Abstract
Dendritic cells (DC) are powerful inducers of primary T-cell responses, but their role in secondary responses has not been extensively analysed. Here, we address the role of two DC subsets derived from human CD16+ (16+ mDC) or CD16– (16– mDC) monocytes on the reactivation of memory responses. CD4+ CD45RA– memory T cells were obtained from adult blood donors, and central (TCM) and effector (TEM) memory T cells were isolated by fluorescence-activated cell sorting with anti-CCR7 antibodies. The 16+ mDC and 16– mDC were cocultured with autologous lymphocytes, either unpulsed or loaded with purified protein derivatives of Mycobacterium tuberculosis (PPD) or tetanus toxoid (TT), and were analysed for up to 8 days. Over a range of doses, 16+ mDC drove stronger T-cell proliferative responses against both antigens. Overall, antigen-specific memory cells tended to acquire a phenotype of TEM at later time-points in the culture, whereas cells that had completed fewer cycles of division were similar to TCM. The 16+ mDC induced higher rates of proliferation on both TCM and TEM lymphocytes than 16– mDC. This phenomenon was not related to the ability of both DC to induce CD25 expression on T cells, to lower secretion of interleukin-2, or to raise production of interleukin-10 during T-cell/16– mDC cocultures. The induction of TCM effector capacity in terms of interferon-γ production was faster and more pronounced with 16+ mDC, whereas both DC had similar abilities with TEM. In conclusion, these data might reveal new potentials in vaccination protocols with 16+ mDC aimed at inducing strong responses on central memory T cells.
Keywords: dendritic cell subsets, interferon-γ production, lymphoproliferation, recall antigens
Introduction
Differentiation of circulating blood monocytes into tissue macrophages and dendritic cells (DC) has been well established in vitro1 and in vivo.2In vivo, they migrate from blood to inflammatory sites where many differentiate to macrophages,2 which apparently fail to initiate T-cell responses.3 Other infiltrating monocytes become DC in the presence of phagocytic or chemoattractant stimuli2,4 and are able to present antigens and prime naive lymphocytes.5In vivo responses of naive T lymphocytes against particulate antigens are mainly dependent on monocyte-derived DC,6 confirming the biological relevance of these cells.
In humans, two monocyte subsets have been identified based on their expression of CD16: the regular CD14hiCD16– subpopulation (referred to as CD16–), accounting for 90–95% of monocytes in healthy individuals, and the minor CD14+/– CD16+ subset (CD16+), comprising the remaining 5–10%.7,8 CD16+ monocytes exhibit certain phenotypic characteristics resembling tissue macrophages, with lower expression of CD11b and CD33 and higher expression of human leucocyte antigen (HLA) class II than classical CD16– monocytes.8 Data suggest an inflammatory nature for CD16+ monocytes. First, they express high levels of surface CD11a, CD11c, intracellular adhesion molecule-1, very-late activation antigen type 4 and CX3CR1, which has been associated with an increased interaction with endothelia and rapid migration into inflamed tissues.8–10 Second, they constitutively produce pro-inflammatory cytokines in patients infected with human immunodeficiency virus11 and secrete low levels of interleukin-10 (IL-10) in vitro12 and third, their numbers are drastically increased in pathological conditions associated with acute or chronic inflammatory responses.13 However, CD16+ monocytes lack CCR2, a chemokine receptor involved in monocyte recruitment to many inflamed tissues,14 so the actually nature of CD16+ monocytes is still unknown. The identification of mouse monocyte subsets that closely resemble human CD16+ (Gr-1lo) and CD16– (Gr-1hi) monocytes15 has allowed their fate in both steady-state conditions and inflammation to be traced. Several lines of evidence suggest that Gr-1hi monocytes are recruited to inflammatory or infection sites and give rise to macrophages and DC, including Langerhans cells,15,16 whereas Gr-1lo monocytes might be precursors for resident DC and/or macrophages in the steady state.15 Remarkably, human CD16+ monocytes preferentially differentiate in vitro to DC in a model of transendothelial trafficking.17 Furthermore, Toll-like receptor 2/1 activation of human monocytes triggers their differentiation to macrophages and DC, but DC differentiation is restricted to the CD16+ subset, while macrophage differentiation is enhanced in CD16– monocytes.18 Though the extent of these findings is unknown, human monocytes, and particularly the CD16+ subset, might be precursors for DC in vivo.
We previously demonstrated that human CD16+ and CD16– monocytes could differentiate to DC (referred as 16+ mDC and 16– mDC) when cultured with granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4.12,19 Both DC types showed similar abilities to induce proliferation of allogeneic naive CD4+ T cells.12 However, 16– mDC induced lower levels of IL-4 on these T cells compared with 16+ mDC, while there were no significant differences in interferon-γ (IFN-γ) induction by both DC subtypes.12,19 Regarding the activation of CD4+ memory T cells, 16+ mDC were found to induce greater responses than 16– mDC in vitro20 in studies performed with cells from patients with metastatic renal cell carcinoma. Patients were vaccinated with total monocyte-derived DC pulsed with tumour lysates and keyhole limpet haemocyanin and matured with tumour necrosis factor-α (TNF-α) and prostaglandin E2. The 16+ mDC induced greater proliferation of CD4+ T lymphocytes against tumour antigens in 70% of patients after treatment, while the remaining 30% showed similar levels of proliferation.20 Additionally, 16– mDC induced higher ratios of IFN-γ : IL-4 among CD4+ T cells against tumour antigens before therapy. However, 16+ mDC reversed that tendency and induced stronger T helper type 1 (Th1) responses after treatment.20
Recent data supported a preferential role of DC in memory T-cell reactivation. Memory CD8+ T-cell responses in lymphoid and non-lymphoid organs are strongly reduced when DC are transiently ablated,3 as previously suggested by others for memory CD4+ lymphocytes in peripheral tissues.21 Several studies have demonstrated that some DC subtypes induce T-cell proliferation more efficiently than others.22 In humans, DC derived from CD34+ progenitors or from CD11c+ blood precursors induced better lymphoproliferative responses against recall antigens than monocyte-derived DC.23,24 Therefore, selective activation of distinct types of DC could explain in part the great heterogeneity of T-cell effector functions or the development of different memory subpopulations. In recent years, two subsets of CD4+ memory T cells have been defined based on their differential expression of the chemotactic receptor CCR7 and the adhesion molecule l-selectin (CD62L): central (TCM) and effector (TEM) lymphocytes.25,26 The TEM (CCR7– CD62L–/+) migrate preferentially from blood into tissues under homeostatic or inflammatory conditions. Upon restimulation, TEM display immediate secretion of effector cytokines (IFN-γ, IL-4, IL-5), which has been attributed to their fully differentiated state and low activation threshold. In contrast, TCM possess only a partial level of differentiation and, upon polyclonal stimulation, are only capable of secreting IL-2. The TCM constitutively express CCR7 and CD62L, which allows them to gain access into secondary lymphoid organs. There, TCM could interact with professional antigen-presenting cells, such as DC, increasing the likelihood that they progress along their differentiation pathway. According to their properties, it is predictable that TEM mount immediate protective responses at sites of antigen entry, whereas TCM need first to travel to secondary lymphoid compartments for commitment to proliferation and differentiation to effector cells.27 The pathways leading to TCM and TEM development are currently controversial, and it is not clear whether they represent separate or interrelated cell lineages.28–32
Since previous evidence suggested that 16– mDC and 16+ mDC differentially affect T-cell memory activation, the present study sought to clarify the role of these DC in the restimulation of secondary responses in healthy individuals against two well-known recall antigens, related to Th1 (purified protein derivative of Mycobacterium tuberculosis; PPD) or Th2 (tetanus toxoid; TT) cytokine patterns. We present evidence that 16+ mDC induced higher proliferative responses of memory CD4+ T cells than 16– mDC. This phenomenon was independent of CD25 expression on these cells or the amount of IL-2 and IL-10 secreted during cultures. Finally, we showed that 16+ mDC elicit stronger IFN-γ responses in memory cells, particularly in TCM.
Materials and methods
Media and reagents
Lymphocytes and monocytes were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated autologous serum, 2 mm l-glutamine, 1 mm sodium pyruvate, 0·1 mm non-essential amino acids (Hyclone Laboratories, Logan, UT), 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μm 2-mercaptoethanol (Gibco BRL, Grand Island, NY), referred to as complete medium. The following reagents were used: human recombinant GM-CSF (hrGM-CSF; 1000 U/ml), rat neutralizing monoclonal antibody (mAb) to IL-10 (JES3–19F1, 5 μg/ml) and its immunoglobulin isotype control (BD PharMingen, San Diego, CA); human IL-2 (5–100 U/ml; Gibco BRL); hrIL-4 (15 ng/ml) and prostaglandin E2 (0·1 μg/ml) (Calbiochem, La Jolla, CA); hrTNF-α (40 ng/ml; R & D Systems, Minneapolis, MN); lipopolysaccharide (LPS) from Escherichia coli 0111:B4 (0·5 μg/106 cells/ml; Sigma-Aldrich, St Louis, MO); peptidoglycan (PGN) from Staphylococcus aureus (10 μg/ml; Fluka, Milwaukee, WI); polyinosinic–polycytidylic acid [poly (I:C), 50 μg/ml; Amersham Life Science, Buckingham, UK]; carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR); TT (0·01–10 μg/ml; kindly supplied by the National Institute of Hygiene, Mexico); PPD (0·01–100 μg/ml; Statens Serum Institut, Copenhagen, Denmark).
Cell separation and differentiation of peripheral blood monocytes
Peripheral blood mononuclear cells were purified from buffy coats of healthy volunteers vaccinated with TT and with a positive tuberculin skin test reaction (induration ≥10 mm at 48 hr; Aventis Pasteur, France). They were isolated using Ficoll-Hypaque (Gibco BRL) density gradient centrifugation. Subsequently, CD56– CD16+ and CD56– CD16– CD14+ cells (referred as CD16+ and CD16– monocytes) were separated by magnetic cell sorting, using MACS isolation kits (Miltenyi Biotec, Bergisch Gladbach, Germany), as described elsewhere.19 Autologous memory CD4+ CD45RA– T lymphocytes from adult blood were separated by negative selection using the MACS CD4+ T-cell isolation kit (Miltenyi Biotec), followed by incubation with MACS anti-CD45RA antibody. Central (CCR7+, TCM) and effector (CCR7–, TEM) memory T cells were isolated from CD4+ CD45RA– lymphocytes by fluorescence-activated cell sorting using anti-CCR7 antibodies (3D12; BD PharMingen).
Isolated monocytes were cultured at 106 cells/ml in complete medium supplemented with GM-CSF and IL-4 to obtain DC. The cultures were fed with fresh medium and cytokines every 2 days. After 6 days, non-adherent DC were harvested and replated at 5 × 105 cells/ml in medium containing GM-CSF and IL-4. Then, different doses of TT or PPD were added to the immature DC, and 8 hr later DC were activated with TNF-α and prostaglandin E2 for two more days.
Cytokine detection in DC culture supernatants
Production of IL-10 in the supernatants of immature DC (day 6) was quantified using an enzyme-linked immunosorbent assay (ELISA) kit from BD PharMingen. Either 16– mDC or 16 + mDC were incubated with LPS, PGN or poly (I:C) at the concentrations mentioned above. Controls were established with cells cultured without additional stimulus. IL-10 production was measured from 24-hr supernatants of 1 × 105 cells/100 μl.
T-cell activation
For T-cell memory proliferation assays, CD4+ CD45RA– lymphocytes were labelled with CFSE as described elsewhere33 and cocultured with either autologous antigen-loaded or unloaded DC, at a ratio of 8 : 1 for up to 8 days. In some experiments, memory lymphocytes were activated for 5 days with CD16– or CD16+ monocytes pulsed with PPD (10 μg/ml) at a ratio of 8 : 1. Occasionally, cocultures with 16– mDC were treated with IL-2 (5–100 U/ml). On different days, lymphocytes were harvested and stained with allophycocyanin-conjugated anti-CD62L (DREG-56), phycoerythrin-labelled anti-CD25 (M-A251), or phycoerythrin-labelled anti-CCR7 (3D12) antibodies (BD PharMingen), and analysed by flow cytometry.
For cytokine production assays, 2 × 105 to 4 × 105 memory T cells were cocultured with either autologous PPD-pulsed or TT-pulsed DC at an 8 : 1 ratio in complete medium. Controls were set with unpulsed DC. Lymphocytes were harvested 2–8 days after priming, according to the different experiments, washed extensively, and restimulated for 6 hr with phorbol 12-myristate 13-acetate (20 ng/ml) plus ionomycin (500 ng/ml) for intracellular staining of IFN-γ, or for 24 hr with immobilized anti-CD3 mAb (5 μg/ml, UCHT1, BD PharMingen) for quantification of IL-10 secretion. For intracellular staining, GolgiPlugTM (BD PharMingen) was added to the cultures 4 hr before the cells were harvested, to prevent cytokine secretion. Then, cells were fixed and permeabilized using a Cytofix/Cytoperm kit (BD PharMingen) and incubated with allophycocyanin-labelled anti-IFN-γ (clone 45–15, Miltenyi Biotec). Quantification of IL-10 secretion in the supernatants was performed with an ELISA kit from BD PharMingen.
Statistical analyses
Data were expressed as mean ±SD of independent experiments. The statistical significance of the data was determined by Student's two-tailed paired t-test, assuming equal variances.
Results
DC subtypes induced differential T-cell memory responses against TT and PPD
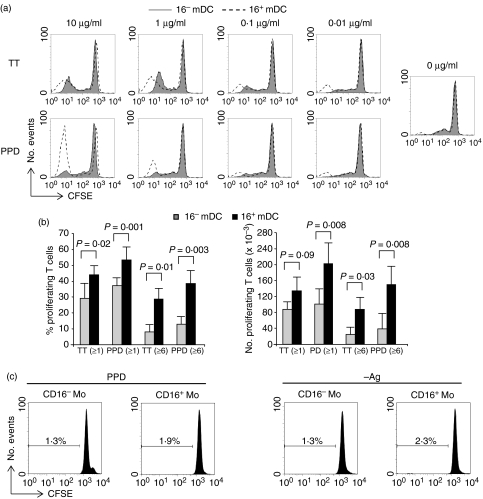
The 16+ mDC showed stronger ability than 16– mDC to induce CD4+ T-cell memory proliferation in response to TT and PPD, over a range of antigen doses (0·01–10 μg/ml) (Fig. 1a,b). Both the percentage and the number of proliferating T lymphocytes were significantly higher when stimulated with 16+ mDC (Fig. 1b). However, the most substantial difference with respect to 16– mDC was observed in cells undergoing intermediate to high numbers of divisions (six or more, Fig. 1b). Overall, the response to PPD was slightly higher than to TT in several independent donors, and differences between 16+ mDC and 16– mDC were usually more marked with PPD. The diminished proliferation induced by 16– mDC was not the result of an enhancement of cell death because we detected more necrotic or apoptotic lymphocytes in cultures with 16+ mDC, probably caused by activation-induced cell death (data not shown).
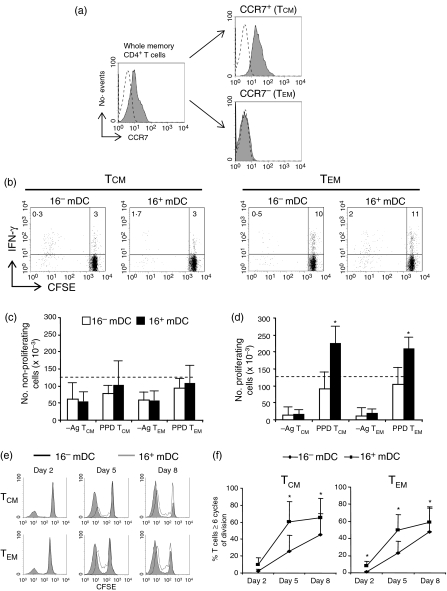
Figure 1.
Lymphoproliferative activity of CD4+ memory T cells against TT and PPD induced by 16– mDC and 16+ mDC. (a) DC were pulsed with different doses (0–10 μg/ml) of TT (top) or PPD (bottom), and cocultured for 5 days with CFSE-labelled CD4+ CD45RA– autologous T lymphocytes. Cell division induced by 16– mDC (solid histograms) and 16+ mDC (empty histograms) was measured by flow cytometry. A representative assay of four is shown. (b) Percentage (left) and number (right) of proliferating T cells with ≥1 or ≥6 cycles or of division after 5 days of coculture with 16– mDC or 16+ mDC pulsed with TT (1 μg/ml) or PPD (10 μg/ml). Cultures were set as above with 2·5 × 105 memory T lymphocytes at 1 : 8 DC : T-cell ratio. After 5 days, lymphocytes were counted and the number and percentage of proliferating cells were calculated by the CFSE dilution method. Data shown are the mean ± SD of six different donors. The values obtained with unpulsed DC were subtracted. Statistical significance of the data is indicated. (c) CD16– and CD16+ monocytes were cultured for 5 days with CFSE-labelled autologous CD4+ CD45RA– memory lymphocytes, in the presence of PPD (10 µg/ml) or in the absence of antigen (– Ag). Cell division was measured by flow cytometry. Percentages shown correspond to cells with one or more cycle of division.
We next asked whether the differences between 16– mDC and 16+ mDC in eliciting secondary responses were also attributable to their monocyte precursors. Both CD16+ and CD16– monocytes were cultured with CD4+ CD45RA– lymphocytes in the presence of 10 μg/ml PPD (Fig. 1c). Under the same conditions where DC induced considerable responses, none of the monocyte subpopulations were able to elicit antigen-driven proliferation of memory lymphocytes.
Effector activity of memory lymphocytes activated by DC
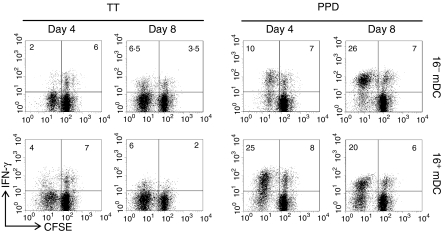
Along with proliferation, memory lymphocytes achieved effector activity, assessed by intracellular IFN-γ production (Fig. 2). Some non-proliferating cells also acquired the ability to produce this cytokine (Fig. 2). The cytokine profile was as expected, i.e. the percentage of PPD-specific IFN-γ-producing lymphocytes was greater with respect to TT-specific cells. The number of both PPD-specific and TT-specific lymphocytes stimulated with 16+ mDC that produced IFN-γ at early time-points was larger compared to those activated by 16– mDC (day 4, Fig. 2). Hence, the kinetics of appearance of IFN-γ+ cells was faster with 16+ mDC. However, at later time-points (day 8) lymphocytes stimulated by 16– mDC occasionally reached the levels observed with 16+ mDC (Fig. 2). On the other hand, at that time, 16+ mDC-activated lymphocytes tended to diminish the mean fluorescence intensity of IFN-γ+ cells, which is associated with an earlier returning to rest (not shown).
Figure 2.
IFN-γ production by CD4+ memory T cells stimulated by 16– mDC (top panel) or 16+ mDC (bottom panel) loaded with TT (1 μg/ml) or PPD (10 μg/ml). Cultures were established as in Fig. 1. After 4 or 8 days of priming, lymphocytes were restimulated with PMA and ionomycin for 6 hr and stained with APC-labelled anti-IFN-γ antibodies. Results are representative of four independent experiments. The numbers represent percentage of cells in the respective quadrant.
Phenotype of memory lymphocytes stimulated by DC
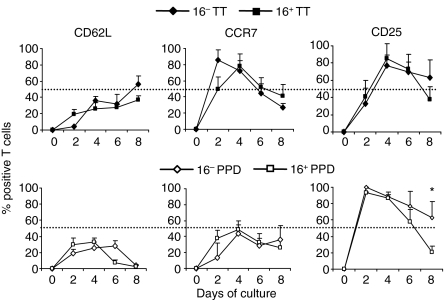
To assess the phenotype of memory T cells upon restimulation with both DC, we performed a kinetic study of secondary lymphoid tissue homing (CCR7, CD62L) and activation receptor (CD25) expression within the proliferating cells. Co-cultures were maintained for an 8-day period and analysed every 2 days. In those assays the total of proliferating cells was represented at every time as 100% so as to compare both DC subtypes while avoiding their differences in T-cell proliferation (Fig. 3). At later time-points, PPD-responding lymphocytes tended to down-regulate CD62L, whereas ∼40% of TT-specific cells conserved the expression of this molecule (Figs 3 and 4c,d). Overall, the levels of CCR7 at earlier time-points (2–4 days) were diminished in PPD-specific compared to TT-specific lymphocytes, but at the end of the assays both types of cells were mostly CCR7– (60–75% of dividing cells were CCR7– at day 8, Figs 3 and 4). Moreover, the maximum percentage of CD25+ in cycling lymphocytes responding to PPD was found at early times (day 2), while those responding to TT reached their peak 2 days later (Fig. 3). These differences could correlate with the more efficient expansion of PPD-specific lymphocytes that was previously observed (Fig. 1b). When we lengthened the time of coculture, lymphocytes responding to TT and PPD down-regulated the expression of CD25, but this tended to occur faster and more prominently with 16+ mDC (Fig. 3; P < 0·05 at day 8 with PPD). Accordingly, cells with more rounds of division, i.e. lymphocytes stimulated with PPD-loaded 16+ mDC, expressed the lowest level of CD25 (Figs 3 and 4d).
Figure 3.
Kinetics of marker expression on CD4+ memory T lymphocytes stimulated with 16– mDC or 16+ mDC. CFSE-labelled lymphocytes were cultured with 16– mDC or 16+ mDC loaded with 1 μg/ml TT or 10 μg/ml PPD for an 8-day period. Lymphocyte phenotype was evaluated every 2 days with anti-CD62L, anti-CCR7 and anti-CD25 antibodies by flow cytometry. Values are the mean ± SD of four independent donors, and were calculated as the percentage of T cells positive for each marker within the proliferating population (100%). Statistical analysis: *P < 0·05.
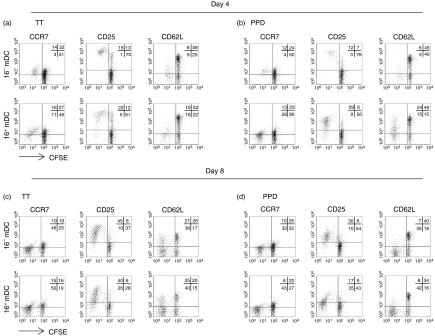
Figure 4.
Phenotypic analysis of antigen-specific CD4+ memory T cells stimulated with 16– mDC or 16+ mDC. DC were pulsed with 1 μg/ml TT (a, c) or 10 μg/ml PPD (b, d) and cocultured with CFSE-labelled T lymphocytes as described in Fig. 1. After 4 (a, b) or 8 (c, d) days, cells were harvested, stained with the corresponding antibodies (anti-CCR7, anti-CD25, anti-CD62L), and analysed by flow cytometry. One representative experiment out of four is shown. Percentage of cells in each quadrant is indicated.
Additionally, we detected that lymphocytes undergoing fewer cycles of division (∼ 3–6) expressed higher levels of CCR7 and CD25, while cells that had experienced more rounds of proliferation down-regulated both markers. Figure 4(a–d) shows a representative example. Nevertheless, down-regulation of CCR7 occurred earlier compared with CD25. Thus, most of the dividing cells converted over time from CCR7+ CD25+, to CCR7– CD25+, to CCR7– CD25–, and this gradual transition paralleled the rate of cell division. Since lymphocytes activated with 16+ mDC usually underwent more extensive proliferation, it was common to find higher numbers of CCR7– CD25– lymphocytes within the proliferating population stimulated with this DC subtype.
Effect of DC subtypes on antigen-driven recall of TCM and TEM subsets
The differences found between 16– mDC and 16+ mDC using total memory CD4+ cells prompted us to examine if they had a distinct effect on the response of the two major memory subsets, TCM and TEM. Thus, both subpopulations were separated by fluorescence-activated cell sorting based on their expression of CCR7 (Fig. 5a) and were activated with PPD-loaded DC. The features of isolated T cells were in agreement with previous reports25,26 as TEM had earlier effector activity than TCM (they had superior capacity to produce IFN-γ before the lymphocytes divided, Fig. 5b). Similar to the observation made with the entire CD4+ memory population, we detected a significantly increased rate of proliferation induced by 16+ mDC in both PPD-specific TCM and TEM (Fig. 5d–f). There were no significant differences in the number of viable non-proliferating lymphocytes upon culture with 16– mDC or 16+ mDC (Fig. 5c). An average of 60–80% of the initial TCM or TEM lymphocytes survived as non-cycling cells when cultured with antigen-pulsed DC (not shown). As cells divide along the culture, the percentage of proliferating lymphocytes reached 50–75% of the recoverable viable cells at day 5 (not depicted). Differences between 16– mDC and 16+ mDC were equally prominent with TCM and TEM (Fig. 5d). Likewise, when the percentage of cells in advanced rounds of division (at least six cycles, Fig. 5e,f) was taken into account, differences were evident. In general, we can conclude that 16+ mDC allowed both TCM and TEM to complete more cycles of division in the same time frame, and the total number of proliferating T cells stimulated with 16+ mDC was greater compared to those stimulated with 16– mDC.
Figure 5.
Interaction of 16– mDC and 16+ mDC with the major subsets of CD4+ memory T cells. (a) Central memory T cells (CCR7+, TCM) and effector memory T cells (CCR7–, TEM) were isolated from CD4+ CD45RA– lymphocytes by fluorescence-activated cell sorting using anti-CCR7 antibodies. Histograms show CCR7 expression on total CD4+ CD45RA– memory lymphocytes and on isolated TCM and TEM cells (solid lines). Dashed lines correspond to an isotype-control mAb. (b) Secretion of IFN-γ by CFSE-labelled TCM and TEM lymphocytes stimulated for 2 days with 16– mDC or 16+ mDC pulsed with 10 μg/ml PPD. Percentage of positive cells in the respective quadrant is indicated. (c) Number of non-proliferating TCM or TEM stimulated with 16– mDC (white) or 16+ mDC (black). Cultures were set with PPD-loaded DC (10 μg/ml) or unpulsed DC (– Ag) and CFSE-labelled lymphocytes (1·25 × 105) at a 1 : 8 DC : T-cell ratio. After 5 days, lymphocytes were counted and the number of non-proliferating cells was obtained using the CFSE dilution method. Data indicate the mean ± SD of five different donors. (d) Number of proliferating cells under the same conditions as in (c). (e) A representative example of the proliferation measured by CFSE dilution of TCM and TEM lymphocytes cultured as in (b) for an 8-day period with 16– mDC (empty black histograms) or 16+ mDC (solid grey histograms). (f) Percentage of TCM or TEM within the viable population with six or more cycles of division cultured for 5 days with 16– mDC or 16+ mDC. Data show the mean ±SD of five independent donors. Statistical analysis: *P < 0·05.
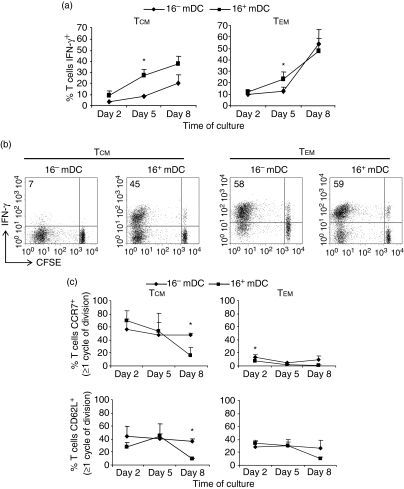
In terms of effector activity, 16+ mDC induced a higher percentage of IFN-γ-producing cells in the TCM subset than did 16– mDC, whereas differences with TEM were less evident (Fig. 6a). By the end of the culture, a higher proportion of TEM lymphocytes showed effector activity with respect to TCM cells. At that time, there were still differences between 16+ mDC and 16– mDC with TCM lymphocytes (although not significant), but the percentage of IFN-γ+ cells activated by both DC was similar in TEM (Fig. 6a,b). Regarding the phenotype of lymphocytes during culture, TEM did not re-express CCR7, and roughly 80% of the cells were CD62L– by the end of the assays, indicating that they maintained a classical TEM phenotype. Most of the 16+ mDC-activated TCM cells acquired a TEM phenotype, while about half of those stimulated with 16– mDC maintained the expression of CCR7.
Figure 6.
Phenotype and kinetics of IFN-γ production by TCM and TEM lymphocytes stimulated with 16– mDC or 16+ mDC. (a) CFSE-labelled lymphocytes were cultured with 16– mDC or 16+ mDC loaded with 10 μg/ml PPD for an 8-day period. The percentage of IFN-γ+ cells was evaluated by flow cytometry. Values were reported as the percentage of IFN-γ+ T cells. (b) A representative example of IFN-γ production upon 8 days of culture. The percentage of IFN-γ+ proliferating cells is indicated. (c) Phenotype of dividing cells cultured as in (a). Values were calculated as the percentage of positive cells within the proliferating population (100%). Data show the mean ± SD of three independent donors in (a) and (c). Statistical analysis: *P < 0·05.
Putative molecules involved in the decreased lymphoproliferation induced by 16– mDC
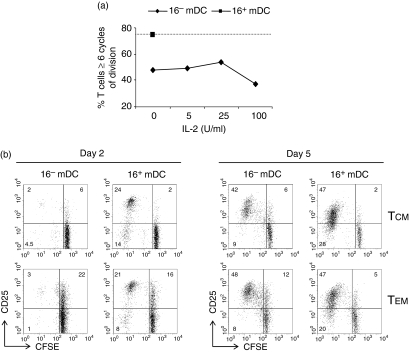
We next investigate whether the low rate of proliferation induced by 16– mDC in memory cells was the result of a failure in the activation of the IL-2/CD25 system.34 To test this hypothesis, we performed cocultures of 16– mDC with CD4+ memory T cells in the presence of graded doses of IL-2. The results showed that lymphocytes activated with 16– mDC and IL-2 were unable to reach the levels of proliferation of cells stimulated by 16+ mDC (Fig. 7a). Moreover, we detected CD25 expression on TCM and TEM when both subsets were activated with PPD-pulsed 16– mDC or 16+ mDC (Fig. 7b). In the case of 16– mDC-activated lymphocytes, CD25 was expressed mostly by non-dividing cells at day 2 of culture, but cycling cells at day 5 expressed normal levels of CD25. It is worth noting that a higher number of TEM expressed CD25 than TCM before division (Fig. 7b).
Figure 7.
The higher lymphoproliferative activity induced by 16+ mDC is independent of IL-2 and CD25 expression. (a) CFSE-labelled CD4+ memory T lymphocytes were cultured for 5 days with 16– mDC or 16+ mDC pulsed with 10 μg/ml PPD, in the absence (both) or presence (16– mDC only) of different doses of IL-2. The percentage of lymphocytes with six or more cycles of division is represented. (b) CFSE-labelled TCM and TEM lymphocytes were cultured as in Fig. 4(b), and after 2 or 5 days CD25 expression was evaluated. Percentage of positive cells in the respective quadrant is indicated. Data are from a representative experiment out of four.
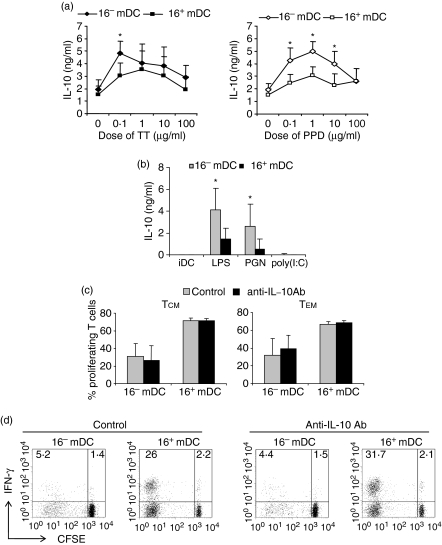
Then, we tested the possibility that the antiproliferative cytokine IL-10 had an effect on the diminished proliferation induced by 16– mDC. We found that higher amounts of IL-10 were secreted during the cocultures of CD4+ memory lymphocytes with 16– mDC compared with 16+ mDC, not only for TT, but particularly for PPD (Fig. 8a). On the other hand, 16– mDC by themselves secreted greater amounts of IL-10 in response to different ligands of Toll-like receptors, such as LPS from E. coli or PGN from S. aureus, than did 16+ mDC (Fig. 8b). To assess the influence of IL-10 on the proliferative ability of PPD-specific TCM and TEM lymphocytes, we blocked this cytokine throughout the cultures. The results indicated that IL-10 secreted during the cultures with 16– mDC or 16+ mDC did not affect the proliferative capacity of either TCM or TEM lymphocytes against PPD (Fig. 8c). Moreover, IL-10 blockade did not increase significantly the percentage of IFN-γ+ cells in TCM (Fig. 8d) or TEM subsets (not depicted). Therefore, molecules other than CD25, IL-2 or IL-10 seem to be involved in the decreased proliferation induced by 16– mDC on CD4+ memory T cells.
Figure 8.
The high levels of IL-10 secreted during cocultures of 16– mDC with T lymphocytes are not responsible for their diminished proliferation. (a) Memory T cells were stimulated with 16– mDC or 16+ mDC loaded with different doses (0–100 μg/ml) of TT and PPD. After 5 days of priming, lymphocytes were restimulated with immobilized anti-CD3 mAb. Analysis of IL-10 secretion on culture supernatants was performed by ELISA and production of cytokines by lymphocytes cultured with unloaded DC was subtracted. Results are the mean ± SD of eight independent experiments. (b) IL-10 secretion by immature 16– mDC and 16+ mDC after 24 hr of stimulation with LPS, PGN or poly (I:C). The iDC represents immature DC in the absence of stimulation. (c) Cocultures of CFSE-labelled TCM or TEM were performed for 5 days in the presence of PPD-pulsed (10 μg/ml) 16– mDC or 16+ mDC. Lymphocytes were maintained in the presence or absence of a neutralizing anti-human IL-10 antibody. Graphs represent the percentage of proliferating cells measured by CFSE dilution. (d) IFN-γ expression in cultures established as in (c) with TCM lymphocytes. The percentages of positive cells in the respective quadrants are shown. Statistical analysis: *P < 0·05.
Discussion
Priming of naive T lymphocytes with DC is influenced by several variables that lead to heterogeneous responses, such as antigen dose, cytokine signalling, duration of T-cell stimulation35,36 or DC subtype;37 it is conceivable that some of these factors also contribute to shape memory T-cell responses. The purpose of the present study was to determine whether human CD4+ memory T cells maintain their acquired phenotypical and effector status upon restimulation, or whether they could be modifiable, particularly by distinct types of DC. Here, we examined the potential of different human DC subsets derived from two monocyte precursors to elicit secondary CD4+ T-cell responses: the regular CD16– monocytes and the ‘non-classical’ CD16+ monocytes. We have previously observed that 16+ mDC seemed to induce greater secondary responses in vitro than 16– mDC.20 However, in that study we evaluated T cells from cancer patients, and the results could be biased as a consequence of the disease. In the present work, by using cells from healthy individuals, we have demonstrated that both DC subtypes were fully competent to stimulate secondary responses, whereas their monocyte precursors were very inefficient under the same conditions (Fig. 1). Nevertheless, 16+ mDC induced more vigorous proliferative responses against the two recall antigens tested here (PPD and TT) at earlier time-points compared to 16– mDC (Figs 1 and 4). In some donors, the total percentage of cycling cells was similar when lymphocytes were stimulated with both DC, but a higher proportion of lymphocytes consistently underwent more rounds of division with 16+ mDC (Figs 1b, 4 and 5e,f). The T-cell effector activity was linked only partially to cell proliferation because non-dividing cells (probably TEM) were able to produce IFN-γ (Figs 2 and 5b). However, the most striking finding was that 16– mDC were relatively inefficient in their stimulation of the effector activity of TCM in comparison with 16+ mDC, while minor differences were evidenced on TEM (Fig. 6).
The cytokine pattern observed for PPD-specific and TT-specific lymphocytes was consistent with the hypothesis that 16+ mDC stimulate stronger Th1 secondary responses (Fig. 2), confirming previous results obtained with tumour antigens.20 Because the acquisition of effector cytokine-producing capacity increases with cell division,38 the higher lymphoproliferative activity of 16+ mDC could correlate with the larger extent of IFN-γ production observed early in the cultures of total memory T cells. At later time-points (8 days), the number of divisions reached by 16– mDC-stimulated lymphocytes might be sufficient to detect similar numbers of IFN-γ-producing cells compared with 16+ mDC, although this varied among donors. Furthermore, Th1 cells are short-lived39 and some of the effectors activated by 16+ mDC may have already died at later time-points.
The memory subsets sorted by CCR7 expression revealed lower activation thresholds for TEM compared with TCM, which is in agreement with previous results.25,26 This affirmation is based on the early detection of a higher percentage of TEM that up-regulated the expression of CD25 upon stimulation (Fig. 7b), linked to a higher frequency of IFN-γ-producing cells before division occurs (Fig. 5b). Effector activity of TCM was only evident after cell division (Fig. 6b). These data are consistent with evidence that freshly isolated TEM have polarized cytokine gene acetylation patterns and show immediate cytokine production. In contrast, TCM are non-polarized cells that carry hypoacetylated or non-acetylated effector cytokine genes, which in turn prevents cytokine production unless restimulation under appropriate conditions takes place.40 Although 16+ mDC induce higher rates of proliferation than 16– mDC in TEM and TCM, differences in IFN-γ production were more apparent in TCM. Thus, many PPD-specific TCM stimulated with 16– mDC remained IFN-γ– irrespective of their number of divisions, indicating that parameters other than cell cycle are playing important roles in TCM differentiation. The alternative, that 16– mDC favour TCM differentiation to Th2 cells, is unlikely because the production of intracellular IL-4 was analysed in some donors and 16+ mDC induce similar or higher frequencies of IL-4+ cells (not depicted). Although the reason why IFN-γ production by TEM was affected only marginally by the DC subtype is not known, it may rely on their previously acquired acetylation at the Ifng promoter,40 which allows immediate cytokine production without further influence of cell division.
The findings discussed above suggest that regardless of the effector activity of the human CD4+ memory T cells before restimulation, it could be susceptible to modification by DC subtypes, at least for some antigens. We have previously observed that secretion of IL-12 by 16+ mDC was significantly lower than that produced by 16– mDC.12,19 However, naive CD4+ T lymphocytes secreted similar amounts of IFN-γ in response to allogeneic 16– mDC and 16+ mDC.12 These data indicate that the low levels of IL-12 produced by 16+ mDC are sufficient to stimulate IFN-γ secretion in naive T cells, at levels comparable with 16– mDC. It is also possible that 16+ mDC produce additional factors that compensate the IL-12 deficiency. In that sense, preliminary microarray data from our group showed an increased number of IL-18 transcripts in 16+ mDC, which might facilitate the deviation toward the Th1 phenotype (unpublished results). Notably, according to the data presented here, both DC appear to behave differently in naive and TEM versus TCM lymphocytes in terms of IFN-γ induction. Those discrepancies may be the result of different requirements of lymphocytes to become effectors.40,41 Moreover, naive and memory cells express distinct cytokine receptors and display differential responsiveness to various cytokines.42 Though we did not provide direct evidence, the distinct sensitivity of these cells to factors produced by 16+ mDC could underlie the efficient differentiation of TCM toward Th1 effectors supported by this DC subset.
Our above observations are consistent with some reports regarding the generation of TEM and TCM from activated naive lymphocytes following a progressive model of development28,43–45 in which TCM would arise in conditions of weaker activation stimulus, without transiting through an effector phase, while TEM precursors would emerge from effector cells. In fact, when compared with human CD4+ TCM, it has been reported that TEM are more differentiated cells, which correlates with a decreased expansion potential.25,42 However, in agreement with other reports,3 we did not observe a diminished replication capacity of TEM with respect to TCM (Fig. 5e), and perhaps this hypothesis should be revisited by testing CD4+ TEM and TCM responses to different recall antigens.
The phenotype of the responding memory T cells upon restimulation was related to the number of cycles they experienced, i.e. cells with more rounds of division tended to down-regulate CCR7 (a classical marker for delineating the human TCM subset), while lymphocytes with fewer cycles of proliferation, presumably those with higher activation thresholds, retained a TCM phenotype profile. Consequently, upon restimulation of memory lymphocytes with their cognate antigen, the final ratio of TCM : TEM specific for this antigen would depend on the number of divisions they previously underwent. Since 16+ mDC had greater lymphoproliferative ability, most of the 16+ mDC-stimulated TCM down-regulated CCR7 and CD62L, whereas a considerable proportion of 16– mDC-activated TCM conserved their phenotype (Fig. 6c). The TEM did not regain a TCM phenotype upon restimulation with both DC in the time-frame evaluated here (Fig. 6c), though some studies have observed a reverse phenotypic differentiation of TEM.29,46 Additionally, because many TCM acquire effector abilities when stimulated with 16+ mDC, but not with 16– mDC (Fig. 6b), the phenotypic changes induced by 16+ mDC could be stable in these cells, as a result of the conversion of TCM to TEM.28 In contrast, TCM that became CCR7– CD62L– upon activation with any DC type, but that did not acquire effector activity, could indeed have a transitory phenotype and regain long-term TCM characteristics. Based on the above results, we propose that DC evoking weaker secondary responses would have the ability to maintain substantial numbers of TCM after reactivation.
Currently, it is unknown why 16+ mDC have superior lymphoproliferative ability in secondary responses compared with 16– mDC. According to our evidence, this characteristic is independent of the amount of IL-2 and IL-10 secreted during DC : T-cell cocultures, as well as of CD25 expression by antigen-specific lymphocytes. Other studies have shown that human and mouse DC subtypes can induce different proliferative responses in the entire CD4+ memory cell population and, for mouse splenic DC subsets, those differences have been attributable to a distinct production of IL-2 during cocultures.23,24,34 A recent study in mice demonstrated a role for the CD28/B7 costimulatory pathway as a regulator of memory T-cell reactivation.47 Blockade of this pathway with cytotoxic T-lymphocyte antigen-4 immunoglobulin (CTLA-4Ig) substantially inhibited antigen-driven memory CD4+ T-cell expansion and IL-2 and IFN-γ production, but early activation (CD25 and CD69 expression) was not affected. Interestingly, treatment with CTLA-4Ig diminished the number of memory cells that underwent more than two or three cycles of division, as well as the generation of TEM.47 Previous results from our group demonstrated that TNF-α/prostaglandin E2-matured 16+ mDC had similar expression of HLA-DR and CD40, but increased levels of CD80 and CD86 compared with 16– mDC.12 Thus, DC subtypes bearing different levels of B7 molecules could affect human memory CD4+ T-cell restimulation in the way described by Ndejembi et al.47 Obviously, because 16– mDC and 16+ mDC expressed B7 molecules, the net effect observed would be less evident Then, some activities might be unaffected (i.e. IL-2 induction), while others might be more influenced: rate of lymphocyte proliferation, number of cells in advanced cycles of division, IFN-γ production and, importantly, the final ratio of TCM : TEM in the responding population. In addition to the potential effect of B7 molecules, preliminary data from our group showed that 16+ mDC had augmented expression of several transcripts of costimulatory molecules belonging to the TNF superfamily, such as TNFSF14 (LIGHT) and TNFSF18 (GITRL) (unpublished results), which could increase the capacity of 16+ mDC to activate T lymphocytes.48,49 Nevertheless, those hypotheses will require further investigation.
In conclusion, because 16+ mDC induce higher proliferative and effector responses in TCM than 16-mDC under the same conditions (i.e. maturation stimulus, antigen dose, time of stimulation), we propose that some types of DC could be more efficient to overcome the threshold for differentiation of TCM to TEM upon antigenic stimulation, while other types would have a major role in maintaining the numbers of TCM and, perhaps, the uncommitted characteristic of some cells within this population. The biological significance of the data provided here is challenging to ascertain. First, it remains to be determined whether blood monocytes have a physiological role in reactivating memory responses in vivo. Further, at present it is unknown whether human monocytes can differentiate in vivo to DC. Nevertheless, it appears that the equivalent mouse monocyte subsets are precursors for DC in vivo15,16 and total human monocytes transferred into severe combined immunodeficiency (SCID) mice acquire the phenotypic characteristics of DC.4 Whether human DC subsets other than 16– mDC and 16+ mDC have a direct influence on memory responses in vivo is yet to be determined. However, our findings could have practical relevance for clinical approaches. In vitro manipulation of TCM and TEM using 16+ mDC and 16– mDC might be useful in certain pathogenic conditions associated with a low proportion of antigen-specific CD4+ T-cell clones, in which the selective expansion and induction of TCM effector activity could aid in defeating the disease.
Acknowledgments
The support of the staff of the Juarez Hospital Blood Bank in providing the blood samples is gratefully acknowledged. We also thank Victor H. Rosales for help with flow cytometry, Julio C. Ramírez for technical assistance, and Ms Ninfa Arreola for her aid in the preparation of the manuscript. Special acknowledgement is made of the blood donors; this study would not be possible without their generosity. This work was supported by a grant to C.S.T. from CONACYT (no. 42712). S.B., H.T., A.R.C and S.R.A. are recipients of CONACYT predoctoral scholarships (nos 144142, 180583, 149529 and 180592, respectively).
Glossary
Abbreviations
- 16– mDC
CD16– monocyte-derived DC
- 16+ mDC
CD16+ monocyte-derived DC
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- DC
dendritic cell
- LPS
lipopolysaccharide
- PGN
peptidoglycan
- poly (I:C)
polyinosinic–polycytidylic acid
- PPD
purified protein derivatives of Mycobacterium tuberculosis
- TCM
central memory T
- TEM
effector memory T
- TT
tetanus toxoid
References
- 1.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–61. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 3.Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–70. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soruri A, Riggert J, Schlott T, Kiafard Z, Dettmer C, Zwirner J. Anaphylatoxin C5a induces monocye recruitment and differentiation into dendritic cells by TNF-α and prostaglandin E2-dependent mechanisms. J Immunol. 2003;171:2631–6. doi: 10.4049/jimmunol.171.5.2631. [DOI] [PubMed] [Google Scholar]
- 5.Parlato S, Santini SM, Lapenta C, et al. Expression of CCR-7, MIP-3beta, and Th-1 chemokines in type I IFN-induced monocyte-derived dendritic cells: importance for the rapid acquisition of potent migratory and functional activities. Blood. 2001;98:3022–9. doi: 10.1182/blood.v98.10.3022. [DOI] [PubMed] [Google Scholar]
- 6.Rotta G, Edwards EW, Sangaletti S, et al. Lipopolysaccharide or whole bacteria block the conversion of inflammatory monocytes into dendritic cells in vivo. J Exp Med. 2003;198:1253–63. doi: 10.1084/jem.20030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–34. [PubMed] [Google Scholar]
- 8.Ziegler-Heitbrock HW, Fingerle G, Ströbel M, et al. The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages. Eur J Immunol. 1993;23:2053–8. doi: 10.1002/eji.1830230902. [DOI] [PubMed] [Google Scholar]
- 9.Steppich B, Dayyani F, Gruber R, Lorenz R, Mack M, Ziegler-Heitbrock HWL. Selective mobilization of CD14+CD16+ monocytes by exercise. Am J Physiol Cell Physiol. 2000;279:C578–86. doi: 10.1152/ajpcell.2000.279.3.C578. [DOI] [PubMed] [Google Scholar]
- 10.Ancuta A, Rao R, Moses A, Mehle A, Shaw SK, Luscinskas FW, Gabuzda D. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J Exp Med. 2003;197:1701–7. doi: 10.1084/jem.20022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thieblemont N, Weiss L, Sadeghi HM, Estcourt C, Haeffner-Cavaillon N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol. 1995;25:3418–24. doi: 10.1002/eji.1830251232. [DOI] [PubMed] [Google Scholar]
- 12.Rivas-Carvalho A, Meraz-Ríos MA, Bajaña S, Santos-Argumedo L, Soldevila G, Moreno-García ME, Sánchez-Torres C. CD16+ human monocyte-derived dendritic cells matured with different and unrelated stimuli promote similar allogeneic Th2 responses. Regulation by pro- and anti-inflammatory cytokines. Int Immunol. 2004;16:1251–63. doi: 10.1093/intimm/dxh127. [DOI] [PubMed] [Google Scholar]
- 13.Scherberich JE, Nockher WA. CD14++ monocytes, CD14+/CD16+ subset and soluble CD14 as biological markers of inflammatory systemic diseases and monitoring immunosuppressive therapy. Clin Chem Laboratory Med. 1999;37:209–13. doi: 10.1515/CCLM.1999.039. [DOI] [PubMed] [Google Scholar]
- 14.Ajuebor MN, Flower RJ, Hannon R, Christie M, Bowers K, Verity A, Perretti M. Endogenous monocyte chemoattractant protein-1 recruits monocytes in the zymosan peritonitis model. J Leukoc Biol. 1998;63:108–16. doi: 10.1002/jlb.63.1.108. [DOI] [PubMed] [Google Scholar]
- 15.Geissmann F, Jung S, Littman D. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 16.Ginhoux F, Tacke F, Angeli V, et al. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265–73. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Randolph GJ, Sanchez-Schmitz G, Liebman R, Schäkel K. The CD16(+) FcgammaRIII(+) subset of human monocytes preferentially becomes migratory dendritic cells in a model tissue setting. J Exp Med. 2002;196:517–27. doi: 10.1084/jem.20011608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krutzik SR, Tan B, Li H, et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med. 2005;11:653–60. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez-Torres C, Garcia-Romo GS, Cornejo-Cortes MA, Rivas-Carvalho A, Sanchez-Schmitz G. CD16+ and CD16– human blood monocyte subsets differentiate in vitro to dendritic cells with different abilities to stimulate CD4+ T cells. Int Immunol. 2001;13:1571–81. doi: 10.1093/intimm/13.12.1571. [DOI] [PubMed] [Google Scholar]
- 20.Arroyo JC, Gabilondo F, Llorente L, Meraz-Ríos MA, Sánchez-Torres C. Immune response induced in vitro by CD16– and CD16+ monocyte-derived dendritic cells in patients with metastatic renal cell carcinoma treated with dendritic cell vaccines. J Clin Immunol. 2004;24:86–96. doi: 10.1023/B:JOCI.0000018067.71622.fb. [DOI] [PubMed] [Google Scholar]
- 21.Lambrecht BN, Salomon B, Klatzmann D, Pauwels RA. Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. J Immunol. 1998;160:4090–7. [PubMed] [Google Scholar]
- 22.Krug A, Veeraswamy R, Pekosz A, Kanagawa O, Unanue ER, Colonna M, Cella M. Interferon-producing cells fail to induce proliferation of naïve T cells but can promote expansion and T helper 1 differentiation of antigen-experienced unpolarized T cells. J Exp Med. 2003;197:899–906. doi: 10.1084/jem.20021091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bai L, Feuerer M, Beckhove P, Umansky V, Schirrmacher V. Generation of dendritic cells from human bone marrow mononuclear cells: advantages for clinical application in comparison to peripheral blood monocyte derived cells. Int J Oncol. 2002;20:247–53. [PubMed] [Google Scholar]
- 24.Osugi Y, Vuckovic S, Hart DNJ. Myeloid blood CD11c+ dendritic cells and monocyte-derived dendritic cells differ in their ability to stimulate T lymphocytes. Blood. 2002;100:2858–66. doi: 10.1182/blood.V100.8.2858. [DOI] [PubMed] [Google Scholar]
- 25.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector function. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 26.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets. Function, generation and maintenance. Annu Rev Immunol. 2004;22:24.1–19. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 27.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4+ T cells in the whole body. Nature. 2001;410:101–5. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 28.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–7. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 29.Wherry EJ, Teichgraber V, Becker C, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–34. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 30.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–9. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baron V, Bouneaud C, Cumano A, Lim A, Arstila TP, Kourilsky P, Ferradini L, Pannetier C. The repertoires of circulating human CD8(+) central and effector memory T cell subsets are largely distinct. Immunity. 2003;18:193–204. doi: 10.1016/s1074-7613(03)00020-7. [DOI] [PubMed] [Google Scholar]
- 32.Lefrancois L, Marzo A. The descent of memory T-cell subsets. Nat Rev Immunol. 2006;6:618–23. doi: 10.1038/nri1866. [DOI] [PubMed] [Google Scholar]
- 33.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Meth. 1994;171:131–7. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 34.Kronin V, Fitzmaurice CJ, Caminschi I, Shortman K, Jackson DC, Brown LE. Differential effect of CD8(+) and CD8(–) dendritic cells in the stimulation of secondary CD4(+) T cells. Int Immunol. 2001;13:465–73. doi: 10.1093/intimm/13.4.465. [DOI] [PubMed] [Google Scholar]
- 35.Langenkamp A, Casorati G, Garavaglia C, Dellabona P, Lanzavecchia A, Sallusto F. T cell priming by dendritic cells. Thresholds for proliferation, differentiation and death and intraclonal functional diversification. Eur J Immunol. 2002;32:2046–54. doi: 10.1002/1521-4141(200207)32:7<2046::AID-IMMU2046>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 36.Iezzi G, Scolett E, Sheidegger D, Lanzavecchia A. The interplay between the duration of TCR and cytokine signalling determines T cell polarization. Eur J Immunol. 1999;29:4092–101. doi: 10.1002/(SICI)1521-4141(199912)29:12<4092::AID-IMMU4092>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 37.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YL. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 38.Bird JJ, Brown DR, Mullen AC, et al. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229–37. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- 39.Wu CY, Kirman JR, Rotte MJ, et al. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat Immunol. 2002;3:852–8. doi: 10.1038/ni832. [DOI] [PubMed] [Google Scholar]
- 40.Messi M, Giacchetto I, Nagata K, Lanzavecchia A, Natoli G, Sallusto F. Memory and flexibility of cytokine gene expression as separable properties of human TH1 and TH2 lymphocytes. Nat Immunol. 2002;4:78–86. doi: 10.1038/ni872. [DOI] [PubMed] [Google Scholar]
- 41.Rivino L, Messi M, Jarrossay D, Lanzavecchia A, Sallusto F, Geginat J. Chemokine receptor expression identifies pre-T helper (Th) 1, pre-Th2 and non-polarized cells among human CD4+ central memory T cells. J Exp Med. 2004;200:725–35. doi: 10.1084/jem.20040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4+ T cells. J Exp Med. 2001;194:1711–19. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation. Impact on priming of TH1, TH2 and non-polarized T cells. Nat Immunol. 2000;1:311–16. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 44.Huster KM, Koffler M, Stemberger C, Schiemann M, Wagner H, Busch DH. Unidirectional development of CD8+ central memory T cells into protective Listeria-specific effector memory T cells. Eur J Immunol. 2006;36:1453–64. doi: 10.1002/eji.200635874. [DOI] [PubMed] [Google Scholar]
- 45.Swain SL, Agrewala JN, Brown DM, et al. CD4+ T-cell memory: generation and multi-faceted roles for CD4+ T cells in protective immunity to influenza. Immunol Rev. 2006;211:8–22. doi: 10.1111/j.0105-2896.2006.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwendemann J, Choi C, Schirrmacher V, Beckhove P. Dynamic differentiation of activated human peripheral blood CD8+ and CD4+ effector memory T cells. J Immunol. 2005;175:1433–9. doi: 10.4049/jimmunol.175.3.1433. [DOI] [PubMed] [Google Scholar]
- 47.Ndejembi MP, Teijaro JR, Patke DS, et al. Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. J Immunol. 2006;177:7698–706. doi: 10.4049/jimmunol.177.11.7698. [DOI] [PubMed] [Google Scholar]
- 48.Tamada K, Shimozaki K, Chapoval AI, et al. LIGHT, a TNF-like molecule, costimulates T cell proliferation and is required for dendritic cell-mediated allogeneic T cell response. J Immunol. 2000;164:4105–10. doi: 10.4049/jimmunol.164.8.4105. [DOI] [PubMed] [Google Scholar]
- 49.Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, Collins M, Shevach EM. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004;173:5008–20. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]