Abstract
Cytokines with anti-inflammatory properties have been implicated in the prevention of inappropriate immune activation by commensal bacteria in the intestinal tract. Here, we analysed receptor expression, cellular signalling, and the inhibitory activity of interleukin (IL)-4, -10, -11, and -13 as well as of transforming growth factor-β on lipopolysaccharide-mediated small intestinal epithelial cell activation. Only IL-4 and IL-13 had a significant inhibitory effect on chemokine secretion and nitric oxide (NO) production in differentiated and polarized cells. Reverse transcription–polymerase chain reaction of primary intestinal epithelial cells obtained by laser-microdissection confirmed expression of the type II IL-4 receptor consisting of the IL-4 receptor α and the IL-13 receptor α1. Also, IL-4 or IL-13 led to rapid signal transducer and activator of transcription 6 phosphorylation, diminished inducible NO synthase expression, and enhanced the antagonistic arginase 1 activity. In conclusion, cytokines such as IL-4 and IL-13 affect intestinal epithelial cells and exhibit a modulating activity on Toll-like receptor-4-mediated epithelial cell activation.
Keywords: Toll-like receptor, mucosal immunology, interleukin, lipopolysaccharide, intestinal mucosa
Introduction
Intestinal epithelial cells are able to recognize microbial pattern molecules such as lipopolysaccharide (LPS), a potent immunostimulatory cell wall component of Gram-negative bacteria.1–3 Recognition is mediated through Toll-like receptor (TLR)4 and leads to cellular activation and the release of proinflammatory mediators. However, the intestinal mucosal surface is constantly exposed to significant concentrations of microbial ligands derived from the intestinal microflora as well as ingested nutrients. Therefore, inhibitory mechanisms must exist to prevent inappropriate immune activation and to maintain the integrity of the mucosal epithelial layer.
An important role of endogenous negative regulatory mediators such as cytokines for the control of inappropriate innate immune recognition and maintenance of gut homeostasis is supported by in vivo studies. Likewise, mice deficient in interleukin (IL)-10 or IL-10R show spontaneous development of chronic intestinal inflammation.4,5 IL-10 has been shown to diminish inflammatory cytokine production in macrophages and to direct the differentiation of T cells towards T helper 2 (Th2) cells.6,7 Similarly, IL-11 exhibits a marked inhibitory effect on LPS-induced nuclear factor (NF)-κB activation and revealed cytoprotection and improved mucosal barrier formation in clinical studies.8,9 Additionally, mice carrying a dominant negative mutant form of the transforming growth factor-β (TGF-β) type II receptor develop spontaneous colitis (unless they are maintained under specific pathogen-free (SPF) conditions) and reduced local TGF-β production was associated with tissue injury in inflammatory bowel disease.10,11 Likewise, TGF-β1-deficient mice succumb to a systemic inflammatory disease within 3 weeks of life, which also involves the gastrointestinal tract.12–14 Finally, a polymorphism (−590T) within the IL-4 gene leading to reduced cytokine expression has been linked to Crohn's disease.15 However, cell type-specific cytokine activity has recently been described and the effect of negative regulatory cytokines on epithelial innate immune recognition has not been systematically evaluated.16
Here, we investigated the influence of IL-4, IL-10, IL-11, IL-13, and TGF-β on lipopolysaccharide (LPS)-mediated cellular stimulation of differentiated intestinal epithelial m-ICcl2 cells. Our study included the analysis of the cytokine receptor expression in laser microdissected primary intestinal epithelial cells and of cytokine-mediated epithelial cell signalling. The data obtained provide clear evidence that IL-4 and IL-13 significantly diminish epithelial LPS-mediated chemokine production and nitric oxide (NO) production through activation of the IL-4Rα/IL-13Rα1 heterodimeric type II IL-4 receptor.
Materials and methods
Antibodies and reagents
Escherichia coli
K12 D31m4 (Re) LPS was purchased from List Biological Laboratories (Campbell, CA). LPS purity was verified by complete inhibition of the stimulatory effect by polymyxin B, absent stimulation of TLR4-deficient macrophages, and no reduction of LPS activity after repeated phenol extraction (data not shown). Carbachol, and bovine liver arginase were ordered from Sigma (Deisenkirchen, Germany). The anti-TLR4/MD2 antibody was kindly provided by Kensuke Miyake (Department Immunology, Saga Medical School, Nabeshima, Saga, Japan). The NF-κB reporter construct (pBIIX-luciferase) encoding two copies of the κB sequences from the immunoglobulin κ (Igκ) enhancer 3′ of the luciferase gene was generously provided by S. Ghosh (Yale University Medical School, New Haven, CT). Recombinant murine or human cytokines were obtained from BD PharMingen (San Diego, CA) (rmIL-12) and from R & D Systems (Minneapolis, MN) (rmIL-4, rmIL-10, rmIL-11, rmIL-13, rmIL-12, rmIL-18, rhTGF-β1, recombinant murine tumour necrosis factor (rmTNF), recombinant murine interferon-γ (rmIFN-γ)). Quantification of membrane inhibitory protein-2 (MIP-2) in cell culture supernatant was performed using an enzyme-linked immunosorbent assay from Nordic BioSite (Täby, Sweden). The unstable NO is rapidly oxidized and accumulates as nitrite (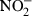 ) in culture supernatant.
) in culture supernatant. 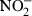 was measured using the Griess assay as described.12 Arginase activity in cell lysates is given as µmol urea/mg protein/min and was determined as described.17
was measured using the Griess assay as described.12 Arginase activity in cell lysates is given as µmol urea/mg protein/min and was determined as described.17
Cell culture and cell stimulation assays
Intestinal epithelial m-ICcl2 cells originally derived from mice transgenic for the simian virus large T antigen were grown in a modified hormonally defined fetal calf serum (FCS)-supplemented (2%) medium and incubated at 37° in a 5% CO2−95% air atmosphere.18 Culture on collagen-coated cell culture plates or on 0·4 µm pore size collagen coated Costar Transwell supports (Corning Inc. Corning, NY) for 6 days with medium changes every second day was previously shown to induce a differentiated and apical–basolateral polarized small intestinal epithelial phenotype.2,18 rmIL-4, rmIL-10, rmIL-11, rmIL-13 (each at 10 ng/ml) or rhTGF-β1 (5 ng/ml) was added prior to or simultaneously with LPS, LPS plus rmIFN-γ, or rmTNF as indicated. Cells were stimulated for 6 hr (MIP-2) or 24 hr (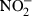 ) and supernatants were stored at −19° until further analysis. m-ICcl2 cells stably transfected with a NF-κB reporter construct were examined after 2·5 hr stimulation with 10 ng/ml LPS and luciferase activity was determined (Promega, Mannheim, Germany). Intestinal epithelial cell (IEC) isolation from C57BL/6 mice (Charles River Breeding Laboratories, Sulzfeld, Germany) was performed as previously described showing less than 1% contaminating CD45-positive cells.3 Peritoneal macrophages were obtained following intraperitoneal injection with 3 ml Brewer thioglycollate broth (Sigma) and seeded at 2 × 105 cells/well in 48-well plates 2 hr prior to stimulation to ensure adherance. RAW 264.7 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and propagated in RPMI-1640 medium (Gibco BRL, Grand Island, NY) supplemented with 20 mm HEPES, 2 mm l-glutamine, and 10% fetal calf serum (FCS).
) and supernatants were stored at −19° until further analysis. m-ICcl2 cells stably transfected with a NF-κB reporter construct were examined after 2·5 hr stimulation with 10 ng/ml LPS and luciferase activity was determined (Promega, Mannheim, Germany). Intestinal epithelial cell (IEC) isolation from C57BL/6 mice (Charles River Breeding Laboratories, Sulzfeld, Germany) was performed as previously described showing less than 1% contaminating CD45-positive cells.3 Peritoneal macrophages were obtained following intraperitoneal injection with 3 ml Brewer thioglycollate broth (Sigma) and seeded at 2 × 105 cells/well in 48-well plates 2 hr prior to stimulation to ensure adherance. RAW 264.7 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and propagated in RPMI-1640 medium (Gibco BRL, Grand Island, NY) supplemented with 20 mm HEPES, 2 mm l-glutamine, and 10% fetal calf serum (FCS).
For Ussing chamber experiments, distal small intestinal fragments from six mice were opened along the mesenteric border, layered on a Millipore® filter (8-µm pores), and mounted as flat sheets in Ussing chambers with an exposed surface area of 0·2 cm2. Tissue surfaces were bathed on both sides with 1 ml of Ringer solution, which was continuously thermostated, circulated, oxygenated, and maintained at pH 7·4 with 5% CO2/95% O2. The mucosal and serosal bathing solutions were connected via agar bridges to calomel electrodes for measurement of the transepithelial potential difference (PD) and to Ag–AgCl electrodes for current (I) application. The tissues were kept under open-circuit conditions for 4 hr, and electrical measurements were performed using a DVC 1000 voltage/current clamp (World Precision Instruments, Aston, UK). The tissue was regularly clamped (every 30 min) at 5 mV to measure I and to calculate the electrical resistance (R) according to Ohm's law (PD = R × I). Preservation of the epithelial barrier during the observed time period is indicated by lack of significant reduction in the transepithelial resistance in control tissue.
Laser microdissection (LMD) and reverse transcription–polymerase chain reaction (RT–PCR) analysis
Isolation of primary epithelial cells by LMD was performed as recently described using the AS LMD Microdissection System (Leica Mikrosystems, Wetzlar, Germany).3 Total RNA from m-ICcl2 cells or primary IECs was isolated using TRIzol® reagent (Gibco BRL) following the manufacturer's protocol. cDNA was synthesized from total mRNA using oligo(dT)18 primer from the first strand cDNA synthesis AMV kit (Roche, Mannheim, Germany). The following primers were used for DNA amplification: Il-4rα (forward: 5′- GGCTTTGCACCAAGTTCCTG-3′, reverse: 5′-TCGGGATGCATGTGAGGTTT-3′), Common γ chain (forward: 5′-ATGGAGCCAGCCTGTCCAC-3′, reverse: 5′-GCCCTTTAGACACACCACTCCA-3′), Il-13 rα1 (forward: 5′-CGAGCTGTTGGTGCTGCTAC-3′, reverse: 5′-CCAGGGGTAATTCCTCTTTACGA-3′), Il-11rα1 (forward: 5′-CAGCACGTGCCTACTGGATG-3′, reverse: 5′-CCCAGCCACAGCATCTGTTA-3′), gp130 (forward: 5′-CTTTGGGCAGATCGAGCAGA-3′, reverse: 5′-GCCATGCTTTGACTGGCAAT-3′), Il-10r2 (forward: 5′-CAGAGTGGTCGGAGGAGCAG-3′, reverse: 5′-GAGAGGGTTGGCTGGGAAGA-3′), Tgf-βrI (forward: 5′-GTCCGCAGCTCCTCATCGT-3′, reverse: 5′-GCCCCTGTTTTTGAAGATGG-3′), Tgf-βrII (forward: 5′-GCTGCATATCGTCCTGTGGA-3′, reverse: 5′-TTCTGGTTGTCGCAAGTGGA-3′), β2-microglobulin (forward: 5′-GCTCGGTGACCCTGGTCTTT-3′, reverse: 5′-AATGTGAGGCGGGTGGAACT-3′), Hprt1 (forward: 5′-TGATCAGTCAACGGGGGACA-3′, reverse: 5′-TTCGAGAGGTCCTTTTCACCA-3′). All primers used in this study were intron spanning as illustrated by the absence of an amplification product from mouse genomic DNA. The specificity of the listed primers was confirmed by sequencing of the PCR products. The quantitative RT–PCR was performed using an ABI Prism 7900 sequence detector (Applied Biosystems, Warrington, UK) in combination with following gene expression assays developed by Applied Biosystems: inducible NO synthase (iNOS, Mm00440485_m1), arginase-1 (Mm00475988_m1), arginase-2 (Mm00477592_m1), and hypoxanthine guanine phosphoribosyl transferase-1 (HPRT1, Mm00446968_m1).
Immunohistochemistry, immunoblotting, and fluorescence-activated cell sorting (FACS) analysis
Immunoblotting was performed using the following primary antibodies: a rabbit antiphospho-NF-κB p65 (Ser536) polyclonal antibody, a rabbit-antiphospho-stress-activated protein kinase (SAPK)/Janus kinase (JNK; Thr183/Tyr185) monoclonal antibody, a rabbit-antiphospho-p38 mitogen-activated protein kinase (Thr180/Tyr182) monoclonal antibody, a rabbit-antiphospho-signal transducer and activator of transcription 6 (STAT6; Tyr641) polyclonal antibody, a rabbit-antiphospho-STAT3 (Tyr705) monoclonal antibody (all from Cell Signaling Technology, Beverly, MA, and used at the recommended concentrations), a rabbit-anti-iNOS polyclonal antibody (1 : 5000),10 as well as rabbit polyclonal antibodies against STAT3 (C20), STAT6 (S20), and heat-shock protein-90 (all from Santa Cruz Biotechnology Inc., and used at the recommended concentrations). Detection was performed using peroxidase-labelled goat anti-rabbit secondary antibodies (Jackson ImmunoResearch, West Grove, PA) in combination with the ECL kit (Amersham Bioscience, Amersham, UK). FACS analysis of TLR4/MD-2 expression was performed using a rat anti-TLR4/MD2 antibody and goat anti-rat fluoroscein isothiocyanate (FITC)-conjugated secondary antibody (Jackson ImmunoResearch Laboratories) following fixation (Cytofix, BD Pharmingen) with permeabilization in Ca2+ and Mg2+-free phosphate-buffered saline (PBS) containing 0·5% saponin and 2% FCS.
The human IL-8 (NT_006216) and the murine MIP-2 (NT_109320) genomic nucleotide sequence was analysed for binding sites for transcription factors using the MatInspector program.21
Statistical analysis
Results are given as the mean ± SD of one representative experiment out of at least three performed in triplicate or quadruplicate. Statistical analyses were performed using the one-way anova test with Tukey's multiple comparison or the Student's t-test as appropriate. A P-value < 0·05 was considered significant. Quantitative RT–PCR analyses (TaqMan) were performed in triplicate and are depicted as relative expression levels (average) including 95% confidential interval normalized to expression of the housekeeping gene HPRT. One representative experiment out of at least three is shown.
Results
IL-4 and IL-13 diminish LPS-mediated cell activation
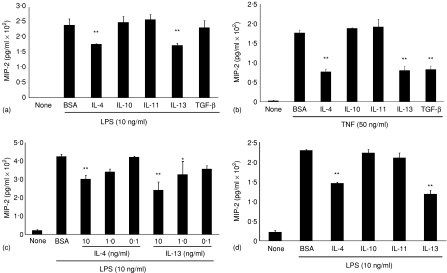
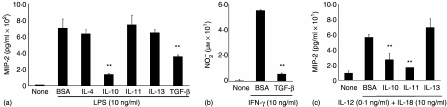
m-ICcl2 cells were exposed to IL-4, IL-10, IL-11, IL-13 (all 10 ng/ml), or TGF-β1 (5 ng/ml), and simultaneously stimulated with LPS (10 ng/ml). A significant reduction (P < 0·05) of LPS-induced cellular activation was only detected for IL-4 and IL-13 (Fig. 1a). A similar inhibitory effect was observed in m-ICcl2 cells grown on transwell supports following basolateral exposure to IL-4 or IL-13 (data not shown). Similar to macrophages, TGF-β1 diminished TNF- but not LPS-induced MIP-2 secretion (Fig. 1b).14 The IL-4- or IL-13-mediated suppressive effect was dose dependent (Fig. 1c) and not enhanced by simultaneous addition of IL-10, IL-11, or TGF-β. No synergistic effect of IL-4 and IL-13 was noted (data not shown). Also, preincubation with IL-4 or IL-13 for 24–48 hr prior to LPS stimulation did not further enhance the inhibitory activity (Fig. 1d). The biological activity of the recombinant cytokine preparations used was verified using primary peritoneal macrophages. Both IL-10 and TGF-β significantly inhibited LPS-mediated cytokine production and addition of TGF-β1 abolished IFN-γ-induced NO production (Fig. 2a and b).14,22 Also, IL-10 and IL-11 significantly diminished MIP-2 secretion in response to combined IL-12 and IL-18 stimulation (Fig. 2c). Thus, IL-4 and IL-13 exhibit an inhibitory activity on LPS-induced MIP-2 secretion by epithelial cells. Interestingly, peritoneal macrophages, as compared to IEC, showed a markedly different pattern of responsiveness.
Figure 1.
Inhibitory cytokine effect on LPS-stimulated IECs. Epithelial cells were simultaneously exposed to (a)LPS (10 ng/ml) or (b) TNF (50 ng/ml) and bovine serum albumin (BSA), IL-4, IL-10, IL-11, or IL-13 or TGF-β1 and MIP-2 was quantified in cell culture supernatant. (c) Dose–response of the inhibitory effect of IL-4 and IL-13. (d) LPS stimulation following preincubation with the indicated cytokines for 48 hr. One representative experiment out of three is shown. *P < 0·05; **P < 0·01.
Figure 2.
Inhibitory cytokine effect on LPS-stimulated peritoneal macrophages. (a) Peritoneal macrophages were simultaneously exposed to LPS (10 ng/ml) and BSA, IL-4, IL-10, IL-11, or IL-13 (each at 10 ng/ml) or TGF-β1 (5 ng/ml) and MIP-2 was quantified in cell culture supernatant after 6 hr incubation at 37°. (b) Peritoneal macrophages were stimulated with interferon-(IFN)γ (10 ng/ml) preincubated in the absence or presence of TGF-β1 (5 ng/ml) and the concentration of 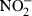 in cell culture supernatant was determined. (c) Peritoneal macrophages were simultaneously stimulated with IL-12 (0·1 ng/ml) and IL-18 (10 ng/ml) in the presence of the indicated cytokines and MIP-2 was quantified after 6 hr incubation at 37°. *P < 0·05; **P < 0·01.
in cell culture supernatant was determined. (c) Peritoneal macrophages were simultaneously stimulated with IL-12 (0·1 ng/ml) and IL-18 (10 ng/ml) in the presence of the indicated cytokines and MIP-2 was quantified after 6 hr incubation at 37°. *P < 0·05; **P < 0·01.
Cytokine receptor expression by intestinal epithelial cells
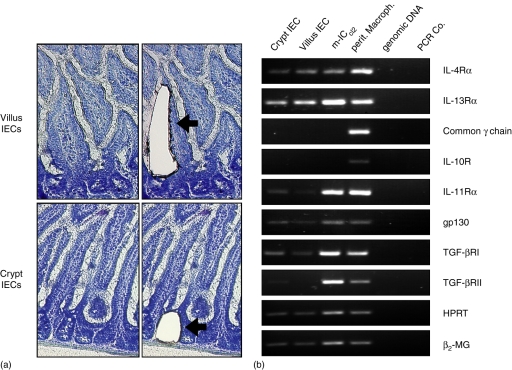
To investigate possible mechanisms, cytokine receptor expression was studied. Primary epithelial cells from the villus region as well as the crypt region were separately collected by LMD and analysed together with m-ICcl2 cells and peritoneal macrophages by RT–PCR (Fig. 3a). Whereas peritoneal macrophages displayed a wide range of cytokine receptors, the pattern expressed by epithelial cells was more limited. Expression of the IL-4Rα chain as well as the IL-13Rα chain were readily detected in all intestinal epithelial cell preparations examined (Fig. 3b). This confirms an earlier report indicating expression of the IL-4Rα by IECs23. The expression of the IL-11Rα chain, gp130, TGF-βRI, and TGF-βRII was also found in primary epithelial cells, although at somewhat lower levels.11,13 No significant difference in the cytokine receptor expression was found between crypt epithelial and villus epithelial cells. In contrast, primary IECs and m-ICcl2 cells completely lacked detectable expression levels of the IL10Rα as well as the common γ-chain (γc). The common γc represents an essential constituent of several cytokine receptors such as the IL-2, type I IL-4, IL-7, and IL-15 receptor and lack of γc expression therefore indicates the absence of relevant numbers of contaminating intraepithelial lymphocytes in the cell preparation. However, the presence of non-epithelial cells in the laser-microdissected preparations cannot be excluded. Of note, the receptor chain expression pattern detected in polarized m-ICcl2 cells completely matched the pattern found in primary IECs supporting their well-preserved intestinal epithelial phenotype.
Figure 3.
RT–PCR analysis of cytokine receptor expression. (a) Primary crypt and villus IECs were obtained from tissue sections by LMD. Illustration of the tissue sections before (left) and after (right) the laser-directed dissection procedure. Magnification ×200. (b) RT–PCR of total RNA-derived cDNA from laser-microdissected IECs, peritoneal macrophages, or m-ICcl2 cells. Genomic DNA and water was added as control for the intron-spanning primer pairs. One representative out of three experiments is shown.
Cytokine effects on transepithelial resistance, TLR4 expression and intracellular signalling
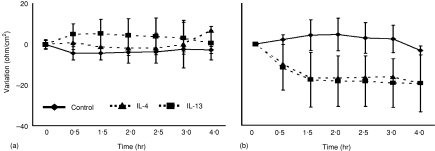
IL-4 and IL-13 were shown to increase paracellular ion flux and fluid transport and to reduce the transepithelial resistance in human colon epithelial T84 cells.24,25 In order to verify responsiveness of intestinal epithelial cells to IL-4 and IL-13 in situ, the transepithelial ion current was measured in primary intestinal tissue using an established model of enteric tissue analysis in Ussing chambers. Indeed, a marked reduction of the transepithelial resistance suggesting an increase of the paracellular permeability was observed after 1·5 hr in the presence of high concentrations (30 ng/ml) of IL-4 and IL-13 (Fig. 4). A similar decrease of the transepithelial resistance was also noted in the presence (100 µm) of the parasympaticomimetic agent carbachol (data not shown). These results confirm the functional responsiveness of epithelial cells to IL-4 and IL-13 and support the results obtained by the cytokine receptor expression analysis.
Figure 4.
Cytokine effect on transepithelial resistance. Intestinal tissue was mounted into Ussing chambers and incubated in the absence or presence of (a) 10 ng/ml or (b) 30 ng/ml IL-4 or IL-13. Transepithelial resistance was measured at the indicated time points. The graphs indicate the variation in resistance over time (ohm/cm2).
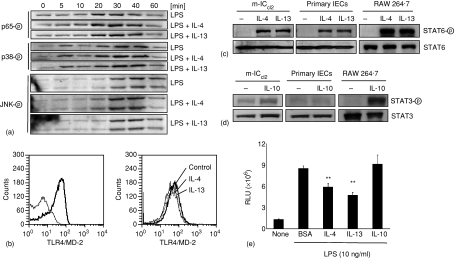
To investigate a possible effect of IL-4 and IL-13 on epithelial TLR signalling, phosphorylation of p38 and the c-Jun NH2 terminal kinase (JNK) as well as the NF-κB subunit p65 was examined. However, no detectable difference was noted between cells stimulated in the absence or presence of IL-4 or IL-13 (Fig. 5a). IL-4 and IL-13 induced down-regulation of TLR4 expression was recently reported in human colon epithelial cells but no significant alteration of the LPS receptor expression was noted in mouse intestinal epithelial m-ICcl2 cells even after prolonged incubation (Fig. 5b).26
Figure 5.
Cytokine mediated effects on cellular signalling and NF-κB activation. (a) m-ICcl2 cells were exposed to LPS (10 ng/ml) in the absence or presence of IL-4 or IL-13 for the indicated time periods and the cell lysate was analysed for phospho-p65, phospho-p38 (left), and phospho-JNK (right). (b) FACS analysis of m-ICcl2 cells stained with isotype control or anti-TLR4/MD-2 antibodies (left). m-ICcl2 cells were left untreated or exposed to IL-4 or IL-13 (10 ng/ml) for 16 hr. m-ICcl2 cells, primary isolated IECs, and macrophage-like RAW 264.7 cells were exposed to IL-4 or IL-13 (10 ng/ml) or IL-10 (10 ng/ml) for 20 min and the cell lysate was examined for total STAT6 as well as a phospho-STAT6 (c) or total STAT3 as well as a phospho-STAT3 (d), respectively. (e) m-ICcl2 cells carrying a stable NF-κB luciferase reporter construct were stimulated with LPS in the absence or presence of the indicated cytokines and the luciferase production was quantified using a luminometric assay. One representative experiment out of three is shown. **P < 0·01.
In line with the differential cytokine receptor expression, m-ICcl2 cells and macrophage-like RAW 264.7 cells rapidly phosphorylated STAT6 in response to IL-4 and IL-13 (Fig. 5c) whereas STAT3 phosphorylation in response to IL-10 stimulation was restricted to macrophages (Fig. 5d). Importantly, rapid STAT6 phosphorylation was also observed in freshly isolated primary IECs demonstrating the presence of functional IL-4 type II receptors on primary gut epithelium (Fig. 5c). Finally, also transcriptional activity of m-ICcl2 cells expressing a NF-κB reporter construct was significantly diminished in the presence of IL-4 or IL-13 (Fig. 5e), indicating a direct cytokine effect on the LPS-induced gene regulation.
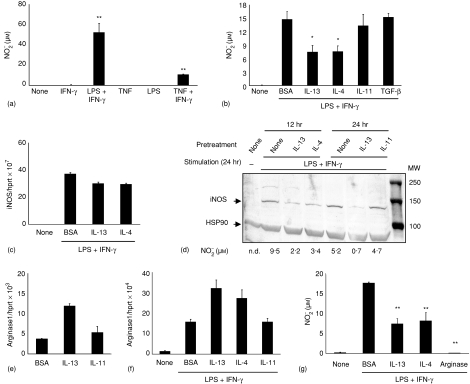
Cytokine effects on epithelial NO production
In addition to chemokine secretion, IFN-γ and/or LPS-induced production of NO as measured by NO2– determination was examined. m-ICcl2 cells readily released significant concentrations of NO upon simultaneous stimulation with LPS or TNF and IFN-γ(Fig. 6a) and epithelial NO secretion was significantly (P < 0·01) diminished after preincubation with IL-4 and IL-13 for 12 h (Fig. 6b). Inducible NO synthase (iNOS or NOS2) metabolizes the amino acid l-arginine in the presence of oxygen to citrulline and NO. iNOS is expressed by multiple cell types and NO exerts a wide range of antimicrobial and regulatory functions.27 In macrophages, iNOS expression is regulated both at the transcriptional and the post-transcriptional level.19 Therefore, the expression of iNOS mRNA, protein and activity in intestinal epithelial cells was quantitatively analysed. Whereas iNOS mRNA was only marginally diminished after 12 h IL-4 or IL-13 incubation (Fig. 6c) the marked induction of iNOS protein by LPS/IFN-γ was severely reduced in the presence of IL-4 or IL-13 but not IL-11 (Fig. 6d). In addition, the production of NO is regulated by metabolism of the iNOS substrate arginine by arginase. Arginase which exists in two isoforms (arginase 1 and arginase 2) degrades l-arginine to urea and ornithine and thereby limits the availability of free arginine.27 Because efficient production of NO was shown to depend on extracellular arginine, arginine depletion may limit NO production. In addition, arginine seems to directly affect translational control of iNOS synthesis.19,28 IL-13 but not IL-11 caused enhanced arginase-1 expression (Fig. 6e). Also, the LPS/IFN-γ induced arginase-1 levels were further increased by preincubation with IL-4 or IL-13 but not IL-11 for 12 hr (Fig. 6f). In contrast, no elevation of arginase 2 mRNA was noted (data not shown). Enhanced arginase metabolic activity was additionally noticed after prolonged IL-4 or IL-13 incubation (data not shown). Also, addition of exogenous arginase (4 U/ml) to the cell culture medium prior to LPS/IFN-γ stimulation caused a potent reduction of 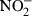 production (Fig. 6g).
production (Fig. 6g).
Figure 6.
Cytokine mediated effects on LPS-induced epithelial 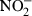 production. (a) NO production in response to stimulation with LPS (100 ng/ml) or TNF (5 ng/ml) and IFN-γ (20 ng/ml) for 24 hr. (b) Reduction of the LPS/IFN-γ induced NO synthesis by preincubation with IL-4 and IL-13 but not IL-11 or TGF-β for 12 hr. The lower total NO production as compared to Fig. 2(e) is explained by the extended culture time.19 (c) Quantitative iNOS mRNA analysis of m-ICcl2 cells preincubated in the absence or presence of IL13 or IL-4 for 12 hr and subsequently stimulated with LPS (100 ng/ml) and IFN-γ (20 ng/ml) for 24 hr. Values are presented as RQ ± RQ maximum and minimum, respectively, and indicate epithelial iNOS mRNA expression normalized to HPRT1. (d) Immunoblot of m-ICcl2 cells pretreated for 12 hr (left) or 24 hr (right) with medium alone, IL-4, IL-13, or IL-11 as indicated and subsequently stimulated with LPS/IFN-γ for 24 hr; 120 µg total cell lysate were loaded in each lane. The results are representative for three independent experiments. The numbers indicate NO concentrations in the cell supernatant from the experiment. Note that because of a different medium volume to cell ratio, reduced NO quantities are found as compared to (b) and (g). (e) Quantitative arginase 1 mRNA analysis of m-ICcl2 cells pretreated for 12 hr with medium, IL-13, IL-11. (f) Quantitative arginase 1 mRNA analysis of m-ICcl2 cells after incubation with medium, IL-13, IL-4, or IL-11 for 24 hr and subsequent stimulation with LPS/IFN-γ for 24 hr. (g) Both addition of 4 U/ml purified arginase or pretreatment with IL-4 or IL-13 for 24 hr to the culture of m-ICcl2 cells stimulated with IFN-γ/LPS for 24 hr led to a reduction of NO production. The results from stimulation assays and Western blot are representative for three independent experiments. *P < 0·05, **P < 0·01.
production. (a) NO production in response to stimulation with LPS (100 ng/ml) or TNF (5 ng/ml) and IFN-γ (20 ng/ml) for 24 hr. (b) Reduction of the LPS/IFN-γ induced NO synthesis by preincubation with IL-4 and IL-13 but not IL-11 or TGF-β for 12 hr. The lower total NO production as compared to Fig. 2(e) is explained by the extended culture time.19 (c) Quantitative iNOS mRNA analysis of m-ICcl2 cells preincubated in the absence or presence of IL13 or IL-4 for 12 hr and subsequently stimulated with LPS (100 ng/ml) and IFN-γ (20 ng/ml) for 24 hr. Values are presented as RQ ± RQ maximum and minimum, respectively, and indicate epithelial iNOS mRNA expression normalized to HPRT1. (d) Immunoblot of m-ICcl2 cells pretreated for 12 hr (left) or 24 hr (right) with medium alone, IL-4, IL-13, or IL-11 as indicated and subsequently stimulated with LPS/IFN-γ for 24 hr; 120 µg total cell lysate were loaded in each lane. The results are representative for three independent experiments. The numbers indicate NO concentrations in the cell supernatant from the experiment. Note that because of a different medium volume to cell ratio, reduced NO quantities are found as compared to (b) and (g). (e) Quantitative arginase 1 mRNA analysis of m-ICcl2 cells pretreated for 12 hr with medium, IL-13, IL-11. (f) Quantitative arginase 1 mRNA analysis of m-ICcl2 cells after incubation with medium, IL-13, IL-4, or IL-11 for 24 hr and subsequent stimulation with LPS/IFN-γ for 24 hr. (g) Both addition of 4 U/ml purified arginase or pretreatment with IL-4 or IL-13 for 24 hr to the culture of m-ICcl2 cells stimulated with IFN-γ/LPS for 24 hr led to a reduction of NO production. The results from stimulation assays and Western blot are representative for three independent experiments. *P < 0·05, **P < 0·01.
Discussion
Dysfunction of inhibitory endogenous regulators has been suggested to contribute to intestinal inflammation in patients with inflammatory bowel disease (IBD) or various animal models of enteric inflammation.29 The presented data support a role of IL-4 and IL-13 in intestinal homeostasis demonstrating type II IL-4 receptor expression, IL-4 and IL-13-induced STAT6 phosphorylation and decrease of gene transcription and proinflammatory chemokine secretion by intestinal epithelial cells. Of note, this work does not exclude an indirect effect of, e.g. IL-10 or TGF-β on epithelial cells exerted by their possible activity on other resident enteric cell types.
Both IL-4 and IL-13 were first described for their suppressive effect on cytokine secretion but were subsequently shown to exert unique features. Whereas IL-4 is mainly associated with the regulation of Th2 responses in the gastrointestinal tract, IL-13 shows an unique importance for airway hyperresponsiveness, allergic inflammation, and protection from helminthic infections, as well as unexpected activities on tumour growth, tissue remodelling, and fibrosis.25,30–35 IL-4 and IL-13 have also been shown to up-regulate mucus production and secretion of the specific goblet cell product trefoil factor-3 (TFF3), an important mediator of mucosal homeostasis.36,37
IL-4 is recognized by two different receptors. In myeloid cells, the IL-4Rα chain associates with the common gamma chain (γc) to form the type I IL-4 receptor. In haematopoietic and non-haematopoietic cells, the type II receptor is constituted by the IL-4Rα chain and the IL-13Rα1 chain.30,38 Importantly, the IL-4 receptor type II (IL-4Rα/IL-13Rα1) is also utilized by IL-13. The absence of γc expression in epithelial cells indicates usage of the type II IL-4 receptor and therefore explains the comparable activity seen using IL-4 and IL-13 as well as the lack of synergy of both cytokines. Importantly, our data are supported by the recent description of a polymorphism (−590T) within the IL-4 gene which led to a reduced cytokine expression and was linked to Crohn's disease.15 However, the role of IL-4 seems to be more complex, since IL-4 was also shown to exacerbate colitis in a CD4+ CD45RBhigh T-cell transfer model of colitis and elevated IL-4 and IL-13 levels were found during the chronic phase of the IL-10-deficient mouse colitis model.39,40
Cytokine binding to the type II IL-4R induces recruitment and activation of the Janus family of receptor associated kinases (JAK1–3, and Tyk2).30,39 JAK1 binding to IL-4Rα induces recruitment and phosphorylation of STAT6. STAT6 then disengages, homodimerizes, and migrates to the nucleus to bind to consensus sequences found within the promotor region of regulated genes. Rapid phosphorylation of the transcription factor STAT6 upon exposure to IL-4 or IL-13 suggested a direct inhibitory effect of STAT6 on Mip-2 transcription. Indeed, similar to the human IL-8 gene (position −1850 bp), a STAT6 binding site was also identified within the promoter region of the Mip-2 gene (position −1720). Because of overlapping binding sites between the NF-κB binding region and a sequence closely related to the STAT6 consensus binding site within the promotor region, STAT6 might additionally exert a competitive inhibitory effect as recently suggested.41 IL-4 and IL-13 also inhibited the IFN-γ/LPS-induced NO production by intestinal epithelial cells. The reduced NO levels were associated with a diminished iNOS expression and enhanced arginase 1 mRNA levels. Thus, the strongly reduced NO production might result from three different levels of action, i.e. from the suppression of iNOS mRNA, the depletion of the iNOS substrate arginine by arginase, and – in analogy to the findings with macrophages19 and astrocytes28– from the reduction of iNOS protein synthesis as a consequence of an arginase-induced lack of arginine.
In conclusion, we present a detailed analysis of cytokine receptor expression and functional responsiveness of LPS-induced intestinal epithelial cells to IL-4, IL-10, IL-11, IL-13 and TGF-β1. We demonstrate that IL-4 and IL-13 via the IL-4 receptor type II (IL-4Rα/IL-13Rα1) exert a modulatory effect on TLR4-induced epithelial cell activation. IL-4 and IL-13 induce rapid cellular signalling, alter expression of proinflammatory genes, and reduce NO production and might thereby contribute to prevent inappropriate host responses of intestinal epithelial cells to luminal microbial substances. However, additional mechanisms such as the recently proposed acquisition of epithelial endotoxin tolerance or negative regulatory circuits must exist to limit cell activation and maintain the enteric mucosal homeostasis.3,42
Acknowledgments
We are indepted to Dr Mathias Kirsch and Andreas Steup, Institute for Anatomie, University of Freiburg for help with the laser microdissection microscope and to Dr Martine Heyman for generous help with the Ussing chamber experiments. This work has been supported by grants from the German Research Foundation (DFG grant HO 2236/5-1 and 5-2; grant SFB620 project A9 to C.B.), the Swedish Research Council (K2003-31P-14792), Cancerfonden, the University of Freiburg, and the Fondation de la Recherche Médical (FRM).
References
- 1.Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–72. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 2.Hornef MW, Frisan T, Vandewalle A, Normark S, Richter-Dahlfors A. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J Exp Med. 2002;195:559–70. doi: 10.1084/jem.20011788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lotz M, Gutle D, Walther S, Menard S, Bogdan C, Hornef MW. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med. 2006;203:973–84. doi: 10.1084/jem.20050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 5.Moore KW, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–90. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 6.Spencer SD, DiMarco F, Hooley J, et al. The orphan receptor CRF2–4 is an essential subunit of the interleukin 10 receptor. J Exp Med. 1998;187:571–8. doi: 10.1084/jem.187.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopydlowski KM, Salkowski CA, Cody MJ, van Rooijen N, Major J, Hamilton TA, Vogel SN. Regulation of macrophage chemokine expression by lipopolysaccharide in vitro and in vivo. J Immunol. 1999;163:1537–44. [PubMed] [Google Scholar]
- 8.Ellis M, Zwaan F, Hedstrom U, Poynton C, Kristensen J, Jumaa P, Wassell J, al-Ramadi B. Recombinant human interleukin 11 and bacterial infection in patients of haematological malignant disease undergoing chemotherapy: a double-blind placebo-controlled randomised trial. Lancet. 2003;361:275–80. doi: 10.1016/s0140-6736(03)12322-7. [DOI] [PubMed] [Google Scholar]
- 9.Shull MM, Ormsby I, Kier AB, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–9. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Zotto B, Mumolo G, Pronio AM, Montesani C, Tersigni R, Boirivant M. TGF-beta1 production in inflammatory bowel disease: differing production patterns in Crohn's disease and ulcerative colitis. Clin Exp Immunol. 2003;134:120–6. doi: 10.1046/j.1365-2249.2003.02250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahm KB, Im YH, Parks TW, Park SH, Markowitz S, Jung HY, Green J, Kim SJ. Loss of transforming growth factor beta signalling in the intestine contributes to tissue injury in inflammatory bowel disease. Gut. 2001;49:190–8. doi: 10.1136/gut.49.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulkarni AB, Huh CG, Becker D, et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–4. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ropeleski MJ, Tang J, Walsh-Reitz MM, Musch MW, Chang EB. Interleukin-11-induced heat shock protein 25 confers intestinal epithelial-specific cytoprotection from oxidant stress. Gastroenterology. 2003;124:1358–68. doi: 10.1016/s0016-5085(03)00282-8. [DOI] [PubMed] [Google Scholar]
- 14.Vodovotz Y, Bogdan C, Paik J, Xie QW, Nathan C. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor beta. J Exp Med. 1993;178:605–13. doi: 10.1084/jem.178.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein W, Tromm A, Griga T, et al. Interleukin-4 and interleukin-4 receptor gene polymorphisms in inflammatory bowel diseases. Genes Immun. 2001;2:287–9. doi: 10.1038/sj.gene.6363779. [DOI] [PubMed] [Google Scholar]
- 16.Lee JH, Kaminski N, Dolganov G, Grunig G, Koth L, Solomon C, Erle DJ, Sheppard D. Interleukin-13 induces dramatically different transcriptional programs in three human airway cell types. Am J Respir Cell Mol Biol. 2001;25:474–85. doi: 10.1165/ajrcmb.25.4.4522. [DOI] [PubMed] [Google Scholar]
- 17.Corraliza IM, Campo ML, Soler G, Modolell M. Determination of arginase activity in macrophages: a micromethod. J Immunol Methods. 1994;174:231–5. doi: 10.1016/0022-1759(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 18.Bens M, Bogdanova A, Cluzeaud F, et al. Transimmortalized mouse intestinal cells (m-ICc12) that maintain a crypt phenotype. Am J Physiol. 1996;270:C1666–74. doi: 10.1152/ajpcell.1996.270.6.C1666. [DOI] [PubMed] [Google Scholar]
- 20.Hornef MW, Normark BH, Vandewalle A, Normark S. Intracellular recognition of lipopolysaccharide by toll-like receptor 4 in intestinal epithelial cells. J Exp Med. 2003;198:1225–35. doi: 10.1084/jem.20022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Gayar S, Thuring-Nahler H, Pfeilschifter J, Rollinghoff M, Bogdan C. Translational control of inducible nitric oxide synthase by IL-13 and arginine availability in inflammatory macrophages. J Immunol. 2003;171:4561–8. doi: 10.4049/jimmunol.171.9.4561. [DOI] [PubMed] [Google Scholar]
- 21.Cartharius K, Frech K, Grote K, et al. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Boinformatics. 2005;21:2933–42. doi: 10.1093/bioinformatics/bti473. [DOI] [PubMed] [Google Scholar]
- 22.Naiki Y, Michelsen KS, Zhang W, Chen S, Doherty TM, Arditi M. Transforming growth factor-beta differentially inhibits MyD88-dependent, but not TRAM- and TRIF-dependent, lipopolysaccharide-induced TLR4 signaling. J Biol Chem. 2005;280:5491–5. doi: 10.1074/jbc.C400503200. [DOI] [PubMed] [Google Scholar]
- 23.Reinecker HC, Podolsky DK. Human intestinal epithelial cells express functional cytokine receptors sharing the common gamma c chain of the interleukin 2 receptor. Proc Natl Acad Sci USA. 1995;92:8353–7. doi: 10.1073/pnas.92.18.8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zund G, Madara JL, Dzus AL, Awtrey CS, Colgan SP. Interleukin-4 and interleukin-13 differentially regulate epithelial chloride secretion. J Biol Chem. 1996;271:7460–4. doi: 10.1074/jbc.271.13.7460. [DOI] [PubMed] [Google Scholar]
- 25.Ceponis PJ, Botelho F, Richards CD, McKay DM. Interleukins 4 and 13 increase intestinal epithelial permeability by a phosphatidylinositol 3-kinase pathway. Lack of evidence for STAT 6 involvement. J Biol Chem. 2000;275:29132–7. doi: 10.1074/jbc.M003516200. [DOI] [PubMed] [Google Scholar]
- 26.Mueller T, Terada T, Rosenberg IM, Shibolet O, Podolsky DK. Th2 cytokines down-regulate TLR expression and function in human intestinal epithelial cells. J Immunol. 2006;176:5805–14. doi: 10.4049/jimmunol.176.10.5805. [DOI] [PubMed] [Google Scholar]
- 27.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–16. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Ryu H, Ferrante RJ, Morris SM, Jr, Ratan RR. Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. Proc Natl Acad Sci USA. 2003;100:4843–8. doi: 10.1073/pnas.0735876100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duchmann R, Neurath MF, Meyer zum Buschenfelde KH. Responses to self and non-self intestinal microflora in health and inflammatory bowel disease. Res Immunol. 1997;148:589–94. doi: 10.1016/s0923-2494(98)80154-5. [DOI] [PubMed] [Google Scholar]
- 30.Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science. 2003;300:1527–8. doi: 10.1126/science.1085458. [DOI] [PubMed] [Google Scholar]
- 31.Rengarajan J, Szabo SJ, Glimcher LH. Transcriptional regulation of Th1/Th2 polarization. Immunol Today. 2000;21:479–83. doi: 10.1016/s0167-5699(00)01712-6. [DOI] [PubMed] [Google Scholar]
- 32.Madden KB, Whitman L, Sullivan C, Gause WC, Urban JF, Jr, Katona IM, Finkelman FD, Shea-Donohue T. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. J Immunol. 2002;169:4417–22. doi: 10.4049/jimmunol.169.8.4417. [DOI] [PubMed] [Google Scholar]
- 33.Zhao A, McDermott J, Urban JF, Jr, et al. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J Immunol. 2003;171:948–54. doi: 10.4049/jimmunol.171.2.948. [DOI] [PubMed] [Google Scholar]
- 34.Interleukin-13. In: Grünig G, de Vries JE, de Waal Malefyt R, editors; Thomson AW, Lotze MT, editors. The Cytokine Handbook. 4. London: Elsevier Science Ltd; 2003. pp. 409–29. [Google Scholar]
- 35.Zhao A, Morimoto M, Dawson H, et al. Immune regulation of protease-activated receptor-1 expression in murine small intestine during Nippostrongylus brasiliensis infection. J Immunol. 2005;175:2563–9. doi: 10.4049/jimmunol.175.4.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blanchard C, Durual S, Estienne M, Bouzakri K, Heim MH, Blin N, Cuber JC. IL-4 and IL-13 up-regulate intestinal trefoil factor expression: requirement for STAT6 and de novo protein synthesis. J Immunol. 2004;172:3775–83. doi: 10.4049/jimmunol.172.6.3775. [DOI] [PubMed] [Google Scholar]
- 37.Dabbagh K, Takeyama K, Lee HM, Ueki IF, Lausier JA, Nadel JA. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J Immunol. 1999;162:6233–7. [PubMed] [Google Scholar]
- 38.Murata T, Noguchi PD, Puri RK. IL-13 induces phosphorylation and activation of JAK2 Janus kinase in human colon carcinoma cell lines: similarities between IL-4 and IL-13 signaling. J Immunol. 1996;156:2972–8. [PubMed] [Google Scholar]
- 39.Fort M, Lesley R, Davidson N, Menon S, Brombacher F, Leach M, Rennick D. IL-4 exacerbates disease in a Th1 cell transfer model of colitis. J Immunol. 2001;166:2793–800. doi: 10.4049/jimmunol.166.4.2793. [DOI] [PubMed] [Google Scholar]
- 40.Spencer DM, Veldman GM, Banerjee S, Willis J, Levine AD. Distinct inflammatory mechanisms mediate early versus late colitis in mice. Gastroenterology. 2002;122:94–105. doi: 10.1053/gast.2002.30308. [DOI] [PubMed] [Google Scholar]
- 41.Bennett BL, Cruz R, Lacson RG, Manning AM. Interleukin-4 suppression of tumor necrosis factor alpha-stimulated E-selectin gene transcription is mediated by STAT6 antagonism of NF-kappaB. J Biol Chem. 1997;272:10212–9. doi: 10.1074/jbc.272.15.10212. [DOI] [PubMed] [Google Scholar]
- 42.Otte JM, Cario E, Podolsky DK. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology. 2004;126:1054–70. doi: 10.1053/j.gastro.2004.01.007. [DOI] [PubMed] [Google Scholar]