used to determine significant differences in the percentage of thymocyte subsets between the control and experimental groups. The significance was determined at P < 0·05. The Kolmogorov–Smirnov statistic was used to evaluate changes in the expression of surface markers. The most representative results are presented on histograms. Statistically significant differences between the experimental and control groups are denoted in the figures.
Results
Occurrence of CD4+ CD25+ SP T cells in young and old thymi
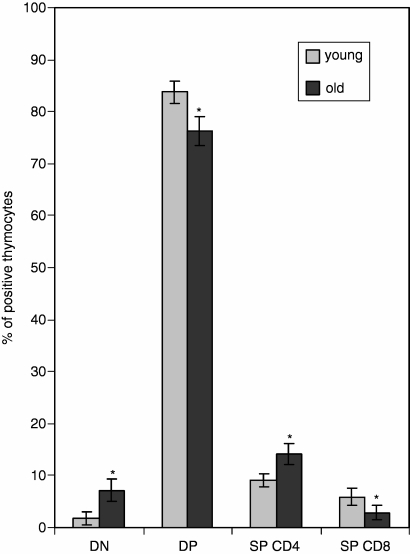
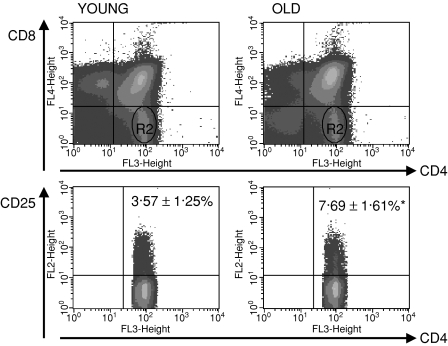
The difference in the distribution of the main thymocyte populations between the old and the young thymi was typical: an increase in the percentage of double-negative (DN) thymocytes, a decrease of double-positive (DP) thymocytes, a decrease of SP CD8+ T cells and an increase of SP CD4+ T cells were all observed in aged mice in comparison to their young counterparts (Fig. 1). The percentage of CD25+ T cells among the SP CD4+ population was higher in the thymi of old mice: 3·57 ± 1·25% and 7·69 ± 1·61%, respectively, for young and old mice (Fig. 2).
Figure 1.
The distribution of thymocyte populations in young and old mice. DN, double-negative thymocytes, CD4– CD8–; DP, double-positive thymocytes, CD4+ CD8+ SP CD4, single-positive thymocytes, CD4+ CD8–; SP CD8, single-positive thymocytes, CD8+ CD4–. Statistically significant difference in the percentage of positive cells between old and young mice.
Figure 2.
CD4+ CD25+ T cells in the thymi of young and old mice. R2, SP CD4+ thymocytes (CD4+ CD8–). Statistically significant difference in the percentage of CD4+ CD25+ in the thymus between old and young mice.
Phenotype characterization of CD4+ CD25+ SP T cells in the thymi of young and old mice
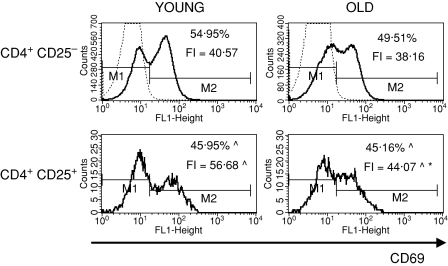
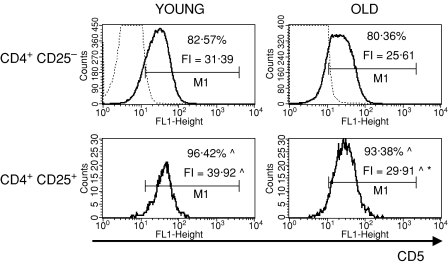
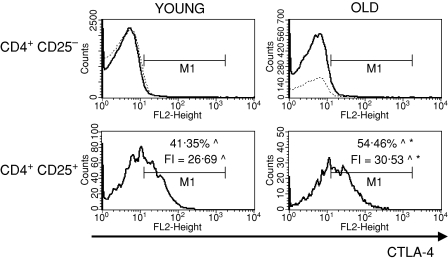
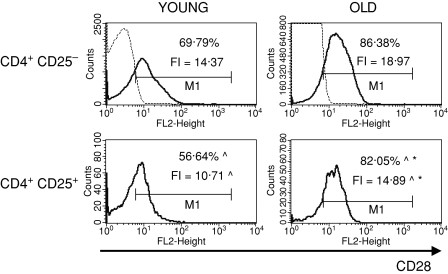
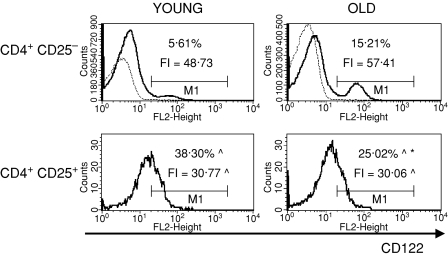
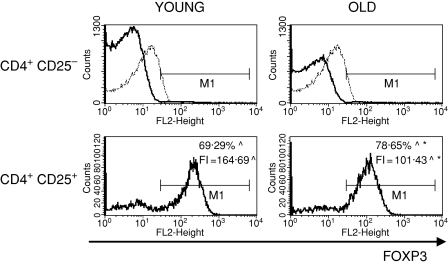
To characterize the CD4+ CD25+ thymocytes, we compared the expression of several surface markers that are usually used to describe the phenotype of nTreg cells: CD69, CD5, CD28, CTLA-4, CD122 and FOXP3 between CD4+ CD25+ and CD4+ CD25– thymocytes. CD69 is a leucocyte early activation molecule. It is transiently expressed on thymocytes during the positive selection process. A lower percentage of CD4+ CD25+ thymocytes with the highest expression of CD69 in comparison with CD4+ CD25– thymocytes was shown both in young and old mice (Fig. 3). Additionally, the expression of CD69 on CD4+ CD25+ T cells was higher than on CD4+ CD25– in both groups of tested animals. The percentage of cells with the highest expression of CD69 among CD4+ CD25+ thymocytes was similar in old and young mice, but the level of its expression was significantly lower in old individuals. CD5, a developmental and an activation-dependent marker, was found on almost all thymocytes. Its expression on CD4+ CD25+ thymocytes was higher than on CD4+ CD25– T cells of both young and old mice (Fig. 4). However, the expression of CD5 on CD4+ CD25+ thymocytes was lower in aged mice. CTLA-4 (cytotoxic T-lymphocyte antigen 4; CD152) is expressed at a low density on mature, activated peripheral T cells, and is involved in the negative regulation of cell-mediated immune responses. CD4+ CD25– thymocytes did not express this molecule, in contrast to CD4+ CD25+ thymocytes (Fig. 5). The percentage of CD4+ CD25+ thymocytes expressing CTLA-4 and the expression of this molecule on the cell surface were both higher in the thymi of old mice. CD28 is involved in T-cell selection, and its expression on mature SP thymocytes was twofold lower than on the immature subsets. The percentage of CD28-positive thymocytes was lower in the CD4+ CD25+ than in the CD4+ CD25– subset (Fig. 6). The percentage of these thymocytes in the subset of CD4+ CD25+ T cells was higher in the thymi of old mice. The expression of this molecule was also higher in the subset of CD4+ CD25+ thymocytes in aged animals. Additionally, it was shown that the sum of the percentages of CD28-positive and CTLA-4-positive nTreg cells of old mice exceeded 100%, which might suggest that both markers are coexpressed on some of the CD4+ CD25+ thymocytes. Moreover, this phenomenon was not shown for young mice. CD122 was expressed on a minor part of CD4+ CD25– thymocytes. It was expressed on a higher percentage of CD4+ CD25+ cells, but its level of expression on these cells was lower. The percentage of CD122+ CD4+ CD25+ thymocytes decreased in old mice, while the level of its expression did not change (Fig. 7). FOXP3 was identified as the key transcription factor in the development and function of Treg cells. Here we demonstrate that FOXP3 was expressed among CD4+ CD25+ thymocytes, in contrast to CD4+ CD25– thymocytes, which did not express this transcription factor. The percentage of thymic nTreg cells expressing FOXP3 in old mice was higher than in their young counterparts. However, the expression of FOXP3 in CD4+ CD25+ thymocytes was lower in aged mice (Fig. 8).
Figure 3.
CD69 expression on CD4+ CD25+ thymocytes in young and old mice. Dotted line, isotype control; solid line, positively stained cells; ^Statistically significant difference in the percentage of positively stained cells or fluorescence intensity (FI) between CD4+ CD25+ and CD4+ CD25– thymocytes in the group of young and old mice. *Statistically significant difference in the percentage of positively stained cells or fluorescence intensity (FI) between old and young mice in the CD4+ CD25+ subset.
Figure 4.
CD5 expression on CD4+ CD25+ thymocytes in young and old mice. See legend to Fig. 3 for key.
Figure 5.
CTLA-4 expression on CD4+ CD25+ thymocytes in young and old mice. See legend to Fig. 3 for key.
Figure 6.
CD28 expression on CD4+ CD25+ thymocytes in young and old mice. See legend to Fig. 3 for key.
Figure 7.
CD122 expression on CD4+ CD25+ thymocytes in young and old mice. See legend to Fig. 3 for key.
Figure 8.
FOXP3 expression in CD4+ CD25+ thymocytes in young and old mice. See legend to Fig. 3 for key.
Proliferation suppressing activity of young and old CD4+ CD25+ SP thymocytes
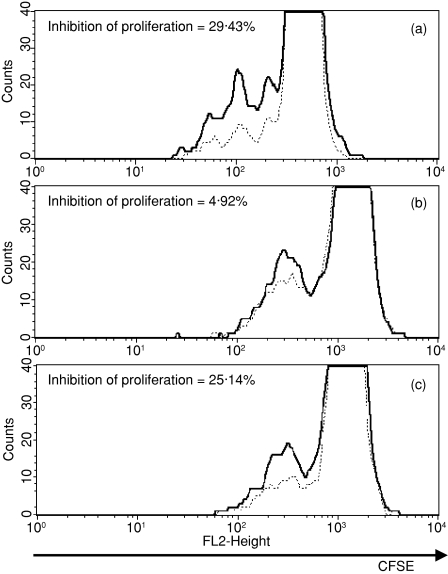
In our experiments we used the two-signal model for lymphocyte activation through TCR and CD28. The TCR signal in conjunction with costimulation through CD28 induces the production of cytokines [including interleukin-2 (IL-2)], antiapoptotic proteins, thereby leading to T-cell clonal expansion.21 The contribution of such costimulation to responding CD4+ CD25– T cells depends on the strength of the TCR signal. To demonstrate the suppressing activity of sorted thymic CD4+ CD25+ nTreg cells the proliferation assay was performed with lymph node cells activated by anti-CD3 and anti-CD28 monoclonal antibodies. In our in vitro model we used this pair of activating monoclonal antibodies at concentrations that were selected for their ability to induce lymph node lymphocyte proliferation. The suppression in cocultures of lymph node cells and sorted CD4+ CD25+ thymocytes was calculated based on the Proliferation Wizard analysis in ModFit LT software. In the first step we used sorted thymic CD4+ CD25+ cells and lymph node cells of young mice. Activating antibodies at the concentrations used in our experiments induced proliferation at the levels of 18–20% and 12–16% of total cells in the culture, respectively, for young and old lymph node cells. The coculture of activated lymph node cells with CD4+ CD25+ thymocytes at a ratio of 10 : 1 reduced the level of proliferation by approximately 29%(Fig. 9a). When we performed the experiment with thymic regulatory cells and lymph node cells, both isolated from old mice, the inhibition of proliferation was below 5% (Fig. 9b). This result may point to the abrogation of the suppressive function of thymic CD4+ CD25+ T cells of old mice, or may suggest a decrease in the sensitivity of the responder lymph node lymphocytes to the suppressing activity of nTreg cells. To address this question, in the next step of our experiment we cocultured activated lymph node cells from young mice with thymic CD4+ CD25+ T cells from old mice. The total level of proliferation was reduced by approximately 25% (Fig. 9c).
Figure 9.
The inhibition of proliferation of activated lymph node lymphocytes by sorted CD4+ CD25+ thymocytes (a) Coculture of lymph node cells of young mice and CD4+ CD25+ thymocytes of young mice. (b) Coculture of lymph node cells of old mice and CD4+ CD25+ thymocytes of old mice. (c) Coculture of lymph node cells of young mice and CD4+ CD25+ thymocytes of old mice. Solid line, CFSE fluorescence intensity of activated lymph node cells cultured without CD4+ CD25+ thymocyte; dotted line, CFSE fluorescence intensity of activated lymph node cells cocultured with CD4+ CD25+ thymocytes.
Discussion
It is generally accepted that a generation of T cells is compromised with ageing because of thymic involution, but is sufficient to maintain the naive T-cell differentiation.22 The 15- to 17-month-old mice used in our experiments exhibited typical age-related changes in the distribution of the main thymocyte populations: the accumulation of DN thymocytes, decrease of the percentage of DP thymocytes, decrease of the percentage of SP CD8+ and increase of SP CD4+ thymocytes. We demonstrated a twofold increase in the percentage of CD4+ CD25+ thymocytes in old mice in comparison with young individuals. Thymic CD4+ CD25+ T cells compared to the CD4+ CD25– subset showed similar changes in the expression of several surface markers as in young animals: a higher expression of CD5, CD69 and lower expression of CD122, CD28. These results are consistent with those presented by other authors for young animals.2 We showed that the surface CTLA-4 was expressed uniquely by CD4+ CD25+ thymocytes. The expression of surface markers usually used to characterize the regulatory phenotype was found to change with ageing. The percentage of CD69-positive cells in the CD4+ CD25+ subset is similar in the thymi of aged and young mice, but its expression is lower in old individuals. Thymocyte CD69 expression is transient during positive selection; however, CD69-deficient mice show normal thymocyte selection. The role of CD69 is still not fully understood.23 The constitutive expression of CD69 might define the pathway of Treg-cell generation. However, other reports showed that functionally mature thymocytes accumulate in the thymic medulla of CD69 transgenic mice and did not migrate to the periphery.24 Thus, according to these data the greater percentage of nTreg cells in the thymi of old mice may result from the impairment of T-cell export to the periphery. The expression of CD5 is lower on thymic CD4+ CD25+ cells of old mice. However, the percentage of CD5-positive cells among this population is similar in old and young mice. CD5 and other regulators of T-cell activation, including CTLA-4, demonstrate an increased expression at the mRNA and protein levels in peripheral Treg cells.25 CD5 functions as a negative regulator of TCR-mediated signalling and its expression is regulated by TCR avidity. High expression of CD5 on regulatory CD4+ CD25+ T cells may inhibit TCR-mediated proliferation.26,27 The decrease in expression of CD5 on thymic CD4+ CD25+ cells of old mice can be related to weakened suppressing signalling leading to a deterioration of their function. However, we cannot exclude that the decrease of CD5 expression on old thymocytes reflects only the developmental pathway related to ageing. This conclusion results from the fact that old CD4+ CD25+ thymocytes showed a suppressive activity similar to that of their young counterparts when cocultured with lymph node cells of young mice. CD5 can play a role in the mechanism allowing the escape from negative selection. These data are in contrast to our results showing a parallel increase in the percentage of CD4+ CD25+ FOXP3 thymocytes and the decrease in the expression of CD5 on these thymocytes in old mice. Our results are similar to those obtained by Bosco et al. in lymphopenic mice.28 We did not observe the elevation of FOXP3-positive DP thymocytes in aged mice (not shown data) which suggests that the increased percentage of nTreg cells in the thymi of old mice results from the re-circulation of peripheral T cells. However, old mice show the increase of DN thymocytes and the decrease of DP thymocytes, which points to a delay in DN to DP transition, and an increase in CD4+ SP thymocyte leading to changes in the developmental pathway rather than to a simple effect of CD4+ re-circulation. CTLA-4 is expressed at a low density on activated T cells, and is involved in the negative regulation of cell-mediated immune responses.29,30 In humans the expression of CTLA-4 on mature peripheral T cells is up-regulated with ageing.31 Constitutive expression of this molecule on murine peripheral Treg cells provides activating signals in Treg-cell-mediated suppression.32 Here we demonstrate that the only cells exhibiting surface expression of CTLA-4 in the thymus are CD4+ CD25+ thymocytes. The percentage of CTLA-4-positive cells, as well as the expression of this molecule in the CD4+ CD25+ subset, is higher in the thymi of aged mice. Thus, a higher percentage of CTLA-4-positive thymocytes in old mice correlates with the higher occurrence of CD4+ CD25+ cells in their thymi. A key role of CTLA-4 in Treg-cell-mediated suppression in vivo and in vitro was recently discussed by Miyara and Sakaguchi.13 Other authors reported that the expression of CD80, CD86 (the natural ligands for CTLA-4 and CD28) and CD40 on dendritic cells of old mice may explain the elevation of the percentage of natural T regulatory cells in the thymus with ageing.33 Low expression of these markers is characteristic for immature DCs, which trigger the generation of T regulatory cells. In addition to thymic DC, the blood-borne dendritic cells can enter the thymus and induce the generation of natural regulatory T cells.34 Thus, we may suggest that the increase of the percentage of T regulatory cells in the thymi of old mice may also result from changes in DC circulation related to ageing. Additionally, the percentage of CD28-positive cells and the expression of CD28 in the CD4+ CD25+ subset is higher in the thymi of aged mice. The involvement of CD28 and CTLA-4 in regulatory T-cell development and function is still controversial.35–37 CD28 is involved in thymocyte development. Recent reports demonstrate a very important role of CD28 in the development of natural regulatory CD4+ CD25+ T cells in the thymus.38 CD28 costimulatory signals transduced to the developing thymocytes through an Lck-binding motif in the cytosolic tail of CD28 induce FOXP3 expression and initiate the differentiation of Treg cells.39–41 FOXP3 can up-regulate the important molecules for the development and function of Treg cells, such as CTLA-4. The results of our studies demonstrated a simultaneous increase in the expression and percentage of CD28 and CTLA-4-positive cells in the CD4+ CD25+ subset in the thymus. This points to the up-regulation of the generation pathway of thymic regulatory cells through CD28–FOXP3–CTLA-4 involvement, which can be supported, at least in part, by the higher expression of CD28 and CTLA-4 on thymic CD4+ CD25+ of aged mice. This hypothesis can also be supported by our results, which demonstrate the increase in the percentage of FOXP3-expressing CD4+ CD25+ thymocytes in old mice. We have yet to find a good explanation for the decrease in expression of FOXP3 in old CD4+ CD25+ thymocytes. Recent experiments indicate a role of FOXP3 in the stabilization of its own expression in a partially IL-2-dependent signaling.12 The ability of activated conventional T cells to produce IL-2 is decreased in old mice (our unpublished data). This may result in diminished expression of this transcription factor. The expression of both CD25 and CD122 during T-cell development in the thymus is transient until the stage of SP thymocytes, when a characteristic stability arises. Most CD4+ CD25+ SP thymocytes demonstrate very low expression of CD122, indicating their low susceptibility to IL-2-induced activation. Weak expression of the IL-2Rβ chain (CD122) despite the constitutive CD25 synthesis results in lack of expression of high-affinity IL-2 receptors as well as the lack of activation-induced proliferation. However, IL-2 signalling is required for the generation and function of nTreg cells.42 CD122 expression is not changed on thymic CD4+ CD25+ T cells of old mice, but the percentage of CD122+ cells in this subset is lowered. This may result in lower sensitivity of nTreg cells from old animals to IL-2 produced by activated T cells. The ability of nTreg cells to suppress activated conventional T lymphocytes is the main functional marker of these cells. Here we demonstrate that mouse thymus-deriving CD4+ CD25+ T cells are functionally mature regulatory cells. Isolated CD4+ CD25+ thymocytes of young mice inhibited the proliferation of activated lymph node cells isolated from young mice by approximately 28%. Their suppressive activity against lymph node lymphocytes demonstrated in an old mouse system (CD4+ CD25+ thymocytes and activated lymph node lymphocytes both of old mice) was very weak. Similar experiments performed by other investigators with human cells demonstrated that the suppressive activity of peripheral CD4+ CD25+ T cells declines with age.43 However, the suppression of proliferation of lymph node lymphocytes isolated from young mice cocultured with CD4+ CD25+ thymocytes of aged mice was inhibited by approximately 25%. We may conclude, therefore, that thymic CD4+ CD25+ cells of aged mice maintain their potential to suppress the proliferation of activated conventional T lymphocytes derived from young individuals. The lack of their suppressive activity against lymph node lymphocytes from old mice did not result from the abrogation of this function, but rather was dependent on the status of the responder cell. Recently, it was shown that the potential of splenic CD4+ CD25+ T cells of aged mice to suppress the proliferation of activated CD4+ CD25– T cells of young individuals was not impaired, suggesting that the age-related decline in T-cell-mediated immune response is dependent on changes in the CD4+ CD25– T-cell population and not on the increase of the suppressive activity of regulatory cells.44 Weak proliferation-suppressing activity of old CD4+ CD25+ thymocytes cocultured with old target lymph node lymphocytes may result from the decrease of the proliferating activity of peripheral lymphocytes with ageing, which may be related to the decrease of the expression of CD28, alterations in the recruitment of signal-transduction proteins to lipid rafts or defects in the TCR-triggered cytoskeletal rearrangement in CD4+ T cells.45–49 Changes in the signal transduction through CD3 and CD28 in lymphocytes from old mice result in poor activation and low level production of IL-2, which is needed for the effector function of Treg cells.50,51 It is also possible that naive T cells of old mice may be functionally impaired in their responsiveness to Treg cells, similar to their impaired sensitivity to costimulatory signals or reduced capacities to proliferate or to secrete cytokines.45,52 It was also demonstrated that the hyporesponsiveness of CD4+ CD25– in aged mice may results from the gain in suppressive activity of these T cells with ageing.53 Here we demonstrate that anti-CD3 and anti-CD28-induced activation of cocultured CD4+ CD25+ thymocytes and lymph node lymphocytes results in the suppression of proliferation of the latter. Our results contradict other findings that demonstrate that the addition of anti-CD28 antibodies to the coculture of Treg cells and responding T cells results in abrogation of the suppressive activity of regulatory CD4+ CD25+ T cells.2,7,54 However, this abrogation resulted from the elevated potential of anti-CD3 activated T responding cells to proliferate after addition of anti-CD28 at high concentration. In our model we used selected concentrations of anti-CD3 and anti-CD28 antibodies, which did not induce the proliferation of responder cells when added separately to the coculture, and did not activate CD4+ CD25+ thymocytes to proliferate when added in combination. Several groups of investigators have demonstrated the suppressive activity of CD4+ CD25+ Treg cells in vitro without antigen-presenting cells. Co-cultures of anti-CD3-activated and anti-CD28-activated human CD4+ CD25– T cells and CD4+ CD25+ Treg cells resulted in the suppression of proliferation.55 Additional results demonstrated that human Treg cells induced to proliferate by anti-CD3 monoclonal antibodies and IL-2 are still capable of suppressing responder CD4+ CD25– T cells.56 Conflicting results relating the requirements for antigen-presenting cells in the induction of suppressive activity of natural Treg cells in vitro have been shown for the murine model.2,7,56
In this study we demonstrated that thymus-deriving CD4+ CD25+ T cells of old mice maintain their suppressing potential. The lack of proliferation-suppressing activity of nTreg cells in an old animal system is related to the decrease of the activation level of responder old lymphocytes rather than to the abrogated function of nTreg cells. This may explain, at least in part, the age-related impairment of the regulation of the immune response.
Acknowledgments
This study was supported by Grant no. 2PO4C 112 29 from the State Committee for Scientific Research (Poland).
Glossary
Abbreviations
- BSA
bovine serum albumin
- DN
double-negative
- DP
double-positive
- PBS
phosphate-buffered saline
- SP
single-positive, nTreg cells, natural regulatory T cells
- TCR
T-cell receptor
References
- 1.Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25): breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;160:1151–64. [PubMed] [Google Scholar]
- 2.Itoh M, Takahashi T, Sakaguchi N, et al. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–26. [PubMed] [Google Scholar]
- 3.Shevach EM. Regulatory T cells in autoimmunity. Annu Rev Immunol. 2000;18:423–49. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 5.Fehervari Z, Sakaguchi S. Development and function of CD4+CD25+ regulatory T cells. Curr Op Immunol. 2004;16:203–8. doi: 10.1016/j.coi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz RH. Natural regulatory T cells and self-tolerance. Nature Immunol. 2005;6:327–30. doi: 10.1038/ni1184. [DOI] [PubMed] [Google Scholar]
- 7.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shevach EM. CD4+CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 9.Shevach EM, McHugh RS, Piccirillo CA, Thornton AM. Control of T-cell activation by CD4+CD25+ suppressor T cells. Immunol Rev. 2001;182:58–67. doi: 10.1034/j.1600-065x.2001.1820104.x. [DOI] [PubMed] [Google Scholar]
- 10.Aspinall R. Age-associated thymic atrophy in the mouse is not associated with a deficiency in the CD44+CD25–CD3–CD4–CD8– thymocyte population. Cell Immunol. 2001;212:150–7. doi: 10.1006/cimm.2001.1848. [DOI] [PubMed] [Google Scholar]
- 11.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor FOXP3. Nature Immunol. 2005;6:331–7. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 12.Zheng Y, Rudensky AY. FOXP3 in control of the regulatory T cell lineage. Nature Immunol. 2007;8:457–62. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 13.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–16. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen non-specific. J Immunol. 2000;164:183–90. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 15.Globerson A, Effros RB. Ageing of lymphocytes and lymphocytes in the aged. Immunol Today. 2000;21:515–21. doi: 10.1016/s0167-5699(00)01714-x. [DOI] [PubMed] [Google Scholar]
- 16.Fry TJ, Mackall CL. Current concepts of thymic aging. Springer Sem Immunopathol. 2002;24:7–22. doi: 10.1007/s00281-001-0092-5. [DOI] [PubMed] [Google Scholar]
- 17.Aspinall R, Andrew D, Pido-Lopez J. Age-associated changes in thymopoiesis. Springer Sem Immunopathol. 2002;24:87–101. doi: 10.1007/s00281-001-0098-z. [DOI] [PubMed] [Google Scholar]
- 18.Montecino-Rodriguez E, Min H, Dorshkind K. Reevaluating current models of thymic involution. Sem Immunol. 2005;17:356–61. doi: 10.1016/j.smim.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Zediak VP, Bhandoola A. Aging and T cell development: interplay between progenitors and their environment. Sem Immunol. 2005;17:337–46. doi: 10.1016/j.smim.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Lyons AB. Analysing cell division in vivo and in vitro using flow cytometry measurement of CFSE dye dilution. J Immunol Meth. 2000;243:147–54. doi: 10.1016/s0022-1759(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 21.McAdam AJ, Schweitzer AN, Sharpe AH. The role of B7 co-stimulation in activation and differentiation of CD4+ and CD8+ T cells. Immunol Rev. 1998;165:231–47. doi: 10.1111/j.1600-065x.1998.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 22.Pawelec G, Remarque E, Barnett Y, Solana R. T cells and ageing. Front Biosci. 1998;3:d59–d99. doi: 10.2741/a266. [DOI] [PubMed] [Google Scholar]
- 23.Sancho D, Gomez M, Sanchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26:136–40. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Feng C, Woodside KJ, Vance BA, et al. A potential role for CD69 in thymocyte emigration. Int Immunol. 2002;14:535–44. doi: 10.1093/intimm/dxf020. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu J, Yamazaki S, Takahashi T, et al. Stimulation of CD4+CD25+ regulatory T cells: through GITR breaks immunological self-tolerance. Nature Immunol. 2002;3:135–42. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 26.Tarakhovsky A, Kanner SB, Hombach J, et al. A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science. 1995;269:535–7. doi: 10.1126/science.7542801. [DOI] [PubMed] [Google Scholar]
- 27.Azzam HS, Grinberg A, Lui K, et al. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998;188:2301–11. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosco N, Agenes F, Rolink AG, Ceredig R. Peripheral T cell lymphopenia and concomitant enrichment in naturally arising regulatory T cells: the case of the pre-Tα gene-deleted mouse. J Immunol. 2006;177:5014–23. doi: 10.4049/jimmunol.177.8.5014. [DOI] [PubMed] [Google Scholar]
- 29.Walunas TL, Lenschow DL, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–13. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 30.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–65. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leng Q, Bentwich Z, Borkow G. CTLA-4 upregulation during aging. Mech Ageing Dev. 2002;123:1419–21. doi: 10.1016/s0047-6374(02)00077-5. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by (CD25+) CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinman RM, Hawiger D, Nussenzwieg MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 34.Goldschneider I, Cone RE. A central role for peripheral dendritic cells in the induction of acquired thymic tolerance. Trends Immunol. 2003;24:77–81. doi: 10.1016/s1471-4906(02)00038-8. [DOI] [PubMed] [Google Scholar]
- 35.Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 36.Tang Q, Boden EK, Henriksen KJ, et al. Distinct roles of CTLA-4 and TGF-β in CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 37.Sansom DM, Walker LS. The role of CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell biology. Immunol Rev. 2006;212:131–48. doi: 10.1111/j.0105-2896.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 38.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces FOXP3 expression and regulatory T cell differentiation independently of interleukin. Nature Immunol. 2005;6:152–62. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 39.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for scurfin in CD4+CD25+ T regulatory cells. Nature Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 40.Fontenot JD, Gavin MA, Rudensky AY. FOXP3 programs the development and function of CD4+CD25+ regulatory T cells. Nature Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 41.Fontenot JD, Rudensky AY. Molecular aspects of regulatory T cell development. Sem Immunol. 2004;16:73–80. doi: 10.1016/j.smim.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Paust S, Cantor H. Regulatory T cells and autoimmune disease. Immunol Rev. 2005;204:195–207. doi: 10.1111/j.0105-2896.2005.00247.x. [DOI] [PubMed] [Google Scholar]
- 43.Tsaknaridis L, Spencer L, Culbertson N, et al. Functional assay for human CD4+CD25+ Treg cells reveals an age-dependent loss of suppressive activity. J Neurosci Res. 2003;74:296–308. doi: 10.1002/jnr.10766. [DOI] [PubMed] [Google Scholar]
- 44.Nishioka T, Shimizu J, Iida R, et al. CD4+CD25+FOXP3+ T cells and CD4+CD25–FOXP3+ T cells in aged mice. J Immunol. 2006;176:6586–93. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- 45.Engwerda CR, Handwerger BS, Fox BS. Aged T cells are hyporesponsive to costimulation mediated by CD28. J Immunol. 1994;152:3740–7. [PubMed] [Google Scholar]
- 46.Larbi A, Douzieh N, Dupuis G, et al. Age-associated alterations in the recruitment of signal-transduction proteins to lipid rafts in human T lymphocytes. J Leukocyte Biol. 2001;75:373–81. doi: 10.1189/jlb.0703319. [DOI] [PubMed] [Google Scholar]
- 47.Garcia GG, Miller RA. Age dependent-defects in TCR-triggered cytoskeletal rearrangement in CD4+ T cells. J Immunol. 2001;169:5021–7. doi: 10.4049/jimmunol.169.9.5021. [DOI] [PubMed] [Google Scholar]
- 48.Gupta S, Su H, Bi R, et al. Life and death of lymphocytes: a key to immunosenescence. Immunity Ageing. 2005;2:12–27. doi: 10.1186/1742-4933-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sadighi Akha AA, Miller RA. Signal transduction in the aging immune system. Curr Op Immunol. 2005;17:486–91. doi: 10.1016/j.coi.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Thornton AM, Piccirillo CA, Shevach EM. Activation requirements for the induction of CD4+CD25+ T cell suppressor function. Eur J Immunol. 2004;34:366–76. doi: 10.1002/eji.200324455. [DOI] [PubMed] [Google Scholar]
- 51.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural FOXP3+CD25+CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–35. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Linton P-J, Haynes L, Klinman NR, Swain SL. Antigen-independent changes in naive CD4 T cells with ageing. J Exp Med. 1996;184:1891–900. doi: 10.1084/jem.184.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shimizu J, Moriizumi E. CD4+CD25– T cells in aged mice are hyporesponsive and exhibit suppressive activity. J Immunol. 2003;170:1675–82. doi: 10.4049/jimmunol.170.4.1675. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi T, Kuniyasu Y, Toda M, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–80. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 55.Jonuleit H, Schmitt E, Stassen M, et al. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levings MK, Sangregorio R, Roncarolo M-G. Human CD25+CD4+ T cells suppress naive and memory T-cell proliferation and can be expanded in vitro without loss of suppressor function. J Exp Med. 2001;193:1295–302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]