Abstract
Systemic lupus erythematosus (SLE) is characterized by the production of autoantibodies directed against nuclear antigens including nucleosomes and DNA. To determine the role of T-cell costimulatory molecule 4-1BB in the regulation of SLE, MRL-Faslpr (lpr) mice deficient in 4-1BB (lpr/4-1BB–/–) were generated and their disease phenotype was compared to that of control lpr mice. The main finding of this study is that the lpr/4-1BB–/– mice had more pronounced skin lesions which appeared earlier, increased lymphadenopathy, increased renal damage, and higher mortality than 4-1BB-intact control lpr mice. The increased severity of lesions in lpr/4-1BB–/– mice was closely associated with increases in CD4+ T, CD3+ B220+ double-negative T cells, serum immunoglobulin, anti-dsDNA autoantibodies, and tissue immunoglobulin deposits. These data suggest that the 4-1BB−4-1BB ligand signalling pathway plays an important role in SLE and that deletion of 4-1BB confers susceptibility to lpr mice, leading to accelerated induction of disease and early mortality.
Keywords: 4-1BB, B cells, autoantibodies, lupus, kidney
Introduction
Systemic lupus erythematosus (SLE) is a complex polygenic autoimmune disease characterized by the presence of pathogenic autoantibodies to a spectrum of nuclear antigens, including nucleosomes and DNA.1 Its clinical heterogeneity originates in chronic activation of the immune system, resulting in tissue damage mediated by autoantibody and inflammatory processes initiated as a consequence of deposition of complement-fixing immune complexes.1 Although it is well established that B2 and T lymphocytes3,4 participate in disease pathogenesis, the production of high titres of autoantibodies is a key hallmark and primary element of SLE, supporting the crucial role of B cells in SLE pathogenesis. T cells, especially CD4+ T cells, are required for full penetrance of the disease, largely via their role in helping to activate autoreactive B cells.1
4-1BB, a 50–55-kDa tumor necrosis factor receptor (TNFR) family member, is an activation-induced costimulatory molecule existing on CD4, CD8 and natural killer (NK) T (NKT) cells, with the exception of CD24+ CD25+ T regulatory cells and dendritic cells, which express this antigen in a constitutive manner.5,6 Through the use of gene-deficient mice and/or antibody-mediated signalling/blocking, the importance of the 4-1BB pathway has been underscored in a variety of clinical settings.7 4-1BB gene-deficient mice have suboptimal NK/NKT numbers8 and dysregulated T-cell division,9,10 and causes acute inflammatory bowel disease in severe combined immunodeficiency (SCID) mice upon adoptive transfer of 4-1BB–/– CD4+ T cells.11
A relationship between autoimmune SLE and 4-1BB signalling has recently been suggested.12,13 To further elucidate the role of the 4-1BB−4-1BB ligand (4-1BBL) pathway in autoimmune-prone MRL-Faslpr (lpr) mice with special reference to SLE, we generated lpr mice deficient in 4-1BB and followed the disease pattern in these mice. Our results suggest that 4-1BB deficiency causes accelerated autoimmune lesions, early mortality, and acute nephritis in lpr mice.
Materials and methods
Mice
MRL/MpJ-Tnfrs6lpr (MRL/MpJ-Faslpr; MRL-Faslpr; lpr) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). The 4-1BB-deficient mice have been previously described.9 In order to generate lpr mice deficient in 4-1BB, we mated the homozygous parents from individual single knock-out backgrounds. Independent colonies were established and backcrossed amongst siblings over nine generations so that MRL background genes were present at >90%. The genotype of mutant mice was assessed by Southern analysis of tail DNA or by polymerase chain reaction (PCR). All mice were housed in the Louisiana State University Health Sciences Center specific pathogen-free animal facility. All experiments were performed using strain-, age-, and sex-matched mice. Animal experimentation protocols were approved by the Louisiana State University Health Sciences Center Institutional Animal Care and Use Committee.
Assessment of skin lesions
Skin lesions were recorded every month until animals were killed at the age of 5 months. Grading of skin lesions was performed as follows: 0, none; 0·5, mild (tip of the nose plus ears); 1, moderate (< 1 cm; tip of the nose plus ears); 2, moderately severe (< 2 cm; tip of the nose plus eyes plus ears plus skin scabs), and 4, severe (> 2 cm; tip of the nose plus eyes plus ears plus skin scabs).
Histological observation
Animals were killed on scheduled days and tissues were frozen in Tissue-Tek O.C.T. embedding medium (Miles, Elkhart, IN). Sections (7 μm) were cut and stained with haematoxylin and eosin (H & E) by routine methods.
Immunohistochemistry
Staining for germinal centres was performed with biotinylated peanut agglutinin (PNA; 1/20) (Vector Laboratories Inc., Burlingame, CA) and visualized with ABC reagents (Vector) followed by haematoxylin counterstaining and cover-slipping. Micrographs were taken with an Eclipse E600 microscope (Nikon, Melville, NY).
Immunofluorescence staining
Sections (7 μm) were cut, air-dried, fixed in chilled acetone, rehydrated in tris buffered saline (TBS) and used in assays. After blocking in 5% normal rabbit serum (Sigma-Aldrich, St Louis, MO), sections were incubated with dilutions of fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG) and the FITC-conjugated goat IgG fraction of mouse compliment receptor 3 (both from the Cappel Laboratory, Malvern, PA) for 60 min at room temperature. Sections were washed three times (3 min each wash) with TBS containing 0·05% T20 followed by cover-slipping. Micrographs were taken with an Eclipse E600 microscope (Nikon). The titres were quantified as the reciprocal value of the highest anti-IgG- and anti-C3-FITC dilution that gave a clear positive reaction.
Flow cytometry analysis
Phenotypic analysis of lymphocytes (1 × 106 cells in 100 µl) was performed at 4° after an initial blocking step with 1 µg of unlabelled anti-FcγR antibody (eBioscience, San Diego, CA). The monoclonal antibodies used included CD3, CD4, CD5, CD8β and B220 (eBioscience).
Determination of serum antibody titres
Serum samples were obtained from mice aged 3 and 5 months. Serially diluted sera were subjected to immunoglobulin analysis by enzyme-linked immunosorbent assay (ELISA). Total IgG1, IgG2a, and IgG2b were quantified using commercially available kits according to the instructions of the manufacturer (Bethyl Laboratories, Montgomery, TX). Analysis of anti-DNA-specific antibodies was performed by coating microtitre plates with 10 µg/ml double-stranded DNA (dsDNA) (Sigma-Aldrich) followed by the addition of serially diluted sera, and bound immune complexes were revealed as above. The TMB peroxidase substrate (eBioscience) was used to detect horseradish peroxidase (HRP) activity by absorbance at 450 nm. Serum antinuclear antibodies (ANoA) were quantified using HEp-2-coated slides (Antibodies Inc., Davis, CA).
Statistical analysis
Each experiment was performed on four to seven mice and was repeated at least three times with similar results. All statistical analyses were conducted using Student's t-test and results were considered significant at P≤0·05.
Results
4-1BB deficiency causes pronounced splenomegaly, lymphadenopathy, and severe skin lesions in lpr mice
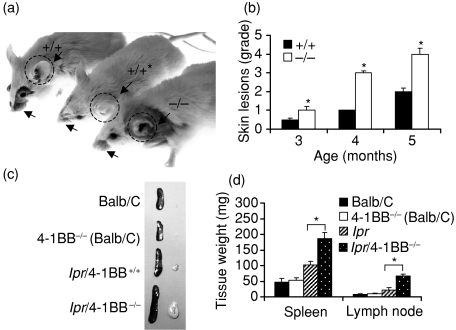
The phenotypes of lpr/4-1BB+/+ and lpr/4-1BB–/– mice were analysed from birth to the age of 5 months. One of the hallmarks of autommmune disease in lpr mice is the development of visible skin lesions (of the back, neck and ears),14,15 which become acute with age and disease progression.16 We analysed the development of pathological symptoms in these mice. The lpr/4-1BB+/+ mice developed the characteristic skin lesions, i.e. around the tip of the nose and around the ears, and such lesions were particularly pronounced in lpr/4-1BB–/– mice (Fig. 1a). Additionally, the lpr/4-1BB–/– but not the lpr/4-1BB+/+ mice showed progressive erosion of the ear pinnae in 60–80% of cases, with onset at 4 months of age (Fig. 1a). The incidence and severity of skin lesions and ear pinna damage were comparable among male and female lpr/4-1BB–/– mice (data not shown). Previous studies suggested that treatment with anti-4-1BB reduces visible skin lesions in lpr mice,12 and in the current study lpr mice treated with agonistic anti-4-1BB monoclonal antibody as a control showed no apparent skin lesions (Fig. 1a; asterisk). Time–course grading of skin lesions gave the same result (Fig. 1b). As splenomegaly and lymphadeopathy are constant features of SLE pathogenesis,16 we evaluated the gross sizes and weights of spleens and axillary lymph nodes of lpr/4-1BB+/+ and lpr/4-1BB–/– mice and found significant spleen and lymph node enlargement in the latter (Figs 1c and d).
Figure 1.
4-1BB deficiency in MRL-Faslpr (lpr) mice causes acute skin lesions, lymphadenopathy, and early mortality. (a) Representative photographic images of 5-month-old lpr/4-1BB+/+ (+/+) and lpr/4-1BB–/– (–/–) mice. *The lpr/4-1BB+/+ (+/+) mice were treated subcutaneously from age 1 month with agonistic anti-4-1BB monoclonal antibody (100 µg twice weekly) and visible skin lesions were assessed at age 5 months. Note the acute skin lesions and ear pinna loss in lpr/4-1BB–/– mice (arrows and dashed circles). (b) Skin lesions in (a) were graded as mentioned in the Materials and methods. (c, d) Size distribution (c) and tissue weights (d) of spleens and lymph nodes in 5-month-old mice. *P < 0·001.
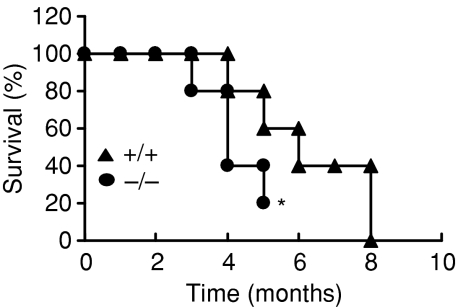
4-1BB gene deletion leads to early mortality in lpr mice
It has been shown that death in lpr mice is chiefly a result of renal disease, with a mean survival time of 6 months.17 Given the severe skin lesions and significant lymphadenopathy and splenomegaly in lpr/4-1BB–/– mice, we tested whether the genetic absence of 4-1BB affected the mortality of lpr mice. We followed the survival patterns of cohorts of 4-1BB-sufficient and -deficient lpr mice (both male and female) and found a mortality rate of 80% in lpr mice deficient in 4-1BB compared with 40% mortality in lpr/4-1BB+/+ mice by the time they reached the age of 5 months (Fig. 2). By 4 months of age, lpr/4-1BB–/– mice were becoming increasingly moribund and showing reductions in cage movement. As per the guidelines of our Institute's Animal Holding facilities, the experiments involving lpr/4-1BB–/– mice were terminated by the time they reached 5 months of age. Collectively, these data strongly suggest that the 4-1BB gene function has an important role in SLE and its deficiency causes early mortality in lpr mice.
Figure 2.
The mice were assessed every month and mortality was monitored. The asterisk indicates termination of experiments because of deterioration of the general health of MRL-Faslpr (lpr)/4-1BB–/– mice.
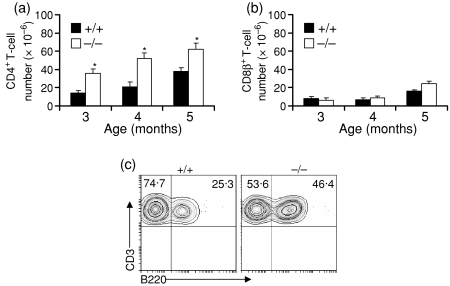
Increased numbers of CD4+ T cells in spleens of lpr/4-1BB–/– mice
Because T cells, and in particular CD4+ T cells, play crucial roles in the development of autoimmune lesions in lpr mice,18–21 and in the light of reports that 4-1BB–/– mice inherently display increased in vitro and in vivo CD4+ T-cell division9,10 and our finding that lpr/4-1BB–/– mice show increased mortality (Fig. 2), we evaluated the T-cell make-up of various treated groups. Our results suggest that the lpr/4-1BB–/– mice showed early increases in CD4+ T-cell numbers in the spleen which continued to rise thereafter (Fig. 3a). However, we did not find any marked alteration in CD8β+ T-cell numbers in any group or at any age (Fig. 3b). Growing evidence suggests that the development of autoimmunity in lpr mice is closely correlated with the appearance of cells that coexpress both T-cell and B-cell phenotypes [CD4– CD8– B220+ CD3+ double-negative (DN) T cells] and that the presence of these cells is linked to disease progression.22 We found increased proportions of DN T cells in the spleens (Fig. 3c) of lpr/4-1BB–/– mice compared with lpr/4-1BB+/+ mice. Taken together, these data suggest that CD4+ T-cell and DN T-cell hyperactivity in lpr/4-1BB–/– mice appears in part responsible for the disease severity and mortality seen in these groups.
Figure 3.
Increased CD4+ T-cell numbers in spleens of MRL-Faslpr (lpr)/4-1BB–/– mice. Single cell suspensions of spleens were prepared and processed as mentioned in the Materials and methods. Cells (1 × 106 per sample) were stained with fluorochrome-labelled anti-CD4 (a) and anti-CD8β (b) antibodies and analysed by flow cytometry. Absolute numbers of individual cell types were calculated from the total cell number. The figure shows mean ± standard deviation (n = 4). (c) Splenocytes from 5-month-old mice were subjected to flow cytometry analysis to determine the percentage of double-negative T cells (CD3 gated). *P < 0·001.
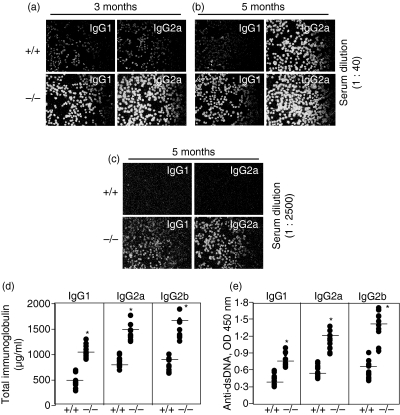
Serum autoantibody titres are higher in lpr/4-1BB–/– mice
One of the key aspects of the lpr mutation correlating with disease severity is the generation of autoantibody production and immunoglobulin accumulation in target organs (especially the kidneys).16,17 Serum was collected by cardiac puncture and antinuclear antibody production was assessed in conjunction with examination of HEp-2-coated slides. The lpr/4-1BB–/– mice, compared with lpr/4-1BB+/+ mice, showed increased serum titres of antinuclear IgG1/2a (Figs 4a–c). Additionally, we analysed serum levels of total immunoglubulins and anti-DNA antibody production in these mice. Consistent with the antinuclear antibody data, levels of circulating immunoglobulins and anti-dsDNA titres were also significantly higher in lpr/4-1BB–/– mice compared with lpr mice (Figs 4d and e).
Figure 4.
Autoantibody production is significantly enhanced in MRL-Faslpr (lpr)/4-1BB–/– mice. (a–c) Three and five months after initial treatment, mice were killed, and sera were collected and applied at indicated dilutions to HEp-2-coated slides for the analysis of serum antinuclear antibody titres; bound immune complexes were visualized using appropriate fluorescein isothiocyanate (FITC)-conjugated anti-immunoglobulin antibodies as mentioned in the Materials and methods. (d) Sera (diluted 1/500) from 5-month-old mice were applied to appropriate anti-immunoglobulin coated microtitre wells and levels of circulating autoantibodies were evaluated by enzyme-linked immunosorbent assay (ELISA) using commercial kits. (e) Sera (diluted 1/500) from 5-month-old mice were incubated in microtitre plates precoated with double-stranded DNA (dsDNA). Immune complexes formed were quantified by ELISA. Magnification ×9·6. OD, optical density.
Increased B-cell function in lpr/4-1BB–/– mice
In the light of the increased serum antibody titres of lpr/4-1BB–/– mice, we investigated whether B-cell number and function were substantially altered in lpr/4-1BB–/– mice in a series of experiments. First, we evaluated germinal centre formation in lpr/4-1BB+/+ and lpr/4-1BB–/– mice at 5 months of age. Results clearly showed greatly increased germinal centre formation (determined by peanut agglutinin reactivity) in lpr/4-1BB–/– mice compared with lpr mice (Fig. 5a). This suggested that some populations of B cells may also be increased in lpr/4-1BB–/– mice. To test this hypothesis, we measured B-cell subsets in both the spleen and the peritoneal cavity. At 5 months of age, the percentages of B1 (B220+ CD5+) and B2 cells (B220+ CD5–) were slightly higher (but not significantly so) in the spleens of lpr/4-1BB–/– mice compared with lpr mice, but the differences were significant in the peritoneums of these mice (Figs 5b and c). These results suggest that 4-1BB deficiency does not lead to relative changes in splenic B cells but affects their function in lpr mice.
Figure 5.
Enhanced B-cell function in MRL-Faslpr (lpr)/4-1BB–/– mice. (a) Frozen sections (7 µm) of spleens from untreated and treated lpr and lpr/4-1BB–/– mice were collected from 5-month-old mice, and incubated with biotin-conjugated peanut agglutinin (PNA) followed by streptavidin–horseradish peroxidase. After colour development with diaminobenzidine substrate, sections were lightly counterstained with haematoxylin. (b, c) Single suspensions of spleens were obtained by collagenase/DNaseI treatment. The peritoneal exudate cells were collected by flushing the peritoneum of individual mice with ice-cold Hanks' balanced salt solution. Red blood cell-free cells were processed for flow cytometry using antibodies to indicated cell populations. Numbers in panels represent the percentage of positive events recorded for each cell type. Magnification ×20. *P < 0·001.
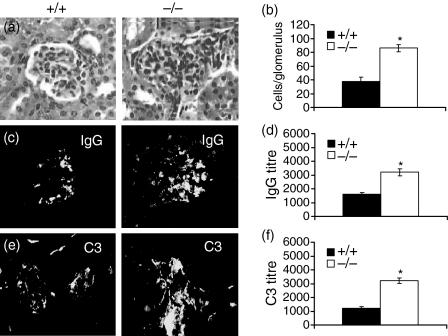
4-1BB deletion exacerbates renal damage in lpr mice
As mortality in lpr mice is often a result of renal failure,17 we evaluated whether 4-1BB deletion in lpr mice also exacerbated renal injury. Histological analyses confirmed that lpr/4-1BB–/– mice showed increased glomerular infiltrates (Figs 6a and b). We also examined whether pathological lesions in the kidneys were affected in lpr/4-1BB–/– mice by evaluating kidney deposition of IgG and complement receptor 3 (C3). The lpr/4-1BB–/– mice revealed increased accumulation of both IgG and C3 in comparison with lpr/4-1BB+/+ mice (Figs 6c and e). Titration experiments further confirmed that the kidneys of lpr/4-1BB–/– mice had increased IgG and C3 deposition (Figs 6d and f), confirming that 4-1BB deficiency in lpr mice causes severe renal damage.
Figure 6.
4-1BB-deletion in MRL-Faslpr (lpr) mice causes acute renal injury. (a, c, e) Frozen sections (7 µm) of kidneys were stained with haematoxylin and eosin (H & E) (a) or with the indicated fluorescein isothiocyanate (FITC)-conjugated antibodies (c, e). (b) Infiltrating cells in each glomerulus were visually counted under a microscope. A minimum of at least 10 glomeruli were counted and expressed as mean ± standard deviation. (d, f) Staining of kidney sections was performed and titres of immunoglobulin G (IgG) and C3 assessed as described in the Materials and methods. Magnification ×18. *P < 0·001.
Discussion
Our study shows that lpr mice develop an SLE-like autoimmune disease characterized by massive lymphoproliferation, acute skin lesions, anti-DNA autoantibodies, immune complex deposition, glomerulonephritis and mortality, and that 4-1BB deficiency dramatically exacerbates this phenotype. The 4-1BB-deficient lpr mice exhibited early and increased immune activation, as demonstrated by increases in lymph node and spleen weights and hypergammaglobulinaemia.
We investigated the basis of the relationship between 4-1BB deficiency and the severe disease phenotype in lpr mice. As CD4+ T-cell activity has been shown to be critical in the pathogenesis of SLE18–21 and lpr/4-1BB–/– mice revealed significantly increased CD4+ T-cell numbers in the present study, such increased CD4+ T-cell activity may in part be responsible for the observed differences between the two groups. The finding of increased CD4+ T cells numbers in lpr/4-1BB–/– mice compared with lpr/4-1BB+/+ mice is consistent with previous reports that 4-1BB–/– mice have inherently increased CD4+ T-cell division.9,10 Recently, it was demonstrated that CD4+ T cells isolated from 4-1BB–/– mice increased the severity of colitis and mortality in SCID mice after adoptive transfer,11 revealing an important characteristic of 4-1BB–/– CD4+ T cells. Taken together, these findings suggest that the hyperproliferative 4-1BB–/– CD4+ T-cell phenotype11 may have further increased the already enhanced CD4+ T-cell responses of lpr mice, resulting in the lpr mutation producing a more acute and susceptible phenotype. Increased percentages of pathogenic double-negative (CD4– CD8– CD3+) T cells expressing the B220 marker in lpr/4-1BB–/– mice seem to be another factor contributing to more severe disease in these mice.
Robust B-cell activation and increased production of autoantibodies are among the factors contributing to disease severity in lpr mice.23,24 Increases in B-cell function but not numbers in lpr/4-1BB–/– mice may in part be responsible for the altered disease severity. Interestingly, the effect on autoantibody production was significant from the age of 3 months onwards in lpr/4-1BB–/– mice, becoming acute in the later stages of life. In this study, we have shown that the percentage of B1 cells was markedly higher in lpr/4-1BB–/– mice compared with lpr controls, especially in the peritoneum. Conventional B cells (B2) are responsible for antibody production and immunoglobulin class switching and have been extensively studied in the lpr model.23,24 The role of B1 cells in the autoimmune-prone lpr mice is still not clear and, although they have not been shown to contribute to the development of autoimmune lesions in lpr mice,25 several lines of research have suggested a role for B1 cells in SLE pathogenesis through the production of low-affinity antibodies, diminished negative regulation and recruitment to the germinal centre, or the production of interleukin-10.26 In lpr mice, B1 cells (which are abundant in the peritoneum) were shown to accumulate in secondary lymphoid organs, suggesting a role for these cells in SLE pathogenesis.27 Interestingly, deletion of B1 cells by hypotonic shock reduced disease severity in BWF1 mice.28 It should be mentioned that control Balb/c 4-1BB–/– mice did not reveal any serum autoantibodies or tissue immunoglobulin deposition at the age groups studied (data not shown), suggesting that the observed B-cell hyperactivity in lpr/4-1BB–/– mice did not stem from the 4-1BB-deficient background but was the result of combined fas and 4-1BB deficiencies.
The present study indicates that the severity of SLE-like symptoms in lpr/4-1BB–/– mice is a result of enhanced CD4+ T-cell (but not forkhead/winged-helix family of transcriptional regulators (Foxp3+) CD4+ regulatory T cell; data not shown) and B-cell function, suggesting that deleting or altering the functions of these cells might alleviate disease symptoms in these mice. We are currently investigating whether adoptive transfer of cells from lpr/4-1BB+/+ mice into lpr/4-1BB–/– mice decreases disease symptoms. We did not examine the cytokine and chemokine make-ups of the two mouse strains, and it will be of interest to determine whether there exist any differences in the above parameters. Unpublished data from our laboratory suggest that there is no difference in IFN-γ production between the T cells of the two mouse strains. Future studies will determine whether regulatory cytokines such as interleukin-10 and transforming growth factor-β have a role in disease severity in lpr/4-1BB–/– mice.
In conclusion, we have demonstrated that endogenous 4-1BB plays a critical role in exacerbating murine SLE through up-regulation of CD4+ T-cell, DN T-cell and B-cell function, in that deletion of 4-1BB accelerates disease resolution and causes acute autoimmune lesions, leading to early mortality in lpr mice.
Acknowledgments
This work was supported in part by US Public Health Service grants R01EY013325 (BSK), KRF-2005-201-E00008 and KRF-2005-084-E00001; the Ulsan Technopark Fund; Science Research Center funds to the Immunomodulation Research Center, University of Ulsan; and the Korean Science and Engineering Fund.
References
- 1.Fairhurst AM, Wandstrat AE, Wakeland EK. Systemic lupus erythematosus: multiple immunological phenotypes in a complex genetic disease. Adv Immunol. 2006;92:1–69. doi: 10.1016/S0065-2776(06)92001-X. [DOI] [PubMed] [Google Scholar]
- 2.Jacobi AM, Diamond B. Balancing diversity and tolerance: lessons from patients with systemic lupus erythematosus. J Exp Med. 2005;202:341–4. doi: 10.1084/jem.20050221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musette P, Pannetier C, Gachelin G, Kourilsky P. The expnasion of a CD4+ T cell population bearing a distinctive β chain in MRL lpr/lpr mice suggests a role for the Fas protein in peripheral T cell selection. Eur J Immunol. 1994;24:2761–6. doi: 10.1002/eji.1830241128. [DOI] [PubMed] [Google Scholar]
- 4.Musette P, Galelli A, Chabre H, Callard P, Peumans W, Truffa-Bachi P, Kourilsky P, Gachelin G. Urtica dioica agglutinin, a Vβ8.3-specific superantigen, prevents the development of the systemic lupus erythematosus-like pathology of MRL lpr/lpr mice. Eur J Immunol. 1996;26:1707–11. doi: 10.1002/eji.1830260807. [DOI] [PubMed] [Google Scholar]
- 5.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity. Nat Rev Immunol. 2003;3:609–20. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 6.Vinay DS, Kwon BS. Dual immunoregulatory pathways of 4–1BB signaling. J Mol Med. 2006;84:726–36. doi: 10.1007/s00109-006-0072-2. [DOI] [PubMed] [Google Scholar]
- 7.Chen L. CD137 Pathway: Immunology and Diseases. 1. New York, NY: Springer; 2006. [Google Scholar]
- 8.Vinay DS, Choi BK, Bae JS, Kim WY, Gebhardt BM, Kwon BS. CD137-deficient mice have reduced NK/NKT cell numbers and function, are resistant to lipopolysaccharide-induced shock syndromes, and have lower IL-4 responses. J Immunol. 2004;173:4218–29. doi: 10.4049/jimmunol.173.6.4218. [DOI] [PubMed] [Google Scholar]
- 9.Kwon BS, Hurtado JC, Lee ZH, et al. Immune responses in 4-1BB (CD137)-deficient mice. J Immunol. 2002;168:5483–90. doi: 10.4049/jimmunol.168.11.5483. [DOI] [PubMed] [Google Scholar]
- 10.Lee SW, Vella AT, Kwon BS, Croft M. Enhanced CD4 T cell responsiveness in the absence of 4-1BB. J Immunol. 2005;174:6803–8. doi: 10.4049/jimmunol.174.11.6803. [DOI] [PubMed] [Google Scholar]
- 11.Maerten P, Kwon BS, Shen C, et al. Involvement of 4-1BB (CD137)−4-1BB ligand interaction in the modulation of CD4 T cell-mediated inflammatory colitis. Clin Exp Immunol. 2006;143:228–36. doi: 10.1111/j.1365-2249.2005.02991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Y, Chen HM, Subudhi SK, Chen L, Koka R, Chen L, Fu YX. Costimulatory molecule-targeted antibody therapy of a spontaneous autoimmune disease. Nature Med. 2003;8:1405–13. doi: 10.1038/nm1202-796. [DOI] [PubMed] [Google Scholar]
- 13.Foell J, Strahotin S, O'Neil SP, et al. CD137 costimulatory T cell receptor engagement reverses acute disease in lupus-prone NZB × NZW F1 mice. J Clin Invest. 2003;111:1505–18. doi: 10.1172/JCI17662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furukawa F, Tanaka H, Sekita K, Nakamura T, Horiguchi Y, Hamashima Y. Dermatopathalogical studies on skin lesions of MRL mice. Arch Dermatol Res. 1984;276:186–94. doi: 10.1007/BF00414018. [DOI] [PubMed] [Google Scholar]
- 15.Chan OT, Paliwal V, McNiff JM, Park SH, Bendelac A, Shlomichik MJ. Deficiency in β2-microglubulin, but not CD1, accelerates spontaneous lupus skin disease while inhibiting nephritis in MRL-Faslpr mice: an example of disease regulation at the organ level. J Immunol. 2001;167:2985–90. doi: 10.4049/jimmunol.167.5.2985. [DOI] [PubMed] [Google Scholar]
- 16.Theofilopoulos AN, Dixon FJ. Etiopathogenesis of murine SLE. Immunol Rev. 1981;55:179–216. doi: 10.1111/j.1600-065x.1981.tb00343.x. [DOI] [PubMed] [Google Scholar]
- 17.Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 18.Peng S, Madaio M, Hughes D, Crispe IN, Owen MJ, Wen L, Hayday AH, Craft J. Murine lupus in the absence of αβ T cells. J Immunol. 1996;156:4041–9. [PubMed] [Google Scholar]
- 19.Ohno K, Takahashi T, Maki K, Ueda M, Taguchi O. Successful transfer of localized autoimmunity with positively selected CD4+ cell to SCID mice lacking functional B cell. Autoimmunity. 1999;29:103–10. doi: 10.3109/08916939908995379. [DOI] [PubMed] [Google Scholar]
- 20.Busser BW, Adair BS, Erikson J, Laufer TM. Activation of diverse repertoires of autoreactive T cells enhances the loss of anti-dsDNA B cell tolerance. J Clin Invest. 1983;112:1361–71. doi: 10.1172/JCI18310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajagopalan S, Zordan T, Tsokos GC, Datta SK. Pathogenic anti-DNA autoantibody-inducing T helper cell lines from patients with active lupus nephritis: isolation of CD4-8-T helper cell lines that express the gamma delta T-cell antigen receptor. Proc Natl Acad Sci USA. 1990;87:7020–4. doi: 10.1073/pnas.87.18.7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–69. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 23.Grammer AC, Donner T, Lipsky PE. Abnormalities in B cell activity and the immunoglobulin repertoire in human systemic lupus erythematosus. Mol Pathol Autoimmun Dis. 2001;2:282–318. [Google Scholar]
- 24.Lipsky PE. Systemic lupus ertymatosus: an autoimmune disease of B cell hyperactivity. Nature Immunol. 2001;2:764–6. doi: 10.1038/ni0901-764. [DOI] [PubMed] [Google Scholar]
- 25.Reap EA, Sobel ES, Jennette JC, Cohen PL, Eisenberg RA. Conventional B cells, not B1 cells, are the source of autoantibodies in chronic graft-versus-host disease. J Immunol. 1993;151:7316–23. [PubMed] [Google Scholar]
- 26.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 27.Ito T, Ishikawa S, Sato T, et al. Defective B1 cell homing to the peritoneal cavity and preferential recruitment of B1 cells in the target organs in a murine model for systemic lupus erythematosus. J Immunol. 2004;172:3628–34. doi: 10.4049/jimmunol.172.6.3628. [DOI] [PubMed] [Google Scholar]
- 28.Murakami M, Yoshioka H, Shirai T, Tsubata T, Honjo T. Prevention of autoimmune symptoms in autoimmune-prone mice by elimination of B-1 cells. Int Immunol. 1995;7:877–82. doi: 10.1093/intimm/7.5.877. [DOI] [PubMed] [Google Scholar]