Abstract
Interleukin (IL)-12p40, a subunit of IL-12p70 and IL-23, has previously been shown to inhibit IL-12p70 activity and interferon-γ (IFN-γ) production. However, recent evidence has suggested that the role of IL-12p40 is more complex. To study the contribution of IL-12p40 to immune responses against mycobacterial infections, we have used transgenic (tg) mice overexpressing IL-12p40 under the control of a major histocompatibility complex-II promoter. The IL-12p40 transgene was expressed during steady state at concentrations of 129 ± 25 ng/ml of serum and 75 ± 13 ng per spleen, while endogenous IL-12p40 was hardly detectable in control littermates. Bacille Calmette–Guérin (BCG) infection strongly induced the expression of IL-12p40 transgene in infected organs, and IL-12p40 monomeric and dimeric forms were identified in spleen of IL-12p40 tg mice. Excessive production of IL-12p40 resulted in a 14-fold increase in IL-12p70 serum levels in tg mice versus non-transgenic mice. IL-23 was also strongly elevated in the serum and spleens of IL-12p40 tg mice through BCG infection. While IFN-γ and tumour necrosis factor protein levels were similar in IL-12p40 tg and non-transgenic mice, Th2 type immune responses were reduced in IL-12p40 tg mice. The number of BCG granulomas and macrophage expressing inducible nitric oxide synthase were similar in IL-12p40 tg and non-transgenic mice. IL-12p40 tg mice were as resistant as non-transgenic mice to BCG and Mycobacterium tuberculosis infections as they could efficiently control bacillary growth. These data show that high amounts of IL-12p40 promotes IL-12p70 and IL-23 formation, but that does not affect T helper 1 type immune responses and granuloma function, thus leading to normal mycobacterial clearance in infected organs.
Keywords: IL-12p40 overexpression, IL-12p70, IL-23, mycobacterial infections
Introduction
The epidemic of tuberculosis has been declared a global emergency by the World Health Organization. The aetiological agent, Mycobacterium tuberculosis, causes clinical disease in 8 million people annually, resulting in 2 million deaths, mostly in developing countries.1 Tuberculosis appears as a localized lung disease in which granulomas contain mycobacteria at least during the early stages of infection, but latent infection is often life-long. The combination of innate immunity and T helper 1 (Th1)-dominant adaptive immune response is critical in host defence against tuberculosis.2
Protective cell-mediated Th1 type immune response against mycobacterial infections involves the formation of granulomas, which consist of antigen-specific cells that activate monocytes/macrophages to acquire the capacity to induce bactericidal mechanisms. Production of interferon-γ (IFN-γ) and tumour necrosis factor (TNF) is essential for the control of mycobacterial proliferation. In their absence, mice are susceptible to both bacille Calmette–Guérin (BCG) and M. tuberculosis infections.3–7 Within granulomas, IFN-γ and TNF synergistically induce activation of bactericidal mechanisms such as inducible nitric oxide synthase (iNOS), which produces nitric oxide, one of the mechanisms responsible for killing the invading mycobacteria.8–10 The key factor for release of IFN-γ by natural killer and Th1 cells is interleukin (IL)-12p70.11 Genetic mutations affecting the IL-12 pathway were shown to compromise human host defence mechanisms against virulent and avirulent mycobacteria.12
IL-12p70 is a heterodimeric cytokine consisting of two subunits, p35 and p40, which is produced by dendritic cells (DCs) and phagocytes after microbial or cytokine stimulation.13,14 IL-12p40 molecules are always secreted in large excess over bioactive IL-12p70 heterodimer and can form a second active cytokine, the homodimeric IL-12(p40)2 molecule which can act as a chemoattractant.15 Moreover, both IL-12p40 monomeric and dimeric forms activate TNF expression in macrophages and microglial cells.16 IL-12(p40)2 may also have an IL-12 antagonistic role by inhibiting the biological activity of IL-12p70.17–19In vivo studies have demonstrated that mice lacking IL-12p40 are more sensitive to M. tuberculosis, M. bovis BCG, and M. avium infections than IL-12p35-deficient mice.20–23 Mycobacterial infection of mice deficient in IL-12p35/IL-12p40 genes has shown that exogenous IL-12(p40)2 may have IL-12 agonistic function and be able to contribute to protective Th1-type immune response.24 These observations are partially the result of the fact that a third cytokine uses the IL-12p40 subunit. The IL-12p40 molecule binds to the p19 subunit to form IL-23, which is a potent activator of memory T cells and mediator of T-cell dependent inflammatory response.25–27 IL-23 is required for protection against M. tuberculosis infection in IL-12p35-deficient mice, but in the presence of IL-12p70, IL-23 seems to play a minor role in host immunity against mycobacterial infection.28,29
The present study investigates how overexpression of IL-12p40 may affect host immune responses against mycobacterial infections. We observe that excess IL-12p40 during BCG infection results in high levels of IL-12p70 and IL-23, which can be redundant in the activation of Th1 type immune responses because IFN-γ production is not modified in vivo; in contrast, Th2 type immune responses are decreased. In addition, TNF expression and bacillary clearance are not affected in mice overexpressing IL-12p40. This suggests that regulatory mechanisms leading to mycobacterial elimination are maintained when IL-12p40- and IL-12p40-dependent cytokines are present in excessive amounts.
Materials and methods
Generation of transgenic mice overexpressing IL-12p40
A HindIII–EcoRI fragment (1050 bp) from the IL-12p40 cDNA (gift from U. Gubler, Hoffmann-La Roche Inc., Nutley, NJ) was inserted into a BamHI site of the pDOI-5 plasmid containing the Eαd promoter (major histocompatibility complex (MHC)-II gene H-2d haplotype)30 and the β-globin gene. A NruI/ApaII fragment containing the IL-12p40 cDNA under the control of Eαd promoter was used to generate tg mice. Microinjection into the male pro-nucleus of C57BL/6 mice was performed by standard procedures.31 Detection of transgenic mouse founders was carried out by PCR on mouse tail DNA using a 5′ primer specific for IL-12p40 (5′-CAA CAT CAA GAG CAG TAG CA-3′) and a-3′ primer specific for β-globin (3′ CAC CAC CTT CTG ATA GGC A 5′), and confirmed by Southern blot analysis. Three founder mice were obtained, and the founder with the highest transgene copy number (about 10 copies) was selected and crossed with C57BL/6 mice for more than 12 generations. IL-12p40 tg mice and negative littermates were maintained under conventional conditions in the animal facility of the Medical Faculty, University of Geneva. Animal experiments were carried out in accordance with institutional guidelines and approved by the local ethical committee on animal experimentation.
Evaluation of cytokines and chemokines in serum and spleen
Blood samples from retro-orbital sinuses were obtained from 4-week-old mice and at different time points after infection. Spleens were homogenized in 0·04% Tween-80/saline buffer (125 mg of tissue/ml of buffer).32 IL-12p40, IL-12p70, IL-23, TNF-α, IFN-γ, IL-4, RANTES (regulated on activation, normal, T-cell expressed, and secreted) and monocyte chemoattractant protein-1 (MCP-1) amounts were evaluated by enzyme-linked immunosorbent assay (ELISA) with a sensitivity of 5–2000 pg/ml (Diaclone, Besançon, France or R & D System, Abingdon, UK or eBioscience, San Diego, CA).
Western blot analysis
Spleens from 4-weeks BCG-infected mice were homogenized (125 mg/ml) in lysis buffer (50 mm Tris-HCl [pH 8·0], 250 mm NaCl, 0·1% sodium dodecyl sulphate (SDS), 1% Triton-X-100, 0·5% Na-deoxycolate, proteases inhibitors), centrifuged (13 000 g, 15 min, 4°) and supernatants collected for analysis. Protein concentration was measured using Bio-Rad protein assay kit. Protein extracts (600 µg) were mixed with sample buffer (125 mm Tris/HCl, pH 6,8; 4% SDS; 20% glycerol, bromophenol blue) for electrophoresis on SDS-polyacrylamide gels and blotted to nitrocellulose membranes (Amersham International, Amersham, UK). Blots were blocked with 5% milk in Tris-buffered saline (TBS)-T buffer (0·2 m Tris [pH 7·4], 1·5 m NaCl, 0·1% Tween-20; 2 hr) and incubated with primary antibody (3 µg/ml, purified rat anti-mouse IL-12p40, C15.6). Peroxidase-conjugated rabbit anti-rat immunoglobulin G antibody (1 : 5000 dilution, gift from D. Hoessli, Faculty of Medicine, Geneva, Switzerland) was the second antibody. Anti-actin antibody was used as control. Bands were visualized by chemiluminescent substrate (ECL; Amersham International).
RNA preparation and reverse transcription–polymerase chain reaction (RT–PCR)
mRNA was isolated using the RNeasy Mini Kit from Qiagen (Hombrechtikon, Switzerland) and cDNA was synthesized using the SuperScript II Reverse Transcriptase (Invitrogen, Basel, Switzerland), following the protocol of the kit. Instead of the Oligo(dT), random nonamers were used (Microsynth, Balgach, Switzerland). cDNA was purified with the QIAquick PCR purification kit from Qiagen. The quantitative real time RT–PCR was done as previously described.33 Primers for RT–PCR were the following: IL-12 p40 F, 5′-GGA AGC ACG GCA GCA GAA TAA-3′, R 5′-CTT GAG GGA GAA GTA GGA ATG-3′, IL-12p35 F 5′-AGG ACT TGA AGA TGT ACC AG-3′, R 5′-CTA TCT GTG TGA GGA GGG-3′, IL-23p19 F 5′-AAT GTG CCC CGT ATC C-3′, R 5′-GGA GGT GTG AAG TTG CT-3′, HPRT F 5′-GTT GGA TAT GCC CTT GAC-3′, R 5′-AGG ACT AGA ACA CCT GCT-3′.
Experimental infection and determination of colony-forming units (CFU) in infected organs
IL-12p40 tg mice and control littermates were infected intravenously (i.v.) with 107 CFU of living BCG strain 1173 P2 or with 105 CFU of virulent M. tuberculosis strain H37Rv. Mice were killed at different time points and bacterial loads were evaluated as previously described.34,35
Histological analyses
Liver, lung and spleen were fixed in 4% buffered formaldehyde and embedded in paraffin for haematoxylin/eosin staining. Tissues were also kept frozen for acid phosphatase reaction.36
Determination of BCG-specific antibodies
BCG antigens were prepared as previously described.37 ELISA plates were coated with BCG antigens (10 µg/ml) overnight at room temperature. Sera (50 µl; starting dilution 1 : 200) were incubated overnight at room temperature and alkaline-phosphatase labelled goat anti-mouse immunoglobulin G1 (IgG1; Rockland Immunochemicals, Philadelphia, PA) was used as detecting antibody. Plates were developed using p-nitrophenyl phosphate substrate (Sigma-Aldrich, Buchs, Switzerland). Absorbance was measured at 405 nm and optical density values were considered for the analysis. Non-specific antibodies were determined in parallel using bovine serum albumin as unrelated antigens, and these values were deducted from those obtained for specific anti-BCG antibodies.
Determination of iNOS activity in spleen proteins
Evaluation of the iNOS activity was done on crude spleen protein extracts of BCG-infected mice measuring the capacity of supernatant to convert radioactive L-(14C)-arginine (Amersham International) to L-(14C)-citrulline as previously described.9,32
iNOS immunohistochemistry
Liver and lung sections were prepared for iNOS staining as described,38 incubated with rabbit anti-mouse iNOS (Calbiochem, San Diego, CA) (60 min) followed by incubation with biotinylated donkey anti-rabbit antibody (Amersham International) and revealed by streptavidin alkaline phosphatase (Boehringer Mannheim, Rotkreuz, Switzerland).35
Antigen-specific release of cytokines from splenocytes
Mice infected with 107 BCG were killed 4 weeks later. Splenocytes were isolated and suspended in Dulbecco's modified Eagle's minimal essential medium containing 10% fetal calf serum and plated at 5 × 105 cells per well in 96-well plates. Cells were cultured for different time points and stimulated with BCG culture filtrate protein extracts (1·7 mg/ml) and living BCG (103 CFU/well) as previously described.34
Statistical analyses
Statistical analyses between experimental groups were evaluated by using the Student t-test. P-values lower than 0·05 were considered as statistically significant.
Results
High levels of IL-12p40 and IL-12p40–dependent cytokines are detected in tg mice during steady state
Transgenic mice expressing high levels of murine IL-12p40 under the control of the Eαd MHC class II promoter were obtained.30,31 These mice exhibited a normal phenotype, comparable to negative littermates. IL-12p40 tg mice used in this study contain IL-12p40 in serum at concentrations ranged from 45 to 293 ng/ml representing a 14 000-fold increase compared to non-transgenic mice (Table 1). Serum and splenic concentrations of IL-12p70 and IL-23, which are IL-12p40-dependent cytokine containing the IL-12p40 dimerized to IL-12p35 or p19 subunits, respectively, were measured at steady state. IL-12p70 as well as IL-23 amounts were significantly higher in serum and spleen of IL-12p40 tg mice compared to control littermates (Table 1).
Table 1.
levels of IL-12p40, IL-12p70 and IL-23 in serum and spleen at steady state in IL-12p40 TG mice and control littermates
| Control mice | IL-12p40 tg mice | |
|---|---|---|
| IL-12p40 | ||
| Serum (ng/ml) | 0·01 ± 0·01 | 129 ± 24** |
| Spleen (ng/organ) | 0·13 ± 0·03 | 75 ± 13* |
| IL-12p70 | ||
| Serum (pg/ml) | <2–3 | 38 ± 8* |
| Spleen (pg/organ) | 29 ± 7 | 47 ± 5* |
| IL-23 | ||
| Serum (pg/ml) | 10 ± 5 | 53 ± 15* |
| Spleen (pg/organ) | 164 ± 66 | 653 ± 198 |
Amounts of cytokines were determined by ELISA in the different strains of mice and are expressed as means ng of IL-12p40 or pg of IL-12p70 and IL-23/ml of serum or/organ ± SEM of 12–15 animals per group
(P < 0·005 and
P < 0·00001).
BCG infection promotes transgenic IL-12p40 synthesis resulting in high levels of IL-12p70 and IL-23
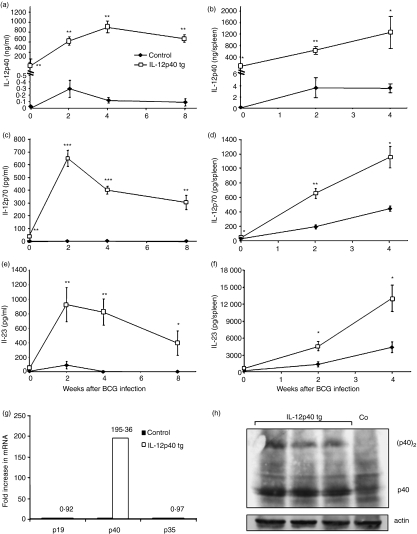
To investigate whether BCG infection activated IL-12p40 transgenic expression, concentrations were assessed at different time points after BCG infection. Serum amounts of IL-12p40 were increased in IL-12p40 tg mice 2 weeks postinfection and maintained at high levels 8 weeks after (Fig. 1a). Splenic amounts of IL-12p40 were also progressively increased during infection in IL-12p40 tg mice (Fig. 1b). IL-12p70 and IL-23 proteins were also determined. Serum and spleen levels of IL-12p70 and IL-23 were strongly increased in IL-12p40 tg mice through BCG infection (Fig. 1c–f). These results suggest that excess of IL-12p40 induced by BCG infection promotes IL-12p40-dependent cytokine formation.
Figure 1.
BCG infection activates transgenic IL-12p40 and results in high concentrations of IL-12p70 and IL-23. Amounts of IL-12p40 (a and b), IL-12p70 (c and d) and IL-23 (e and f) were measured at day 0 and at different time points of BCG infection in IL-12p40 tg mice and control littermates. Cytokines were evaluated in serum (a, c and e) (n = 12–15 mice/group) and in spleen extracts (b, d and f) (n = 5–6 mice/group). Data are represented, in a and b, as means ng of protein per ml of serum or spleen ± SEM or, in c–f, as means pg per serum or spleen. *P < 0·002, **P < 0·00004, ***P < 0·00000002. (g) IL-12p19, IL-12p40, and IL-12p35 mRNA expression in spleen of 2 weeks BCG-infected mice. Relative levels of mRNA were determined by quantitative RT–PCR. Results are expressed as fold increase in IL-12 subunit mRNA expression in IL-12p40 tg mice as compared to non-transgenic mice (n = 3 mice/group). (h) Identification of homodimeric (p40)2 and monomeric p40 in spleen of IL-12p40 tg mice at 4 weeks of BCG infection by Western blot. Only the monomeric form p40 is detected in non-transgenic mice. Spleen proteins were also blotted with an anti-actin antibody as control for protein loading. These results are representative of two or three independent experiments.
Analysis by RT–PCR showed that IL-12p40 mRNA expression was 195-fold higher in the spleen of IL-12p40 tg mice when compared to negative littermates 2 weeks after BCG infection. In contrast, no difference in IL-12p35 and p19 mRNA expression was observed by RT–PCR in the spleen of IL-12p40 tg and control mice (Fig. 1g). Comparable results were obtained by RT–PCR analyses using IL-12p40 tg mouse liver RNA (data not shown). Analysis by Western blot revealed that transgenic IL-12p40 was present as both monomeric and homodimeric forms in the spleen of IL-12p40 tg mice whereas only the monomeric form was detected in the spleen of control mice during BCG infection (Fig. 1h).
Th1 and Th2 immune responses in IL-12p40 tg mice after BCG infection
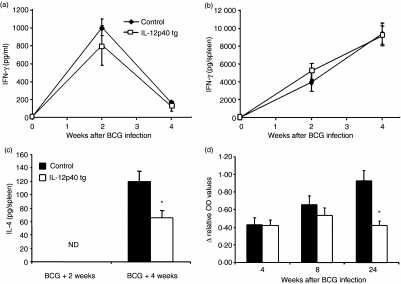
Mycobacterial infection induces a protective Th1 type immune response characterized by IFN-γ production required for host defence. To determine if IL-12p40 overexpression modified local and systemic expression of IFN-γ, we measured its concentration in serum and in the spleen during BCG infection. IFN-γ levels in sera and spleen were comparable in IL-12p40 tg and non-transgenic mice (Figs 2a, b). To determine whether BCG-induced Th2 cytokine expression was affected by overexpression of IL-12p40, we quantified IL-4 during BCG infection. Serum levels of IL-4 were lower than 5 pg/ml, which is the detection limit of the ELISA. IL-4 spleen concentrations were undetectable before and at 2 weeks after BCG infection, but, after 4 weeks of infection, IL-4 amounts were significantly lower in IL-12p40 tg mice compared to non-transgenic mice (Fig. 2c). Serum concentrations of IgG1 anti-BCG antibodies were found to be equivalent in IL-12p40 tg and non-transgenic mice in the early phase of infection, but, at the late stage of infection, serum amounts of IgG1 anti-BCG antibodies were reduced in IL-12p40 tg mice compared to non-transgenic mice (Fig. 2d). These results show that high concentrations of IL-12p40 do not modify the in vivo production of IFN-γ but may negatively influence IL-4 and the humoral Th2 type immune response characterized by IgG1.
Figure 2.
Activation of IFN-γ, IL-4 and IgG1 anti-BCG antibodies by BCG infection and influence of IL-12p40 overexpression. Kinetics of serum (a) and spleen (b) levels of IFN-γ induced by BCG infection. Amounts of IL-4 (c) in spleen of IL-12p40 tg and control mice after BCG infection. IL-4 was not detectable (ND) at 2 weeks of infection. Data are represented as means ± SEM (pg of protein/ml of serum or pg of protein/spleen) (n = 5–9 mice/group). Kinetics of IgG1 anti-BCG (d) antibodies at different time points after BCG infection. Results are represented as relative OD values after subtraction of unspecific OD corresponding to serum before infection. Data are represented as means ± SEM (n = 5–6 mice/group). These results are representative of two independent experiments (a to c) or one experiment (d). *P < 0·006.
Overexpression of IL-12p40 does not affect TNF and chemokine production
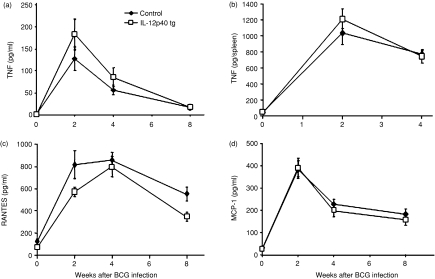
As TNF is one of the main cytokines induced by BCG infection, we investigated whether IL-12p40 overexpression affected BCG-dependent TNF activation during infection. Serum and spleen levels of TNF in IL-12p40 tg mice during BCG infection were comparable to those found in control mice (Fig. 3a, b). TNF is a regulator of chemokine induction after mycobacterial infection which is essential for cell migration to infection sites and granuloma formation.39 Analysis of the serum levels of two chemokines, RANTES and MCP-1, showed that BCG-induced chemokine production were comparable in IL-12p40 tg and non-transgenic mice (Fig. 3c, d).
Figure 3.
BCG-induced TNF and chemokines are not modified by transgenic IL-12p40. Serum (a) (n = 8–15 mice/group) and spleen (b) (n = 5 mice/group) TNF were measured at different time points after infection. Amounts of RANTES (c) and MCP-1 (d) were measured in serum at different time points after BCG infection (n = 8–15 mice/group). Data are expressed as means ± SEM. These results are representative of two independent experiments.
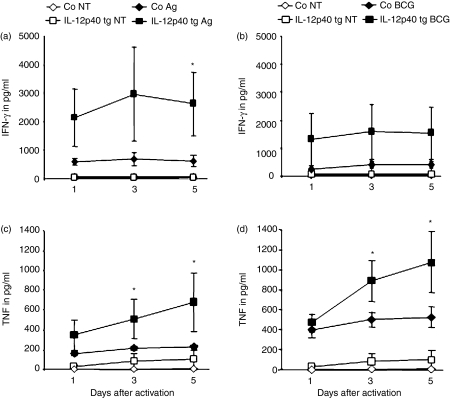
Antigen-induced production of IFN-γ and TNF by splenocytes derived from BCG infected mice
To determine whether the presence of IL-12p40 affected antigen-specific production of IFN-γ and TNF, their amounts were determined at different time points after activating splenocytes from infected animals with either BCG culture filtrate proteins or living BCG. Splenocytes from IL-12p40 tg infected mice activated in vitro with BCG proteins produced higher levels of IFN-γ and TNF than those from non-transgenic mice (Fig. 4a, c). Addition of viable BCG induced increased cytokine levels, and mainly TNF, in IL-12p40 tg mouse splenocytes (Fig. 4b, d). This result suggests that overexpression of IL-12p40 enhances ex vivo antigen-specific IFN-γ and TNF responses by splenocytes.
Figure 4.
Antigen-induced IFN-γ and TNF production by splenocytes from 4 weeks BCG-infected mice. IFN-γ (a and b) and TNF (c and d) were evaluated in the culture supernatant from cells incubated with medium (non treated: NT), or activated with BCG culture filtrate proteins (BCG antigens: Ag) (a and c) or with viable BCG (BCG) (b and d). Values are represented as means ± SEM from four to five mice/group (assay in duplicate). *P < 0·05.
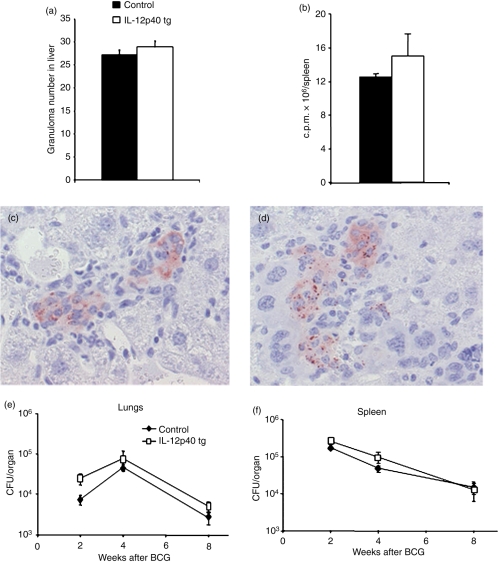
Granuloma formation, iNOS activity and bacillary clearance are not altered during BCG infection in IL-12p40 tg mice
To determine whether IL-12p40 tg mice were able to form normal bactericidal granulomas, the number and the phenotype of granulomas were assessed 4 weeks after BCG infection. IL-12p40 tg mice showed well-formed liver granulomas containing large and differentiated multinucleated giant cells as observed in control littermates. Comparable number of liver granulomas 4 weeks after BCG infection was found in IL-12p40 tg and in non-transgenic mice (Fig. 5a). The acid phosphatase staining, a marker for macrophage activation, evaluated on liver frozen sections and its quantification showed the same pattern of macrophage activation in the two groups of mice (data not shown). Macrophage iNOS activity in spleen proteins showed a similar pattern of activity in IL-12p40 tg mice and non-transgenic mice 4 weeks after BCG infection (Fig. 5b). INOS immunostaining of liver granulomas 4 weeks after BCG infection showed a similar pattern in IL-12p40 tg and non-transgenic mice (Fig. 5c, d).
Figure 5.
BCG granuloma formation, iNOS activity and bacillary loads are similar in IL-12p40 tg and non-transgenic mice. The number of liver granulomas (a) at 4 weeks of BCG infection were counted in IL-12p40 tg and in non-transgenic mice (n = 5 mice/group). Data are represented as means ± SEM of the number of granulomas per mm2 tissue section. These results are representative of two independent experiments. Macrophage iNOS activity (b) was assessed in spleen protein extracts of IL-12p40 tg and control mice 4 weeks after BCG infection. Data are represented as means of c p.m. (± SEM) of four to five mice per group. These results are representative of two independent experiments. iNOS immunostaining of liver sections of control (c) and IL-12p40 tg mice (d) at 4 weeks of BCG infection. Pictures in this figure are representative of five mice per group. Magnifications: 400×. (e and f), IL-12p40 mice are able to control and eliminate BCG in infected organs. Bacterial loads were determined in lungs (e) and spleen (f) 2, 4, and 8 weeks after i.v. inoculation with 107 CFU of BCG. The figure represents means ± SEM from five mice per group. Experiments were repeated twice with comparable results.
To assess whether IL-12p40 tg mice were protected from BCG infection, survival studies were carried out. After an intravenous infection with 107 living bacilli, IL-12p40 tg mice were able to control BCG infection and no mortality was observed even months (24 weeks) after infection (n = 10/per group). IL-12p40 tg mice showed no weight differences through infection when compared to control littermates (data not shown). In addition, bacterial loads in infected organs as quantified by colony assay at different time points of infection showed comparable amounts of living BCG in spleen and lungs of IL-12p40 tg mice and control littermates (Fig. 5e, f).
IL-12p40 tg mice are able to control M. tuberculosis infection
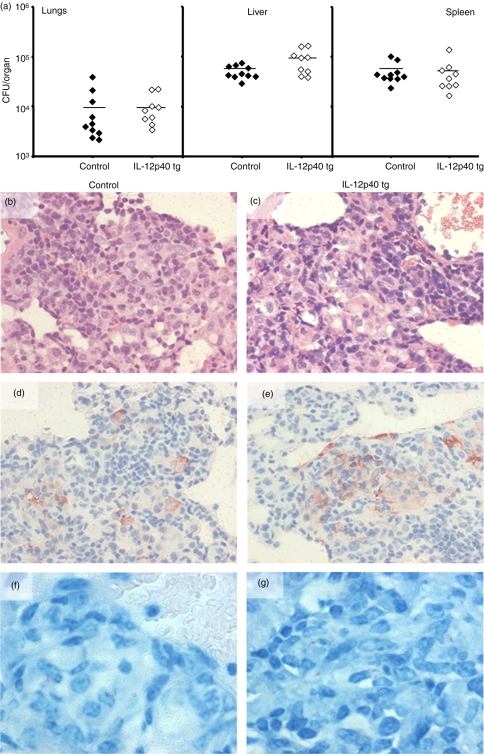
We also studied the capacity of IL-12p40 tg mice to control an i.v. infection by M. tuberculosis. Susceptibility of IL-12p40 tg mice to M. tuberculosis (H37Rv, 105 CFU) was assessed by bacterial counts in infected organs 4 weeks after infection. Bacterial loads of IL-12p40 tg mouse infected lungs, liver, and spleen were similar to those of non-transgenic mice (Fig. 6a). Lung granuloma morphology of IL-12p40 tg mice 4 weeks after M. tuberculosis infection was comparable to those of control littermates (Fig. 6b, c). Pulmonary caseous necrotic lesions which characterize the human lung immunopathology were not observed in either the IL-12p40 tg or the control mice. The iNOS expression was mainly located in macrophages forming granulomas and was equivalent in IL-12p40 tg and non-transgenic mice (Fig. 6d, e). Acid-fast bacillus content in granulomas was low in number and comparable in IL-12p40 tg and non-transgenic mice (Fig. 6f, g). These data demonstrate that overexpression of IL-12p40 did not change host resistance against systemic M. tuberculosis infection.
Figure 6.
IL-12p40 tg mice are resistant to M. tuberculosis infection and able to form granulomas expressing iNOS. (a) Bacterial loads were determined in lungs, liver and spleen 4 weeks after i.v. inoculation with 105 CFU of virulent M. tuberculosis strain H37Rv. Data represent individual values of infected mice (n = 9–10 mice/group). (b and c) Lung sections were stained with H&E at 4 weeks after M. tuberculosis inoculation from control (b) and IL-12p40 tg mice (c) (d and e) Illustration of iNOS immunostaining of lungs at 4 weeks of M. tuberculosis infection and cells expressing iNOS in granulomas from control (d) and IL-12p40 tg mice (e) Ziehl-Neelsen staining of lung lesions showing very low number of acid fast bacilli in control (f) and IL-12p40 tg mice (g) These figures are representative of one experiment with 9–10 mice per group. Original magnification: 400× (b–e) and 800× (f–g).
Discussion
The present work investigates cell-mediated immune responses mounted against M. bovis BCG and M. tuberculosis infections in mice overexpressing IL-12p40, and provides evidence that IL-12p40 promotes IL-12p40-dependent cytokine formation, does not inhibit Th1 type immune responses, and does not affect bacillary elimination during both BCG and M. tuberculosis infections. These data show that overexpression of IL-12p40 leads to the presence of increased levels of IL-12p70 and also of IL-23 which can be found in the circulation and in a minor extent localized in the spleen of IL-12p40 tg mice. A previous publication has shown that in transgenic mice expressing high amounts of systemic IL-12p40, the IL-12p70 serum concentration was equivalent to that found in control littermates.40 In a more recent study, lung IL-12p70 protein level was comparable in tg and non-transgenic mice when IL-12p40 transgene was produced by epithelial pulmonary cells.41 In our model system, IL-12p40 overexpression not only enhances IL-12p70 and IL-23 formation at steady state, but strong concentrations of these cytokines were activated by BCG infection in IL-12p40 tg mice clearly demonstrating that excess of IL-12p40 promotes IL-12p70 and IL-23 expression. Nevertheless, excess of IL-12p40, IL-12p70 and IL-23 did not influence BCG-induced IFN-γ protein concentrations in vivo as IFN-γ levels were comparable in IL-12p40 tg and non-transgenic mice. Only IL-4 production was found to be reduced in IL-12p40 tg mice. Analysis of the humoral immune response showed that serum levels of anti-BCG IgG1 antibodies were also lower in IL-12p40 tg than in non-transgenic mice. Our data show that IL-12p40 overexpression does not affect in vivo the Th1 type while the Th2 type immune responses are decreased during BCG infection.
TNF is one of the main cytokines induced by BCG infection. Although monomeric and dimeric IL-12p40 have been shown to induce TNF expression in macrophages,16 our results did not show any change in TNF expression upon BCG infection in IL-12p40 tg mice compared to negative littermates. In addition, BCG-activated chemokines such as RANTES and MCP-1 were not altered by overexpression of IL-12p40. This indicates that in vivo excess of IL-12p40 does not have a great effect on BCG-induced macrophage activity. Indeed, the macrophage iNOS activity was comparable in both the IL-12p40 tg and non-transgenic mice after BCG infection, and this suggests that excess of IL-12p40, but also of IL-12p70 and IL-23, appears to be redundant and does not modify BCG or M. tuberculosis control. This demonstrates that the presence of high concentrations of IL-12p40 is not detrimental for host immune responses against mycobacteria and this differs from previous data showing that transgenic pulmonary expression of IL-12p40 could negatively influence M. tuberculosis infection. Thus, mice expressing IL-12p40 transgene under the control of the SP-C lung promoter showed increase bacillary burden in the lung after intranasal infection by M. tuberculosis.41 In a previous study, mice expressing high serum levels of IL-12p40 under the control of a liver promoter presented a reduction in Th1 type of immune responses after immunization with keyhole limpet haemocyanin and ex vivo stimulation with antigens.40 The detrimental effects of IL-12p40 depicted in these two papers contrast with those obtained in mice deficient in IL-12p40 or IL-12p35. Administration of exogenous IL-12p40 to BCG infected IL-12p40–/–/IL-12p35–/– mice reduced bacillary load and restored antigen-specific T-cell responses.24 Recently, it has been reported that IL-12p40 treatment of pulmonary DC from IL-12p40–/– mice restored M. tuberculosis-induced DC migration and the ability of these DC to activate naïve T cells.42 In the absence of IL-12p70, antigen specific T-cell activation and Th1 immune responses were shown to be dependent on IL-23 thus showing that IL-23 can compensate for the absence of IL-12p70.42 However, in the presence of IL-12p70, IL-23 is dispensable for protection and susceptibility to BCG infection, suggesting that IL-23 only plays a role in mycobacterial infection when IL-12p70 cannot be produced.29
The IL-12 antagonist role of mouse IL-12p40 in in vitro experiments has been previously explained by the capacity to bind to the mouse IL-12 receptor thereby inhibiting IL-12-dependent cellular responses.17 However, more complex results were obtained in vivo on IL-12 non-mutated mice showing that exogenous IL-12p40 could have an IL-12 antagonist as well as agonistic role depending on the animal model system. Administration of high amounts (40–100 µg) of IL-12p40 inhibited IFN-γ production and prevented mortality after LPS without modification of TNF or IL-12p70 expression.43,44 In contrast, IL-12p40 did not prevent mortality after lipopolysaccharide/galactosamine challenge, but IFN-γ levels were not shown in the study.44 These studies illustrate the complexity of the activities of IL-12p40 in the presence of native molecules. One can consider that these distinct activities can be related to the animal model systems. In our model system, we do not observe any IL-12 antagonistic activity of IL-12p40 or any effect on in vivo Th1-type immune responses or on bacterial elimination and this differs from the previous IL-12p40 transgenic mouse studies indicating the IL-12 antagonist role of IL-12p40. The differences with the previous works can be explained by specificity and up-regulation of MHC class II promoter in immune responses and by systemic IL-12p40 production compared to local expression suggesting differences in promoter regulatory sequences upon an infection or by differences in local and systemic transgene expression levels.
MHC class II promoter is up-regulated by BCG infection, consequently, transgenic IL-12p40 production is increased resulting in progressive enhanced amounts of IL-12p70 and IL-23. Previous reports have shown that IL-12p70 secretion by PBMC correlates with increase of p40 mRNA but not p35 mRNA.13 We also observed that whereas IL-12p70 and IL-23 proteins increased through the infection, only IL-12p40 mRNA was up-regulated in IL-12p40 tg when compared to control mice, indicating that formation of IL-12p70 and IL-23 proteins does not involve up-regulation of p35 or p19 mRNA expression.
In conclusion, the present work shows that overexpression of IL-12p40 promotes IL-12p70 and IL-23 formation which do not influence in vivo BCG-induced IFN-γ and TNF whereas these cytokines are highly produced on ex vivo stimulation. In addition, IL-12p40 overexpression does not modify granuloma activity and mycobacterial elimination suggesting that excess of IL-12p40 and dependent cytokines are redundant in immunocompetent host.
Acknowledgments
This work was supported by grant 3200B0-105914 (to I.G) from the Swiss National Foundation for Scientific Research and by grant from Sir Jules Thorn Charitable Overseas Trust Foundation.
The authors are grateful to Dr Frederic Lajaunias for taking care of the IL-12p40 tg mice. We thank Ms G. Levraz, J. Stalder, M. Coassin, P. Gindre, Mr T. Le Minh and P. Chavarot for technical analyses, and the laboratory of the Professor C. Barazzone-Argiroffo: Dr S. Carnesecchi, Dr I. Metrailler-Ruchonnet, Dr A. Pagano and Mr Y. Donati for important assistance in Western blot method. We are grateful to Dr Arun Kamath and Ms Cecilia Ong for critical reading of the manuscript.
References
- 1.WHO. Report: Global Tuberculosis Control. Geneva: World Health Organization; 2003. [Google Scholar]
- 2.Salgame P. Host innate and Th1 responses and the bacterial factors that control Mycobacterium tuberculosis infection. Curr Opin Immunol. 2005;17:374–80. doi: 10.1016/j.coi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Adams LB, Mason CM, Kolls JK, Scollard D, Krahenbuhl JL, Nelson S. Exacerbation of acute and chronic murine tuberculosis by administration of a tumor necrosis factor receptor-expressing adenovirus. J Infect Dis. 1995;171:400–5. doi: 10.1093/infdis/171.2.400. [DOI] [PubMed] [Google Scholar]
- 4.Bean AG, Roach DR, Briscoe H, France MP, Korner H, Sedgwick JD, Britton WJ. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999;162:3504–11. [PubMed] [Google Scholar]
- 5.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia I, Miyazaki Y, Marchal G, Lesslauer W, Vassalli P. High sensitivity of transgenic mice expressing soluble TNFR1 fusion protein to mycobacterial infections. synergistic action of TNF and IFN-gamma in the differentiation of protective granulomas. Eur J Immunol. 1997;27:3182–90. doi: 10.1002/eji.1830271215. [DOI] [PubMed] [Google Scholar]
- 7.Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–40. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 8.Chan J, Tanaka K, Carroll D, Flynn JL, Bloom BR. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995. pp. 736–40. [DOI] [PMC free article] [PubMed]
- 9.Garcia I, Guler R, Vesin D, et al. Lethal Mycobacterium bovis bacillus Calmette–Guerin infection in nitric oxide synthase 2-deficient mice: cell-mediated immunity requires nitric oxide synthase 2. Lab Invest. 2000;80:1385–97. doi: 10.1038/labinvest.3780146. [DOI] [PubMed] [Google Scholar]
- 10.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–8. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–9. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 12.Fieschi C, Casanova JL. The role of interleukin-12 in human infectious diseases: only a faint signature. Eur J Immunol. 2003;33:1461–4. doi: 10.1002/eji.200324038. [DOI] [PubMed] [Google Scholar]
- 13.D'Andrea A, Rengaraju M, Valiante NM, et al. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–98. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trinchieri G. Interleukin-12 and its role in the generation of TH1 cells. Immunol Today. 1993;14:335–8. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- 15.Russell TD, Yan Q, Fan G, Khalifah AP, Bishop DK, Brody SL, Walter MJ. IL-12 p40 homodimer-dependent macrophage chemotaxis and respiratory viral inflammation are mediated through IL-12 receptor beta 1. J Immunol. 2003;171:6866–74. doi: 10.4049/jimmunol.171.12.6866. [DOI] [PubMed] [Google Scholar]
- 16.Jana M, Dasgupta S, Saha RN, Liu X, Pahan K. Induction of tumor necrosis factor-alpha (TNF-alpha) by interleukine-12 p40 monomer and homodimer in microglia and macrophages. J Neurochem. 2003;86:519–28. doi: 10.1046/j.1471-4159.2003.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillessen S, Carvajal D, Ling P, et al. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995;25:200–6. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 18.Ling P, Gately MK, Gubler U, et al. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J Immunol. 1995;154:116–27. [PubMed] [Google Scholar]
- 19.Mattner F, Fischer S, Guckes S, et al. The interleukin-12 subunit p40 specifically inhibits effects of the interleukin-12 heterodimer. Eur J Immunol. 1993;23:2202–8. doi: 10.1002/eji.1830230923. [DOI] [PubMed] [Google Scholar]
- 20.Cooper AM, Kipnis A, Turner J, Magram J, Ferrante J, Orme IM. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J Immunol. 2002;168:1322–7. doi: 10.4049/jimmunol.168.3.1322. [DOI] [PubMed] [Google Scholar]
- 21.Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehlers S, Lehmann J, Mossmann H, Alber G, Holscher C. Interleukin-12p40 mediates transient protection against Mycobacterium avium infection in the absence of interleukin-12. Immunobiology. 2005;210:217–27. doi: 10.1016/j.imbio.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Wakeham J, Wang J, Magram J, et al. Lack of both types 1 and 2 cytokines, tissue inflammatory responses, and immune protection during pulmonary infection by Mycobacterium bovis bacille Calmette–Guerin in IL-12-deficient mice. J Immunol. 1998;160:6101–11. [PubMed] [Google Scholar]
- 24.Holscher C, Atkinson RA, Arendse B, Brown N, Myburgh E, Alber G, Brombacher F. A protective and agonistic function of IL-12p40 in mycobacterial infection. J Immunol. 2001;167:6957–66. doi: 10.4049/jimmunol.167.12.6957. [DOI] [PubMed] [Google Scholar]
- 25.Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–8. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 26.Murphy CA, Langrish CL, Chen Y, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–7. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 28.Chackerian AA, Chen SJ, Brodie SJ, Mattson JD, McClanahan TK, Kastelein RA, Bowman EP. Neutralization or absence of the IL-23 pathway does not compromise immunity to mycobacterial infection. Infect Immun. 2006;74:6092–9. doi: 10.1128/IAI.00621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khader SA, Pearl JE, Sakamoto K, et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol. 2005;175:788–95. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 30.Ibnou-Zekri N, Iwamoto M, Gershwin ME, Izui S. Protection of murine lupus by the Ead transgene is MHC haplotype-dependent. J Immunol. 2000;164:505–11. doi: 10.4049/jimmunol.164.1.505. [DOI] [PubMed] [Google Scholar]
- 31.Garcia I, Miyazaki Y, Araki K, et al. Transgenic mice expressing high levels of soluble TNF-R1 fusion protein are protected from lethal septic shock and cerebral malaria, and are highly sensitive to Listeria monocytogenes and Leishmania major infections. Eur J Immunol. 1995;25:2401–7. doi: 10.1002/eji.1830250841. [DOI] [PubMed] [Google Scholar]
- 32.Guler R, Olleros ML, Vesin D, et al. Inhibition of inducible nitric oxide synthase protects against liver injury induced by mycobacterial infection and endotoxins. J Hepatol. 2004;41:773–81. doi: 10.1016/j.jhep.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 33.Otten LA, Tacchini-Cottier F, Lohoff M, et al. Deregulated MHC class II transactivator expression leads to a strong Th2 bias in CD4 (+) T lymphocytes. J Immunol. 2003;170:1150–7. doi: 10.4049/jimmunol.170.3.1150. [DOI] [PubMed] [Google Scholar]
- 34.Olleros ML, Guler R, Corazza N, et al. Transmembrane TNF induces an efficient cell-mediated immunity and resistance to Mycobacterium bovis bacillus Calmette–Guerin infection in the absence of secreted TNF and lymphotoxin-alpha. J Immunol. 2002;168:3394–401. doi: 10.4049/jimmunol.168.7.3394. [DOI] [PubMed] [Google Scholar]
- 35.Olleros ML, Guler R, Vesin D, et al. Contribution of transmembrane tumor necrosis factor to host defence against Mycobacterium bovis bacillus Calmette–guerin and Mycobacterium tuberculosis infections. Am J Pathol. 2005;166:1109–20. doi: 10.1016/S0002-9440(10)62331-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucas R, Tacchini-Cottier F, Guler R, et al. A role for lymphotoxin beta receptor in host defence against Mycobacterium bovis BCG infection. Eur J Immunol. 1999;29:4002–10. doi: 10.1002/(SICI)1521-4141(199912)29:12<4002::AID-IMMU4002>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 37.Power CA, Wei G, Bretscher PA. Mycobacterial dose defines the Th1/Th2 nature of the immune response independently of whether immunization is administered by the intravenous, subcutaneous, or intradermal route. Infect Immun. 1998;66:5743–50. doi: 10.1128/iai.66.12.5743-5750.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guler R, Olleros ML, Vesin D, Parapanov R, Vesin C, Garcia I. Differential effects of total and partial neutralization of tumor necrosis factor on cell-mediated immunity to Mycobacterium bovis BCG infection. Infect Immun. 2005;73:3668–76. doi: 10.1128/IAI.73.6.3668-3676.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 2002;168:4620–7. doi: 10.4049/jimmunol.168.9.4620. [DOI] [PubMed] [Google Scholar]
- 40.Yoshimoto T, Wang CR, Yoneto T, et al. Reduced T helper 1 responses in IL-12 p40 transgenic mice. J Immunol. 1998;160:588–94. [PubMed] [Google Scholar]
- 41.Leemans JC, Wieland CW, Florquin S, van der Poll T, Vervoordeldonk MJ. Mice overexpressing p40 in lungs have reduced leucocyte influx and slightly impaired resistance during tuberculosis. Immunology. 2006;117:409–18. doi: 10.1111/j.1365-2567.2005.02315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khader SA, Partida-Sanchez S, Bell G, et al. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J Exp Med. 2006;203:1805–15. doi: 10.1084/jem.20052545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heinzel FP, Hujer AM, Ahmed FN, Rerko RM. In vivo production and function of IL-12 p40 homodimers. J Immunol. 1997;158:4381–8. [PubMed] [Google Scholar]
- 44.Mattner F, Ozmen L, Podlaski FJ, Wilkinson VL, Presky DH, Gately MK, Alber G. Treatment with homodimeric interleukin-12 (IL-12) p40 protects mice from IL-12-dependent shock but not from tumor necrosis factor alpha-dependent shock. Infect Immun. 1997;65:4734–7. doi: 10.1128/iai.65.11.4734-4737.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]