Abstract
Coupling between certain pathogen-associated molecular patterns and corresponding pattern recognition receptors of endothelial cells is important for the mediation of vascular inflammatory responses. Mannose-binding lectin (MBL) recognizes certain carbohydrate structures of microbes and subsequently activates the complement system as well as facilitates the phagocytosis of targets. We investigated whether MBL can intervene in the interaction between bacterial lipopolysaccharide (LPS) and endothelial cells to modulate subsequent inflammatory responses. In response to LPS, human umbilical vein endothelial cells (HUVEC) produced various cytokines/chemokines. Addition of the purified human MBL/MBL-associated serine proteases (MASP) complex or recombinant human MBL enhanced LPS-mediated cytokine/chemokine secretion by HUVEC, including interleukin-8 (IL-8), IL-6 and monocyte chemoattractant protein-1 in a dose-dependent manner. This enhancing effect was ameliorated by the addition of anti-MBL antibody or mannan. Among the cytokines/chemokines we analysed, IL-6 showed the greatest increase of secretion in the presence of native MBL/MASP complex or recombinant MBL. MBL, regardless of its association with MASP, alters LPS-mediated cytokine/chemokine secretion of HUVEC. Besides the well-known functions of MBL, to activate the lectin–complement pathway and to facilitate clearance of targets, alteration of cytokine/chemokine secretion may provide an additional role for MBL in modulating vascular inflammation.
Keywords: chemokines/monokines, endothelial cells, interleukin-6, lipopolysaccharide, mannose-binding lectin
Introduction
Innate immunity is a crucial host defence mechanism against microbial infection in animals. The ability of a host to distinguish between self and non-self remains a central hallmark of innate immunity.1 Pathogenic microbes possess distinct pathogen-associated molecular patterns (PAMPs), such as the lipopolysaccharide (LPS) of Gram-negative bacteria, peptidoglycan (PGN) of Gram-positive bacteria,1,2 and β-1,3-glucan of fungi.3 The recognition of these PAMPs is achieved by a group of germ-line encoded receptors and soluble proteins.2 Membrane-associated receptors, such as Toll-like receptors (TLRs), detect specific PAMPs and influence cytokine expression, contributing to the subsequent immune response and inflammation.4,5 Secreted soluble proteins like mannose-binding lectin (MBL) bind to microbes and flag them for destruction by either the complement system or by phagocytic cells.6 The endothelium is the first line of defence to confront invading blood-borne pathogenic microbes and it plays a key role in the defence system by orchestrating various defensive responses including coagulation, vascular permeability and recruitment and activation of inflammatory cells. Endothelial cells are known to respond to various bacterial PAMPs through TLRs.7 Bacterial LPS induces triggering of TLR4 and subsequent activation of downstream signalling pathways causing endothelial cells to release pro-inflammatory cytokines or express abundant adhesion molecules.8 The link between a PAMP and its recognition receptor on an endothelial cell is a key step in the mediation of vascular inflammatory responses.
Mannose-binding lectin is a complement protein and has a unique ability to discriminate between self and non-self by recognizing carbohydrate structures of microbial cells. It is an oligomer of polypeptide chains, each of which comprises a globular head, a carbohydrate recognition domain (CRD) and a collagenous tail domain.9 The CRD of the MBL protein recognizes specific PAMPs on the surfaces of microbes. In human plasma, this protein is associated with MBL-associated serine proteases (MASP) and circulates as an MBL/MASP complex.9 Upon recognition of microbes, MBL/MASP activates the lectin–complement pathway to promote their elimination.9 It has also been demonstrated that MBL can facilitate phagocytosis of targets by binding to its cell surface receptor,10,11 like other collagenous defence proteins including C1q,12 surfactant A,13 surfactant D14 and ficolin.15 Based on the functions of MBL, both as a circulating PAMP-recognition molecule and as an opsonin, we hypothesized that the collagenous defence proteins might intervene in the interaction between PAMPs and endothelial cells. To address this, the optimal PAMP for the stimulation of endothelial cells was selected through a cytokine array, and the effect of MBL on cytokine production of human umbilical vein endothelial cells (HUVEC) in response to the PAMP was investigated in comparison with that of other collagenous defence proteins, C1q and ficolin.
Materials and methods
Isolation of human MBL/MASP, ficolin/MASP and recombinant human MBL
All chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless specified otherwise. MBL/MASP and ficolin/MASP were purified using Staphylococcus aureus PGN-coupled Sepharose 4B columns as described previously.16 In brief, a PGN-coupled Sepharose 4B column was loaded with human serum precipitate by polyethyleneglycol 4000. The column was eluted with 0·3 m mannose and 0·3 mN-acetylglucosamine (MBL/MASP and ficolin/MASP, respectively) and each of the eluates containing MBL/MASP and ficolin/MASP, respectively, was passed through protein A and subsequently anti-immunoglobulin M columns for further purification. Final pass-through fractions were dialysed and concentrated by ultrafiltration through a membrane filter (YM10, Amicon Plastics, Houston, TX). Purified recombinant MBL (rMBL) was provided by the Dobeel Corporation (Seongnam, Korea). Briefly, human MBL cDNA was cloned into an expression vector (pMSG vector) and then the pMSG-MBL DNA was transfected into Chinese hamster ovary (CHO) cells. The MBL-expressing CHO cells were cultured in HyQ SFM4 CHO medium, and then the culture supernatant was subjected to anion exchange chromatography with Q-Sepharose Fast Flow. The pass-through fraction was loaded on a mannan–agarose column and eluted with 20 mm Tris–HCl/150 mm NaCl containing 5 mm ethylenediaminetetraacetic acid (EDTA). The eluate was dialysed and concentrated. The purity and functional intactness of these proteins were confirmed by 12% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) under reducing and non-reducing conditions and by C4 cleavage assay, respectively.
C4 cleavage assay
The ability of both MBL/MASP and rMBL to activate the lectin-complement pathway was determined by the C4 cleavage assay as described previously.17 The purified MBL/MASP (0·1 μg/ml) or rMBL (1 μg/ml) in gelatin veronal buffer++ (0·1% gelatin, 0·5 mm MgCl2, 2 mm CaCl2, 142 mm NaCl, 0·1% sodium diethyl barbiturate, pH 7·4) was added to each 100 μg/ml mannan-coated microplate well (Nunc, Roskilde, Denmark), and the plates were incubated at 4° for 2 hr. After washing with gelatin veronal buffer++, 100 ng human C4 (Advanced Research Technologies, San Diego, CA) was added to each well, and the plates were incubated at 37° for 1 hr. The wells were then washed, and C4 deposition was detected by incubation with a horseradish peroxidase-conjugated polyclonal anti-C4 antibody (Quidel Corporation, San Diego, CA) at 37° for 1 hr and a subsequent colour development reaction. The amount of C4 deposition was determined by measuring the absorbance at 405 nm. To confirm the specificity of the reaction, MBL/MASP (0·1 μg/ml) mixed with mannose (100 mm) was assayed for C4 deposition in parallel. To examine the functional intactness of rMBL, rMBL reconstituted with human MBL-deficient serum containing <0·005 μg/ml functional MBL was also assayed.
Stimulation of HUVEC with PAMPs
The HUVEC (Cambrex, Walkersville, MD) were cultured with full growth medium, EGM-2. The medium was changed every day, and cells were passaged in a 1 : 3 ratio using trypsin/EDTA (0·025%/0·2 mm). Cells between the fifth and ninth passages were used for the experiments. HUVEC (2 × 104 cells) were seeded into each well of 96-well plates. After incubation at 37° for 24 hr to reach confluence, EGM-2 was changed to serum-free EBM-2 medium from the same company, containing human soluble CD14 (1 μg/ml, R & D Systems, Inc., Minneapolis, MN) at least 2 hr before an experiment was started.
Cells were stimulated with each PAMP (300 ng/ml) for 24 hr: LPS from Escherichia coli 0114:B4, soluble S. aureus PGN prepared by sonication and curdlan (β-1,3-glucan polymer, Wako, Osaka, Japan). The supernatants were harvested and used for subsequent cytokine measurement. To investigate the effect of collagenous defence proteins on LPS-mediated cytokine production, MBL/MASP, rMBL, purified C1q (Advanced Research Technologies, San Diego, CA) or purified ficolin/MASP was added to each well 30 min before LPS stimulation. In some experiments, monoclonal anti-MBL antibodies (1 μg/ml, Dobeel Corp.) or mannan (100 μg/ml) were added to each well along with rMBL and MBL/MASP to inhibit MBL binding to PAMPs. Cells were also stimulated with 10 ng/ml human tumour necrosis factor-α (TNF-α; R & D Systems) for 24 hr in the presence of rMBL or MBL/MASP and as a control, in the absence of them.
To block the interaction between MBL and calreticulin on HUVEC, goat polyclonal antibodies (1 μg/ml) against N-terminal and C-terminal epitopes of calreticulin (Santa Cruz Biotechnology, Santa Cruz, CA) were added into the cell culture. Each experiment was performed in triplicate.
Cytokine analysis
To screen the change of cytokine secretion of HUVEC, a cytokine antibody array system, the RayBio® Human Cytokine Antibody Array I (RayBiotech, Inc., Norcross, GA) was used. For subsequent quantitative determination of chemokine/cytokine in each supernatant, interleukin-8 (IL-8), IL-6, and monocyte chemoattractant protein-1 (MCP-1) enzyme-linked immunosorbent assay (ELISA, R & D Systems) was performed according to the manufacturer's instruction.
Statistics
Data are expressed as mean and standard error of the mean of at least three independent experiments. Differences between the groups were compared by a one-way analysis of variance with post hoc testing (Student–Newman–Keuls test). A P-value less than 0·05 was considered statistically significant.
Results
LPS functions as a PAMP reacting to HUVEC
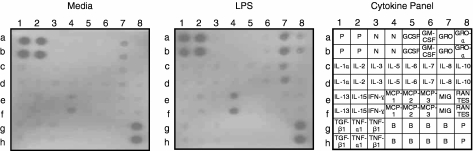
To select the appropriate PAMP to stimulate HUVEC, HUVEC were incubated with well-known microbial PAMPs including LPS, soluble PGN and β-1,3-glucan for 24 hr, and then the supernatant was harvested and assayed for 23 human cytokines using a cytokine array system. As shown in Fig. 1, LPS certainly promoted the secretion of a few cytokines including MCP-1, IL-8, growth-related oncogene (GRO) and GRO-α. Soluble PGN or β-1,3-glucan increased the secretion of MCP-1 from HUVEC but their effects were not as noticeable as that of LPS (data not shown). Through this screening procedure, we concluded that LPS is the best activator for endothelial cells among the three types of PAMPs and LPS used in the following steps of this study.
Figure 1.
Cytokine array of culture supernatant of LPS-stimulated HUVEC. HUVEC were cultured in soluble CD14-containing serum-free media with or without 300 ng/ml LPS for 24 hr. LPS increases the production of some cytokines: IL-8 (7c, 7d), MCP-1 (4e, 4f), GRO (7a, 7b) and GRO-α (8a, 8b). P, positive; N, negative; B, blank.
Purified MBL/MASP and rMBL are functionally intact
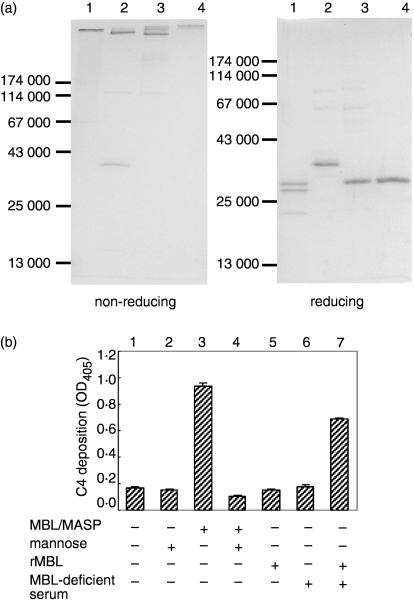
To check the purity and integrity of the collagenous defence proteins we obtained, we loaded MBL/MASP, rMBL, C1q and ficolin/MASP on SDS–PAGE under non-reducing and reducing conditions. We observed high-molecular-weight bands on the 12% non-reducing gel and one or three subunit polypeptide(s) of each protein on the reducing one (Fig. 2a). This result indicated that these proteins were well-purified and that the high-molecular-weight structures remained intact.
Figure 2.
Purity and functional assay of purified MBL/MASP and rMBL. (a) SDS–PAGE pattern of the purified collagenous defence proteins under non-reducing and reducing conditions: Lanes 1, C1q; lanes 2, ficolin/MASP; lanes 3, MBL/MASP; and lanes 4, rMBL. (b) Mannan-coated well was incubated with MBL/MASP (0·1 μg/ml) or rMBL (1 μg/ml) in various conditions for 2 hr and subsequently with C4 (100 ng) for 1 hr. C4 deposition on the well was detected by ELISA using anti-C4 antibody and expressed in the absorbance at 405 nm (mean ± standard error).
To confirm the functional intactness of the purified MBL/MASP and rMBL, their C4 cleavage activity on a mannan-coated well was examined. Incubation of 0·1 μg/ml MBL/MASP on mannan-coated wells and subsequent addition of C4 resulted in abundant C4 deposition on the wells, indicating intact C4 cleavage activity (column 3 in Fig. 2b). Addition of mannose into the reaction mixture abolished C4 deposition, assuring the specificity of the reaction (column 4 in Fig. 2b). Meanwhile, rMBL was not able to deposit substantial amounts of C4 on the mannan-coated wells (column 5 in Fig. 2b). However, rMBL, together with MBL-deficient serum containing unbound MASP, recovered the C4 deposition activity (column 7 in Fig. 2b). This result demonstrated that the rMBL we obtained is able to associate with MASP in the fluid phase to comprise a functional MBL/MASP in contact with its appropriate ligand.
MBL/MASP and rMBL enhance LPS-mediated chemokine/cytokine secretion of HUVEC
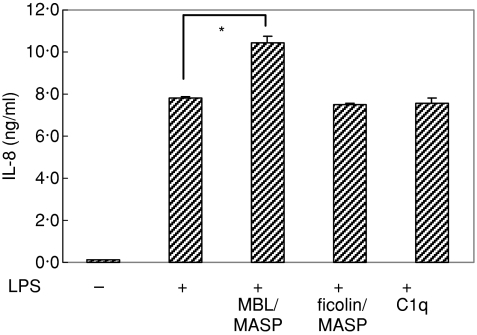
To determine whether collagenous defence proteins can modulate LPS-mediated stimulation of HUVEC, the cells were stimulated with LPS for 24 hr in the presence and absence of each of the collagenous defence proteins (0·5 μg/ml), MBL/MASP, ficolin/MASP and C1q. After incubation, the IL-8 level in the supernatants was measured by ELISA. The presence of MBL/MASP promoted IL-8 secretion compared with IL-8 secretion in its absence (P = 0·013) while the presence of same amount of C1q or ficolin/MASP had no effect on that activity (Fig. 3), suggesting that MBL/MASP can enhance LPS-mediated chemokine secretion of HUVEC.
Figure 3.
Effect of collagenous defence proteins on LPS-mediated IL-8 secretion of HUVEC. IL-8 level was measured by ELISA in the culture supernatants of HUVEC which were stimulated with 300 ng/ml LPS for 24 hr in the absence and presence of 0·5 μg/ml each of collagenous defence proteins, MBL/MASP, ficolin/MASP and C1q (*P < 0·05).
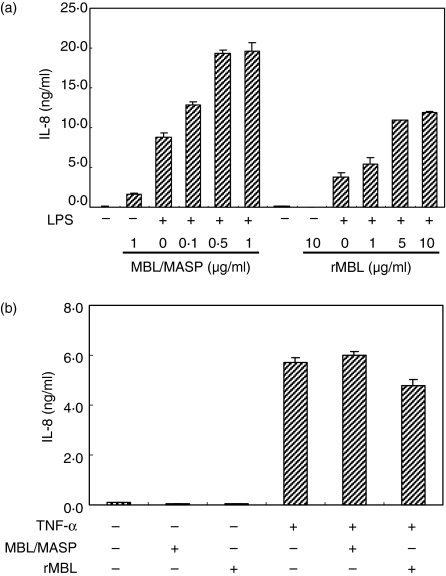
We could confirm the effect of MBL/MASP on LPS-mediated chemokine secretion because increasing concentrations of MBL/MASP enhanced IL-8 secretion in a dose-dependent manner, reaching a plateau at 0·5 μg/ml MBL/MASP (Fig. 4a). To clarify whether this enhancing effect is dependent on the serine protease activity of MBL/MASP, IL-8 secretion in the presence of rMBL which is devoid of MASP, was studied. Surprisingly, rMBL also enhanced IL-8 secretion in a dose-dependent manner, although its enhancing effect was weaker than that of MBL/MASP and a higher concentration was required to demonstrate the effect (Fig. 4a). This result suggested that MASP is not an essential requirement for MBL to enhance LPS-mediated cytokine secretion.
Figure 4.
Effect of MBL on IL-8 secretion of HUVEC in response to LPS or TNF-α. (a) Dose-dependent effect of MBL on LPS-mediated IL-8 secretion of HUVEC. IL-8 level was measured in the culture supernatants of HUVEC which were incubated with/without LPS for 24 hr in the presence of different concentrations of MBL/MASP or rMBL. (b) HUVEC were stimulated with TNF-α (10 ng/ml) with/without MBL/MASP (0·5 μg/ml) or rMBL (5 μg/ml) and then IL-8 concentration was measured in their supernatants.
To check if this enhancing effect is specific for LPS stimulation, the cells were stimulated with TNF-α (10 ng/ml) in the presence and absence of each of MBL/MASP (0·5 μg/ml) and rMBL (5 μg/ml). Stimulation of TNF-α induced IL-8 secretion from HUVEC as well as did LPS stimulation, but addition of either MBL/MASP or rMBL to the cells stimulated with TNF-α did not enhance IL-8 secretion significantly (Fig. 4b), indicating that the enhancing effect of MBL is specifically linked to LPS stimulation.
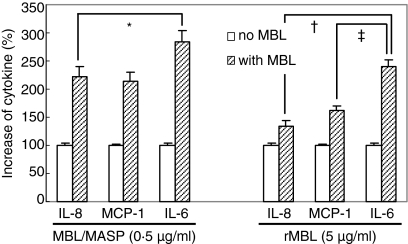
Additionally, to address if MBL can change the profile of LPS-mediated cytokine secretion of HUVEC, representative chemokines of endothelial cells such as IL-8 and MCP-1, and the immunoregulatory cytokine IL-6, were assayed by ELISA in the supernatants of HUVEC stimulated with LPS in the presence and absence of MBL/MASP (0·5 μg/ml) and rMBL (5 μg/ml). In the presence of MBL/MASP, the secreted amounts of IL-8, MCP-1 and IL-6 were 223 ± 18%, 213 ± 161% and 283 ± 21% of that in the absence of MBL, and in the presence of rMBL they were 135 ± 9%, 162 ± 9% and 240 ± 12%, respectively (Fig. 5). The presence of MBL/MASP or rMBL significantly increased the secretion of all three cytokines compared with their absence (P < 0·05). However, when we compared the relative increase in the secretion of each cytokine linked to the presence of MBL/MASP or rMBL, the increase in IL-6 secretion was significantly greater than that of IL-8 and MCP-1 secretion (P < 0·05), suggesting a possible immunoregulatory role for MBL in the LPS-mediated inflammatory responses of endothelial cells.
Figure 5.
Relative increase of LPS-mediated IL-8, MCP-1 and IL-6 secretion in response to MBL. IL-8, MCP-1 and IL-6 levels were measured in the culture supernatants of LPS-stimulated HUVEC in the absence and presence of 0·5 μg/ml MBL/MASP or 5 μg/ml rMBL. Per cent increase of cytokine secretion in the presence of each MBL was calculated from each cytokine level in the absence of MBL set as 100%. *P < 0·05; †P < 0·05; and ‡P < 0·05.
Enhancing effect on chemokine/cytokine production is dependent on binding of MBL to LPS
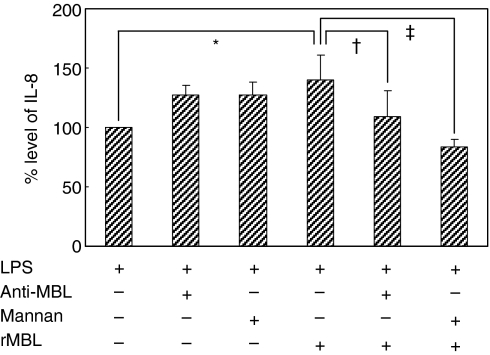
To determine whether the binding of MBL to LPS is involved in enhancing chemokine/cytokine secretion, an anti-MBL monoclonal antibody or mannan was added to cell cultures in the presence and absence of rMBL. After LPS stimulation, IL-8 secretion was measured. Anti-MBL antibody and mannan in a given concentration were shown to inhibit MBL-mediated C4 deposition on mannan-coated wells (data not shown). The rMBL increased LPS-mediated IL-8 secretion of HUVEC (P < 0·001) but addition of anti-MBL antibody or mannan into the reaction mixture abolished this enhancing effect (P = 0·001 and P < 0·001, Fig. 6); in the absence of rMBL, it increased LPS-mediated IL-8 secretion slightly. This indicated that the binding of MBL to LPS through its CRD is mandatory for this modulating effect of MBL.
Figure 6.
Effect of anti-MBL antibody or mannan on the MBL-mediated enhancement of IL-8 secretion of HUVEC. IL-8 level was measured in the culture supernatants of LPS-stimulated HUVEC in the absence and presence of rMBL with and without anti-MBL antibody or mannan. IL-8 level in the absence of rMBL was set as 100%. *P < 0·001; †P = 0·001; ‡P < 0·001.
Finally, to clarify how LPS-bound MBL can interact with HUVEC, we examined the interaction between MBL and calreticulin on HUVEC. It has been reported that calreticulin is a common receptor for C1q and MBL,18–20 and calreticulin is known to be expressed on the surface of endothelial cells.18,21 We assumed that LPS-bound MBL would expose the collagenous tail in an aggregated state to calreticulin on HUVEC to transfer the signal intracellularly. When we added polyclonal anti-calreticulin antibodies into wells of HUVEC to block the putative receptor for the MBL collagenous tail, we observed a significant amount of IL-8 secretion by the HUVEC, even without LPS stimulation (data not shown), suggesting that co-ligation of calreticulin by the anti-calreticulin antibody may activate HUVEC. A direct interaction between the collagenous tail of MBL and calreticulin of endothelial cells could not, therefore, be confirmed.
Discussion
Lipopolysaccharide activates several immune cells through interaction with TLR4, which transfers the signal into the cell through the nuclear factor-κB pathway. MBL, as a soluble PAMP-binding protein, binds to certain carbohydrates found on bacterial surfaces or necrotic or apoptotic cells by CRD.9,22 It is well-known that MBL binds to the mannose-enriched portion of LPS through CRD and its binding mediates lectin–complement pathway activation.23–25 Thus, we expected that binding of MBL to LPS may interfere with the interaction between LPS and TLR4 on the cell surface. However, in this study, MBL enhanced, rather than inhibited, the cytokine secretion and unexpectedly, rMBL without the aid of MASP enhanced the LPS-mediated cytokine secretion of HUVEC, in a similar manner to MBL/MASP.
There have been a series of reports demonstrating that MBL can modulate the secretion of cytokines in response to bacterial PAMPs. Peptidoglycan increases the secretion of several cytokines from phorbol myristate acetate-stimulated human monocytic U937 cells and the presence of MBL together with PGN increases the secretion of IL-8 but reduces that of TNF-α.26 Another report has shown that both C1q and MBL contribute signals to human peripheral blood mononuclear cells, leading to the suppression of LPS-induced secretion of IL-1α and IL-1β, and an increase in the secretion of IL-10, the receptor antagonist IL-1, MCP-1, and IL-6.27 However, these studies have been confined to the effect on mononuclear phagocytic cells and it has been unclear if the regulatory effect of MBL is dependent on the MBL protein itself or on active MASP because only purified MBL/MASP complexes have been used before. It has been speculated that MBL binding to LPS might lead to the activation of the MBL/MASP complex, further transmitting the signal through complement cascades or cell surface molecules. Here, we clearly demonstrate that rMBL, as well as MBL/MASP, can alter LPS-mediated cytokine secretion of HUVEC. This suggests a direct role for the MBL molecule in the modulation of the inflammatory responses of endothelial cells. We speculate that LPS-bound MBL may interact with HUVEC through its receptor, and the presence of active MASP may provide an additional signal to cells or other complement components.
Mannose-binding lectin enhances phagocytosis of microbes or apoptotic cells through binding to targets by CRD and interaction with the surface receptors of phagocytic cells by a collagenous tail. Calreticulin has been suggested as a potential corresponding receptor for collagenous defence proteins.19,20 Gardai et al.28 have demonstrated that calreticulin/CD91 is essential for the enhancing effect of C1q and lung collectins on pro-inflammatory responses induced by LPS or necrotic cells. They proposed a mechanism in which collagenous defence proteins, in the presence of foreign organisms, apoptotic cells or cell debris, present the collagenous tails in an aggregated state to calreticulin on the cells, initiating phagocytosis and/or pro-inflammatory responses. It has been reported that calreticulin is expressed on HUVEC in both resting and activated states, and MBL and C1q bind to HUVEC competitively.18,21 Rocco et al.29 have shown that C1q alone without any PAMP can stimulate HUVEC, resulting in the up-regulation of IL-8, IL-6 and MCP-1 production, which is dependent on the collagenous tail fraction of C1q. They suggested that C1q might induce the production of inflammatory cytokines through binding to calreticulin on HUVEC. However, we could not detect any significant increase in IL-8 secretion from HUVEC in the presence of MBL/MASP or rMBL without LPS stimulation. Moreover, there was no enhancing effect of MBL on IL-8 secretion from the cells stimulated with TNF-α, which also activates NF-κB pathway via its distinct receptor. These results suggest that the enhancing effect of MBL is linked to the interaction between HUVEC and LPS. A group of researchers reported that TLR4 is not expressed on the surface of human coronary artery endothelial cells and the LPS-binding protein (LBP) facilitates the uptake of LPS/CD14 complexes and their delivery to intracellular TLR4/MD-2.30 We speculate that MBL may facilitate the transfer of LPS to intracellular TLR4/MD-2 through binding to LPS by its CRD and interaction with the corresponding cell receptor by its collagenous tails.
We investigated the effect of MBL on chemokine/cytokine production of HUVEC in response to LPS stimulation. When we compared the production of three cytokines, IL-8, MCP-1 and IL-6, in the absence and presence of MBL, MBL enhanced the secretion of all three cytokines and among them IL-6 showed the greatest enhancement. Interleukin-6 is known to block neutrophil accumulation at the site of inflammation and also to promote neutrophil apoptosis.31,32 It is now defined as a resolution factor for acute inflammation that balances pro- and anti-inflammatory outcomes.33 Therefore, it is suggested that MBL may promote the rapid resolution of acute inflammation by increased production of IL-6 as well as by the rapid clearance of foreign microbes and necrotic debris, which provoke acute inflammatory responses.
Several reports have suggested an association between MBL and vascular events. Ischaemic injury on endothelial cells initiates lectin-complement pathway activation and pretreatment with anti-MBL antibody reduces post-ischaemic myocardial reperfusion injury.34,35 Thus far, epidemiological evidence looks more complicated. Systemic lupus erythematosus patients with variant MBL genotypes who showed decreased levels of multimeric functional MBL had a higher risk of arterial thrombosis than those with the wild MBL genotype.36 In a population-based cohort, variant MBL genotypes were associated with increased risk for coronary artery disease and another cohort study also showed that a high level of MBL was associated with a decreased odds ratio for myocardial infarction.37,38 In contrast, a prospective study of patients who underwent carotid endarterectomy indicated that female patients with variant MBL genotypes had a slower progression to early restenosis.39 These results contradict each other and it is not yet conclusive if MBL functions harmfully or beneficially in terms of cardiovascular inflammation. With the invasion of an infectious organism, the MBL/MASP complex can activate the lectin–complement pathway, which is important for killing pathogenic microbes and mediating pro-inflammatory responses in the vascular system. On the other hand, MBL itself can help the clearance of infectious agents and up-regulate the production of IL-6, promoting early termination of the acute inflammatory process and rapid transition to adaptive immune process. Such multiple roles for MBL provide an explanation for contradictory epidemiological data about the association between MBL deficiency and vascular events.
Acknowledgments
This study was supported by a grant from the Korea Health 21 R & D Project, Ministry of Health & Welfare, Republic of Korea (Project No. A040004).
Glossary
Abbreviations
- CHO
Chinese hamster ovary
- CRD
carbohydrate recognition domain
- EDTA
ethylenediaminetetraacetic acid
- ELISA
enzyme-linked immunosorbent assay
- GRO
growth-related oncogene
- HUVEC
human umbilical vein endothelial cells
- IL
interleukin
- LPS
lipopolysaccharide
- MASP
mannose-binding lectin-associated serine proteases
- MBL
mannose-binding lectin
- MCP
monocyte chemoattractant protein
- NF
nuclear factor
- PAMP
pathogen-associated molecular pattern
- PGN
peptidoglycan
- rMBL
recombinant human mannose-binding lectin
- SDS–PAGE
sodium dodecyl sulphate–polyacrylamide gel electrophoresis
- TLR
Toll-like receptor
- TNF
tumour necrosis factor
References
- 1.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 3.Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197:1119–24. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 5.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K, Ip WE, Michelow IC, Ezekowitz RA. The mannose-binding lectin: a prototypic pattern recognition molecule. Curr Opin Immunol. 2006;18:16–23. doi: 10.1016/j.coi.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grandel U, Grimminger F. Endothelial responses to bacterial toxins in sepsis. Crit Rev Immunol. 2003;23:267–99. doi: 10.1615/critrevimmunol.v23.i4.20. [DOI] [PubMed] [Google Scholar]
- 8.Faure E, Equils O, Sieling PA, et al. Bacterial lipopolysaccharide activates NF-kappaB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J Biol Chem. 2000;275:11058–63. doi: 10.1074/jbc.275.15.11058. [DOI] [PubMed] [Google Scholar]
- 9.Gadjeva M, Takahashi K, Thiel S. Mannan-binding lectin – a soluble pattern recognition molecule. Mol Immunol. 2004;41:113–21. doi: 10.1016/j.molimm.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Hartshorn KL, Sastry K, White MR, Anders EM, Super M, Ezekowitz RA, Tauber AI. Human mannose-binding protein functions as an opsonin for influenza A viruses. J Clin Invest. 1993;91:1414–20. doi: 10.1172/JCI116345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arora M, Munoz E, Tenner AJ. Identification of a site on mannan-binding lectin critical for enhancement of phagocytosis. J Biol Chem. 2001;276:43087–94. doi: 10.1074/jbc.M105455200. [DOI] [PubMed] [Google Scholar]
- 12.Bobak DA, Gaither TA, Frank MM, Tenner AJ. Modulation of FcR function by complement: subcomponent C1q enhances the phagocytosis of IgG-opsonized targets by human monocytes and culture-derived macrophages. J Immunol. 1987;138:1150–6. [PubMed] [Google Scholar]
- 13.Tenner AJ, Robinson SL, Borchelt J, Wright JR. Human pulmonary surfactant protein (SP-A), a protein structurally homologous to C1q, can enhance FcR- and CR1-mediated phagocytosis. J Biol Chem. 1989;264:13923–8. [PubMed] [Google Scholar]
- 14.Bufler P, Schmidt B, Schikor D, Bauernfeind A, Crouch EC, Griese M. Surfactant protein A and D differently regulate the immune response to nonmucoid Pseudomonas aeruginosa and its lipopolysaccharide. Am J Respir Cell Mol Biol. 2003;28:249–56. doi: 10.1165/rcmb.4896. [DOI] [PubMed] [Google Scholar]
- 15.Matsushita M, Endo Y, Taira S, Sato Y, Fujita T, Ichikawa N, Nakata M, Mizuochi T. A novel human serum lectin with collagen- and fibrinogen-like domains that functions as an opsonin. J Biol Chem. 1996;271:2448–54. doi: 10.1074/jbc.271.5.2448. [DOI] [PubMed] [Google Scholar]
- 16.Ma YG, Cho MY, Zhao M, Park JW, Matsushita M, Fujita T, Lee BL. Human mannose-binding lectin and 1-ficolin function as specific pattern recognition proteins in the lectin activation pathway of complement. J Biol Chem. 2004;279:25307–12. doi: 10.1074/jbc.M400701200. [DOI] [PubMed] [Google Scholar]
- 17.Petersen SV, Thiel S, Jensen L, Steffensen R, Jensenius JC. An assay for the mannan-binding lectin pathway of complement activation. J Immunol Meth. 2001;257:107–16. doi: 10.1016/s0022-1759(01)00453-7. [DOI] [PubMed] [Google Scholar]
- 18.Peerschke EI, Malhotra R, Ghebrehiwet B, Reid KB, Willis AC, Sim RB. Isolation of a human endothelial cell C1q receptor (C1qR) J Leukoc Biol. 1993;53:179–84. doi: 10.1002/jlb.53.2.179. [DOI] [PubMed] [Google Scholar]
- 19.Sim RB, Moestrup SK, Stuart GR, Lynch NJ, Lu J, Schwaeble WJ, Malhotra R. Interaction of C1q and the collectins with the potential receptors calreticulin (cC1qR/collectin receptor) and megalin. Immunobiology. 1998;199:208–24. doi: 10.1016/s0171-2985(98)80028-4. [DOI] [PubMed] [Google Scholar]
- 20.Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, Henson PM. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–95. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oroszlan M, Daha MR, Cervenak L, Prohaszka Z, Fust G, Roos A. MBL and C1q compete for interaction with human endothelial cells. Mol Immunol. 2007;44:1150–8. doi: 10.1016/j.molimm.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Nauta AJ, Raaschou-Jensen N, Roos A, et al. Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur J Immunol. 2003;33:2853–63. doi: 10.1002/eji.200323888. [DOI] [PubMed] [Google Scholar]
- 23.Brade L, Brade H. A 28,000-dalton protein of normal mouse serum binds specifically to the inner core region of bacterial lipopolysaccharide. Infect Immun. 1985;50:687–94. doi: 10.1128/iai.50.3.687-694.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ihara S, Takahashi A, Hatsuse H, Sumitomo K, Doi K, Kawakami M. Major component of Ra-reactive factor, a complement-activating bactericidal protein, in mouse serum. J Immunol. 1991;146:1874–9. [PubMed] [Google Scholar]
- 25.Devyatyarova-Johnson M, Rees IH, Robertson BD, Turner MW, Klein NJ, Jack DL. The lipopolysaccharide structures of Salmonella enterica serovar typhimurium and Neisseria gonorrhoeae determine the attachment of human mannose-binding lectin to intact organisms. Infect Immun. 2000;68:3894–9. doi: 10.1128/iai.68.7.3894-3899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nadesalingam J, Dodds AW, Reid KB, Palaniyar N. Mannose-binding lectin recognizes peptidoglycan via the N-acetyl glucosamine moiety, and inhibits ligand-induced proinflammatory effect and promotes chemokine production by macrophages. J Immunol. 2005;175:1785–94. doi: 10.4049/jimmunol.175.3.1785. [DOI] [PubMed] [Google Scholar]
- 27.Fraser DA, Bohlson SS, Jasinskiene N, Rawal N, Palmarini G, Ruiz S, Rochford R, Tenner AJ. C1q and MBL, components of the innate immune system, influence monocyte cytokine expression. J Leukoc Biol. 2006;80:107–16. doi: 10.1189/jlb.1105683. [DOI] [PubMed] [Google Scholar]
- 28.Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115:13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]
- 29.van den Berg RH, Faber-Krol MC, Sim RB, Daha MR. The first subcomponent of complement, C1q, triggers the production of IL-8, IL-6, and monocyte chemoattractant peptide-1 by human umbilical vein endothelial cells. J Immunol. 1998;161:6924–30. [PubMed] [Google Scholar]
- 30.Dunzendorfer S, Lee HK, Soldau K, Tobias PS. Toll-like receptor 4 functions intracellularly in human coronary artery endothelial cells. Roles of LBP and sCD14 in mediating LPS responses. Faseb J. 2004;18:1117–19. doi: 10.1096/fj.03-1263fje. [DOI] [PubMed] [Google Scholar]
- 31.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–20. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLoughlin RM, Witowski J, Robson RL, et al. Interplay between IFN-gamma and IL-6 signaling governs neutrophil trafficking and apoptosis during acute inflammation. J Clin Invest. 2003;112:598–607. doi: 10.1172/JCI17129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175:3463–8. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- 34.Collard CD, Vakeva A, Morrissey MA, et al. Complement activation after oxidative stress: role of the lectin complement pathway. Am J Pathol. 2000;156:1549–56. doi: 10.1016/S0002-9440(10)65026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jordan JE, Montalto MC, Stahl GL. Inhibition of mannose-binding lectin reduces postischemic myocardial reperfusion injury. Circulation. 2001;104:1413–18. doi: 10.1161/hc3601.095578. [DOI] [PubMed] [Google Scholar]
- 36.Ohlenschlaeger T, Garred P, Madsen HO, Jacobsen S. Mannose-binding lectin variant alleles and the risk of arterial thrombosis in systemic lupus erythematosus. N Engl J Med. 2004;351:260–7. doi: 10.1056/NEJMoa033122. [DOI] [PubMed] [Google Scholar]
- 37.Best LG, Davidson M, North KE, et al. Prospective analysis of mannose-binding lectin genotypes and coronary artery disease in American Indians: the Strong Heart Study. Circulation. 2004;109:471–5. doi: 10.1161/01.CIR.0000109757.95461.10. [DOI] [PubMed] [Google Scholar]
- 38.Saevarsdottir S, Oskarsson OO, Aspelund T, Eiriksdottir G, Vikingsdottir T, Gudnason V, Valdimarsson H. Mannan binding lectin as an adjunct to risk assessment for myocardial infarction in individuals with enhanced risk. J Exp Med. 2005;201:117–25. doi: 10.1084/jem.20041431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rugonfalvi-Kiss S, Dosa E, Madsen HO, et al. High rate of early restenosis after carotid eversion endarterectomy in homozygous carriers of the normal mannose-binding lectin genotype. Stroke. 2005;36:944–8. doi: 10.1161/01.STR.0000160752.67422.18. [DOI] [PubMed] [Google Scholar]