Abstract
Type 1 interferon-β (T1IFN-β) is an innate cytokine and the first-choice therapy for multiple sclerosis (MS). It is still unclear how T1IFN-β, whose main function is to promote innate immunity during infections, plays a beneficial role in autoimmune disease. Here we show that T1IFN-β promoted the expansion and function of invariant natural killer (iNKT) cells, an innate T-cell subset with strong immune regulatory properties that is able to prevent autoimmune disease in pre-clinical models of MS and type 1 diabetes. Specifically, we observed that T1IFN-β treatment significantly increased the percentages of Vα24+ NKT cells in peripheral blood mononuclear cells of MS patients. Furthermore, iNKT cells of T1IFN-β-treated individuals showed a dramatically improved secretion of cytokines (interleukins 4 and 5 and interferon-γ) in response to antigenic stimulation compared to iNKT cells isolated from the same patients before T1IFN-β treatment. The effect of T1IFN-β on iNKT cells was mediated through the modulation of myeloid dendritic cells (DCs). In fact, DCs modulated in vivo or in vitro by T1IFN-β were more efficient antigen-presenting cells for iNKT cells. Such a modulatory effect of T1IFN-β was associated with up-regulation on DCs of key costimulatory molecules for iNKT (i.e. CD80, CD40 and CD1d). Our data identified the iNKT cell/DC pathway as a new target for the immune regulatory effect of T1IFNs in autoimmune diseases and provide a possible mechanism to explain the clinical efficacy of T1IFN-β in MS.
Keywords: autoimmunity, CD1d-restricted natural killer T cells, cytokines/interferons, dendritic cells, human studies
Introduction
Type 1 interferon-β (T1IFN-β) therapy represented a major breakthrough for the treatment of multiple sclerosis (MS), a T-cell-mediated autoimmune disease of the central nervous system that affects 2·5 million people worldwide. Indeed, the administration of T1IFN-β to patients with the relapsing–remitting form of MS (RR-MS) changed the natural history of the disease by dramatically reducing the incidence of relapses.1–3 Although T1IFN-β is the first-choice drug for treatment of RR-MS, its mechanism of action remains unclear. In particular, it is puzzling how an innate cytokine, whose primary function is to enhance antiviral T-cell immunity, can counter-regulate T-cell autoimmunity in MS. It was originally proposed that T1IFN-β therapy could reduce the number of viral infections that trigger autoimmune relapses in MS. Thus far, there is neither direct evidence to prove that T1IFN-β-treated MS patients react more efficiently to common infections nor has a direct link between viral infections and MS relapses been confirmed. Recently, new modulatory functions for T1IFNs were demonstrated, revealing that those innate cytokines affect almost every cell type and immune function and exert multiple actions on innate and adaptive immunity.4,5 The pleiotropic properties of T1IFNs are accounted by the fact that T1IFN receptors are broadly expressed on most cells and that signalling through them modulates the expression of a large variety of genes.6 Specifically, T1IFNs are capable of shaping the responses of natural killer (NK) and CD8+ T cells,7,8 augment the secretion of key cytokines and growth factors for T cells such as interleukin-15 (IL-15)9,10 and regulate dendritic cell (DC) maturation and function.11–13 The recently revealed modulatory role of T1IFNs suggested the hypothesis that T1IFN-β could prevent autoimmunity in MS by promoting regulatory mechanisms. Specifically, it could enhance the function of innate T cells with immune regulatory ability, such as invariant NK T (iNKT) cells, that are capable of preventing autoimmune diseases in preclinical models of MS and type 1 diabetes.14–19
The iNKT cells represent an unusual regulatory T-cell subset with NK cell markers and an invariant T-cell receptor α chain (human Vα24 and murine Vα14) that recognizes self-glycolipid antigens presented by the restriction major histocompatibility complex (MHC) class I-like molecule CD1d.20 The idea that iNKT cells are key immune regulators for the prevention of T-cell autoimmunity originated from the observation that patients affected by different T-cell-mediated autoimmune diseases, including MS,21 type 1 diabetes22 and rheumatoid arthritis,23 carry a functional defect of the iNKT cell subset. Although more recent findings have weakened the hypothesis that an iNKT cell defect is linked to the onset of autoimmunity in humans, at least in type 1 diabetes,24 studies in pre-clinical models of autoimmune diseases provided direct evidence that iNKT cells are critical to counter-regulate T-cell autoimmunity. For example, iNKT cell expansion in transgenic mice that carried an invariant NK T-cell receptor,14 through systemic administration of their model antigen α-galactosylceramide (αGalCer)15–19 or by up-regulation of the CD1d expression at the site of autoimmunity,25 averted autoimmune disease in pre-clinical models of MS and type 1 diabetes. On the other hand, lack of iNKT cells in CD1d-deficient NOD (non-obese diabetic) mice worsened autoimmune diabetes.26 Since iNKT cells are fundamental to prevent autoimmune disease, a positive effect of T1IFN-β on iNKT cell growth and function could underlie the therapeutic efficacy of this cytokine in MS. There are previous findings suggesting that T1IFNs could regulate iNKT cell activity. For example, the observation that T1IFN-α/β-associated transcription factor, IRF-1, is necessary for iNKT cell development and function.27 In addition, there is a recent report showing that T1IFN-β is responsible for the up-regulation of CD1d on DCs infected by Listeria monocytogenes and the improvement of the antigen-presenting ability of DCs on iNKT cells.28 With the intent to establish whether T1IFN-β exerts a key modulatory effect on iNKT cells and specifically promotes their activation and regulatory function, we measured percentages and cytokine secretion of iNKT cells in individuals receiving T1IFN-β as treatment for MS. The percentages of iNKT cells in peripheral blood mononuclear cells (PBMC) of those individuals before and after treatment with T1IFN-β were compared. We found that T1IFN-β significantly increased the iNKT cell number and improved NKT cell cytokine release in response to antigenic stimulation with α-GalCer. The action of T1IFN-β on the iNKT cell subset differed from that on other innate lymphocytes such as NK cells. In fact, T1IFN-β did not directly induce NKT cell clonal expansion and cytokine secretion. Conversely, T1IFN-β modulated myeloid DCs both in vivo in MS patients and in vitro and significantly increased their antigen-presenting capacity upon iNKT cells. Such an improvement of the antigen-presenting function was associated with a selective maturation of T1IFN-β-modulated DCs. The addition of T1IFN-β during in vitro differentiation of myeloid DCs up-regulated the expression of costimulatory molecules that are crucial for iNKT cell activation such as the restriction molecule CD1d and the costimulatory molecules CD80 and CD40.
Our results suggest that T1IFN-β boosted innate immunity conditioning myeloid DCs, which in turn promoted the expansion and function of regulatory iNKT cells.
Materials and methods
Monoclonal antibodies and phenotypic analysis
Invariant NKT cells were simultaneously stained with anti-Vα24 monoclonal antibody (mAb; clone C15) from Immunotech (Warrenale, PA) and anti-CD3 mAb (clone UCHT1) from BD Biosciences (San Jose, CA). In some experiments NKT cells were simultaneously stained with anti-Vα24 mAb and human CD1d tetramers (kindly provided by Dr M. Kronenberg, La Jolla Institute for Allergy and Immunology, La Jolla, CA) previously loaded with αGalCer (KRN7000, 100 ng/ml, kindly provided by Kirin Brewery, Gunma, Japan). Analysis of the DC phenotype was performed with anti-CD11c, anti-CD80 (clones BU15 and MEM-233 from Caltag, Burlingame, CA), anti-CD40 (clone LOB7/6 from ValterOcchiena, Torino, Italy) and anti-CD1d (clone CD1d42 from BD Biosciences) mAbs. In all experiments dead cells were excluded from the analysis by staining with propidium iodide (Sigma, St. Louis, MO). Flow cytometric experiments were performed using fluorescence-acitvated cell sorter (FACS) Vantage and FACSCalibur instruments and data were analysed by CellQuest software (Becton Dickinson, Mountain View, CA).
DC derivation and culture
DCs were derived from peripheral blood monocytes. Briefly, PBMC isolated from blood using a Ficoll gradient were kept for 2 hr at 37° and 5% CO2 in RPMI-1640 with 10% fetal calf serum and non-adherent cells were washed away with warm RPMI-1640. Adherent cells were cultured for 5 days in the presence of recombinant human granulocyte–macrophage colony-stimulating factor (rhGM-CSF; 400 U/ml) and rhIL-4 (200 U/ml) from Strathmann Biotec (Hamburg, Germany). In indicated experiments recombinant human IFN-β (PBL Biomedical Laboratories, Piscataway, NJ) was added to the DC or iNKT cell cultures at 1000 U/ml.
iNKT cell cultures and proliferation assay
Invariant NKT cells were expanded from PBMC of MS patients by culturing total PBMC in the presence of iNKT cell antigen, αGalCer (100 ng/ml), rhIL-7 (500 U/ml, R & D Systems, Minneapolis, MN) and rhIL-15 (20 ng/ml, R & D Systems) in culture medium (RPMI-1640 supplemented with 10% fetal calf serum, 100 U/ml penicillin/streptomycin, 2 mm glutamine, 1 mm sodium pyruvate, 1% non-essential amino acids and 50 µm 2-β-mercaptoethanol). After 4 weeks, iNKT cells were purified by magnetic beads selection (Miltenyi Biotec, Bergisch Gladbach, Germany) with anti-Vα24 mAbs and bead-conjugated secondary antibody against murine immunoglobulin G. Purified iNKT cells were stimulated with DCs previously pulsed with antigen (αGalCer, 100 ng/ml) for 18 hr and irradiated (3500 rads). Supernatants were collected for cytokine measurement and proliferation assay was performed by adding 1 μCi [3H]thymidine per well in the last 16 hr of the 3-day culture period and measuring thymidine incorporation by liquid scintillation counting.
Cytokine assays
The concentrations of the cytokines IFN-γ, IL-10, IL-5, IL-4 and IL-2 were measured simultaneously from the same sample through the use of a multiplexed flow cytometric assay. A commercial Human Th1/Th2 Cytokine Kit, BD Cytometric Bead Array, a FACSCalibur flow cytometry and bd cba software (BD Immunocytometry Systems, San Jose, CA and BD Biosciences Pharmingen, San Diego, CA) were used for the analysis according to the manufacturer's instructions. The concentration of cytokines was interpolated from standard curves obtained with recombinant human cytokines in the range of 0–10 000 pg/ml.
Statistical analysis
Data were expressed as mean +SEM. Statistical analysis of iNKT cell number in untreated individuals or T1IFN-β-treated MS patients was performed with a non-parametric Mann–Whitney test. Statistical analyses of in vitro experiments were performed with Student's unpaired t-tests. P-values <0·05 were considered statistically significant.
Subjects
The group of RR-MS patients included in our study was heterogeneous for age, disease duration and severity, but all the patients responded to T1IFN-β treatment and showed no clinical or radiological relapses of MS after 2 years of therapy (Table 1). The patients were enrolled between 10 October 2002 and 4 September 2003 and were followed clinically up to the end-point of the study, which was 30 September 2005 (2 years of clinical follow-up). All patients gave their written consent according to our Institution's review board guidelines (Azienda Ospedaliera S. Maria della Misericordia); the informed consent was prepared according to the rules of the local Ethical Committee and signed by all participating subjects. PBMC were collected from RR-MS patients before and at two time-points (3 and 8 months) after the initiation of the treatment with T1IFN-β and were tested by flow cytometry in a single experiment for a comparative analysis of Vα24+ CD3+ iNKT cell percentages.
Table 1.
Information on MS patients (n = 10) included in the study and comparison of numbers of MS relapses in the 2 years before and after starting treatment with T1IFN-β
| MS relapses/year | |||||
|---|---|---|---|---|---|
| Patient | Age (year) | Gender | Disease duration (year) | Pre- IFN-β | Post- IFN-β |
| MSNKT006 | 26 | F | 5 | 1 | none |
| MSNKT009 | 33 | F | 3 | 0 | none |
| MSNKT010 | 40 | F | 1·5 | 2 | none |
| MSNKT015 | 33 | F | 15 | 1 | 1* |
| MSNKT016 | 30 | F | 1 | 2 | none |
| MSNKT017 | 46 | M | 2 | 1 | none |
| MSNKT018 | 26 | F | 5 | 1 | none |
| MSNKT019 | 48 | F | 34 | 0 | none |
Results
Treatment with T1IFN-β enriched the Vα24+ iNKT cell subset
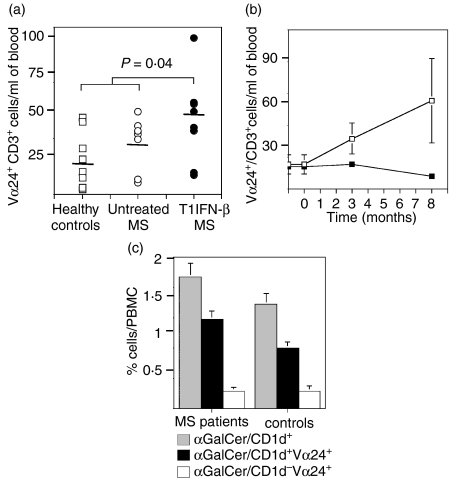
Despite the limited number of iNKT cells in PBMC, we measured a significant increase in the percentages of invariant Vα24+ CD3+ NKT cells in the PBMC of T1IFN-β-treated patients compared to PBMC samples collected from the same patients before treatment or healthy controls (Fig. 1a).
Figure 1.
Percentage of Vα24+ CD3+ NKT cells increased in PBMC of MS patients after treatment with T1IFN-β. (a) Cumulative data of percentages of iNKT in healthy individuals and MS patients before and after treatment with T1IFN-β are presented. Total peripheral blood mononuclear cells (PBMC) were stained with PE-conjugated anti-CD3 and FITC-conjugated anti-Vα24 monoclonal antibodies and analysed by flow cytometry. Percentages of CD3+ Vα24+ iNKT cells significantly increased in T1IFN-β-treated patients (n = 8) compared to PBMC samples from untreated individuals (untreated MS n = 8 and healthy controls, n = 8) (non-parametric Mann–Whitney test, P = 0·04). (b) Percentages of Vα24+ CD3+ iNKT cells increased progressively in the PBMC of MS patients after treatment with T1IFN-β. PBMC samples of each patient (open squares) and controls (closed squares) collected at different time-points were stained and analysed in a single experiment as in (b) (c) The majority of invariant Vα24+ T cells were αGalCer/CD1d+. Total PBMC of MS patients (n = 3) and controls (n = 3) were double-stained with PE-conjugated αGalCer-loaded CD1d tetramers and FITC-conjugated anti-Vα24 mAb. The small percentage (5–15%) of Vα24+ T cells that were αGalCer/CD1d– could represent CD1d-restricted iNKT cells that did not recognize αGalCer as their glycolipid antigen.
The T1IFN-β effect on iNKT cell expansion was progressive and the percentage of iNKT cells was higher at 8 months than 3 months post-treatment (Fig. 1c). Percentages of innate lymphocytes like iNKT cells in peripheral blood could vary in time according to environmental conditions. To exclude the possibility that the observed increase of iNKT cells in T1IFN-β-treated MS patients was the result of accidental environmental factors, we assessed variations of iNKT cell percentages in PBMC collected from untreated individuals at different time-points. In those individuals we did not detect significant changes of iNKT cell percentages over time (Fig. 1c), confirming that the iNKT cell expansion in MS patients was integrally related to T1IFN-β therapy. The phenotype of iNKT cells was confirmed by staining PBMC with αGalCer-loaded CD1d tetramers, showing that the majority of invariant Vα24+ NKT cells were αGalCer/CD1d+ (> 90%) both in MS patients and healthy controls (Fig. 1b). The percentage of Vα24+ CD3+ cells that were αGalCer/CD1d– were included in our analysis because they could represent invariant NKT cells with CD1d restriction but antigen specificity for a glycolipid antigen different from αGalCer.29
T1IFN-β treatment promoted function of regulatory iNKT cells
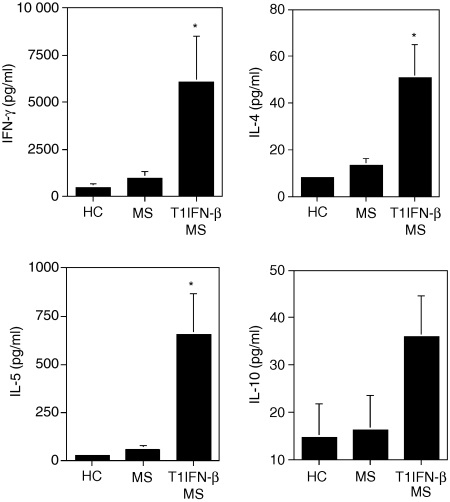
The regulatory function of iNKT cells is related to their rapid and diverse secretion of cytokines like IFN-γ, IL-4, IL-5 and IL-10 upon T-cell receptor stimulation. To demonstrate that the treatment with T1IFN-β favoured iNKT cell function, we tested secretion of cytokines by iNKT cells isolated from the PBMC of MS patients before and after treatment with T1IFN-β. For those experiments, Vα24+ CD3+ iNKT cells were purified from the PBMC of MS patients collected before and 3 months after starting treatment with T1IFN-β and were stimulated in vitro with their model antigen, α-GalCer. We observed that iNKT cells isolated from the PBMC of T1IFN-β-treated MS patients secreted significantly larger amount of IL-5, IL-4 and IFN-γ upon antigenic stimulation compared to their counterparts purified from PBMC collected before treatment (Fig. 2). Secretion of IL-10 was also slightly increased from iNKT cells of treated patients (Fig. 2) but no significant release of other cytokines such as IL-2 from iNKT cells was detected in either of the groups of patients (data not shown). Our findings suggested that treatment with T1IFN-β conditioned iNKT cells in vivo and rendered them more effective in cytokine release upon antigenic stimulation.
Figure 2.
T1IFN-β treatment in MS patients promoted iNKT cell function. The iNKT cells purified from T1IFN-β-treated MS patients (T1IFN-β MS; n = 8) secreted significantly larger amounts of IL-4 (P ≤ 0·01), IL-5 (P ≤ 0·01) and IFN-γ (P ≤ 0·05) compared to their counterparts from untreated healthy controls (n = 8) or the same MS patients before they started treatment (untreated MS; n = 8). IL-10 secretion increased slightly, but not significantly, in T1IFN-β-treated MS patients compared to untreated controls or MS patients (P ≤ 0·09). Vα24+ iNKT cells were expanded by culturing PBMC with αGalCer (100 ng/ml) and cytokines (recombinant human IL-7 and IL-15) and purified by magnetic bead separation with anti-Vα24 monoclonal antibody. iNKT cells (2 × 104 per well) were plated on a 96-well plate and stimulated with 2 × 105αGalCer-pulsed DCs. Supernatants were collected after 7 days and cytokines were simultaneously measured in the supernatants of iNKT cell cultures by a multiplexed flow cytometric assay (Cytometric Bead Array).
T1IFN-β did not have a direct effect on iNKT cell growth and function
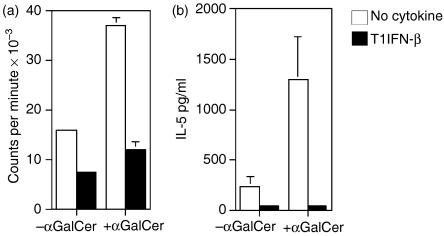
To assess the mechanism by which T1IFN-β modulates iNKT cell activation, we tried to assess whether the cytokine acts directly on the iNKT cell subset. The direct effect of T1IFN-β on lymphocytes is conflicting; on one hand they promote blastogenesis and the STAT cascade for cytokine production in NK cells, while on the other, they inhibit proliferation and antigenic stimulation of T cells.7 To test the direct effect of T1IFN-β on iNKT cells, we added recombinant human T1IFN-β to iNKT cells during antigenic stimulation with αGalCer and measured their proliferation and cytokine secretion. In those experiments, the addition of T1IFN-β completely blocked both proliferation (Fig. 3a) and cytokine secretion (Fig. 3b) of iNKT cells. We concluded that iNKT cells behave more like T lymphocytes than NK cells in response to T1IFN-β and that the beneficial effect of the cytokine on iNKT cell expansion and function observed in vivo in MS patients was probably the result of an indirect action.
Figure 3.
T1IFN-β did not directly enhance NKT cell proliferation and cytokine secretion. The addition of T1IFN-β to iNKT cell cultures inhibited both antigen-specific proliferation and cytokine secretion. Purified Vα24+ iNKT cells (2 × 104; previously expanded in vitro from PBMC of untreated individuals) were stimulated with unpulsed (– αGalCer) or antigen-pulsed (+ αGalCer), irradiated DCs (2 × 105) in 96-well plates with/without rhT1IFN-β (1000 U/ml). (a) Proliferation was measured by 3[H]thymidine incorporation in the last 16 hr of a 3-day culture. (b) Supernatants were collected after 7 days and cytokine release (IL-5) was measured by CBA assay.
T1IFN-β modulated DCs and improved their antigen-presenting capacity upon iNKT cells
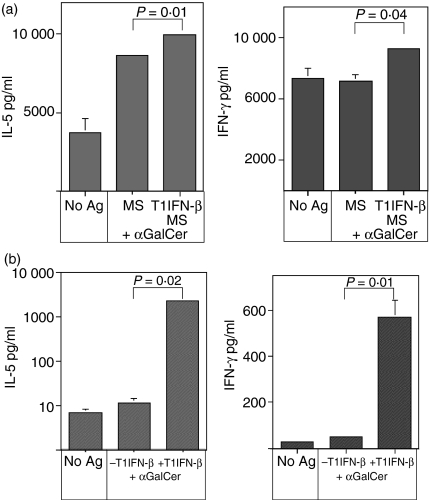
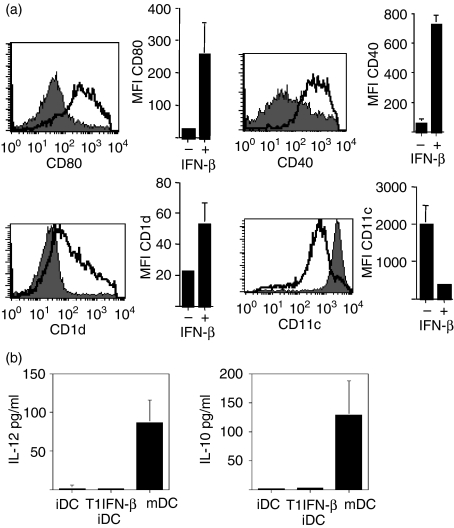
T1IFN-β could promote iNKT cell activation through modulation of myeloid dendritic cells (DCs), a subset of antigen-presenting cells that is essential for iNKT cell maturation and function.30 Hence, we tested whether T1IFN-β acted on DCs by increasing their stimulatory capacity upon iNKT cells. First, we evaluated the ability of T1IFN-β-modulated DCs derived from treated MS patients to trigger iNKT cell activation. In those experiments, DCs were derived from PBMC of T1IFN-β-treated or untreated MS patients, pulsed with the iNKT cell antigen αGalCer and added to purified autologous Vα24+ iNKT cells. We observed that DCs differentiated from PBMC of T1IFN-β-treated patients were more effective for iNKT cell activation than were the DCs of untreated MS patients (Fig. 4a; P ≤ 0·05), as if the preconditioning of monocytes with T1IFN-β favoured their differentiation towards an iNKT cell-stimulating phenotype. Such a modulatory effect of T1IFN-β on DCs was confirmed in vitro. In fact, human DCs, derived from untreated individuals and differentiated in vitro in the presence of recombinant human T1IFN-β, were significantly more efficient antigen-presenting cells for iNKT cells compared to their non-T1IFN-β-modulated counterparts (Fig. 4b; P ≤ 0·01). In addition, we found that conditioning of immature DCs with T1IFN-β, in the absence of any other maturation stimuli, induced their partial maturation with significant up-regulation of the iNKT cell restriction molecule, CD1d, and costimulatory molecules CD80 and CD40 (Fig. 5a) but not CD86 (data not shown). Up-regulation of the expression levels of restriction and costimulatory molecules that are fundamental for iNKT cell activation could explain the increased antigen-presenting function of T1IFN-β-modulated DCs on iNKT cells. However, although T1IFN-β-conditioned DCs up-regulated the expression of maturation markers, they were not fully mature, down-regulated the expression of CD11c (Fig. 5a) and failed to secrete cytokines (Fig. 5b). Our data showed that innate cytokines such as T1IFN-β could function as natural adjuvant for DCs, induced their partial maturation and improved their capacity to stimulate iNKT cells.
Figure 4.
T1IFN-β acted on DC by increasing their stimulatory capacity upon iNKT cells. (a) Pre-conditioning of monocytes with T1IFN-β in treated MS patients favoured differentiation of DCs with an enhanced antigen-presenting capacity upon iNKT cells compared to DCs from untreated MS patients or healthy controls (P ≤ 0·05). PBMC were collected from MS patients before (MS) and 3 months after treatment with T1IFN-β (T1IFN-β/MS) and kept for 2 hr at 37°, in 5% CO2 in RPMI-1640 with 10% fetal calf serum. Adherent cells were cultured for 5 days with rhGM-CSF (400 U/ml) and rhIL-4 (200 U/ml) to differentiate DCs. DCs were either unpulsed or were pulsed with the iNKT cell antigen (± αGalCer, 100 ng/ml), irradiated and cultured at 2 × 105 cells/well in a 96-well plate together with 2 × 104 purified Vα24+ iNKT cells previously expanded from autologous PBMC. Supernatants were collected after 7 days and iNKT cell stimulation was evaluated as the mean of cytokine release (IL-5 and IFN-γ) measured by CBA assay. Data are presented as mean ± SEM of duplicate determinations (SEM <5% are not shown). One representative experiment out of two is shown. (b) DCs differentiated in vitro in the presence of T1IFN-β were more effective antigen-presenting cells for iNKT cells compared to non-T1IFN-β-modulated DCs (P ≤ 0·01). DCs were differentiated from PBMC of untreated individuals (healthy donors) with rhGM-CSF and rhIL-4 with/without rhT1IFN-β, pulsed with αGalCer (100 ng/ml), irradiated and added to autologous purified Vα24+ iNKT cells. Unpulsed DCs were used as negative control (– αGalCer). Supernatants were collected after 7 days and iNKT cell stimulation was evaluated as mean of cytokine release (IL-5 and IFN-γ). Data are presented as mean ± SEM of duplicate determinations. One representative experiment out of three is shown.
Figure 5.
T1IFN-β induced semi-maturation of resting DCs. (a) T1IFN-β up-regulated the expression of CD1d and maturation markers (CD80, CD40) on resting immature DCs. Myeloid DCs were derived from PBMC of healthy individuals with rhGM-CSF (400 U/ml) and rhIL-4 (200 U/ml) in the presence (open histogram) or absence (shaded histogram) of rhT1IFN-β (1000 U/ml). Expression of CD11c, maturation markers CD80 and CD40 and CD1d was determined by flow cytometric analysis. CD80, CD40 and CD1d histograms refer to gated CD11c+ cells. The graph at the right side of each histogram represents cumulative data of three separate determinations ±SEM. (b) T1IFN-β-conditioned DCs were not fully mature and did not secrete cytokines (IL-12 and IL-10). Cytokine release by immature DCs derived from PBMC without (iDC) or with rhT1IFN-β (T1IFN-β iDC) was assessed by CBA on supernatants of 5-day DC cultures. As positive controls, we used fully mature DCs (mDC) obtained by adding LPS (1 μg/ml) to the last 24 hr of culture.
Discussion
Type 1 interferons are innate cytokines essential to fight infections. They contribute to the clearance of pathogens either by directly inhibiting viral replication in infected cells or by boosting up the innate immunity through the modulation of different cell types including NK cells and myeloid DCs.4–13 The effect of T1IFNs on myeloid DCs is so remarkable that those innate cytokines can be considered as endogenous adjuvants for the activation of DCs.11 In fact, T1IFNs, in the absence of pathogen-related signals, are sufficient to promote DC maturation and increase the expression of the restriction molecules MHC class I and II and costimulatory molecules CD40, CD80, CD86 and CD83.31–36 Since T1IFNs are secreted by plasmacytoid DCs in response to infection, those cytokines generally enhance the function of mature myeloid DCs, previously activated by pathogen-related signals through Toll-like receptors. The ultimate effect of the modulation of maturing myeloid DCs by T1IFNs is an increase of their antigen-presenting function on effector T cells as well as of the secretion of inflammatory cytokines like IL-12 aimed to amplify antiviral immunity.
In treated MS patients, because T1IFN-β was not secreted in response to infection but was administered exogenously, it interacted with resting immature DCs. The physiological role of T1IFN modulation on myeloid DCs under ‘steady-state’ conditions (i.e. in the absence of pathogens) is unclear. A recent report in T1IFN-β-treated MS patients suggested that modulation of T1IFNs on immature DCs up-regulates the expression of costimulatory molecules37 that are crucial for the homeostasis and activation of regulatory T cells, including invariant NKT cells.38,39 Our data confirmed that T1IFN-β induced selective DC maturation with the up-regulation of costimulatory molecules CD80 and CD40 and also found an increased expression of the iNKT cell restriction molecule, CD1d. In addition, we demonstrated that T1IFN-β-modulation of myeloid DCs dramatically improved their antigen-presenting function upon regulatory iNKT cells and favoured their activation and expansion both in vitro and in vivo in T1IFN-β-treated MS patients.
Invariant NKT cells are innate lymphocytes that play a dual role in the modulation of T-cell immunity. On one hand, they can inhibit T-cell immunity and induce tolerance for the prevention of autoimmune diseases like classical regulatory T cells. For example, iNKT cells induce systemic tolerance when antigens are administered through the eye.40 Moreover, expansion or activation of the iNKT cell subset protected mice against different experimental models of autoimmune diseases such as the experimental autoimmune encephalomyelitis, the model of MS, and type 1 diabetes.14–19 On the other hand, it is clear that iNKT cells belong to innate immune networks and play a key role in front-line defence against parasites,41,42 bacteria,43,44 viruses45,46 and antitumour responses47,48 by inducing an inflammatory cytokine environment. Hence, iNKT cells balance their adjuvant/regulatory function between the necessity to build an effective inflammatory immune response against pathogens and prevention of autoimmunity. Differentiation of iNKT cells toward a regulatory or effector phenotype could depend upon the phenotype DCs that stimulate iNKT cells. By secreting the inflammatory cytokine IL-12, mature DCs could favour the differentiation of effector/inflammatory iNKT cells that secrete exclusively inflammatory cytokines (IFN-γ and IL-12). Conversely, semimature DCs that up-regulate CD1d and costimulatory molecules without secreting IL-12 differentiate iNKT cells toward a cytokine profile that is characterized by the release of a diverse array of cytokines (IL-4, IL-5 and IL-10 together with IFN-γ) and is clearly linked to iNKT cell-mediated protection from autoimmune diseases.16–19 Type 1 IFN-β could improve the activation and function of both effector and regulatory iNKT cells. During infection, T1IFN-β (secreted by plasmacytoid DCs) modulated mature IL-12-secreting DCs and enhanced their ability to activate effector iNKT cells (IFN-γ-secreting), as recently reported.28 In contrast, exogenous administration of T1IFN-β to MS patients under ‘steady-state’ conditions, in the absence of pathogens, induced immature DCs toward a semimature phenotype that up-regulated the expression of CD1d and costimulatory molecules without secreting IL-12 and triggered iNKT cells with a regulatory cytokine phenotype (IL-4-, IL-5-, IFN-γ- and IL-10-secreting).
The physiological antigens for iNKT cell activation are the self-glycolipids that are found in peripheral tissues. Interestingly, CD1b-restricted T cells specific for a monosialoganglioside (GM1) were found in MS patients, suggesting that T-cell responses against self-glycolipid arise in the course of autoimmune diseases like MS.49 In treated MS patients, T1IFN-β could modulate immature DCs that reside in peripheral tissue where they bind and present self-glycolipid antigens to iNKT cells. The final outcome could be the expansion and activation of the iNKT cell population that we observed in T1IFN-β-treated MS patients. Also, iNKT cells from T1IFN-β-treated MS patients showed a significantly higher response to antigenic stimulation in terms of cytokine secretion. Those data imply that T1IFN-β treatment preactivated iNKT cells so that they could respond more rapidly and efficiently to self-glycolipid antigen stimulation and hamper effector T-cell responses at the time and site of autoimmunity (i.e. the white matter in the brains of MS patients).
Our data showed that T1IFN-β promoted cell growth and function of regulatory iNKT cells and provided a mechanism to explain the therapeutic efficacy of T1IFN-β for the prevention of autoimmune diseases like MS. A primary goal in the field of autoimmunity is to find a therapeutic regimen that inhibits self-reactive T cells while improving the function of regulatory T cells. Our data showed that T1IFN-β clearly inhibited T-cell proliferation and promoted regulatory iNKT cell function. We believe that our findings suggest new modulatory effect of T1IFNs and provide the rationale to propose treatment with innate cytokines as a therapeutic approach for other T-cell-mediated autoimmune diseases like type 1 diabetes and rheumatoid arthritis.
Acknowledgments
The authors thank Veronique Condolo and Lorenza Persello for their assistance and the patients of the Centre for Multiple Sclerosis of Udine, Italy (S. Maria della Misericordia Hospital) for their generosity and support. We thank Kirin Brewery for providing α-GalCer and Massimo Degano and Ehud Hauben for helpful discussions. These studies were supported by a grant from the Italian Ministry of Health (to G.G.).
References
- 1.The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing–remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43:655–61. doi: 10.1212/wnl.43.4.655. [DOI] [PubMed] [Google Scholar]
- 2.Paty D, Li DK UBC/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing–remitting multiple sclerosis. II. MRI analysis of a multicenter, randomized, double-blind, placebo controlled trial. Neurology. 1993;43:662–7. doi: 10.1212/wnl.43.4.662. [DOI] [PubMed] [Google Scholar]
- 3.Hafler DA, Slavik JM, Anderson DE, O'Connor KC, De Jager P, Baecher-Allan C. Multiple sclerosis. Immunol Rev. 2005;204:208–31. doi: 10.1111/j.0105-2896.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- 4.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type 1 interferons (α/β) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–36. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 5.Biron CA. Interferons α and β as immune regulators – a new look. Immunity. 2001;14:661–4. doi: 10.1016/s1074-7613(01)00154-6. [DOI] [PubMed] [Google Scholar]
- 6.Der SD, Zhou A, Williams BR, Silverman RH. Identification of genes differentially regulated by interferon α, β, or γ using oligonucleotide arrays. Proc Natl Acad Sci USA. 1998;95:15623–8. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense. Function and regulation by innate cytokines. Ann Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 8.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–50. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–9. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 10.Durbin JE, Fernandez-Sesma A, Lee CK, et al. Type I IFN modulates innate and specific antiviral immunity. J Immunol. 2000;164:4220–8. doi: 10.4049/jimmunol.164.8.4220. [DOI] [PubMed] [Google Scholar]
- 11.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–55. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 12.Ito T, Amakawa R, Inaba M, Ikehara S, Inaba K, Fukuhara S. Differential regulation of human blood dendritic cell subsets by IFNs. J Immunol. 2001;166:2961–9. doi: 10.4049/jimmunol.166.5.2961. [DOI] [PubMed] [Google Scholar]
- 13.Santini SM, Lapenta C, Logozzi M, Parlato S, Spada M, Di Pucchio T, Belardelli F. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777–88. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehuen A, Lantz O, Beaudoin L, et al. Overexpression of natural killer T cells protects Vα14-Jα281 transgenic nonobese diabetic mice against diabetes. J Exp Med. 1998;188:1831–9. doi: 10.1084/jem.188.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyamoto K, Miyake S, Yamamura TA. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing Th2 bias of natural killer T cells. Nature. 2001;413:531–4. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 16.Jahng A, Maricic I, Pedersen B, et al. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1789–99. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh A, Wilson M, Hong S, et al. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1801–11. doi: 10.1084/jem.194.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong S, Wilson MT, Serizawa I, et al. The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med. 2001;7:1052–6. doi: 10.1038/nm0901-1052. [DOI] [PubMed] [Google Scholar]
- 19.Sharif S, Arreaza G, Zucker P, et al. Activation of natural killer T cells by α-galactosylceramide treatment prevents the onset and recurrence of autoimmune type-1 diabetes. Nat Med. 2001;7:1057–62. doi: 10.1038/nm0901-1057. [DOI] [PubMed] [Google Scholar]
- 20.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;26:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 21.Illes Z, Kondo T, Newcombe J, Oka N, Tabira T, Yamamura T. Differential expression of NKT cell Vα24JαQ invariant TCR chain in the lesions of multiple sclerosis and chronic inflammatory demyelinating polyneuropathy. J Immunol. 2000;164:4375–81. doi: 10.4049/jimmunol.164.8.4375. [DOI] [PubMed] [Google Scholar]
- 22.Wilson S, Kent S, Patton K, et al. Extreme Th1 bias of invariant Vα24JαQ T cells in type 1 diabetes. Nature. 1998;391:177–81. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- 23.Yanagihara Y, Shiozawa K, Takai M, Kyogoku M, Shiozawa S. Natural killer (NK) T cells are significantly decreased in the peripheral blood of patients with rheumatoid arthritis (RA) Clin Exp Immunol. 1999;118:131–6. doi: 10.1046/j.1365-2249.1999.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee PT, Putnam A, Benlagha K, Teyton L, Gottlieb PA, Bendelac A. Testing the NKT cell hypothesis of human IDDM pathogenesis. J Clin Invest. 2002;110:793–800. doi: 10.1172/JCI15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falcone M, Facciotti F, Ghidoli N, et al. Up-regulation of CD1d expression restores the immunoregulatory function of NKT cells and prevents autoimmune diabetes in nonobese diabetic mice. J Immunol. 2004;172:5908–16. doi: 10.4049/jimmunol.172.10.5908. [DOI] [PubMed] [Google Scholar]
- 26.Shi FD, Flodstrom M, Balasa B, Kim SH, Van Gunst K, Strominger JL, Wilson SB, Sarvetnick N. Germ line deletion of the CD1 locus exacerbates diabetes in the NOD mouse. Proc Natl Acad Sci USA. 2001;98:6777–82. doi: 10.1073/pnas.121169698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohteki T, Yoshida H, Matsuyama T, Duncan GS, Mak TW, Ohashi PS. The transcription factor interferon regulatory factor 1 (IRF-1) is important during the maturation of natural killer 1.1+ T cell receptor-alpha/beta+ (NK1+ T) cells, natural killer cells, and intestinal intraepithelial T cells. J Exp Med. 1998;187:967–72. doi: 10.1084/jem.187.6.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raghuraman G, Geng Y, Wang C-R. IFN-β-mediated up-regulation of CD1d in bacteria-infected APCs. J Immunol. 2006;177:7841–8. doi: 10.4049/jimmunol.177.11.7841. [DOI] [PubMed] [Google Scholar]
- 29.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol. 2004;4:231–7. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 30.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002;3:867–74. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 31.Luft T, Pang KC, Thomas E, Hertzog P, Hart DN, Trapani J, Cebon J. Type 1 IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–53. [PubMed] [Google Scholar]
- 32.Paquette RL, Hsu NC, Kiertscher SM, Park AN, Tran L, Roth MD, Glaspy JA. Interferon-alpha and granulocyte–macrophage colony-stimulating factor differentiate peripheral blood monocytes into potent antigen-presenting cells. J Leukoc Biol. 1998;64:358–67. doi: 10.1002/jlb.64.3.358. [DOI] [PubMed] [Google Scholar]
- 33.Radvanyi LG, Banerjee A, Weir M, Messner HA. Low levels of interferon-alpha induce CD86 (B7.2) expression and accelerate dendritic cell maturation from human peripheral blood mononuclear cells. Scand J Immunol. 1999;50:499–509. doi: 10.1046/j.1365-3083.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- 34.Wang C, Al-Omar HM, Radvanyi L, Banerjee A, Bouman D, Squire J, Messner HA. Clonal heterogeneity of dendritic cells derived from patients with chronic myeloid leukaemia and enhancement of their T-cells stimulatory activity by IFN-alpha. Exp Hematol. 1999;27:1176–84. doi: 10.1016/s0301-472x(99)00055-7. [DOI] [PubMed] [Google Scholar]
- 35.Santini SM, Lapenta C, Logozzi M, Parlato S, Spada M, Di Pucchio T, Belardelli F. Type 1 interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBMC-SCID mice. J Exp Med. 2000;191:1777–88. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montoya M, Schiavoni G, Mattei F, Gresser I, Belardelli F, Borrow P, Tough DF. Type 1 interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 2002;99:3263–71. doi: 10.1182/blood.v99.9.3263. [DOI] [PubMed] [Google Scholar]
- 37.Marckmann S, Wiesemann E, Hilse R, Trebst C, Stangel M, Windhagen A. Interferon-β up-regulates the expression of co-stimulatory molecules CD80, CD86 and CD40 on monocytes: significance for treatment of multiple sclerosis. Clin Exp Immunol. 2004;138:499–506. doi: 10.1111/j.1365-2249.2004.02624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salomon B, Lenshow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 39.Hayakawa Y, Takeda K, Yagita H, van Kaer L, Saiki I, Okumura K. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. J Immunol. 2001;166:6012–8. doi: 10.4049/jimmunol.166.10.6012. [DOI] [PubMed] [Google Scholar]
- 40.Sonoda K-H, Exley M, Snapper S, Balk S, Stein-Streilein J. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J Exp Med. 1999;190:1215–26. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Denkers EY, Scharton-Kersten T, Barbieri S, Caspar P, Sher A. A role for CD4+ NK1.1+ T lymphocytes as major histocompatibility complex class II independent helper cells in the generation of CD8+ effector function against intracellular infection. J Exp Med. 1996;184:131–9. doi: 10.1084/jem.184.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonzalez-Aseguinolaza GC, de Oliveira C, Tomaska M, et al. Alpha-galactosylceramide-activated Vα14 natural killer T cells mediate protection against murine malaria. Proc Natl Acad Sci USA. 2000;97:8461–6. doi: 10.1073/pnas.97.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flesch IE, Wandersee A, Kaufmann SH. IL-4 secretion by CD4+ NK1+ T cells induces monocyte chemoattractant protein-1 in early listeriosis. J Immunol. 1997;159:7–10. [PubMed] [Google Scholar]
- 44.Emoto M, Emoto Y, Buchwalow IB, Kaufmann SH. Induction of IFN-gamma-producing CD4+ natural killer T cells by Mycobacterium bovis bacillus Calmette–Guérin. Eur J Immunol. 1999;29:650–9. doi: 10.1002/(SICI)1521-4141(199902)29:02<650::AID-IMMU650>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 45.Kakimi K, Guidotti LG, Koezuka Y, Chisari FV. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med. 2000;192:921–30. doi: 10.1084/jem.192.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grubor-Bauk B, Simmons A, Mayrhofer G, Speck PG. Impaired clearance of herpes simplex virus type 1 from mice lacking CD1d or NKT cells expressing the semivariant V alpha 14-J alpha 281 TCR. J Immunol. 2003;170:1430–4. doi: 10.4049/jimmunol.170.3.1430. [DOI] [PubMed] [Google Scholar]
- 47.Cui J, Shin T, Kawano T, et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–6. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 48.Park SH, Kyin T, Bendelac A, Carnaud C. The contribution of NKT cells, NK cells, and other gamma-chain-dependent non-T non-B cells to IL-12-mediated rejection of tumors. J Immunol. 2003;170:1197–201. doi: 10.4049/jimmunol.170.3.1197. [DOI] [PubMed] [Google Scholar]
- 49.Shamshiev A, Donda A, Carena I, Mori L, Kappos L, De Libero G. Self glycolipids as T-cell autoantigens. Eur J Immunol. 1999;29:1667–75. doi: 10.1002/(SICI)1521-4141(199905)29:05<1667::AID-IMMU1667>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]