Abstract
The effect of infection history on the immune response is ignored in most models of infectious disease and in preclinical vaccination studies. No one, however, is naïve and repeated microbial exposure, in particular during childhood, shapes the immune system to respond more efficiently later in life. Concurrent or sequential infections influence the immune response to secondary unrelated pathogens. The involvement of cross-reactive acquired immunity, in particular T-cell responses, is extensively documented. In this review, we discuss the impact of successive infections on the infected tissue itself, with a particular focus on the innate response of the respiratory tract, including a persistent alteration of (1) epithelial or macrophage expression of Toll-like receptors or adherence molecules used by subsequent bacteria to invade the host, (2) the responsiveness of macrophages and neutrophils and (3) the local cytokine milieu that affects the activation of local antigen-presenting cells and hence adaptive immunity to the next infection. We emphasize that such alterations not only occur during coinfection, but are maintained long after the initial pathogen is cleared. As innate responses are crucial to the fight against local pathogens but are also involved in the maintenance of the homeostasis of mucosal tissues, dysregulation of these responses by repeated infections is likely to have a major impact on the outcome of infectious or allergic disease.
Keywords: infection history, resolution of inflammation, innate response, coinfection, lung imprinting, respiratory viruses, heterologous immunity
Introduction
One of the first observations that diseases are often the result of concurrent or sequential infections dates from the early 19th century, when cases of pneumoniae correlated with the influenza (flu) epidemic.1,2 Subsequent studies confirmed that secondary bacterial infections were a major cause of severe flu-associated illness and death (for an historical review, see Brundage3). Most epidemiological studies indicate that the time lapse between flu infection and bacterial pneumoniae is around 5–10 days, in particular in the context of highly virulent influenza strains such as in 1918. However, in some cases, increased susceptibility to bacteria is seen even several weeks after the resolution of the viral infection, suggesting a long-term impact on the lung.4,5 The interaction between viruses and bacteria is particularly relevant as it is involved in many diseases, such as pneumoniae, sinusitis, otitis media and gastroenteritis. Other examples are the impact of human immunodeficiency virus (HIV) or parasite infections on tuberculosis, hepatitis, opportunistic infections and even vaccination efficacy, which are all important health issues in developing countries.6 In addition, infections or exposure to microbial products can also impact the development of autoimmune and allergic diseases. For example, some respiratory infections exacerbate asthma, while living in less hygienic conditions during childhood or chronic parasite infection protects against the development of allergic atopy.7 The link between infections and allergy is beyond the scope of this review, but some causative mechanisms may also apply to infectious diseases (for further reading, see Schaub et al.,8 Maizels9 and Kamradt et al.10). In addition to epidemiological data, the development of animal models has been instrumental in deciphering the mechanisms involved in polymicrobial infections (Table 1 and reviewed by Bakaletz11). Taken together, these experimental and epidemiological reports show that one pathogen and its associated immune response can influence the way a second unrelated pathogen is handled by the host.
Table 1.
Important parameters emerging from studies on mouse models of sequential infections and their outcomes
| First infection | Secondary infection | Outcome | |
|---|---|---|---|
| Detrimental outcome | |||
| Acute | Influenza | MCMV12,13 | Increases viral load and pathology |
| S. pneumoniae2,29,57,98 | Enhances susceptibility | ||
| Neisseria meningitides56 | idem | ||
| LCMV | Listeria37 | idem | |
| Chronic | Schistosoma mansoni | Vaccinia99 | idem |
| Toxoplasma gondii | Helicobacter felis100 | Potentiates type I immunopathology | |
| Helicobacter felis | Toxoplasma gondii100 | Suppresses the Type 1 protective response | |
| Helminth | Citrobacter rodentium47 | Decreases the gut protective Th1 response | |
| Virus46 | Impairs protective response | ||
| Beneficial outcome | |||
| Acute | Influenza | RSV49 | Reduces immune pathology |
| Influenza | Vaccinia virus12 | Reduces viral titre and pathology | |
| LCMV | |||
| Citrobacter | C. neoformans101 | Reduced Th2 driven eosinophilia | |
| Chronic | Schistosoma mansoni | Trichuris muris102 | Resolves infection, Th2-switch |
| M. tuberculosis | C. neoformans95 | Reduces type 2 response | |
| Trichinella spiralis | Influenza virus103 | Reduces immunopathology | |
| Helminth | Helicobacter felis48 | Suppresses Type 1 responses | |
| Malaria | Hepatitis B51 | Inhibits viral replication | |
LCMV, Lymphocytic choriomeningitis virus; MCMV, murine cytomegalovirus; RSV, respiratory syncitial virus.
Example of mouse models of sequential infections and their outcome. Several important parameters emerge from these studies:
(1) Chronic versus acute infection. Acute infection is characterized by an inflammatory phase followed by resolution where the inflammation is dampened down, the pathogen cleared and the infected tissue repaired. In contrast, the persistence of an infectious agent during chronic infection changes the homeostasis of the tissue, such as the formation of granulomas by Mycobacterium species. This also provides a sustained source of soluble anti-microbial or pro-inflammatory mediators and modifies the phenotype and functions of immune cells.
(2) Site of infection. It is expected that tissue damage and inflammatory mediators induced by an ongoing infection can condition the response to another pathogen at the same site. However, this also occurs at distant mucosal sites suggesting that soluble mediators or migrating cells are involved. For example, infection with the gut-restricted bacterium Citrobacter rodentium modulates the lung immunopathology induced by Cryptococcus neoformans.101
(3) Timing. The timing between two successive infections is critical. The quality and magnitude of inflammation, pathogen load and immune pathology vary during the course of an infection, and this is likely to influence the way a second pathogen is handled (see Fig. 1). In some cases, the effect can be maintained long after the initial infection has resolved.49
(4) Outcome. The outcome of co-infection is not always detrimental for the host but is protective in many cases. The same infection may also have an opposite effect, depending on the nature of the secondary pathogen. For example, influenza infection predisposes to Streptococcus pneumoniae but protects from RSV- or Listeria-induced immunopathology.49,57,104 Analysing of infections in which the outcome is improved compared to those where the outcome is worse should point to specific innate pathways that are altered.
One way in which the host copes with continuous exposure to pathogens is by developing a memory response to microbes in the form of antibodies and memory T cells. Of importance is the ability of this memory response to cross-react and recognize related pathogens. For example, a pool of memory T cells generated during a first viral infection can cross-react with antigens from a heterologous virus and modulate related immunopathology.12,13 Cross-reactive adaptive immunity, however, does not explain all, as even one single infection may influence the response to totally unrelated pathogens. An alteration of the expression or downstream signalling of innate receptors which are known to recognize a broad range of micro-organisms may provide an alternative explanation for these observations. Although innate immunity does not classically develop the memory phenotype we ascribe to adaptive immunity, it is possible that innate pathways may remain altered after resolution of infection. The aim of this review is to discuss evidence of long-term modulation of innate cell functions that is directly induced by pathogens or indirectly influenced by activated cells generated by infection.
As the mucosa is a major portal of entry and replication site for many pathogens, studying the interaction between microbes and the host mucosa is crucial to our understanding of heterologous immunity. Mucosal tissues are continuously exposed to pathogens or allergens and are specially equipped with efficient mechanisms to prevent them from entering the host. These include mechanical clearance (exclusion by the mucus layer and epithelial cell shedding) and neutralization (by antibodies and antimicrobial factors), but also rely on the activation of sentinel cells such as epithelial cells and macrophages. Although they share common features, mucosal tissues are highly specialized and therefore have their own innate effectors and level of immune homeostasis.14 For example, while the respiratory tract is basically sterile below the larynx, the gut carries a microbial flora that interferes with more pathogenic micro-organisms. In this review, we will focus on mucosal responses with a particular emphasis on the respiratory tract. Many investigators have studied the relationship between respiratory viruses and bacteria, which is not surprising given that respiratory infections represent the leading cause of mortality world-wide.15 The mechanisms emerging from these studies may also apply to other pathogen combinations or to other mucosal tissues.
Direct alteration of innate cell function
Changes in pathogen recognition
Specific structures expressed by pathogens, called microbe-associated molecular patterns (MAMPs), are recognized by mucosal sentinel cells via pattern-recognition receptors (PRRs) such as Toll-like receptors (TLRs). The engagement of TLRs results in the activation of nuclear factor (NF)-κB and the production of pro-inflammatory and antimicrobial signals.16 An alteration in the level of PRR expression or cellular re-localization of these receptors as the result of an inflammatory episode will lead to abnormal innate signalling and associated inflammatory responses. For example, TLR expression and response are compartmentalized in the gut epithelium to prevent continual recognition of commensal flora,17,18 although such compartmentalization has not been described thus far in the respiratory tract. Lung epithelial cells do, however, up-regulate TLRs, in particular TLR3 and TLR2, upon respiratory syncitial virus (RSV) and influenza infection, which may be mediated by the release of interferon (IFN) by infected macrophages.19–21 Whether such up-regulation has an impact on secondary infection is unclear.22 Because many of these receptors share components of their signalling pathways, changes in the expression level or phosphorylation status of molecules within these pathways may also interfere with the recognition of subsequent pathogens. This may synergize and exacerbate the inflammatory responses, as recently shown during bacterial coinfection,23 or could lead to an attenuation of the signal. TLR signalling is inhibited by several negative regulators downstream of the receptor.24 For example, increased expression of one of these regulators, IL-1 receptor associated kinase (IRAK-M), correlates with the unresponsiveness of alveolar macrophages to lipopolysaccharide (LPS) in a mouse model of abdominal sepsis.25 Commensals also inhibit TLR responses by directly interfering with the signalling pathways in epithelial cells.26,27 Clearly, a previous infectious event could impact significantly on responsiveness to the next, depending on how long the first influence lasts and whether the subsequent pathogen triggers similar signalling pathways.
Changes in epithelial adherence and antimicrobial defences
Enhanced adherence of bacteria in the context of viral infection has been extensively studied.28 In the case of influenza and pneumococcus synergism, increased bacterial adherence is proposed to be mediated by influenza-expressed neuraminidase which exposes cryptic receptors via the cleavage of sialic acids from surface glycoconjugates29 (Fig. 1). Adenoviruses also enhance bacterial adherence.30 Various antimicrobial molecules are present in the lumen of the gut (beta-defensins)31 or in the airways (collectins)32 that have multifunctional properties, including the ability to suppress macrophage responses and to target different types of micro-organisms.33 Most of them are produced at steady state but can also be up-regulated by TLR ligands.34,35 Altered expression of such molecules with a broad range of specificity may explain why one infection has a similar impact on multiple secondary pathogens independent of antigenic cross-reactivity.
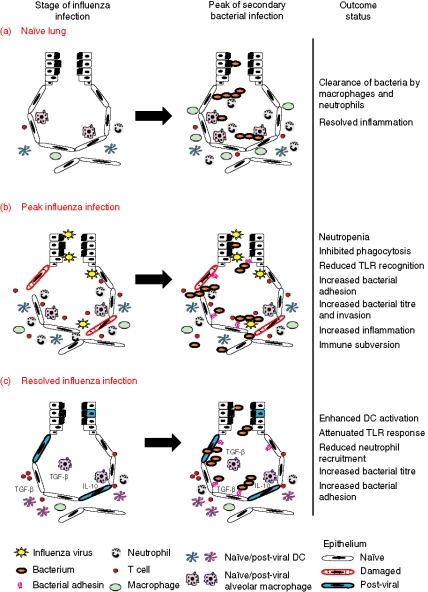
Figure 1.
Model of secondary bacterial superinfection at different stages after influenza infection. On the left a schematic representation of the lung at different stages of influenza infection is shown. The result of a secondary bacterial infection is shown on the right. (a) In naïve lungs, bacteria are cleared rapidly as a result of the antimicrobial activities of alveolar macrophages and neutrophils that are recruited in the airways. In this case, the integrity of the epithelium is preserved which prevents the bacteria spreading systemically. (b) At the peak of influenza infection (day 7 to day 10 post infection), the entry of the bacteria into the lung parenchyma and the systemic compartment is facilitated by the damaged epithelium and increased adhesion of the bacteria. The function of neutrophils and macrophages and the level of Toll-like receptor (TLR) expression are decreased as a result of the viral infection, so that both the recognition and the clearance of the bacteria are impaired. Overall, bacteria-induced immunopathology and bacterial load are increased and usually sustained as a result of the viral coinfection. (c) After the resolution of the initial viral infection, the TLR response of alveolar macrophages and epithelial cells is attenuated. This leads to an impaired recruitment of neutrophils in the airways which may explain the increased bacterial load observed in post-influenza lungs. In the meantime, dendritic cells (DCs) remain in the lungs several weeks after the infection is cleared and as a result of a more activated phenotype they may enhance the generation of an adaptive immune response.
Modulation of neutrophil and macrophage function
It is well established that viral infection reduces monocyte and neutrophil function (for example the reduction of chemotaxis and phagocytosis), which may affect the clearance of secondary bacterial infections. Neutrophil apoptosis occurs during influenza36 and lympho choriomeningitis virus (LCMV) infection,37 and suppression of monocyte and polymorphonuclear cell chemotaxis occurs in humans and in animal models of influenza and herpes simplex virus infection.38–40 Secondary infections may be further affected by the inhibition of macrophage activation and phagocytosis by influenza.41 RSV infection is known to induce cytokine production and the up-regulation of costimulatory molecules by alveolar macrophages, but reduces their antimicrobial function upon bacterial challenge.42 In addition, innate cells, including macrophages, and epithelium are susceptible to tolerance, which in the short term compromises subsequent inflammatory responses. A good example of this phenomenon is cross-tolerance between TLR agonists,43 which could explain why patients with sepsis develop an immunosuppressive state characterized by the hyporesponsiveness of their monocytes and increased susceptibility to pulmonary bacteria.44 A more extensive description of how the macrophage phenotype is modified by the local environment can be found in a recent review by Gordon and Taylor.45
Modification of the cytokine milieu and implications for the innate response
Immune polarization
The adaptive immune response to micro-organisms is often characterized by the polarization of the cytokine response. A type 1 cytokine response to viruses is characterized by the production of IFN-γ, tumour necrosis factor (TNF)-α, interleukin (IL)-12 and cytotoxic CD8+ T cells. A type 2 cytokine response, in contrast, is associated with IL-4, IL-5 and IL-13 and is predominant in parasitic and fungal infections. Type 1 cytokines are known to suppress type 2 responses and vice versa. Pathogens that strongly polarize the immune response may modify the type 1 and type 2 cytokine balance and/or effectors and consequently the local environment in which immunity to a concurrent micro-organism develops. For example, chronic helminth infection induces a strong systemic type 2 response that impairs the protective response to viruses46 and enteric bacteria47 and alleviates immunopathology associated with detrimental type 1 responses induced by Helicobacter.48 There is also evidence that memory T cells may also influence the local environment through bystander activation. For example, although they do not cross-react with RSV, influenza-specific CD8+ T cells migrate to the RSV-infected lungs, produce IFN-γ and may therefore influence RSV-mediated eosinophil recruitment and associated immunopathology.49 Polarizing cytokines will also modulate the function of antigen-presenting cells (APCs; see below) but also differentially activate resident mucosal cells. For example, TNF-α activates epithelial cells and together with IL-1 increases adherence of bacteria.50 Production of pro-inflammatory cytokines will also increase innate cell recruitment at the infection site. For example, improved hepatitis B virus clearance in mice coinfected with plasmodium is caused by an augmentation of innate effector cells in the liver induced by the parasite.51
Taken together, these studies highlight the importance of changes occurring in the local cytokine milieu during an ongoing infection and its effect on subsequent local responses.
Immunosuppressive cytokines: the role of IL-10 and transforming growth factor (TGF)-β
The immunosuppressive role of IL-10 and TGF-β is well documented in the gut, where they promote an inhibitory environment that prevents excessive response to commensals. These cytokines reduce cell recruitment and down-regulate cytokine production by innate cells such as macrophages (for reviews, see Moore et al.52 and Fitzpatrick and Bielefeldt-Ohmann53). Several studies point to a role for IL-10 in the unresponsiveness of macrophages during sepsis.54,55 Lung IL-10 production is also enhanced after a secondary pneumococcal or meningococcal challenge in mice that have experienced an influenza infection resulting in impaired neutrophil-mediated clearance of bacteria.56,57 Interestingly, IL-10 appears late in influenza infection and is sustained after viral clearance. TGF-β expression directly suppresses eosinophilic disease driven by Cryptococcus neoformans,58 is crucial for repair and remodelling of tissues, and may play a role in the resolution phase of the infection (see below). Raz and colleagues recently proposed a homeostatic cross-talk between epithelial cells and alveolar macrophages which is controlled by TGF-β.59 Therefore, increased local levels of TGF-β upon infection may alter mucosal homeostasis and sentinel cell responses.
What is the source of these immunosuppressive cytokines? Natural regulatory T cells (Tregs) are known to accumulate at sites of infection and are thought to regulate excessive pathology or prevent autoimmune diseases via the secretion of IL-10 and TGF-β, possibly after stimulation by TLR ligands during secondary challenge.60,61 A direct beneficial role for these cells is evident during bacteria-induced colitis or during chronic helminth infection.62,63 Long-term suppression of allergen-induced eosinophil recruitment induced by heat-killed mycobacterium correlates with the induction of Tregs.64 However, classical CD4 T cells or macrophages can also secrete these cytokines provided that they receive the appropriate signal. Clearly, sustained expression of immunosuppressive cytokines or retention of Tregs within the mucosa may have an impact on subsequent immunity, especially as Tregs may act in a bystander fashion.
Modulation of mucosal APC function
Ongoing infection may provide an adjuvant effect for subsequent responses through the induction of costimulatory molecules and increased APC recruitment. For example, respiratory infection induces dendritic cell (DC) maturation and enhances immunity to an innocuous antigen inhaled concurrently.65 The activation of APCs is conditioned by the local environment in which they are primed, and this influences the way in which they control T helper type 1/type 2 (Th1/Th2) development.66 Interestingly, purified microbial products, such as TLR ligands or toxins, induce the sustained activation of APCs in the lungs and provide generic protection to subsequent pathogenic insults by ameliorating secondary T-cell priming.67–69 DC migration is also modulated by infection. For example, tracking of DCs in the draining lymph node by direct labelling shows that migration is increased during the first 24 hr of influenza infection but then impaired, despite stimulation by the TLR9 ligand CpG.70 This demonstrates, together with the evidence described above, that infection directly affects antigen presentation and therefore may modulate adaptive responses to concurrent or subsequent pathogens.
Long-term modification of the microenvironment after resolution of a primary acute infection
Sustained modification of the tissue microenvironment after injury
Tissue damage caused by some severe acute infections leads to a repair process that modifies the matrix composition of the lung (such as collagen and fibronectin deposition), which may provide additional binding sites for bacteria71 but also alter the tissue structure itself. To preserve barrier function the epithelium needs to be regenerated either from bone marrow progenitors or from local stem cells. This repair process is controlled by dynamic and bidirectional cross-talk with mesenchymal cells such as fibroblasts, which could also be potentially altered by a previous infection.72 Whether the regenerated epithelium responds differently once renewed is unknown. Airway remodelling is very often restricted to chronic conditions such as asthma or fibrosis, but some must occur after acute infection in view of the level of tissue damage observed. Interestingly, remodelling is classically controlled by TGF-β, a molecule also involved in immune suppression (see above). In addition, repair mechanisms are controlled by NF-κB, and this may interfere with pro-inflammatory signals that are also dependent on NF-κB activation. Matrix metalloproteinases (MMPs), which are usually induced by infection or injury, may be central in the alteration of homeostasis after resolution of infection. Indeed, they play a dual role by participating in repair mechanisms (for example by activating TGF-β73) but also by modulating inflammatory responses (reviewed by Parks et al.74). For instance, matrilysin (MMP7) is essential for the regeneration of the airway epithelium after mucosal injury75 but also directly activates defensins76 and modulates the chemokine gradient and neutrophil transmigration.77 Whether MMP expression is sustained after resolution of one infection in order to modulate successive infections remains to be formally proven. However, should a second pathogen enter the lung during resolution and repair of the first then the immunological and pathological outcome is likely to be severely altered.
Infection also induces persistent lymphatic vessel hyperplasia.78 Mycoplasma pulmonis infection induces a robust lymphangiogenesis driven by vascular endothelial growth factor (VEGF) produced by inflammatory cells migrating into the airways. After resolution of the infection by antibiotic treatment, the network of newly synthesized lymphatic vessels persists in the airways several weeks after the infection. A change in the lymphatic network is likely to influence the outcome of a subsequent infection as this provides conduits for enhanced drainage and migration of activated APCs to the draining lymph nodes. Further studies are needed to look at the role of angiogenesis and lymphangiogenesis in sequential infection models.
Maintenance of cell populations in the mucosa after infection
Concomitant with the resolution phase of infection is an increase in CD11c+ cells in the lungs of infected animals, as shown for respiratory viruses.79,80 In the case of influenza infection, DCs, which remain in the lungs several weeks after the infection is cleared, are more activated and have an enhanced ability to promote T-cell priming, a process that is dependent on IFN-γ81 (Fig. 1). The increase in DCs and macrophages may be controlled in part by cytotoxic γδ T cells recruited during the resolution phase of a bacterial infection.82,83 The number of alveolar macrophages is also increased after acute bacterial infection.84,85 Alveolar macrophages and DCs are often depleted during infection and it is possible that newly recruited cells differentiating into mature APCs acquire a novel phenotype as a result of the modification of the microenvironment.
T lymphocytes specific for respiratory viruses are also maintained long after infection.86–88 Their retention may be mediated by collagen-binding α1β1 integrin expressed on memory T cells89 and correlate with the persistence of antigen after resolution of infection or inflammation.90–92 This is, however, a matter of debate.93 Some of these persisting T cells are found in lymphoid structures in the lung parenchyma called inducible bronchus-associated lymphoid tissue (iBALT).81,94 iBALT is visible up to 6 months after influenza infection in mice (AD, unpublished data) and is potentially important for the local re-stimulation of memory T cells and B cells and their potential bystander effect on subsequent heterologous infection. Interestingly, iBALT shares some features with Peyer's patches present in the gut or isolated follicles found in the colon. Because Peyer's patch formation is in part controlled by commensals, the development of iBALT shows that successive pathogen exposure can induce the neo-formation of ectopic immunological structures and consequently shape the mucosal immune system.
Long-term desensitization to inflammatory signals
We have recently described several examples of how successive infections result in the modulation of subsequent immunopathology.49,67,68,95 A common observation in all these studies is the reduction in cell emigration into the airways, which is a crucial risk factor for airway damage. We recently found that alveolar macrophages are desensitized to TLR stimulation after influenza infection and that the effect is maintained long after inflammation is resolved (Fig. 1). TLR-induced production of chemokines is reduced in post-flu lungs and this impairs the recruitment of cells into the airways. While such desensitization may be beneficial for the host in alleviating overall immunopathology, the reduced neutrophil recruitment in particular compromises the clearance of respiratory Streptococcus and Pseudomonas infection.96 Interestingly, similar results were found in the gut as responsiveness of intestinal epithelial cells to TLR activation is impaired immediately after birth by exposure to exogenous endotoxin.97 While there is no bacterial flora in the distal lung, repetitive exposure to microbial products may induce a similar beneficial hyporesponsiveness. Whether resident sentinel cells are directly affected by infection or subsequent remodelling events and/or whether this is maintained through cell renewal remains to be determined.
Concluding remarks
Many mechanisms can explain why the host responds differently to heterologous pathogens, from a simple breach of the mucosal barrier to subtle alterations in immune modulatory mechanisms. However, recent evidence reviewed here indicates that the innate immune system is also shaped by a history of infection. In addition to the necessity of preserving the integrity of the mucosal barrier, mucosal sites are astonishingly plastic and are therefore likely to evolve through successive experiences (Fig. 1). Although studies of systemic infection with high dose of viruses may be informative, the challenge in this field is to investigate the cellular and molecular mechanisms at mucosal sites, where the education of the immune system is crucial for efficient protection against pathogens. In particular, identifying the molecules involved in increased susceptibility or protection against subsequent infection may have a significant impact on therapeutic strategies to tackle infectious diseases.
References
- 1.Laënnec R. De l'Auscultation Médiate ou Traité du Diagnostic des Maladies des Poumon et du Coeur. 1. Paris: Brosson & Chaudé Édition; 1819. [Google Scholar]
- 2.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571–82. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brundage JF. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect Dis. 2006;6:303–12. doi: 10.1016/S1473-3099(06)70466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cartwright KAV, Jones DM, Smith AJ, Stuart JM, Kaczmarski EB, Palmer SR. Influenza A and meningococcal disease. Lancet. 1991;338:554–7. doi: 10.1016/0140-6736(91)91112-8. [DOI] [PubMed] [Google Scholar]
- 5.Hubert B, Watier L, Garnerin P, Richardson S. Meningococcal disease and influenza-like syndrome: a new approach to an old question. J Infect Dis. 1992;166:542–5. doi: 10.1093/infdis/166.3.542. [DOI] [PubMed] [Google Scholar]
- 6.Kamal SM, El Sayed Khalifa K. Immune modulation by helminthic infections: worms and viral infections. Parasite Immunol. 2006;28:483–96. doi: 10.1111/j.1365-3024.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- 7.Gern JE, Busse WW. Relationship of viral infections to wheezing illnesses and asthma. Nat Rev Immunol. 2002;2:132–8. doi: 10.1038/nri725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaub B, Lauener R, von Mutius E. The many faces of the hygiene hypothesis. J Allergy Clin Immunol. 2006;117:969–77. doi: 10.1016/j.jaci.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Maizels RM. Infections and allergy – helminths, hygiene and host immune regulation. Curr Opin Immunol. 2005;17:656–61. doi: 10.1016/j.coi.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Kamradt T, Goggel R, Erb KJ. Induction, exacerbation and inhibition of allergic and autoimmune diseases by infection. Trends Immunol. 2005;26:260–7. doi: 10.1016/j.it.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Bakaletz LO. Developing animal models for polymicrobial diseases. Nat Rev Microbiol. 2004;2:552–68. doi: 10.1038/nrmicro928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen HD, Fraire AE, Joris I, Welsh RM, Selin LK. Specific history of heterologous virus infections determines anti-viral immunity and immunopathology in the lung. Am J Pathol. 2003;163:1341–55. doi: 10.1016/S0002-9440(10)63493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selin LK, Brehm MA, Naumov YN, Cornberg M, Kim SK, Clute SC, Welsh RM. Memory of mice and men. CD8+ T-cell cross-reactivity and heterologous immunity. Immunol Rev. 2006;211:164–81. doi: 10.1111/j.0105-2896.2006.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raz E. Organ-specific regulation of innate immunity. Nat Immunol. 2007;8:3–4. doi: 10.1038/ni0107-3. [DOI] [PubMed] [Google Scholar]
- 15.Mizgerd JP. Lung infection – a public health priority. PLoS Med. 2006;3 doi: 10.1371/journal.pmed.0030076. e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawai T, Akira S. Pathogen recognition with Toll-like receptors. Cur Opin Immunol. 2005;17:338–44. doi: 10.1016/j.coi.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Mo JH, Katakura K, et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006;8:1327–36. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- 18.Sirard JC, Bayardo M, Didierlaurent A. Pathogen-specific TLR signaling in mucosa: mutual contribution of microbial TLR agonists and virulence factors. Eur J Immunol. 2006;36:260–3. doi: 10.1002/eji.200535777. [DOI] [PubMed] [Google Scholar]
- 19.Groskreutz DJ, Monick MM, Powers LS, Yarovinsky TO, Look DC, Hunninghake GW. Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J Immunol. 2006;176:1733–40. doi: 10.4049/jimmunol.176.3.1733. [DOI] [PubMed] [Google Scholar]
- 20.Guillot L, Le Goffic R, Bloch S, Escriou N, Akira S, Chignard M, Si-Tahar M. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem. 2005;280:5571–80. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]
- 21.Miettinen M, Sareneva T, Julkunen I, Matikainen S. IFNs activate toll-like receptor gene expression in viral infections. Genes Immun. 2001;2:349–55. doi: 10.1038/sj.gene.6363791. [DOI] [PubMed] [Google Scholar]
- 22.Dessing MC, van der Sluijs KF, Florquin S, Akira S, van der Poll T. Toll-like receptor 2 does not contribute to host response during postinfluenza pneumococcal pneumonia. Am J Respir Cell Mol Biol. 2007;36:609–14. doi: 10.1165/rcmb.2006-0166OC. [DOI] [PubMed] [Google Scholar]
- 23.Ratner AJ, Lysenko ES, Paul MN, Weiser JN. Synergistic proinflammatory responses induced by polymicrobial colonization of epithelial surfaces. Proc Natl Acad Sci USA. 2005;102:3429–34. doi: 10.1073/pnas.0500599102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liew FY, Xu D, Brint EK, O'Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–58. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 25.Deng JC, Cheng G, Newstead MW, Zeng X, Kobayashi K, Flavell RA, Standiford TJ. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest. 2006;116:2532–42. doi: 10.1172/JCI28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly D, Campbell JI, King TP, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol. 2004;5:104–12. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 27.Neish AS, Gewirtz AT, Zeng H, et al. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science. 2000;289:1560–3. doi: 10.1126/science.289.5484.1560. [DOI] [PubMed] [Google Scholar]
- 28.Peltola VT, McCullers JA. Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr Infect Dis J. 2004;23:S87–S97. doi: 10.1097/01.inf.0000108197.81270.35. [DOI] [PubMed] [Google Scholar]
- 29.McCullers JA, Bartmess KC. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J Infect Dis. 2003;187:1000–9. doi: 10.1086/368163. [DOI] [PubMed] [Google Scholar]
- 30.Hakansson A, Kidd A, Wadell G, Sabharwal H, Svanborg C. Adenovirus infection enhances in vitro adherence of Streptococcus pneumoniae. Infect Immun. 1994;62:2707–14. doi: 10.1128/iai.62.7.2707-2714.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller CA, Autenrieth IB, Peschel A. Innate defenses of the intestinal epithelial barrier. Cell Mol Life Sci. 2005;62:1297–307. doi: 10.1007/s00018-005-5034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogan MP, Geraghty P, Greene CM, O'Neill SJ, Taggart CC, McElvaney NG. Antimicrobial proteins and polypeptides in pulmonary innate defence. Respir Res. 2006;7:29. doi: 10.1186/1465-9921-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 34.Froy O. Regulation of mammalian defensin expression by Toll-like receptor-dependent and independent signalling pathways. Cell Microbiol. 2005;7:1387–97. doi: 10.1111/j.1462-5822.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- 35.Vora P, Youdim A, Thomas LS, et al. Beta-defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J Immunol. 2004;173:5398–405. doi: 10.4049/jimmunol.173.9.5398. [DOI] [PubMed] [Google Scholar]
- 36.Colamussi ML, White MR, Crouch E, Hartshorn KL. Influenza A virus accelerates neutrophil apoptosis and markedly potentiates apoptotic effects of bacteria. Blood. 1999;93:2395–403. [PubMed] [Google Scholar]
- 37.Navarini AA, Recher M, Lang KS, et al. Increased susceptibility to bacterial superinfection as a consequence of innate antiviral responses. Proc Natl Acad Sci USA. 2006;103:15535–9. doi: 10.1073/pnas.0607325103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kleinerman ES, Snyderman R, Daniels CA. Depressed monocyte chemotaxis during acute influenza infection. Lancet. 1975;2:1063–6. doi: 10.1016/s0140-6736(75)90432-8. [DOI] [PubMed] [Google Scholar]
- 39.Craft AW, Reid MM, Low WT. Effect of virus infections on polymorph function in children. Br Med J. 1976;1:1570. doi: 10.1136/bmj.1.6025.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin RR, Couch RB, Greenberg SB, Cate TR, Warr GA. Effects of infection with influenza virus on the function of polymorphonuclear leukocytes. J Infect Dis. 1981;144:279–80. doi: 10.1093/infdis/144.3.279. [DOI] [PubMed] [Google Scholar]
- 41.Nickerson CL, Jakab GJ. Pulmonary antibacterial defenses during mild and severe influenza virus infection. Infect Immun. 1990;58:2809–14. doi: 10.1128/iai.58.9.2809-2814.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franke Ullmann G, Pfortner C, Walter P, Steinmuller C, Lohmann Matthes ML, Kobzik L, Freihorst J. Alteration of pulmonary macrophage function by respiratory syncytial virus infection in vitro. J Immunol. 1995;154:268–80. [PubMed] [Google Scholar]
- 43.Bagchi A, Herrup EA, Warren HS, Trigilio J, Shin HS, Valentine C, Hellman J. MyD88-dependent and MyD88-independent pathways in synergy, priming, and tolerance between TLR agonists. J Immunol. 2007;178:1164–71. doi: 10.4049/jimmunol.178.2.1164. [DOI] [PubMed] [Google Scholar]
- 44.Munoz C, Carlet J, Fitting C, Misset B, Bleriot JP, Cavaillon JM. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Invest. 1991;88:1747–54. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 46.Actor JK, Shirai M, Kullberg MC, Buller RM, Sher A, Berzofsky JA. Helminth infection results in decreased virus-specific CD8+ cytotoxic T-cell and Th1 cytokine responses as well as delayed virus clearance. Proc Natl Acad Sci USA. 1993;90:948–52. doi: 10.1073/pnas.90.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen CC, Louie S, McCormick B, Walker WA, Shi HN. Concurrent infection with an intestinal helminth parasite impairs host resistance to enteric Citrobacter rodentium and enhances Citrobacter-induced colitis in mice. Infect Immun. 2005;73:5468–81. doi: 10.1128/IAI.73.9.5468-5481.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fox JG, Beck P, Dangler CA, Whary MT, Wang TC, Shi HN, Nagler-Anderson C. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000;6:536–42. doi: 10.1038/75015. [DOI] [PubMed] [Google Scholar]
- 49.Walzl G, Tafuro S, Moss P, Openshaw PJ, Hussell T. Influenza virus lung infection protects from respiratory syncytial virus-induced immunopathology. J Exp Med. 2000;192:1317–26. doi: 10.1084/jem.192.9.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–8. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 51.Pasquetto V, Guidotti LG, Kakimi K, Tsuji M, Chisari FV. Host–virus interactions during malaria infection in hepatitis B virus transgenic mice. J Exp Med. 2000;192:529–36. doi: 10.1084/jem.192.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 53.Fitzpatrick DR, Bielefeldt-Ohmann H. Transforming growth factor beta in infectious disease: always there for the host and the pathogen. Trends Microbiol. 1999;7:232–6. doi: 10.1016/s0966-842x(99)01498-5. [DOI] [PubMed] [Google Scholar]
- 54.Steinhauser ML, Hogaboam CM, Kunkel SL, Lukacs NW, Strieter RM, Standiford TJ. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. J Immunol. 1999;162:392–9. [PubMed] [Google Scholar]
- 55.Reddy RC, Chen GH, Newstead MW, Moore T, Zeng X, Tateda K, Standiford TJ. Alveolar macrophage deactivation in murine septic peritonitis: role of interleukin 10. Infect Immun. 2001;69:1394–401. doi: 10.1128/IAI.69.3.1394-1401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alonso JM, Guiyoule A, Zarantonelli ML, et al. A model of meningococcal bacteremia after respiratory superinfection in influenza A virus-infected mice. FEMS Microbiol Lett. 2003;222:99–106. doi: 10.1016/S0378-1097(03)00252-0. [DOI] [PubMed] [Google Scholar]
- 57.van der Sluijs KF, van Elden LJ, Nijhuis M, et al. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J Immunol. 2004;172:7603–9. doi: 10.4049/jimmunol.172.12.7603. [DOI] [PubMed] [Google Scholar]
- 58.Williams AE, Humphreys IR, Cornere M, Edwards L, Rae A, Hussell T. TGF-beta prevents eosinophilic lung disease but impairs pathogen clearance. Microbes Infect. 2005;7:365–74. doi: 10.1016/j.micinf.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 59.Takabayshi K, Corr M, Hayashi T, et al. Induction of a homeostatic circuit in lung tissue by microbial compounds. Immunity. 2006;24:475–87. doi: 10.1016/j.immuni.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 60.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–60. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 61.Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express Toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–11. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kullberg MC, Jankovic D, Gorelick PL, Caspar P, Letterio JJ, Cheever AW, Sher A. Bacteria-triggered CD4(+) T regulatory cells suppress Helicobacter hepaticus-induced colitis. J Exp Med. 2002;196:505–15. doi: 10.1084/jem.20020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilson MS, Taylor MD, Balic A, Finney CAM, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med. 2005;202:1199–212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zuany-Amorim C, Sawicka E, Manlius C, et al. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat Med. 2002;8:625–9. doi: 10.1038/nm0602-625. [DOI] [PubMed] [Google Scholar]
- 65.Brimnes MK, Bonifaz L, Steinman RM, Moran TM. Influenza virus-induced dendritic cell maturation is associated with the induction of strong T cell immunity to a coadministered, normally nonimmunogenic protein. J Exp Med. 2003;198:133–44. doi: 10.1084/jem.20030266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–93. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 67.Williams AE, Edwards L, Humphreys IR, Snelgrove R, Rae A, Rappuoli R, Hussell T. Innate imprinting by the modified heat-labile toxin of Escherichia coli (LTK63) provides generic protection against lung infectious disease. J Immunol. 2004;173:7435–43. doi: 10.4049/jimmunol.173.12.7435. [DOI] [PubMed] [Google Scholar]
- 68.Edwards L, Williams AE, Krieg AM, Rae AJ, Snelgrove RJ, Hussell T. Stimulation via Toll-like receptor 9 reduces Cryptococcus neoformans-induced pulmonary inflammation in an IL-12-dependent manner. Eur J Immunol. 2005;35:273–81. doi: 10.1002/eji.200425640. [DOI] [PubMed] [Google Scholar]
- 69.Juffermans NP, Leemans JC, Florquin S, et al. CpG oligodeoxynucleotides enhance host defense during murine tuberculosis. Infect Immun. 2002;70:147–52. doi: 10.1128/IAI.70.1.147-152.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Legge KL, Braciale TJ. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity. 2003;18:265–77. doi: 10.1016/s1074-7613(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 71.de Bentzmann S, Plotkowski C, Puchelle E. Receptors in the Pseudomonas aeruginosa adherence to injured and repairing airway epithelium. Am J Respir Crit Care Med. 1996;154:S155–62. doi: 10.1164/ajrccm/154.4_Pt_2.S155. [DOI] [PubMed] [Google Scholar]
- 72.Torday JS, Rehan VK. The evolutionary continuum from lung development to homeostasis and repair. Am J Physiol Lung Cell Mol Physiol. 2007;292:L608–11. doi: 10.1152/ajplung.00379.2006. [DOI] [PubMed] [Google Scholar]
- 73.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–76. [PMC free article] [PubMed] [Google Scholar]
- 74.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–29. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 75.Dunsmore SE, Saarialho-Kere UK, Roby JD, Wilson CL, Matrisian LM, Welgus HG, Parks WC. Matrilysin expression and function in airway epithelium. J Clin Invest. 1998;102:1321–31. doi: 10.1172/JCI1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilson CL, Ouellette AJ, Satchell DP, et al. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–7. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 77.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–46. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 78.Baluk P, Tammela T, Ator E, et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–57. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamamoto N, Suzuki S, Shirai A, Suzuki M, Nakazawa M, Nagashima Y, Okubo T. Dendritic cells are associated with augmentation of antigen sensitization by influenza A virus infection in mice. Eur J Immunol. 2000;30:316–26. doi: 10.1002/1521-4141(200001)30:1<316::AID-IMMU316>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 80.Gill MA, Palucka AK, Barton T, Ghaffar F, Jafri H, Banchereau J, Ramilo O. Mobilization of plasmacytoid and myeloid dendritic cells to mucosal sites in children with respiratory syncytial virus and other viral respiratory infections. J Infect Dis. 2005;191:1105–15. doi: 10.1086/428589. [DOI] [PubMed] [Google Scholar]
- 81.Dahl ME, Dabbagh K, Liggitt D, Kim S, Lewis DB. Viral-induced T helper type 1 responses enhance allergic disease by effects on lung dendritic cells. Nat Immunol. 2004;5:337–43. doi: 10.1038/ni1041. [DOI] [PubMed] [Google Scholar]
- 82.Kirby A, Newton D, Carding S, Kaye P. Pulmonary dendritic cells and alveolar macrophages are regulated by gammadelta T cells during the resolution of S. pneumoniae-induced inflammation. J Pathol. 2007;212:29–37. doi: 10.1002/path.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carding SR, Egan PJ. The importance of gamma delta T cells in the resolution of pathogen-induced inflammatory immune responses. Immunol Rev. 2000;173:98–108. doi: 10.1034/j.1600-065x.2000.917302.x. [DOI] [PubMed] [Google Scholar]
- 84.Kirby AC, Raynes JG, Kaye PM. CD11b regulates recruitment of alveolar macrophages but not pulmonary dendritic cells after pneumococcal challenge. J Infect Dis. 2006;193:205–13. doi: 10.1086/498874. [DOI] [PubMed] [Google Scholar]
- 85.Amano H, Morimoto K, Senba M, et al. Essential contribution of monocyte chemoattractant protein-1/C-C chemokine ligand-2 to resolution and repair processes in acute bacterial pneumonia. J Immunol. 2004;172:398–409. doi: 10.4049/jimmunol.172.1.398. [DOI] [PubMed] [Google Scholar]
- 86.Wiley JA, Hogan RJ, Woodland DL, Harmsen AG. Antigen-specific CD8(+) T cells persist in the upper respiratory tract following influenza virus infection. J Immunol. 2001;167:3293–9. doi: 10.4049/jimmunol.167.6.3293. [DOI] [PubMed] [Google Scholar]
- 87.Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med. 2001;193:981–6. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Bree GJ, van Leeuwen EM, Out TA, Jansen HM, Jonkers RE, van Lier RA. Selective accumulation of differentiated CD8+ T cells specific for respiratory viruses in the human lung. J Exp Med. 2005;202:1433–42. doi: 10.1084/jem.20051365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ray SJ, Franki SN, Pierce RH, et al. The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity. 2004;20:167–79. doi: 10.1016/s1074-7613(04)00021-4. [DOI] [PubMed] [Google Scholar]
- 90.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Julia V, Hessel EM, Malherbe L, Glaichenhaus N, O'Garra A, Coffman RL. A restricted subset of dendritic cells captures airborne antigens and remains able to activate specific T cells long after antigen exposure. Immunity. 2002;16:271–83. doi: 10.1016/s1074-7613(02)00276-5. [DOI] [PubMed] [Google Scholar]
- 92.Zammit DJ, Turner DL, Klonowski KD, Lefrancois L, Cauley LS. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity. 2006;24:439–49. doi: 10.1016/j.immuni.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kohlmeier JE, Miller SC, Woodland DL. Antigen is not required for the activation and maintenance of virus-specific memory CD8+ T cells in the lung airways. J Immunol. 2007;178:4721–5. doi: 10.4049/jimmunol.178.8.4721. [DOI] [PubMed] [Google Scholar]
- 94.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10:927–34. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 95.Walzl G, Humphreys IR, Marshall BG, Edwards L, Openshaw PJ, Shaw RJ, Hussell T. Prior exposure to live Mycobacterium bovis BCG decreases Cryptococcus neoformans-induced lung eosinophilia in a gamma interferon-dependent manner. Infect Immun. 2003;71:3384–91. doi: 10.1128/IAI.71.6.3384-3391.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Didierlaurent A, Goulding J, Patel S, Snelgrove R, Low L, Bebien M, Lawrence T, Van Rijt LS, Lambrecht BN, Sirard J-C, Hussell T. Sustained de-sensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J Exp Med. 2007 doi: 10.1084/jem.20070891. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lotz M, Gutle D, Walther S, Menard S, Bogdan C, Hornef MW. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med. 2006;203:973–84. doi: 10.1084/jem.20050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.LeVine AM, Koeningsknecht V, Stark JM. Decreased pulmonary clearance of S.pneumoniae following influenza A infection in mice. J Virol Meth. 2001;94:173–86. doi: 10.1016/s0166-0934(01)00287-7. [DOI] [PubMed] [Google Scholar]
- 99.Actor JK, Marshall MA, Eltoum IA, Buller RM, Berzofsky JA, Sher A. Increased susceptibility of mice infected with Schistosoma mansoni to recombinant vaccinia virus: association of viral persistence with egg granuloma formation. Eur J Immunol. 1994;24:3050–6. doi: 10.1002/eji.1830241220. [DOI] [PubMed] [Google Scholar]
- 100.Stoicov C, Whary M, Rogers AB, et al. Coinfection modulates inflammatory responses and clinical outcome of Helicobacter felis and Toxoplasma gondii infections. J Immunol. 2004;173:3329–36. doi: 10.4049/jimmunol.173.5.3329. [DOI] [PubMed] [Google Scholar]
- 101.Williams AE, Edwards L, Hussell T. Colonic bacterial infection abrogates eosinophilic pulmonary disease. J Infect Dis. 2006;193:223–30. doi: 10.1086/498915. [DOI] [PubMed] [Google Scholar]
- 102.Curry AJ, Else KJ, Jones F, Bancroft A, Grencis RK, Dunne DW. Evidence that cytokine-mediated immune interactions induced by Schistosoma mansoni alter disease outcome in mice concurrently infected with Trichuris muris. J Exp Med. 1995;181:769–74. doi: 10.1084/jem.181.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Furze RC, Hussell T, Selkirk ME. Amelioration of influenza-induced pathology in mice by coinfection with Trichinella spiralis. Infect Immun. 2006;74:1924–32. doi: 10.1128/IAI.74.3.1924-1932.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gardner ID. Suppression of antibacterial immunity by infection with influenza virus. J Infect Dis. 1981;144:225–31. doi: 10.1093/infdis/144.3.225. [DOI] [PubMed] [Google Scholar]