Abstract
Type I interferons (IFNs), IFN-α and IFN-β, are widely used for treating chronic hepatitis C. Although retrospective studies have suggested that type I IFNs have direct antifibrotic effects, little is known about these mechanisms. The present study was designed to clarify the preventive mechanisms of type I IFNs in the progression of fibrosis for the establishment of a more effective therapy. A murine fibrosis model comprising immunological reactions was induced by the administration of concanavalin A (0·3 mg/body) into mice once a week for 4 weeks. Liver injury and the degree of fibrosis were determined by measuring the serum alanine aminotransferase activities and liver hydroxyproline contents with or without IFN-β pretreatment. IFN-β suppressed the hepatocellular injury and increased the hydroxyproline content induced by repeated concanavalin A injections, but had no effect on established fibrosis. Furthermore, IFN-β reduced the expressions of transforming growth factor-β, basic fibroblast growth factor, collagen type I A2 and tissue inhibitor of metalloproteinase 1 messenger RNAs, which are related to the progression of liver fibrosis. The IFN-β reduced the liver injury and fibrosis induced by immunological reactions. These data suggest that type I IFNs suppress the progression of cirrhosis through inhibition of repeated hepatocellular injury and/or factors that promote the liver fibrosis induced by hepatitis virus infection.
Keywords: cirrhosis, concanavalin A, fibrosis, hepatitis, interferon-β
Introduction
Hepatic fibrosis is a common feature of chronic hepatitis and chronic liver injury, and leads to liver cirrhosis. Its frequent association with hepatocellular carcinoma, especially in cirrhotic patients infected with hepatitis C virus (HCV), is an important clinical issue that has been difficult to deal with.1,2 Although type I interferons (IFNs), IFN-α and IFN-β, are now widely used for the treatment of chronic hepatitis C to eradicate HCV and reduce serum alanine aminotransferase (ALT) levels, only a limited number of patients actually respond to type I IFN therapy in terms of HCV clearance.1–5 However, it remains important to suppress progressive fibrosis and prevent the subsequent occurrence of hepatocellular carcinoma in patients who do not respond to type I IFN therapy.6,7
Therapy with IFN-α has been reported to improve the serum levels of fibrotic markers, such as the N-terminal propeptide of procollagen type III, not only in patients who respond to the therapy but also in those who do not.8,9 In addition, quantitative histopathological analyses of paired biopsy specimens have indicated that there is some improvement in the degree of fibrosis following IFN-α therapy, which is irrespective of the initial virological response.4 These results suggest that IFN-α may have a direct antifibrotic effect in addition to its antiviral activity. However, little is known about the mechanisms responsible for the antifibrotic effects of type I IFNs.
Concanavalin A (Con A)-induced hepatitis is known to be caused by immunological mechanisms through the up-regulation of inflammatory cytokines, such as IFN-γ and tumour necrosis factor-α (TNF-α). Furthermore, a recent study revealed that repeated administration of Con A causes hepatic fibrosis, mainly via up-regulation of transforming growth factor-β1 (TGF-β1) expression in the liver.10 On the basis of these results, we investigated the mechanisms responsible for the antifibrotic effects of type I IFNs using an experimental murine fibrosis model induced by repeated administration of Con A.
We demonstrate that pretreatment with IFN-β strongly suppressed Con A-induced fibrosis and hepatocellular injury. Furthermore, IFN-β reduced the expression of the messenger RNAs (mRNAs) of TGF-β1 and tissue inhibitor of metalloproteinase 1 (TIMP-1), which are related to the progression of liver fibrosis. These findings indicate that type I IFNs may suppress the progression of cirrhosis through inhibition of continuous hepatocellular injury and/or factors that promote liver fibrosis.
Materials and methods
Mice and reagents
The Con A was purchased from Sigma-Aldrich Japan (Tokyo, Japan). It was dissolved in pyrogen-free phosphate-buffered saline (PBS; Sigma-Aldrich Japan) at a concentration of 1 mg/ml. Seven- to 10-week-old female BALB/c mice, 25–30 g in weight (Charles River Laboratories Japan Inc., Kanagawa, Japan), were used for the Con A-induced hepatitis and fibrosis model. (In male mice, the degree of hepatitis and fibrosis induced by Con A administration was more irregular and weaker than in female mice so female mice were used for this model.) All animals used in the present study received humane care in compliance with the National Institutes of Health guidelines for the care and use of laboratory animals. Recombinant murine IFN-β and IFN-α derived from Escherichia coli were obtained from Kamakura Techno-Science Inc. (Kanagawa, Japan) and PBL Biomedical Laboratories (Piscataway, NJ), respectively.
Induction of fibrosis
Five mice in each group [eight mice for the polymerase chain reaction (PCR)] were intravenously (i.v) injected with Con A at a dose of 0·3 mg/body once a week for up to 4 weeks. Liver injury was determined by measuring the plasma ALT activities. Heparinized plasma samples from individual mice were obtained from the oculi vein at the indicated time-points after each Con A injection, and the ALT activities were measured using a DRI-CHEM system (Fujifilm Corp., Tokyo, Japan).
Analysis of liver injury and fibrosis
Liver injury and fibrosis were determined by histological analyses and measurement of the liver hydroxyproline content. For the histological analyses, the liver tissues were fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned at 5-μm thickness and stained with haematoxylin & eosin for light microscopy evaluation. In some experiments, Azan staining was performed for detection of fibrosis. For the determination of hydroxyproline content, left liver lobe samples were dried, hydrolysed in 1 ml 12 m HCl for 24 hr at 110° and analysed using the hydroxyproline colorimetric assay described by Woessner.11 All assays were performed in triplicate.
RNA preparation
Total RNA was isolated from liver tissues by the guanidinium isothiocyanate–acidic phenol–chloroform method using Trizol (Invitrogen Corp., Carlsbad, CA) according to the manufacturer's instructions. Total RNA concentrations were estimated spectrophotometrically by the absorbance at 260 nm.
Quantitative real-time reverse transcription–PCR analysis
Aliquots (1 μg) of total RNA were reverse-transcribed into cDNA using Superscript II (Invitrogen) according to the manufacturer's instructions and then subjected to real-time kinetic PCR on a LightCycler™ with SYBR Green I as a double-stranded DNA-specific binding dye (Roche Diagnostics, Mannheim, Germany) to quantify mRNA expressions for basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF), TGF-α, TGF-β1, IFN-γ, TNF-α, TIMP-1 and collagen type I A2 (COL1A2). The PCR conditions were 40–45 cycles (denaturation at 95° for 10 seconds, annealing at 61° for 10 seconds, and extension at 72° for 6–11 seconds) in 20-μl volumes of reaction mixture. Data analysis was performed using LightCycler™ Software (Roche Diagnostics) with quantification and melting curve options. The acquired fluorescence signal was quantified by the second derivative maximum method using LightCycler data analysis software to obtain crossing-point values and slope. Melting curve analyses were confirmed to verify single products (data not shown). The levels of gene expression were calculated as a ratio to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The PCR primers used, which had been checked for sequence homology against known genes by a BLAST search, are listed in Table 1. The primers for IFN-γ and TNF-α, and for TGF-β1 and GAPDH were purchased from Takara Bio Inc. (Shiga, Japan) and Nihon Gene Research Lab's Inc. (Miyagi, Japan), respectively.
Table 1.
Nucleotide sequences of PCR primers used and expected size of PCR products for each targeted mRNA
| Target mRNA | Sequences of primers | Expected amplicon size | |
|---|---|---|---|
| bFGF | sense: | 5′-TCA AAC TAC AAC TCC AAG CA-3′ | 149 bp |
| antisense: | 5′-AGA TTC CAG TCG TTC AAA GA-3′ | ||
| HGF | sense: | 5′-CAA GAA AAC GAT GCT ACT GG-3′ | 136 bp |
| antisense: | 5′-TTA TAG CTG CCT CCT TTA CC-3′ | ||
| TGF-α | sense: | 5′-CCA GAA GAA GCA AGC CAT C-3′ | 111 bp |
| antisense: | 5′-TCA CAG TGT TTG CGG AGC-3′ | ||
| TGF-β1 | sense: | 5′-AAC AAC GCC ATC TAT GAG-3′ | 294 bp |
| antisense: | 5′- TAT TCC GTC TCC TTG GTT-3′ | ||
| IFN-γ | sense: | 5′-CGG CAC AGT CAT TGA AAG CCT A-3′ | 199 bp |
| antisense: | 5′-GTT GCT GAT GGC CTG ATT GTC-3′ | ||
| TNF-α | sense: | 5′-AAG CCT GTA GCC CAC GTC GTA-3′ | 122 bp |
| antisense: | 5′-GGC ACC ACT AGT TGG TTG TCT TTG-3′ | ||
| TIMP-1 (46) | sense: | 5′-GGA ACG GAA ATT TGC ACA TC-3′ | 270 bp |
| antisense: | 5′-CCC CAA GGG ATC TCC AGG TG-3′ | ||
| COLIA2 (19) | sense: | 5′-CAC CCC AGC GAA GAA CTC ATA-3′ | 75 bp |
| antisense: | 5′-GCC ACC ATT GAT AGT CTC TCC TAA C-3′ | ||
| GAPDH | sense: | 5′-TGA ACG GGA AGC TCA CTG G-3′ | 307 bp |
| antisense: | 5′-TCC ACC ACC CTG TTG CTG TA-3′ |
The specific primer pair was designed according to a published primer set and previously reported nucleotide sequences.
bFGF, basic fibroblast growth factor; HGF, hepatocyte growth factor; TGF, transforming growth factor; IFN, interferon; TNF-α, tumour necrosis factor-α; TIMP-1, tissue inhibitor of metalloproteinase 1; COLIA2, collagen type I A2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Measurement of intrahepatic blood flow
Five mice in each group were given Con A. Three hours after Con A injection, with pretreatment of saline or IFN-β, the abdomen was opened under ether anaesthesia. The intrahepatic blood flow per square unit of liver surface was quantified in the median lobe using a non-contact laser–Doppler blood flowmeter (ALF21N: Advance Co., Ltd, Tokyo, Japan). Data are expressed as a percentage of the blood flow of untreated animals.
Statistical analysis
Values were expressed as the mean ± SEM. The statistical significance of differences among several groups in dose-dependence and time–course studies in ALT levels or hydroxyproline content determination was evaluated by Dunnett's test. A comparison of ALT levels, hydroxyproline contents, mRNA expression levels and intrasinusoidal blood flows between two groups, untreated or saline-treated mice and Con A-treated mice, or Con A-treated mice and mice treated with IFN-β plus Con A, was performed using a non-paired t-test or Welch test. Values of P less than 0·05 were considered significant.
Results
Effect of IFN-β on the liver injury induced by repeated Con A injections
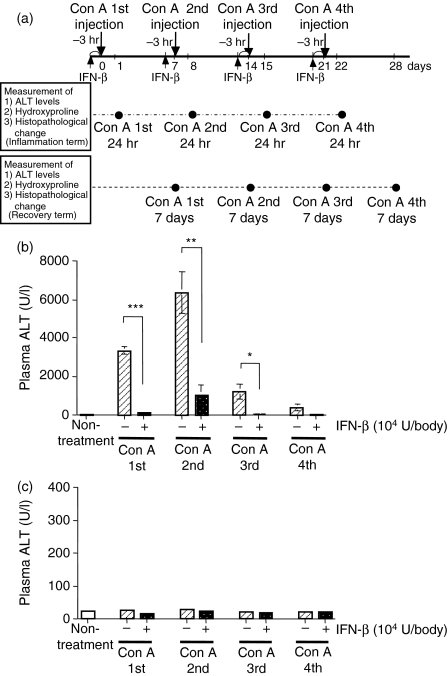
First of all, we confirmed the effects of IFN-α and IFN-β on the Con A-induced hepatitis. As shown in Table 2, IFN-β reduced the hepatitis more than IFN-α, as measured by plasma ALT levels. Therefore, we thought IFN-β was suitable for evaluation. When Con A (0·3 mg/body) was injected i.v. into mice once a week for 4 weeks, the plasma ALT activity 24 hr after the injection reached a peak after the second Con A injection (Fig. 1b). When the same Con A injection procedure was carried out with a previous injection of IFN-β (104 U/body) 3 hr before each Con A injection, the plasma ALT activities were reduced at 24 hr after all the Con A injections (Fig. 1b). Moreover, focal necrosis (indicated by arrows in Fig. 2a) and mononuclear cell infiltration were observed 24 hr after the first Con A injection (Fig. 2a), and IFN-β administration 3 hr before the Con A injection suppressed these pathological changes (Fig. 2b). These data indicate that IFN-β is able to inhibit the liver injury induced by Con A. Following the Con A injection procedure, the plasma ALT activities were normal at 7 days after each injection (Fig. 1c), and pretreatment with IFN-β (104 U/body) 3 hr before each injection had no effect on these activities (Fig. 1c).
Table 2.
Comparison of IFN-α and IFN-β pretreatment in Con A-induced liver injury
| Treatment | Plasma ALT activity (U/l) |
|---|---|
| Non-treatment | 39·5 ± 2·9 |
| Con A | 9777 ± 1336 |
| +IFN-α (dose) | |
| 103 U/body | 9798 ± 1495 |
| 104 U/body | 2040 ± 395** |
| +IFN-β (dose) | |
| 103 U/body | 2290 ± 576** |
| 104 U/body | 970 ± 190*** |
The plasma ALT activities were determined at 24 hr after Con A injection (0·3 mg/body) with or without IFN pretreatment 3 hr before a Con A injection. Each value represents the mean ± SEM. (n = 10).
P < 0·01
P < 0·001, versus Con A treatment without IFNs by Dunnett's test.
Figure 1.
Pretreatment with IFN-β suppresses Con A-induced liver injury. (a) Schema for the Con A-induced hepatic fibrosis model. Con A (0·3 mg/body) was injected once a week for 4 weeks. In some mice, IFN-β was administered at 3 hr before each Con A injection. The closed circles indicate the time-points of blood collection. The plasma ALT activities were determined 24 hr (b) and 7 days (c) after each weekly Con A injection (0·3 mg/body) with or without IFN-β (104 U/body) pretreatment at 3 hr before each Con A injection. The ALT activities were determined as described in the Materials and methods section. Each value represents the mean ± SEM (n = 5). *P < 0·05, **P < 0·01, ***P < 0·001 versus without IFN-β by Welch test.
Figure 2.
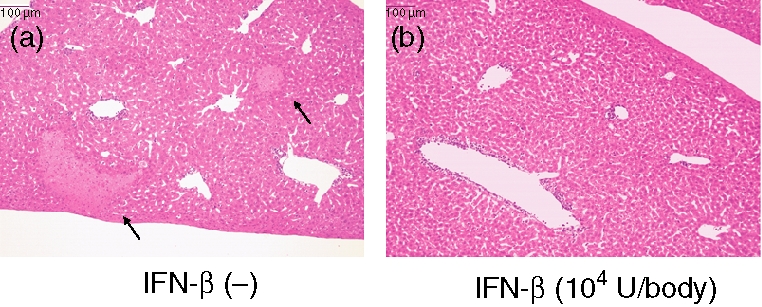
Effects of IFN-β on the liver injury induced by Con A injection. Liver tissues were collected from mice 24 hr after a Con A injection (0·3 mg/body) without (a) or with (b) a previous injection of IFN-β (104 U/body) at 3 hr before the Con A administration. The liver tissues were fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned at 5 μm thickness and stained with haematoxylin and eosin for light microscopy evaluation (×400). Arrows in (a) indicate focal necrosis.
Effects of IFN-β on the liver fibrosis induced by repeated Con A injections
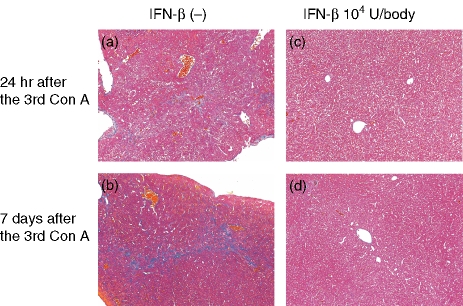
To assess the evolution of the pathological changes in the liver following the weekly Con A injections, liver tissues were collected from mice 24 hr or 7 days after three Con A injections with or without a 3-hr pretreatment with IFN-β (104 U/body) before each Con A injection. At 24 hr after the third Con A injection, hepatocellular necrosis and granuloma were widespread throughout the lobule and fibrosis was detected in the parenchyma (Fig. 3a). In contrast, 24 hr after the third Con A injection with a 3-hr IFN-β pretreatment before each injection, the hepatocellular necrosis, granuloma formation and fibrosis were decreased (Fig. 3c). At 7 days after the third Con A injection, no hepatocellular necrosis and granuloma were observed, although fibrosis was still detected in the parenchyma (Fig. 3b). In contrast, at 7 days after the third Con A injection with a 3-hr IFN-β pretreatment before each Con A injection, the fibrosis was decreased (Fig. 3d). No fibrosis was observed 24 hr after up to the second Con A injection (data not shown).
Figure 3.
Effects of IFN-β on the liver fibrosis induced by repeated Con A injections. Con A was administered to mice (0·3 mg/body) once a week without (a and b) or with (c and d) IFN-β (104 U/body) pretreatment 3 hr before each Con A administration. The livers (right lobes) were collected at 24 hr (a and c) or 7 days (b and d) after the third Con A administration. The liver tissues were fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned at 5-mm thickness and multistained with haematoxylin & eosin and Azan for light microscopy evaluation (×400).
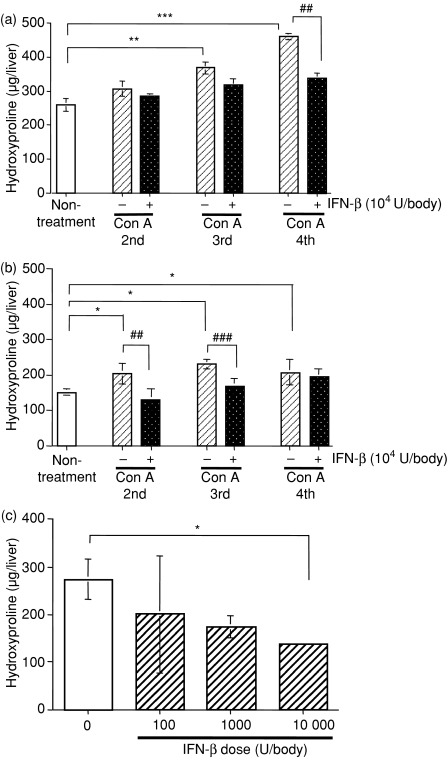
Effects of IFN-β on the liver hydroxyproline contents induced by repeated Con A injections
Following i.v. injections of Con A (0·3 mg/body) into mice once a week for 4 weeks, the liver hydroxyproline content at 24 hr after each injection increased gradually until the fourth Con A injection (Fig. 4a). In contrast, the hydroxyproline content in the liver 7 days after each Con A injection was not increased (Fig. 4b). When the same Con A injection procedure was carried out with a pretreatment injection of IFN-β (104 U/body) 3 hr before each Con A injection, the liver hydroxyproline content at 24 hr after the fourth Con A injection was decreased (Fig. 4a). Furthermore, the hydroxyproline content was suppressed by IFN-β in a dose-dependent manner (Fig. 4c). These data indicate that IFN-β reduced the increased liver hydroxyproline contents induced by the repeated Con A injections.
Figure 4.
Effects of IFN-β on the amount of hydroxyproline in the left liver lobe after repeated Con A injections. Left liver lobes were collected from mice at 24 hr (a) and 7 days (b) after the indicated weekly Con A injections (0·3 mg/body) with or without IFN-β (104 U/body) pretreatment. (c) Left liver lobes were collected from mice at 7 days after three weekly Con A injections (0·3 mg/body) following pretreatment with the indicated concentrations of IFN-β. The amount of hydroxyproline was determined as described in the Materials and methods section. Each value represents the mean ± SEM (n = 5). *P < 0·05, **P < 0·01, ***P < 0·001 versus non-treatment (a and b) or no IFN-β treatment (c) as control by Dunnett's test. ##P < 0·01, ###P < 0·001 versus Con A treatment without IFN-β (a and b) by t-test
Effects of IFN-β on dissolving established liver fibrosis
To determine whether or not IFN-β influences fibrosis that has already been established, the degree of liver fibrosis was assessed with or without IFN-β administration. When Con A (0·3 mg/body) was injected i.v. into mice once a week for 3 weeks followed by administration of either IFN-β (104 U/body) or vehicle at 2, 4 and 6 days after the third Con A injection, IFN-β did not improve the fibrosis (Fig. 5a,b). Therefore, IFN-β did not stimulate dissolution of fibrosis that had already been established.
Figure 5.

Effects of IFN-β on established fibrosis induced by repeated Con A injections. Mice were i.v. injected with three doses of Con A (0·3 mg/body) once a week, and then administered either vehicle (a) or IFN-β (104 U/body) (b) at 2, 4 and 6 days after the final Con A injection. The liver tissues were then fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned at 5-μm thickness and multistained with hematoxylin & eosin and Azan for light microscopy evaluation (×400).
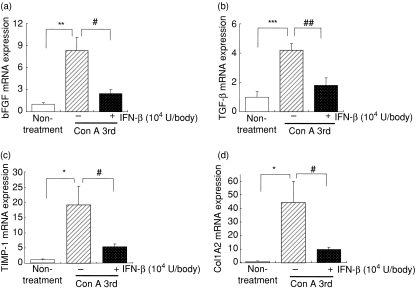
Effects of IFN-β on the expressions of growth factors, cytokines, TIMP-1 and COL1A2 mRNAs in the liver following repeated Con A injections
To clarify whether IFN-β influences the expressions of growth factors, cytokines, TIMP-1 and COL1A2 mRNAs in the liver following repeated Con A injections, Con A (0·3 mg/body) was injected i.v. into mice once a week for 3 weeks, with or without a 3-hr IFN-β (104 U/body) pretreatment before each Con A injection, and RNA was extracted from the liver 24 hr after the third Con A injection. As shown in Fig. 6, IFN-β significantly reduced the expression levels of bFGF, TGF-β1, TIMP-1 and COL1A2 mRNAs, but had no effect on the expression levels of HGF, TGF-α, IFN-γ and TNF-α mRNAs (data not shown). Of these mRNAs, it should be noted that COL1A2 mRNA was increased 45-fold in Con A-treated mice and IFN-β dramatically abrogated the up-regulation.
Figure 6.
Effects of IFN-β on the expression levels of bFGF, TGF-β1, TIMP-1 and COL1A2 mRNAs in the liver fibrosis induced by repeated Con A injections. The livers were collected 24 hr after the third Con A injections with or without IFN-β (104 U/body) pretreatment at 3 hr before each Con A administration. The levels of bFGF (a), TGF-β1 (b), TIMP-1 (c) and collagen type I A2 (d) mRNAs in the liver were determined by real-time PCR assay using a LightCycler instrument as described in the Materials and methods section. Data represent the mean ± SEM (n = 8). *P < 0·05, **P < 0·01 and ***P < 0·001 non-treatment group versus vehicle + Con A group, and #P < 0·05 and ##P < 0·01 IFN-β + Con A group versus vehicle + Con A group.
Effects of IFN-β on intrasinusoidal haemostasis induced by Con A administration
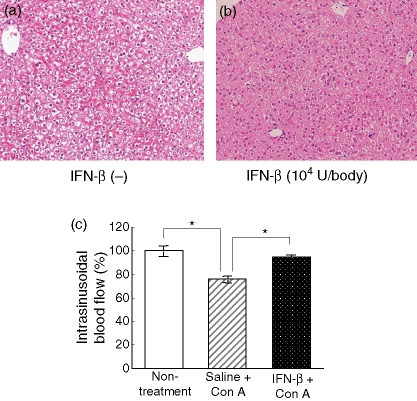
Con A administration led to prominent intrasinusoidal haemostasis, comprising erythrocyte aggregation, lymphocytes sticking to endothelial cells, and platelet aggregation and degranulation, resulting in a marked decrease in the intrahepatic blood flow, and then confluent hepatic necrosis occurred within the congested area of liver parenchyma.12 Therefore, the effects of IFN-β on intrasinusoidal haemostasis induced by Con A administration were evaluated. Four hours after Con A administration, the intrasinusoidal haemostasis was induced. This was reduced by 3-hr pretreatment of IFN-β (104 U/body) (Fig. 7a,b). At that time, intrahepatic blood flow was assessed. The decrease of intrahepatic blood flow induced by Con A administration was inhibited by a 3-hr pretreatment with IFN-β (Fig. 7c).
Figure 7.
Effects of IFN-β on intrasinusoidal haemostasis induced by Con A administration. Paraffin sections from the livers removed at 3 hr after Con A injection with pretreatment of saline (a) or IFN-β (b) were stained with haematoxylin & eosin (original magnification: ×400). Under ether anaesthesia, intrasinusoidal blood flow was measured using a laser–Doppler flow meter at 3 hr after Con A injection with IFN-β administration, and data were expressed as a percentage on the non-treated mice (c) Data represent the mean ± SEM (n =5). *P < 0·05 non-treatment group or IFN-β + Con A group versus saline + Con A group by t-test.
Discussion
Recent reports indicate the importance of suppressing the progressive fibrosis and preventing the subsequent occurrence of hepatocellular carcinoma in patients with chronic hepatitis C who do not respond to type I IFN therapy.6,7 However, little is known about the mechanisms responsible for the antifibrotic effects of type I IFNs. In contrast, the inhibitory actions of type II IFN, IFN-γ, have been extensively studied using a murine pulmonary fibrosis model induced by bleomycin. For example, IFN-γ has been reported to show inhibitory effects on fibroblast proliferation and collagen production.13,14 IFN-β has also been reported to inhibit fibroblast proliferation and collagen production in bleomycin-induced pulmonary fibrosis, but the mechanisms remain to be clarified.15 In the present paper, we tried to clarify the preventive mechanisms of type I IFNs based on the progression of liver fibrosis induced by viral infection. It is important to select this type of experimental model, because the fibrosis in HCV-infected livers is induced by continuous liver injury, which itself is mediated by antigen-specific and antigen-independent immunological reactions.16 There are several liver fibrosis models induced by carbon tetrachloride (CCl4),17 porcine serum,18 or ligation of the bile duct.19 Suppression of liver fibrosis by IFN-α has been reported in the CCl4 model.20 However IFN-α did not show an inhibitory effect in bile-duct-ligation models.20,21 These experimental models are not suitable for our purpose for the reasons outlined above. Considering these points, we chose to use a Con A-induced liver fibrosis model because the fibrosis is caused by continuous hepatocellular injury and is based on an immunological mechanism.10
First, we assessed the effects of IFN-β on the Con A-induced hepatocellular injury, and found that it reduced the amount of hepatocellular injury and markedly suppressed the fibrosis induced by the repeated injections. It should be noted that the doses of IFN-β used in this study (104 U/body) were approximately the same doses in clinical usage (6 × 106 IU/body). Since continuous hepatocellular injury is largely responsible for the progression of fibrosis22 these data suggest that one of the antifibrotic mechanisms of IFN-β is to reduce such continuous hepatocellular injury. In a Con A-induced hepatitis model, immunological responses, such as CD4 T-cell activation,23 NKT cell activation,24 TNF-α production,25 IFN-γ production26 and IL-4 production27 play important roles. On the other hand, IFN-β has been reported to show therapeutic effects in multiple sclerosis by modulating the immunological responses.28–31 Suppressing mononuclear infiltration into the central nervous system is thought to be one of the mechanisms by which IFN-β modulates such immunological responses. Taken together, these findings suggest that IFN-β reduces the Con A-induced hepatocellular injury by modulating immunological responses.
Next, we assessed the effects of IFN-β on established fibrosis, and found that it did not reduce the amount of collagen induced by the repeated Con A injections. These data suggest that IFN-β has no effect on established fibrosis.
Type I collagen, the major extracellular matrix component in fibrotic liver, is a heterotrimer composed of two α1 chains and one α2 chain. These chains are encoded by two distinct genes designated COL1A1 and COL1A2, respectively.32 In general, collagen gene expression is strictly controlled by a large number of growth factors and cytokines33,34 and TGF-β1 is the most important factor for stimulating its expression.33 Furthermore, TIMP-1 contributes to the progression of liver fibrosis by promoting collagen synthesis.34,35 In fact, the level of hepatic TIMP-1 in patients with primary biliary cirrhosis and primary sclerosing cholangitis is related to the stage of fibrosis.34,36 It has been reported that TIMP-1 is responsible for reducing the amount of collagen after cessation of CCl4 administration in a rat fibrosis model.37,38 Up-regulation of bFGF, HGF, TGF-α and TGF-β mRNA expression, closely related to fibrogenesis, in a Con A-induced hepatic fibrosis model, was also previously reported.10 To clarify the inhibitory mechanisms of IFN-β in detail, we assessed the effects of IFN-β on the expression levels of bFGF, HGF, TGF-α, TGF-β1, IFN-γ, TNF-α, TIMP-1 and COL1A2 mRNAs, and found that it reduced the expressions of bFGF, TGF-β1, TIMP-1 and COL1A2 mRNAs but had no effect on the expression of HGF, TGF-α, IFN-γ and TNF-α mRNAs. Down-regulation of COL1A2 mRNA is consistent with a previous report that showed another type I IFN, IFN-α, repressing COL1A2 transcription in hepatic stellate cells.22 TGF-β1 is not only the most important factor for stimulating type I collagen expression but also increases the level of bFGF mRNA in myofibroblastic liver cells.39 Since we found that IFN-β reduced the expression of TGF-β1 mRNA 24 hr after the third Con A injection, down-regulation of bFGF and COL1A2 mRNA expression may result from the reduction of expression of TGF-β1 mRNA by IFN-β. These findings indicate that the inhibitory mechanisms of IFN-β on liver fibrosis are based on the suppression of TGF-β1 and TIMP-1 with simultaneous suppression of continuous liver injury.
Both IFN-γ and TNF-α have been reported to be responsible for the hepatocellular injury induced by Con A.25,26 Accordingly, we assessed the amounts of IFN-γ and TNF-α mRNAs in the liver at 24 hr after the third Con A injection with or without IFN-β. However, IFN-γ mRNA was not detected (data not shown). On the other hand, there was no significant difference between the amounts of TNF-α mRNA induced by Con A with or without IFN-β pretreatment (data not shown). Furthermore, pretreatment with IFN-β did not reduce the amounts of TNF-α and IFN-γ in the serum at 1, 2 and 3 hr after Con A injections (data not shown). These data suggest that one of the inhibitory mechanisms of IFN-β on Con A-induced hepatocellular injury is to modulate the reaction induced by IFN-γ and TNF-α, but not to reduce the production of IFN-γ and TNF-α from T cells,40 natural killer T cells27 and Kupffer cells.41 To prove the inhibitory mechanisms of IFN-β on the Con A-induced hepatitis, the haemostasis and decrease of hepatic blood flow induced by Con A administration was assessed, because it has been reported that those reactions are induced by IFN-γ and TNF-α stimulation.16 As shown in Fig. 7(a–c), the formation of aggregations of erythrocytes, platelets and leucocytes and the decrease of hepatic blood flow by Con A administration were inhibited by a 3-hr pretreatment with IFN-β. These data suggest that one of the IFN-β inhibitory mechanisms on hepatic injury is to improve the haemostasis and reverse the Con A-reduced hepatic blood flow. The adhesion of endothelial cells and lymphocytes induced by haemostasis was caused via E-selectin, P-selectin, vascular cell adhesion molecule 1 and intercellular adhesion molecule 1.42,43 The expressions of those adhesion molecules were induced and/or accelerated by IFN-γ and TNF-α.44–47 Furthermore, it has been reported that IFN-β reduced the expression of intercellular adhesion molecule 1 by TNF-α and human leucocyte antigen-DRα by IFN-γ on human vascular endothelial cells.48 These data suggest the possibility that IFN-β would reduce the expression of adhesion molecules induced by IFN-γ and TNF-α on the endothelial cells.
In summary, the present study is the first to demonstrate that IFN-β suppresses liver fibrosis following repeated administration of Con A. The mechanism for this inhibitory effect at least involves the suppression of hepatocellular injury through the inhibition of hepatic haemostasis induced by IFN-γ and TNF-α and the expressions of bFGF, TGF-β1, TIMP-1 and COL1A2 mRNAs with simultaneous suppression of continuous liver injury. These findings indicate that type I IFN may suppress the progression of cirrhosis in HCV-infected patients through the inhibition of continuous hepatocellular injury and/or factors that promote liver fibrosis. Further studies are required to clarify the mechanisms of type I IFNs in antifibrosis and antihepatic injury using this model as well as investigations of patient biopsy samples.
Acknowledgments
The authors would like to express their sincere thanks to Dr Tetsuya Ishikawa and Dr Shinichi Kakumu (Department of Gastroenterology, Aichi Medical University) for their valuable discussions regarding this study and Mr Kouhei Ueda (Kamakura Techno-Science) for his technical help in preparing the histological sections.
References
- 1.Kobayashi K, Tanaka E, Sodeyama T, Urushihara A, Matsumoto A, Kiyosawa K. The natural course of chronic hepatitis C. A comparison between patients with genotypes 1 and 2 hepatitis C viruses. Hepatology. 1996;23:695–9. doi: 10.1053/jhep.1996.v23.pm0008666319. [DOI] [PubMed] [Google Scholar]
- 2.Yano M, Kumada H, Kaga M, et al. The long-term pathological evolution of chronic hepatitis C. Hepatology. 1996;23:1334–40. doi: 10.1002/hep.510230607. [DOI] [PubMed] [Google Scholar]
- 3.Ikeda F, Shimomura H, Miyake M, et al. Early clearance of circulating hepatitis C virus enhanced by induction therapy with twice-a-day intravenous injection of IFN-beta. J Interferon Cytokine Res. 2000;20:831–6. doi: 10.1089/10799900050151102. [DOI] [PubMed] [Google Scholar]
- 4.Izumi N, Kumada H, Hashimoto N, Harada H, Imawari M, Zeniya M, Toda G. Rapid decrease of plasma HCV RNA in early phase of twice daily administration of 3 MU doses interferon-beta in patients with genotype 1b hepatitis C infection: a multicenter randomized study. Dig Dis Sci. 2001;46:516–23. doi: 10.1023/a:1005686829416. [DOI] [PubMed] [Google Scholar]
- 5.Nomura H, Sou S, Nagahama T, Hayashi J, Kashiwagi S, Ishibashi H. Efficacy of early retreatment with interferon beta for relapse in patients with genotype Ib chronic hepatitis C. Hepatol Res. 2004;28:36–40. doi: 10.1016/j.hepres.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Alric L, Duffaut M, Selves J, et al. Maintenance therapy with gradual reduction of the interferon dose over one year improves histological response in patients with chronic hepatitis C with biochemical response: results of a randomized trial. J Hepatol. 2001;35:272–8. doi: 10.1016/s0168-8278(01)00110-6. [DOI] [PubMed] [Google Scholar]
- 7.Omata M, Shiratori Y. Long-term effects of interferon therapy on histology and development of hepatocellular carcinoma in hepatitis C. J Gastro Hepatol. 2005;15(Suppl.):E134–40. doi: 10.1046/j.1440-1746.2000.02115.x. [DOI] [PubMed] [Google Scholar]
- 8.Suou T, Hosho K, Kishimoto Y, Horie Y, Kawasaki H. Long-term decrease in serum N-terminal propeptide of type III procollagen in patients with chronic hepatitis C treated with interferon alpha. Hepatology. 1995;22:426–31. [PubMed] [Google Scholar]
- 9.Hiramatsu N, Hayashi N, Kasahara A, et al. Improvement of liver fibrosis in chronic hepatitis C patients treated with natural interferon alpha. J Hepatol. 1995;22:135–42. doi: 10.1016/0168-8278(95)80420-x. [DOI] [PubMed] [Google Scholar]
- 10.Kimura K, Ando K, Ohnishi H, Ishikawa T, Kakumu S, Takemura M, Muto Y, Moriwaki H. Immunopathogenesis of hepatic fibrosis in chronic liver injury induced by repeatedly administered concanavalin A. Int Immunol. 1999;11:1491–500. doi: 10.1093/intimm/11.9.1491. [DOI] [PubMed] [Google Scholar]
- 11.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–7. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 12.Miyazawa Y, Tsutsui H, Mizuhara H, Fujiwara H, Kanda K. Involvement of intrasinusoidal hemostasis in the development of concanavalin A-induced hepatic injury in mice. Hepatology. 1997;27:497–506. doi: 10.1002/hep.510270225. [DOI] [PubMed] [Google Scholar]
- 13.Elias JA, Jimenez SA, Freundlich B. Recombinant gamma, alpha, and beta interferon regulation of human lung fibroblast proliferation. Am Rev Respir Dis. 1987;135:62062–5. doi: 10.1164/arrd.1987.135.1.62. [DOI] [PubMed] [Google Scholar]
- 14.Stephenson ML, Krane SM, Amento EP, McCroskery PA, Byrne M. Immune interferon inhibits collagen synthesis by rheumatoid synovial cells associated with decreased levels of the procollagen mRNAs. FEBS Lett. 1995;180:43–50. doi: 10.1016/0014-5793(85)80227-1. [DOI] [PubMed] [Google Scholar]
- 15.Azuma A, Li YJ, Abe S, et al. Interferon-beta inhibits bleomycin-induced lung fibrosis by decreasing transforming growth factor-beta and thrombospondin. Am J Respir Cell Mol Biol. 2005;32:93–8. doi: 10.1165/rcmb.2003-0374OC. [DOI] [PubMed] [Google Scholar]
- 16.Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946–52. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- 17.Milani S, Herbst H, Schuppan D, Hahan EG, Stein HA. In situ hybridization for procollagen types I, III and VI Mrna in normal and fibrotic rat liver: evidence for predominant expression in nonparenchymal liver cells. Hepatology. 1989;10:84–92. doi: 10.1002/hep.1840100117. [DOI] [PubMed] [Google Scholar]
- 18.Rubin E, Hutterer F, Popper H. Experimental hepatic fibrosis without hepatocellular regeneration. A kinetic study. Am J Pathol. 1968;52:111–20. [PMC free article] [PubMed] [Google Scholar]
- 19.Milani S, Herbst H, Schuppan D, Kim KY, Riecken EO, Stein H. Procollagen expression by nonparenchymal rat liver cells in experimental biliary fibrosis. Gastroenterology. 1990;98:175–84. doi: 10.1016/0016-5085(90)91307-r. [DOI] [PubMed] [Google Scholar]
- 20.Fort J, Pilette C, Veal N, Oberti F, Gallois Y, Douay O, Rosenbaum J, Cales P. Effects of long-term administration of interferon alpha in two models of liver fibrosis in rats. J Hepatol. 1998;29:263–70. doi: 10.1016/s0168-8278(98)80012-3. [DOI] [PubMed] [Google Scholar]
- 21.Tarcin O, Avsar K, Demirturk L, et al. In vivo inefficiency of pentoxifylline and interferon-alpha on hepatic fibrosis in biliary-obstructed rats: assessment by tissue collagen content and prolidase activity. J Gastroenterol Hepatol. 2003;18:437–44. doi: 10.1046/j.1440-1746.2003.03004.x. [DOI] [PubMed] [Google Scholar]
- 22.Inagaki Y, Nemoto T, Kushida M, et al. Interferon alpha down-regulates collagen gene transcription and suppresses experimental hepatic fibrosis in mice. Hepatology. 2003;38:890–9. doi: 10.1053/jhep.2003.50408. [DOI] [PubMed] [Google Scholar]
- 23.Tiegs G, Hentschel J, Wendel AA. T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest. 1992;90:196–203. doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeda K, Hayakawa Y, Kaer LV, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci USA. 2000;97:5498–503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gantner F, Leist M, Lohse AW, Germann PG, Tieg G. Concanavalin A-induced T-cell-mediated hepatic injury in mice: the role of tumor necrosis factor. Hepatology. 1995;21:190–8. doi: 10.1016/0270-9139(95)90428-x. [DOI] [PubMed] [Google Scholar]
- 26.Mizuhara H, Uno M, Seki N, et al. Critical involvement of interferon gamma in the pathogenesis of T-cell activation-associated hepatitis and regulatory mechanisms of interleukin-6 for the manifestations of hepatitis. Hepatology. 1996;23:1608–15. doi: 10.1053/jhep.1996.v23.pm0008675184. [DOI] [PubMed] [Google Scholar]
- 27.Toyabe S, Seki S, Iiai T, et al. Requirement of IL-4 and liver NK1+ T cells for concanavalin A-induced hepatic injury in mice. J Immunol. 1997;159:1537–42. [PubMed] [Google Scholar]
- 28.Muraro PA, Liberati L, Bonanni L, et al. Decreased integrin gene expression in patients with MS responding to interferon-β treatment. J Neuroimmunol. 2004;150:123–31. doi: 10.1016/j.jneuroim.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Gniadek P, Aktas O, Wandinger KP, et al. Systemic IFN-β treatment induces apoptosis of peripheral immune cells in MS patients. J Neuroimmunol. 2003;137:187–96. doi: 10.1016/s0165-5728(03)00074-2. [DOI] [PubMed] [Google Scholar]
- 30.Kraus J, Bauer R, Chatzimanolis N, et al. Interferon-β1b leads to a short-term increase of soluble but long-term stabilization of cell surface bound adhesion molecules in multiple sclerosis. J Neurol. 2004;251:464–72. doi: 10.1007/s00415-004-0358-7. [DOI] [PubMed] [Google Scholar]
- 31.Zang YC, Yang D, Hong J, Tejada-Simon V, Rivera V, Zhang JZ. Immunoregulation and blocking antibodies induced by interferon beta treatment in MS. Neurology. 2000;55:397–404. doi: 10.1212/wnl.55.3.397. [DOI] [PubMed] [Google Scholar]
- 32.Inagaki Y, Truter S, Ramirez F. Transforming growth factor-beta stimulates alpha 2(I) collagen gene expression through a cis-acting element that contains an Sp1-binding site. J Biol Chem. 1994;269:14828–34. [PubMed] [Google Scholar]
- 33.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–50. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 34.Gressner AM. Cytokines and cellular crosstalk involved in the activation of fat-storing cells. J Hepatol. 1995;22(Suppl.):28–36. [PubMed] [Google Scholar]
- 35.Benyon RC, Iredale JP, Goddard S, Winwood PJ, Arthur MJ. Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology. 1996;110:821–31. doi: 10.1053/gast.1996.v110.pm8608892. [DOI] [PubMed] [Google Scholar]
- 36.Olaso E, Friedman SL. Molecular regulation of hepatic fibrosis. J Hepatol. 1998;29:836–47. doi: 10.1016/s0168-8278(98)80269-9. [DOI] [PubMed] [Google Scholar]
- 37.Iredale JP, Benyon RC, Arthur MJ, Ferris WT, Alcolado R, Winwood PJ, Clark N, Murphy G. Tissue inhibitor of metalloproteinase-1 messenger RNA expression is enhanced relative to interstitial collagenase messenger RNA in experimental liver injury and fibrosis. Hepatology. 1996;24:176–84. doi: 10.1002/hep.510240129. [DOI] [PubMed] [Google Scholar]
- 38.Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;192:538–49. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenbaum J, Blazejewski S, Preaux AM, Mallat A, Dhumeaux D, Mavier P. Fibroblast growth factor 2 and transforming growth factor beta 1 interactions in human liver myofibroblasts. Gastroenterology. 1995;109:1986–96. doi: 10.1016/0016-5085(95)90767-x. [DOI] [PubMed] [Google Scholar]
- 40.Ajuebor MN, Hogaboam CM, Le T, Proudfoot AE, Swain MG. CCL3/MIP-1alpha is proinflammatory in murine T cell-mediated hepatitis by recruiting CCR1-expressing CD4(+) T cells to the liver. Eur J Immunol. 2004;34:2907–18. doi: 10.1002/eji.200425071. [DOI] [PubMed] [Google Scholar]
- 41.Gantner F, Leist M, Kusters S, Vogt K, Volk HD, Tiegs G. T cell stimulus-induced crosstalk between lymphocytes and liver macrophages results in augmented cytokine release. Exp Cell Res. 1996;229:137–46. doi: 10.1006/excr.1996.0351. [DOI] [PubMed] [Google Scholar]
- 42.Morikawa H, Hachiya K, Mizuhara H, Fujiwara H, Nishiguchi S, Shiomi S, Kuroki T, Kaneda K. Sublobular veins as the main site of lymphocyte adhesion/transmigration and adhesion molecule expression in the porto-sinusoidal-hepatic venous system during concanavalin A-induced hepatitis in mice. Hepatology. 2000;31:83–94. doi: 10.1002/hep.510310115. [DOI] [PubMed] [Google Scholar]
- 43.Massaguer A, Perez-del-Pulgar S, Engel P, Serratosa J, Bosch J, Pizcueta P. Concanavalin-A-induced liver injury is severely impaired in mice deficient in P-selectin. J Leuko Biol. 2002;72:262–70. [PubMed] [Google Scholar]
- 44.Neumann B, Machleidt T, Lifka A, Pfeffer K, Vestweber D, Mak TW, Holzmann B, Kronke M. Crucial role of 55-kilodalton TFN receptor in TNF-induced adhesion molecular expression and leukocyte organ infiltration. J Immunol. 1996;156:1587–93. [PubMed] [Google Scholar]
- 45.Conway JG, Popp JA, Thurman RG. Microcirculation in periportal and pericentral regions of lobule in perfused rat liver. Am J Physiol. 1985;249:G449–56. doi: 10.1152/ajpgi.1985.249.4.G449. [DOI] [PubMed] [Google Scholar]
- 46.Todoroki N, Watanabe Y, Akaike T, et al. Enhancement of IL-1β and IFN-γ of platelet activation. Adhesion to leukocytes via GMP-140/PADGEM protein (CD62) Biochem Biophys Res Commun. 1991;179:756–61. doi: 10.1016/0006-291x(91)91881-c. [DOI] [PubMed] [Google Scholar]
- 47.Pober JS, Cotran RS. The role of endothelial cells in inflammation. Transplantation. 1990;50:537–44. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- 48.Miller A, Lanir N, Shapiro S, Revel M, Honiqman S, Kinarty A, Lahat N. Immunoregulatory effects of IFN-β and interacting cytokines on human vascular endothelial cells. J Neuroimmunol. 1996;64:151–61. doi: 10.1016/0165-5728(95)00164-6. [DOI] [PubMed] [Google Scholar]