Abstract
Nasal and small intestinal mucosae are the first sites of contact with infectious agents and the sites of T-cell-mediated and secreted immunoglobulin A (IgA)-mediated defences against pathogens. We investigated the factors controlling the infiltration of CD3+ T lymphocytes and surface IgA+ (sIgA+) B lymphocytes into swine epithelium and lamina propria (LP) within and between these two mucosal effector sites. Vascular addressins, vascular cell adhesion molecule 1 and mucosal addressin cell adhesion molecule-1 were reciprocally expressed in both mucosae. Strong expression of α4β1 relative to α4β7 was characteristic of CD3+ T cells in nasal mucosa LP and epithelium and of sIgA+ cells in nasal mucosa epithelium. The same profile was observed on corresponding blood cells. Conversely, higher levels of integrins β7 and α4β7 than α4β1 were characteristic of CD3+ T cells and sIgA+ cells in the small intestine. However, about 40% of the LP-activated sIgA+ cells displayed sIgAhigh, integrin α4  and integrin α4
and integrin α4 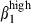 expression. Whereas CCL19, CXCL12, CCL21 and CCL28 messenger RNAs were similarly expressed in both mucosae, CCL25 messenger RNA was only expressed in the small intestine. Thus, the nasal and small intestine mucosae represent separate compartments for infiltration by CD3+ T cells and sIgA+ effector cells, with the exception of a population of small intestine activated sIgA+ cells, which may gain access to both mucosae.
expression. Whereas CCL19, CXCL12, CCL21 and CCL28 messenger RNAs were similarly expressed in both mucosae, CCL25 messenger RNA was only expressed in the small intestine. Thus, the nasal and small intestine mucosae represent separate compartments for infiltration by CD3+ T cells and sIgA+ effector cells, with the exception of a population of small intestine activated sIgA+ cells, which may gain access to both mucosae.
Keywords: B cells, chemokines, migration, mucosal immunity, T cells
Introduction
The mucosal immune system is the major component of defences against pathogens invading the body through mucosal surfaces. This system consists of inductive and effector sites in the lamina propria (LP) and the epithelium, to which activated T and B cells are relocated to express their effector functions. The lymphoid tissues associated with the upper respiratory tract, nasal-associated lymphoid tissue in rodents and Waldeyer's ring in other mammals,1 were believed to be equivalent to the Peyer's patch because there are many structural similarities. The effector sites of both mucosae also share similarities, leading to the suggestion that these mucosae form a common mucosal system. Accordingly, cells activated at one mucosal site can home to any mucosal site.2 However, by varying the mucosal route of immunization, the immune response shows various degrees of compartmentalization, depending on the route of administration and the species-specific inductive site. For example, the intranasal route of immunization in rodents3–5 induces immunoglobulin A (IgA) responses more efficiently in the nasal mucosa than in the intestine,6 whereas the oral route induces a stronger IgA response in the gut than in the respiratory tract. In humans7,8 and swine, intranasal immunization generates an IgA response locally and at remote sites other than the gut,9–12 whereas intestinal immunization induces responses in both the intestine and the respiratory tract.13,14 These results suggest different effector tissues according to the species-specific inductor site, antigen dissemination or both. As differential homing pathways of T and B cells with surface IgA (sIgA+) between mucosae may result from divergence in the adhesion pathway, we wished to explore the cellular and molecular trafficking mechanisms in relation to these inductive sites.1 Lymphocytes activated in the Peyer's patches express integrin α4β7, which interacts with the mucosal addressin cell adhesion molecule-1 (MadCAM-1)15 in the small intestine (SI) effector sites. Then, the transmigration is mediated by the interaction of SI epithelial chemokines with the corresponding lymphocyte receptor, the CC chemokine ligand 25 (CCL25) with CCR9 on the majority of T lymphocytes and some sIgA-secreting cells and CCL28 with CCR10 on most sIgA+ cells and some T cells.16 However, little is known about the integrins and chemokines of nasal mucosa effector sites, although it has been suggested that the ability of sIgA+ cells to migrate to mucosal tissues that are distant from the gut is a result of their responsiveness to vascular cell adhesion molecule 1 (VCAM-1)/integrin α4 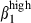 and CCL28/CCR10 interactions.17,18
and CCL28/CCR10 interactions.17,18
To determine whether the process involved in T and sIgA+ lymphocyte recruitment in the nasal mucosa differed from that in the SI, we compared the expression of adhesion molecules and epithelial chemokines in both mucosae in pigs. The pig has a number of advantages for studies of the nasal and intestinal mucosal immune systems: it is a large monogastric omnivore in which nasal and intestinal cells can be compared in a normal single individual, which is not the case in humans. Furthermore, LP and intraepithelial (IE) lymphocytes from inductive sites can be analysed separately, which is not possible in rodents. These elements may facilitate the design of vaccines and help in our understanding of the dissemination of the immune response between mucosal sites after intranasal or gut immunization.
Materials and methods
Animals and tissue samples
Healthy, histocompatible, 6- to 9-month-old miniature pigs (SLAd/d) were obtained from an enzootic-pneumonia-free herd (INRA, Tours). Pigs were electrocuted and exsanguinated in a local slaughterhouse. Cross- sections of the snout were cut at the level of the first premolar tooth. The part of the snout containing the nasal turbinates, septum and lateral walls was cut off and then the entire nasal mucosa covering the dorsal and ventral scroll of the turbinate bones was removed and weighed. Part of the gut (jejunum, 15–20 cm between two Peyer's patches) was excised and cut open longitudinally. The nasal and intestinal mucosae were washed several times with Hanks' balanced salt solution (Gibco BRL, Grand Island, NY) supplemented with 10% fetal calf serum and 1% penicillin/streptomycin (Gibco BRL) and gently blotted against dry paper to remove mucus and blood.
Isolation of lymphocytes from the epithelium and lamina propria of nasal and intestinal mucosae
Small intestine intraepithelial lymphocytes (SI-IEL) and SI-LP lymphocytes (SI-LPL) were isolated as previously described with some modifications.19–21
After ethylene diamine tetraacetic acid (EDTA) treatment, the supernatants, containing epithelial cells and IEL, were quickly filtered through nylon wool (Polyscience, INC, Warrington, PA), to remove epithelial cell clusters, and centrifuged (600 g, 45 min, room temperature) over a Percoll gradient prepared with 2 ml 40% (v/v) and 2 ml 75% (v/v) (Sigma, St Louis, MO). Then, cells at the interphase of the gradient were collected and washed and the red blood cells were lysed with 0·155 m NH4Cl, pH 7·4.
The LPL were isolated from nasal and intestinal mucosae, using a modified version of a published protocol.22,23 Briefly, EDTA-treated nasal and intestinal mucosae were cut (< 3 mm pieces) and digested three times by incubation at 37° for 1 hr with 4 mg/ml collagenase (CLS 3, 237 U/mg, Worthington, Lakewood, NJ) for nasal mucosa and 2 mg/ml collagenase for gut mucosa. After filtration through gauze, the released cells, comprising glandular epithelial cells and LPL, were pooled and centrifuged on a Percoll gradient, as described above. Red blood cells were lysed and nasal and intestinal IEL and LPL were fixed with Stabilcyte (BioE, St Paul, MN), according to the manufacturer's instructions. Lymphocytes were then prepared for cytocentrifugation, May–Grünwald Giemsa staining and fluorescence-activated cell sorting (FACS) analysis.
Antibodies
The following primary antibodies were used for flow cytometry analysis and immunohistochemistry:1 hybridoma supernatants anti-CD3 [BB23 8E6, BB23 8E6-fluorescein isothiocyanate (FITC)]; anti-CD4 (74-12-4); anti-CD8 (76-2-11); anti-IgA (K61-1B4); anti-IgM (K513D1); anti-IgG (K138 4C12); anti-secretory component (K60-IF1); anti-SWC3a; anti-mouse integrin β7 (FIB27) (Pharmingen, San Jose, CA), anti-human wCD29 (β1; UCP1D2); anti-human integrin α4β7 (ACT-1); anti-human integrin α4β1 (P4G) (Dako, Glostrup, Denmark); anti-human l-selectin (LAM1-3); anti-endothelial cells (MIL11); anti-murine peripheral lymph node addressin (PNAd; MECA79); anti-human MadCAM-1 (7G11); anti-VCAM-1 (3F4) and as isotype control R35-95 for rat IgG2a (Pharmingen); X0942 for IgM (Dako), X0943 for IgG2a (Dako), FX0944 for IgG2b (Dako) and X0931 for IgG1 (Dako). An FITC-conjugated goat immunoglobulin (A100–102F, Bethyl Laboratories, Inc, Montgomery, TX) was used against IgA.
Flow cytometric analysis
Flow cytometric analysis was performed as previously described.1 Antibodies directed against porcine SWC3a and secretory component were used to identify granular cells and epithelial cells and the corresponding cell area was excluded. In the forward-scatter versus side-scatter dot plot, the electronic gate was set so that only small and large lymphocytes were included. Data were then analysed with winmidi 3·8 software.
Immunohistochemical staining of tissue sections
Serial 7-μm sections of the nasal mucosa and gut were dried, fixed by incubation in cold acetone for 10 min, dried again for 1 hr and stored at −70°. Immunohistochemical staining was performed as previously described.1
Tissue distribution of chemokine transcripts
Double immunofluorescence staining of endothelial cells for MIL11 and VCAM-1 and semiquantitative reverse transcription–polymerase chain reaction (RT-PCR) were performed as previously described.1 Amplification products were separated by electrophoresis in a 2% Tris 44·57 mM, Borate 44·57 mm, EDTA 1 mm (TBE) agarose gel stained with ethidium bromide and visualized on an ultraviolet transilluminator. We determined the fluorescence intensity of bands with an Alpha Fluorchem Imager Gel Analysis System version 2·00 (Alpha Innotech Corporation, San Leandro, CA). The cyclophilin gene was used as constitutively expressed ‘housekeeping’ gene control, to check the uniformity of the reverse transcription reactions.
Statistical analysis
The Kolmogorov–Smirnov test was used to determine whether the values obtained followed a Gaussian distribution. The mean percentages of cells positive for the various markers or homing receptor (HR) ± SEM in different organs were compared by variance analysis, with Bonferroni correction for multiple testing, to check for significant differences. Paired Student's t-tests were used to compare the percentages of cells positive for various HRs within a given organ and differences were considered significant if P < 0·05. Mean fluorescence intensity (MFI) values were obtained by determining mean fluorescence peak channel numbers for cells expressing each HR, normalized with respect to the isotype control (log MFI HR − log MFI isotype control). Chemokine messenger RNA (mRNA) levels are presented as the mean ± SEM of the ratio of the band intensity of the chemokine RT-PCR product over that of the corresponding cyclophilin (log chemokine − log cyclophilin).
Results
Distribution of CD3+ T cells and sIgA+ B cells in nasal and small intestine mucosal effector sites
The proportion of CD3+ T cells was much higher in the SI than in the nose (P < 0·05, Table 1) regardless of the tissue layer considered: epithelium (∼ 85% and 63%) or LP (∼ 79% and ∼54%, Table 1). In addition, CD3+ T cells in the epithelia were much more enriched in CD8 (Table 1) than those in the LP, with a CD8 : CD4 ratio of 4 in the nasal epithelium and of ∼6 in the intestinal epithelium; the corresponding LP contained fewer CD8+ cells, and the CD8 : CD4 ratio was only ∼3 in the nasal LP and ∼2 in the gut LP. There was about the same proportions of sIg or cytoplasmic immunoglobulin in both mucosae and about twice as much cytoplasmic IgA and IgM as IgG cells in the nose and SI, regardless of the tissue location: epithelium or LP (Table 1). In tissue sections, the secretory component was expressed by nasal and intestinal epithelial cells (Fig. 1a, i–vi). Furthermore, sIgA+ cells were found in clusters between the glands in the nose (data not shown), whereas they tended to be located in the basal (i.e. facing the crypts) rather than in the villous LP in the gut.
Table 1.
T-cell and B-cell surface phenotype from epithelium and LP of nasal and SI mucosae and from PBL
| Nasal mucosa (n = 4) | Intestinal mucosa (n = 4) | ||||
|---|---|---|---|---|---|
| Markers | IEL | LPL | IEL | LPL | PBL (n = 3) |
| CD3 | 63·2 ± 3·1 | 54·1 ± 4·1 | 85·1 ± 1·7 | 79·1 ± 1·3 | 72·8 ± 1·4 |
| CD4 | 12·8 ± 0·6* | 14·2 ± 1·0* | 10·1 ± 2·2* | 29·6 ± 1·6* | 33·6 ± 1·8 |
| CD8 | 51·2 ± 3·9**a | 37·5 ± 3·6**b | 78·0 ± 2·3**a | 48·8 ± 2·7**b | 35·0 ± 5·0 |
| CD8/CD4 | 4·00 ± 0·29 | 2·66 ± 0·23 | 6·45 ± 0·18 | 1·67 ± 0·17 | 1·03 ± 0·09 |
| sIgA | 13·3 ± 1·6 | 19·3 ± 1·1 | 12·9 ± 2·3 | 13·4 ± 1·4 | 16·8 ± 1·5 |
| sIgM | 14·8 ± 1·6 | 12·5 ± 3·0 | 10·2 ± 1·3 | 9·6 ± 1·7 | 13·7 ± 0·1 |
| sIgA/IgM | 0·90 ± 0·04 | 1·82 ± 0·40 | 1·33 ± 0·24 | 1·59 ± 0·41 | 1·33 ± 0·05 |
| sIgG | 3·8 | 4·2 ± 0·8 | 4·4 ± 2·5 | 5·5 ± 2·1 | ND |
| c-IgA1 | 0 − 8 | 13·3 − 41·7 | 0 − 0·5 | 11·2 ± 1·3 | ND |
| c-IgM1 | 0 − 6·5 | 0 − 11·5 | 0 | 9·3 ± 0·9 | ND |
| c-IgG1 | 0 − 4 | 6·3 − 26·3 | 0 | 2·3 ± 0·2 | ND |
| c-SC1 | < 1 | 16 − 20 | < 1 | < 1 | ND |
Results are presented as mean percentage ± SEM.
Percentage of IgA, IgM, IgG, secretory component-containing cells in IEL and LPL of nasal and SI mucosae after immunohistochemical staining on cytocentrifuged cell suspensions. Results are represented as the range of the percentage of positive cells to total cells in cytocentrifugated slides for nasal IEL and LPL and mean ± SEM for gut LPL (n = 3–4).
Different lowercase letters in superscript in a same line indicate organs with statistically significant difference (P < 0·05), by t-test.
Different lowercase letters in superscript in a same line indicate organs with statistically significant difference (P < 0·05), by t-test.
In a same column, asterisk indicates that the proportions of cells expressing two different markers are statistically different (P < 0·05), paired t-test.
In a same column, asterisk indicates that the proportions of cells expressing two different markers are statistically different (P < 0·05), paired t-test.
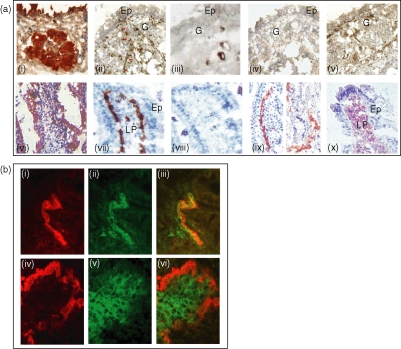
Figure 1.
Expression of the secretory components MadCAM-1 and VCAM-1 in tissue sections from nasal and intestinal nasal mucosae. (a) Nasal (i–v) and SI mucosae (vi–x). Secretory component (i, vi), blood vessels (ii, arrows and vii), PNAd (iii and viii) MadCAM-1 (iv and ix) and VCAM-1 (v and x). Peroxidase and alkalinephosphatase activities were revealed by DAB (brown, i–v) and by AEC (red, vi–x). Cryosections were counterstained with Mayer's haematoxylin. PNAd is expressed in some large vessels in N-LP but not in SI (viii); MadCAM-1 is not expressed onto blood vessels in nasal mucosa (iv), in contrast to SI mucosa (vii), inside and at the basis of the villus; VCAM-1 is expressed on blood vessels in nasal mucosa (v), whereas, in the gut, VCAM-1 is expressed by extravascular cells in the villi LP (x) Ep, epithelium; LP, lamina propria; G, glandular tissue. (b) Dual immunofluorescence of blood vessels (i and iv, red) and VCAM-1 (ii and v, green) in cryosections of nasal (i–iii) and intestinal mucosae (iv–vi). MIL11 and VCAM-1 staining colocalized in nasal mucosa (iii, orange) but not in intestinal mucosa (vi). MIL11 staining of cryostat sections was detected with biotin-conjugated rat anti-mouse IgE and streptavidin/rhodamine; VCAM-1 staining was detected with FITC-conjugated goat anti-mouse IgG1. Pictures were taken with a Sony 3CCD camera, using analysis software, and were overlaid to reveal double staining.
Thus, nasal and intestinal mucosae are comparable mucosal effector sites, with LP comprising sIgA+ and CD3+ T cells and epithelia containing more CD8+ than CD4+ IE T lymphocytes (Fig. 1 and Table 1).
Reciprocal patterns of expression for VCAM-1 and MadCAM-1 on blood vessels in the nasal and small intestinal mucosae
Vascular addressins and corresponding homing receptors are involved in the extravasation of lymphocytes from blood at mucosal sites. Moreover, some of these addressins may also be expressed by non-endothelial cells.
In the nasal and SI mucosae, labelled endothelial cells were present in the capillary blood vessels running either under the surface epithelium or around the glandular epithelium in the nasal-LP (N-LP; Fig. 1a, ii) and in a deeper position in the SI-LP (Fig. 1a, vii).
The peripheral addressin, PNAd, was expressed by some medium-sized blood vessels in the N-LP (Fig. 1a, iii) but not by intestinal blood vessels (Fig. 1a, viii).
The MadCAM-1, which was not detected on blood vessels in N-LP (Fig. 1a, iv), was expressed in SI-LP (Fig. 1a, ix) on the capillaries' flat endothelium beneath the epithelial basement membrane in the upper and basal portions of the villi.
The VCAM-1 was expressed on the capillaries' endothelium beneath the basement membrane of the surface epithelium and in the glandular epithelium in both organs (Fig. 1a, v), but staining was diffuse (Fig. 1a, v x). Then, we investigated the distribution of VCAM-1 in the endothelium by carrying out MIL11/VCAM-1 double-staining. MIL11 is a specific marker of endothelial cells. The colocalization of VCAM-1 and MIL11 staining was observed on blood vessels in the nasal mucosa (Fig. 1b, iii), whereas no such colocalization was observed in the SI-LP, with only an expression of VCAM-1 in the stromal cells of the intestinal villi (Fig. 1b, vi).
In conclusion, VCAM-1 and MadCAM-1 displayed diametrically opposite distributions on the nasal and intestinal LP blood capillaries.
Differential expression of integrins by CD3+ and sIgA+ cells from nasal and small intestine mucosae
Flow cytometry profiles of HR staining for the total lymphocyte populations from the N and SI tissue layers, revealed that l-selectin, the recirculating lymphocyte- specific HR, was at the same MFI in both IE and LP (data not shown). No major difference in the integrin α4β1 distribution was found between the same compartments of each mucosa (∼ 58% for LPL and ∼67% for IEL), but the integrin α4β1 MFI was higher for N-IEL than SI-IEL (1·6 versus 1·1) and higher for most N-LPL than SI-LPL (1·4 versus 1·0). Conversely the distribution of integrin β7 (∼ 85% versus 53%) and integrin α4β7 (80% versus 66%) was higher among SI-IEL than among N-IEL. Additionally, SI-LPL tended to express a higher level of integrin β7 than integrin α4β1 (1·17 versus 1·0) so the integrins α4β1, β7 and/or α4β7 discriminated N and SI lymphocytes whatever the compartment (epithelium or LP).
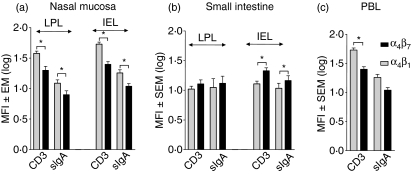
We then investigated whether these differences depended on lymphocyte subsets using two-colour immunofluorescence flow cytometry to assess the correlated expression of HR on CD3+ T and sIgA+ B cells (Figs 2–4). Irrespective of the epithelium or LP cell location and of T-cell or sIgA+ cell lineages, in nasal mucosa the MFI for integrin α4β1 was significantly higher (P < 0·05) than that of integrin α4β7 (Fig. 2). In contrast, in SI mucosa, there was a trend for higher integrin α4β7 than α4β1 MFI (Fig. 2). In blood, there was the same integrin α4β7/integrin α4β1 profile as in nasal mucosa, higher α4β1 than α4β7 MFI (Fig. 2). These data define α4β1 and α4β7 as primary integrins, whose MFI profiles correlate with CD3+ and sIgA+ cells infiltrating the nose and the SI.
Figure 2.
Mean fluorescence intensity level (MFI) of CD3+ T and sIgA+ B cells from nasal mucosa epithelium and lamina propria (a), small intestine (b) and peripheral blood lymphocytes (c). Values were obtained by determining the mean fluorescence channel numbers for cells expressing each HR relatively to the isotype control (Log IFI- Log Isotype control) and results are expressed as the mean MFI ± SEM (n = 3–4).
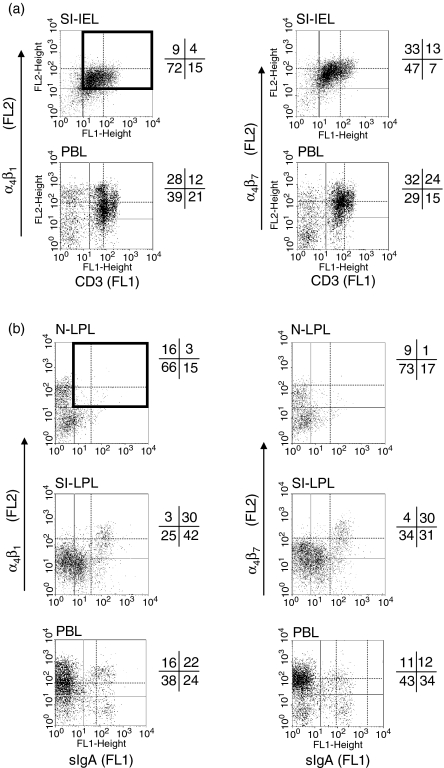
Figure 4.
Comparison with PBL of integrin α4β1 and α4β7 heterodimer expression on IE (a) and LP (b) CD3high T cells and sIgAhigh cells from the nose and gut, by two-colour flow cytometry. The number in the upper right quadrant corresponds to the percentage of very bright CD3+ T cells and sIgA+ cells expressing high level of HR whereas the number beneath it corresponds to the complement percentage to the total positive cells.
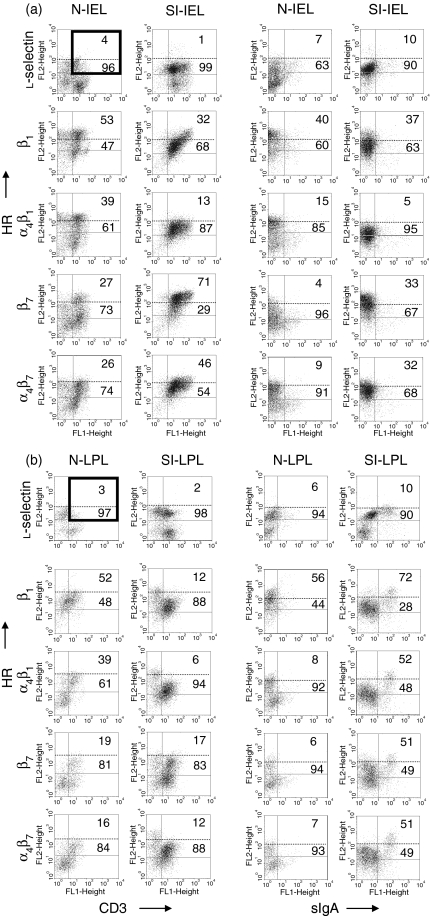
In epithelia, consistent with the predominant integrin α4β1 or α4β7 MFI, there was higher integrin α4 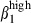 cell frequency among CD3+ T cells in nasal than in the SI epithelium (39% and 13%, respectively, Fig. 3a, left) and slightly more integrin α4
cell frequency among CD3+ T cells in nasal than in the SI epithelium (39% and 13%, respectively, Fig. 3a, left) and slightly more integrin α4 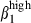 among sIgA+ cells in nasal than in SI epithelium (15% versus 5%, Fig. 3a, right): conversely, integrin α4β7 was expressed on more SI-IE than N-IE sIgA+ cells (32% versus 9%, Fig. 3a, right). In addition, SI-IE CD3+ T cells, which expressed high levels of CD3 (CD3high, MFI > 102) and of integrins β7 and α4β7, also more frequently expressed the
among sIgA+ cells in nasal than in SI epithelium (15% versus 5%, Fig. 3a, right): conversely, integrin α4β7 was expressed on more SI-IE than N-IE sIgA+ cells (32% versus 9%, Fig. 3a, right). In addition, SI-IE CD3+ T cells, which expressed high levels of CD3 (CD3high, MFI > 102) and of integrins β7 and α4β7, also more frequently expressed the 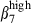 integrin chain than the integrin α4
integrin chain than the integrin α4 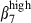 heterodimer (71% and 46%, Fig. 3a, left). This could be because of the expression of a new integrin αEβ7 heterodimer (∼ 20%). In contrast, the corresponding N-IE CD3+ T cells would be largely negative for the αE chain because there were the same proportions of integrins
heterodimer (71% and 46%, Fig. 3a, left). This could be because of the expression of a new integrin αEβ7 heterodimer (∼ 20%). In contrast, the corresponding N-IE CD3+ T cells would be largely negative for the αE chain because there were the same proportions of integrins 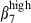 and α4
and α4 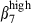 (27% and 26%, Fig. 3a, left). Comparatively, in the LP of both mucosae, consistent with the predominance of integrin α4β1 heterodimers in N-LP over SI-LP CD3+ T cells (83% and 68%, P < 0·05, Table 2), there were also more integrin α4
(27% and 26%, Fig. 3a, left). Comparatively, in the LP of both mucosae, consistent with the predominance of integrin α4β1 heterodimers in N-LP over SI-LP CD3+ T cells (83% and 68%, P < 0·05, Table 2), there were also more integrin α4 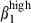 cells among N-LP than SI-LP T cells (Fig. 3b and 39% and 6%). However, these CD3+ T cells comprised similar proportions of integrin α4
cells among N-LP than SI-LP T cells (Fig. 3b and 39% and 6%). However, these CD3+ T cells comprised similar proportions of integrin α4 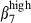 (16% and 12%, Fig. 3b). In contrast to CD3+ cells, most of the SI-LP sIgA+ cells expressed integrins α4β1 and/or α4β7, about twice more than N-LP sIgA+ cells (α4β1: 62·5% in SI-LP and 33·4% in N-LP cells; α4β7: 59·9% in SI-LP and 28·1% in N-LP cells; Table 2). This can be accounted for by the presence in the gut of activated SI-LP sIgA+ cells expressing simultaneously high levels of sIgA+ (MFI > 102), α4
(16% and 12%, Fig. 3b). In contrast to CD3+ cells, most of the SI-LP sIgA+ cells expressed integrins α4β1 and/or α4β7, about twice more than N-LP sIgA+ cells (α4β1: 62·5% in SI-LP and 33·4% in N-LP cells; α4β7: 59·9% in SI-LP and 28·1% in N-LP cells; Table 2). This can be accounted for by the presence in the gut of activated SI-LP sIgA+ cells expressing simultaneously high levels of sIgA+ (MFI > 102), α4 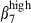 and α4
and α4 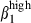 (Fig. 3b, right). In contrast, only 7% and 8% of N-LP sIgA+ cells expressed α4
(Fig. 3b, right). In contrast, only 7% and 8% of N-LP sIgA+ cells expressed α4 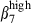 and α4
and α4 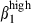 (Fig. 3b, right).
(Fig. 3b, right).
Figure 3.
Expression of l-selectin and integrins β1, α4β1, β7 and α4β7 on IEL (a) and LP (b) CD3+ T (left panel) and sIgA+ cells (right panel) from the nose and gut, by two-colour flow cytometry. (a) Isolated IEL cells from nasal and gut mucosae were analysed with the FITC- conjugated mouse anti-pig CD3 (FL1, x-axis), combined with an anti-l-selectin, anti-β1, anti-α4β1, anti-β7 or anti-α4β7. Antibody binding was detected with biotinylated goat anti-mouse or rabbit anti-rat immunoglobulin and RPE–streptavidin (FL2, y-axis). Quadrants with solid lines delimit the negative and positive populations. The upper number in the bold quadrant, containing a horizontal dashed line, corresponds to the percentage of CD3+ cells expressing the HR at high level. (b) Isolated LPL from nasal and gut mucosa cells were analysed with the FITC-conjugated goat anti-pig IgA (FL1, x-axis), combined with an anti-l-selectin, anti-β1, anti-α4β1, anti-β7 or anti-α4β7. Antibody binding was detected with biotinylated goat anti-mouse or rabbit anti-rat immunoglobulin and RPE–streptavidin (FL2, y-axis). Quadrants delimit negative and positive populations. The numbers in the bold quadrant with the horizontal dashed line correspond to the percentage of sIgA+ cells expressing the HR at high level (the horizontal dashed lines indicate the threshold used to define ‘a high level’) (for three or four animals).
Table 2.
Expression of l-selectin and integrins among CD3+ and sIgA+ cells from nasal mucosa as compared to SI and PBL
| Nasal mucosa (n = 4) | Intestinal mucosa (n = 4) | |||||
|---|---|---|---|---|---|---|
| Markers | IEL | LPL | IEL | LPL | PBL (n = 3) | |
| l-selectin | CD3 | 36·7 ± 4·11 | 68·7 ± 6·9 | 32·4 ± 2,5 | 71·6 ± 4·9 | 48·9 ± 5·9 |
| sIgA | 68·2 ± 3·5 | 82·9 ± 2·5 | 55·6 ± 5·2 | 66·1 ± 5·1 | 46·2 ± 7·7 | |
| Integrin β7 | CD3 | 64·7 ± 2,9a | 50·8 ± 3·9b | 90·6 ± 1·9c | 55·7 ± 6·3ab | 80·1 ± 3·3 |
| sIgA | 29·2 ± 4·2a | 25·7 ± 5·7a | 54·2 ± 5·8b | 57·0 ± 6·0b | 45·0 ± 4·2 | |
| Integrin α4β7 | CD3 | 78·5 ± 4,6a | 64·6 ± 6·1b | 90·2 ± 2·9a | 66·1 ± 8·7ab | 89·7 ± 0·9 |
| sIgA | 31·8 ± 3·8ab | 28·1 ± 3·6a | 49·8 ± 7·9bc | 59·9 ± 8·1c | 50·6 ± 1·2 | |
| Integrin α42 | CD3 | 81·4 ± 3,6 | 85·4 ± 2·5 | 90·0 ± 2·4 | 83·4 ± 3·4 | 90·9 ± 2·2 |
| sIgA | 40·6 ± 3·2a | 39·5 ± 4·3a | 51·6 ± 8·2ab | 74·4 ± 5·8b | 62·1 ± 0·5 | |
| Integrin β1 | CD3 | 88·0 ± 4,5 | 89·6 ± 2·3 | 85·8 ± 2·5 | 79·8 ± 2·2 | 85·8 ± 2·3 |
| sIgA | 71·5 ± 2·5* | 77·0 ± 3·3* | 57·9 ± 5·5* | 74·8 ± 4·0* | 66·6 ± 5·0 | |
| Integrin α4β1 | CD3 | 77·2 ± 4,1ab | 83·0 ± 3·8a | 78·0 ± 4·9ab | 68·1 ± 4·1b | 87·7 ± 2·7 |
| sIgA | 34·7 ± 1·8a** | 33·4 ± 5·9ac** | 46·2 ± 5·1bc** | 62·5 ± 5·4b** | 59·2 ± 0·8 | |
Results are presented as the mean percentage ± SEM of CD3+ T or sIgA+ B cells. The relative representation of selectin or integrin was calculated from FACS plots of CD3+ or sIgA+ gated using CellQuest software; the values represent the percentage of gated CD3+ or sIgA+ lymphocyte in each designated region as drawn on representative FACS plot in Fig. 3. Background staining with isotype control antibodies was substracted.
Percentage of cells expressing integrin α4 is similar to that of cells expressing integrins α4β1 or α4β7 indicating the presence of both heterodimers on the same cell.
Different lowercase letters in superscript in the same line indicate organs with statistically significant difference (P < 0·05), by t-test.
Different lowercase letters in superscript in the same line indicate organs with statistically significant difference (P < 0·05), by t-test.
In a same column, asterisk indicates that the proportions of cells expressing two different markers are statistically different (P < 0·05), paired t-test.
In a same column, asterisk indicates that the proportions of cells expressing two different markers are statistically different (P < 0·05), paired t-test.
These results indicate that the defined integrin α4 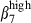 /α4β1 or α4β7/α4
/α4β1 or α4β7/α4 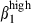 subset colocalized with MadCAM-1 and VCAM-1, in the respective mucosae. The integrin α4
subset colocalized with MadCAM-1 and VCAM-1, in the respective mucosae. The integrin α4 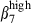 /α4
/α4 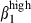 subset was mainly present in the SI.
subset was mainly present in the SI.
Among the peripheral blood lymphocytes (PBL), the levels of α4 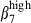 and α4
and α4 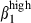 on CD3high T cells and sIgAhigh B cells allowed the definition of four subsets in relation to the corresponding subset in both mucosae (Fig. 4a,b). Both PBL and SI-IEL contained more double CD3high α4
on CD3high T cells and sIgAhigh B cells allowed the definition of four subsets in relation to the corresponding subset in both mucosae (Fig. 4a,b). Both PBL and SI-IEL contained more double CD3high α4 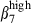 than CD3high α4
than CD3high α4 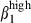 (24% versus 12% and 13% versus 4%, Fig. 4a), indicating a relative enrichment into CD3high α4
(24% versus 12% and 13% versus 4%, Fig. 4a), indicating a relative enrichment into CD3high α4 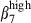 by MadCAM-1 selection. Although SI-LPL comprised the same proportions of α4
by MadCAM-1 selection. Although SI-LPL comprised the same proportions of α4 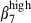 sIgAhigh and sIgAhigh α4
sIgAhigh and sIgAhigh α4 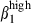 they were relatively enriched in comparison to the proportions of the same subsets in blood at a ratio of ∼2·5 (30 : 12%) versus 1·4 (30 : 22%) (Fig. 4b) indicating a selection via MadCAM-1 blood vessels. The proportion of double-positive cells was very low in nasal mucosal epithelium and LP. However, as expected from the presence of VCAM-1, the proportion of CD3high α4
they were relatively enriched in comparison to the proportions of the same subsets in blood at a ratio of ∼2·5 (30 : 12%) versus 1·4 (30 : 22%) (Fig. 4b) indicating a selection via MadCAM-1 blood vessels. The proportion of double-positive cells was very low in nasal mucosal epithelium and LP. However, as expected from the presence of VCAM-1, the proportion of CD3high α4 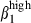 cells in the nose was higher than that of CD3high α4
cells in the nose was higher than that of CD3high α4 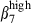 in both the epithelium and the LP (Fig. 3a,b).
in both the epithelium and the LP (Fig. 3a,b).
Chemokine mRNA profiles in nasal mucosa and small intestine
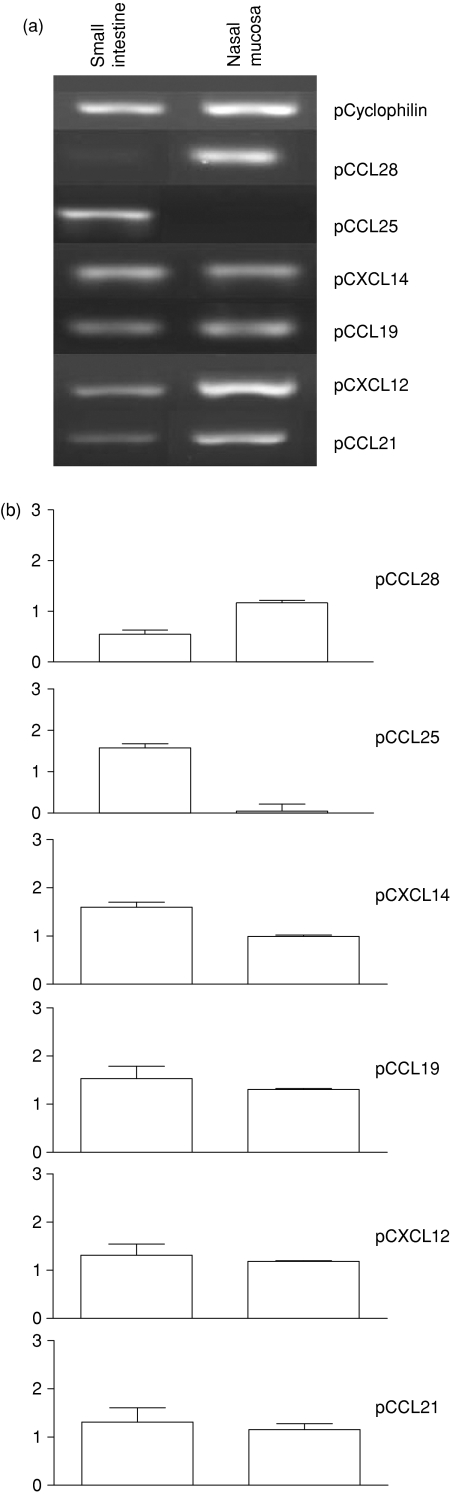
In both mucosae, we investigated the expression of CXCL14, epithelial (CCL28, CCL25) and lymphoid (CCL19, CXCL12 and CCL21) chemokines involved in T and sIgA+ lymphocyte migration.1
CCL28 mRNA levels were higher in the nasal mucosa than in the SI mucosa (P < 0·05, Fig. 5). In contrast, the CCL25 signal was strongest in the SI, and was weak, if detectable, in the nasal mucosa (P < 0·05, Fig. 5). However, we found no significant difference in mRNA levels for CCL19, CXCL12 and CCL21 between the two mucosae, whereas CXCL14 mRNA levels were higher in the SI than in nasal mucosa (Fig. 5).
Figure 5.
Chemokine mRNA expression in porcine tissues. Total RNA samples were prepared from nasal and small intestine mucosae obtained from 6- to 9-month-old SLAd/d pigs. (a) Amplicons from one pig are presented in both tissues; (b) the relative signal intensities from three independent experiments are shown as means ±SEM. Signals were normalized with cyclophilin signal. The fluorescence intensity of bands was determined with an Alpha Fluorchem Imager Gel Analysis System version 2·00 (Alpha Innotech Corporation, San Leandro, CA).
Thus, chemokine mRNA profiles showed opposite patterns of CCL28 and CCL25 expression in the nose and the gut.
Discussion
Our data for the predominance of CD3+ T cells on sIg B cells in both mucosae (regardless of IE or LP locations) are consistent with findings for the human nasal mucosa,24,25 but conflict with findings for mice, in which the proportions of nasal T and sIg B cells are similar.22 In pig and mouse nasal and SI LP, CD8+ are more abundant than CD4+ T lymphocytes,19 whereas the reverse is true in human nasal mucosa24–26 and SI-LP.26 This difference may be the result of the high prevalence of the CD8 subset in swine, even in blood.21 In the N-LP and SI-LP, sIgA+ largely predominated over sIgM+ and sIgG+ cells, which is consistent with previous studies.22,24,27,28 The expression of secretory component shows that the nasal mucosa, like the SI mucosa, is a mucosal secretory site. The presence of B cells in the gut epithelium has already been reported in mice,29 and we provide additional data here regarding sIgA+ cells.
In pigs, most nasal blood vessels express VCAM-1 and very few express PNAd, whereas in humans both PNAd30 and VCAM-131 are each expressed on about 25% of cells.32 However, MadCAM-1 was expressed in blood vessels of SI-LP in pigs as in humans33 and mice.34 Vascular addressins displayed diametrically opposite patterns of expression in nasal and SI mucosae, indicating that these mucosae correspond to two different mucosal effector sites. The patterns of high integrin expression defined highly consistent CD3+ T-cell and sIgA+ B-cell subsets in nasal and SI mucosae. According to the predominance of integrin α4 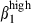 or α4
or α4 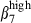 , these subsets are distributed in relation to the corresponding vascular addressin. The proportion of circulating sIgA+ cells expressing integrin α4
, these subsets are distributed in relation to the corresponding vascular addressin. The proportion of circulating sIgA+ cells expressing integrin α4 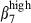 in pigs was similar to that in humans, and these cells also displayed α4
in pigs was similar to that in humans, and these cells also displayed α4 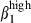 expression.35 Circulating sIgA+ cell fractions concentrated in tissues as a function of homing receptor/vascular addressin interactions.
expression.35 Circulating sIgA+ cell fractions concentrated in tissues as a function of homing receptor/vascular addressin interactions.
Our results extend our knowledge of integrin α4β1/VCAM-1 interactions governing memory T-cell migration from the bronchus-associated lymphoid tissue36 to the nasal mucosa and to the sIgA+ cell subset. Selective infiltration with integrin α4 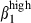 or
or 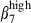 /α4
/α4 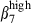 cells is accounted for by the interaction of the circulating subset with the corresponding vascular addressin, but some discrepancies in expression level were apparent between nasal and SI epithelia or between nasal and SI LP. We found a much lower proportion of integrin αEβ7 cells (20%) in the swine SI than reported in humans37 or in mice29 and no αEβ7 cells (less than in humans) in the nasal mucosa (whereas 80% of IEL and 20% of LPL in humans express αEβ7)26 possibly because of weaker up-regulation in pigs than in other species. It remains unclear whether this is because of a lack of transforming growth factor-β as an inducer of integrin αEβ738–41 in the epithelium or to kinetic differences. No difference in frequency or integrin α4
cells is accounted for by the interaction of the circulating subset with the corresponding vascular addressin, but some discrepancies in expression level were apparent between nasal and SI epithelia or between nasal and SI LP. We found a much lower proportion of integrin αEβ7 cells (20%) in the swine SI than reported in humans37 or in mice29 and no αEβ7 cells (less than in humans) in the nasal mucosa (whereas 80% of IEL and 20% of LPL in humans express αEβ7)26 possibly because of weaker up-regulation in pigs than in other species. It remains unclear whether this is because of a lack of transforming growth factor-β as an inducer of integrin αEβ738–41 in the epithelium or to kinetic differences. No difference in frequency or integrin α4 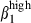 intensity was found between the nasal CD3+ T cells located in the LP or IE. The SI CD3+ T cells expressed integrin α4
intensity was found between the nasal CD3+ T cells located in the LP or IE. The SI CD3+ T cells expressed integrin α4 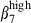 more strongly in the epithelium than in the LP. However sIgA+ cells from the SI-LP site expressed integrin α4
more strongly in the epithelium than in the LP. However sIgA+ cells from the SI-LP site expressed integrin α4 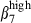 at the same level as in the epithelium, indicating that this HR was specifically down-regulated on SI LP CD3+ T cells. This may reflect the existence of fundamentally different sets of effector T cells in the SI, cells with a more advanced memory status.19 The higher proportion of sIgAhigh α4
at the same level as in the epithelium, indicating that this HR was specifically down-regulated on SI LP CD3+ T cells. This may reflect the existence of fundamentally different sets of effector T cells in the SI, cells with a more advanced memory status.19 The higher proportion of sIgAhigh α4 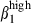 /α4
/α4 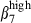 cells in the SI-LP than in the N-LP may be the result of a stronger continuous mucosal immune response to SI-exposed antigens, including the commensal flora, leading to constant antigenic stimulation and trafficking. Furthermore, although close to blood vessels devoid of VCAM-1, SI-activated LP sIgA+ cells unexpectedly express integrin α4
cells in the SI-LP than in the N-LP may be the result of a stronger continuous mucosal immune response to SI-exposed antigens, including the commensal flora, leading to constant antigenic stimulation and trafficking. Furthermore, although close to blood vessels devoid of VCAM-1, SI-activated LP sIgA+ cells unexpectedly express integrin α4 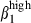 in addition to α4
in addition to α4 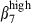 . The α4
. The α4 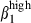 expression contributes to the retention of sIgA+ cells42 in contact with the VCAM-1 expressed by interstitial cells (Fig. 1). In contrast, most nasal sIgA+ cells lack integrin α4
expression contributes to the retention of sIgA+ cells42 in contact with the VCAM-1 expressed by interstitial cells (Fig. 1). In contrast, most nasal sIgA+ cells lack integrin α4 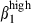 , consistently with the absence of VCAM-1 on nasal interstitial cells. The restricted expression of CCL25 in SI mucosa at induction1 and effector sites defines another divergence between the nasal and SI mucosae. Based on results obtained in mice, humans and sheep, CCL25 should recruit only SI CCR9 T cells43,44 and some sIgA-secreting cells. However, the high level of expression of CCL28 in nasal mucosa indicates that this chemokine may be involved in the nasal homing of sIgA+ via its receptor, CCR10, in conjunction with integrin α4
, consistently with the absence of VCAM-1 on nasal interstitial cells. The restricted expression of CCL25 in SI mucosa at induction1 and effector sites defines another divergence between the nasal and SI mucosae. Based on results obtained in mice, humans and sheep, CCL25 should recruit only SI CCR9 T cells43,44 and some sIgA-secreting cells. However, the high level of expression of CCL28 in nasal mucosa indicates that this chemokine may be involved in the nasal homing of sIgA+ via its receptor, CCR10, in conjunction with integrin α4 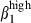 /VCAM-1 interactions, whereas CD3+ T-cell homing in the nasal mucosa may depend on integrin α4
/VCAM-1 interactions, whereas CD3+ T-cell homing in the nasal mucosa may depend on integrin α4 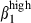 /VCAM-1 interaction and, probably, SDF-1/CXCR4 interaction.45 Furthermore, as CCL28 chemoattracts IgA+ plasmablasts derived from intestinal and extra-intestinal tissues (tonsils, salivary glands),18,45 our results indicate that sIgA+ α4β7/α4
/VCAM-1 interaction and, probably, SDF-1/CXCR4 interaction.45 Furthermore, as CCL28 chemoattracts IgA+ plasmablasts derived from intestinal and extra-intestinal tissues (tonsils, salivary glands),18,45 our results indicate that sIgA+ α4β7/α4 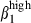 cells may use this chemokine to gain access to nasal and SI mucosae. We are currently testing our hypotheses concerning the trafficking patterns of intestinal and nasal lymphocytes by investigating the expression of chemokine receptors on sIgA+ and CD3+ T lymphocytes from nasal and SI mucosae in pigs.46
cells may use this chemokine to gain access to nasal and SI mucosae. We are currently testing our hypotheses concerning the trafficking patterns of intestinal and nasal lymphocytes by investigating the expression of chemokine receptors on sIgA+ and CD3+ T lymphocytes from nasal and SI mucosae in pigs.46
Recent data have suggested that HR and chemokine receptor up-regulation is tissue-specific,47 with mesenteric lymph node being the site of both α4 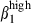 and α4
and α4 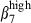 imprinting, whereas Peyer's patches contain only integrin α4
imprinting, whereas Peyer's patches contain only integrin α4 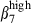 cells, and both mesenteric lymph nodes and Peyer's patches expressing CCL28.1,46,48 Similarly, pharyngeal tonsil and bronchial lymph nodes seem to be the induction sites of the nasal mucosa, as they have sIgA+α4
cells, and both mesenteric lymph nodes and Peyer's patches expressing CCL28.1,46,48 Similarly, pharyngeal tonsil and bronchial lymph nodes seem to be the induction sites of the nasal mucosa, as they have sIgA+α4 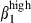 cells1 and CCL28 expression. The joint patterns of integrin α4
cells1 and CCL28 expression. The joint patterns of integrin α4 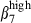 and α4
and α4 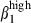 expression, together with CCL28 expression, may help to account for the oral immunization of pigs inducing an immune response in the respiratory tract and the lack of an intestinal immune response after intranasal immunization13,14 because initial selection in the intestinal tract involves MadCAM-1/α4
expression, together with CCL28 expression, may help to account for the oral immunization of pigs inducing an immune response in the respiratory tract and the lack of an intestinal immune response after intranasal immunization13,14 because initial selection in the intestinal tract involves MadCAM-1/α4 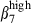 cell interactions. Conversely, nasal immunization leads to a more restricted distribution of antigen-specific IgA cells in the upper respiratory tract tissue and the mammary gland,6 in which VCAM-1 and CCR10 are detected.1
cell interactions. Conversely, nasal immunization leads to a more restricted distribution of antigen-specific IgA cells in the upper respiratory tract tissue and the mammary gland,6 in which VCAM-1 and CCR10 are detected.1
The appropriate induction of expression of these receptors for IgA-secreting plasma cell trafficking may be an important criterion for the success of vaccination protocols designed to provide protection at mucosal surfaces.
Acknowledgments
This work was supported by grants from INRA and Conseil Régional du Centre. We are very grateful to F.J. Bourne, M. Briskin, W. Fodor, K. Haverson, M.D. Pescovitz, T. Tedder, F.A. Zuckermann and Alexion Pharmaceuticals for generously donating monoclonal antibodies and UE PRC and UE IASP for animal care.
References
- 1.Bourges D, Wang CH, Chevaleyre C, Salmon H. T and IgA B lymphocytes of the pharyngeal and palatine tonsils: differential expression of adhesion molecules and chemokines. Scand J Immunol. 2004;60:338–50. doi: 10.1111/j.0300-9475.2004.01479.x. [DOI] [PubMed] [Google Scholar]
- 2.McDermott MR, Bienenstock J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979;122:1892–8. [PubMed] [Google Scholar]
- 3.Kuper CF, Koornstra PJ, Hameleers DM, Biewenga J, Spit BJ, Duijvestijn AM, van Breda Vriesman PJ, Sminia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992;13:219–24. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- 4.Wu HY, Nguyen HH, Russell MW. Nasal lymphoid tissue (NALT) as a mucosal immune inductive site. Scand J Immunol. 1997;46:506–13. doi: 10.1046/j.1365-3083.1997.d01-159.x. [DOI] [PubMed] [Google Scholar]
- 5.Wu HY, Nikolova EB, Beagley KW, Russell MW. Induction of antibody-secreting cells and T-helper and memory cells in murine nasal lymphoid tissue. Immunology. 1996;88:493–500. doi: 10.1046/j.1365-2567.1996.d01-690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao YL, Kurono Y, Ichimiya I, Mogi G. Induction of antigen-specific antibody-secreting cells in the nasal mucosa by intranasal immunization of BALB/c mice. Acta Otolaryngol. 1998;118:124–30. doi: 10.1080/00016489850155251. [DOI] [PubMed] [Google Scholar]
- 7.Childers NK, Tong G, Michalek SM. Nasal immunization of humans with dehydrated liposomes containing Streptococcus mutans antigen. Oral Microbiol Immunol. 1997;12:329–35. doi: 10.1111/j.1399-302x.1997.tb00734.x. [DOI] [PubMed] [Google Scholar]
- 8.Kantele A, Hakkinen M, Moldoveanu Z, Lu A, Savilahti E, Alvarez RD, Michalek S, Mestecky J. Differences in immune responses induced by oral and rectal immunizations with Salmonella typhi Ty21a: evidence for compartmentalization within the common mucosal immune system in humans. Infect Immun. 1998;66:5630–5. doi: 10.1128/iai.66.12.5630-5635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallichan WS, Woolstencroft RN, Guarasci T, McCluskie MJ, Davis HL, Rosenthal KL. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J Immunol. 2001;166:3451–7. doi: 10.4049/jimmunol.166.5.3451. [DOI] [PubMed] [Google Scholar]
- 10.Hibrand-Saint Oyant L, Bourges D, Chevaleyre C, Raze D, Locht C, Salmon H. Role of Bordetella bronchiseptica adenylate cyclase in nasal colonization and in development of local and systemic immune responses in piglets. Vet Res. 2005;36:63–77. doi: 10.1051/vetres:2004056. [DOI] [PubMed] [Google Scholar]
- 11.Johansson EL, Wassen L, Holmgren J, Jertborn M, Rudin A. Nasal and vaginal vaccinations have differential effects on antibody responses in vaginal and cervical secretions in humans. Infect Immun. 2001;69:7481–6. doi: 10.1128/IAI.69.12.7481-7486.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mielcarek N, Nordstrom I, Menozzi FD, Locht C, Holmgren J. Genital antibody responses in mice after intranasal infection with an attenuated candidate vector strain of Bordetella pertussis. Infect Immun. 2000;68:485–91. doi: 10.1128/iai.68.2.485-491.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.VanCott JL, Brim TA, Lunney JK, Saif LJ. Contribution of antibody-secreting cells induced in mucosal lymphoid tissues of pigs inoculated with respiratory or enteric strains of coronavirus to immunity against enteric coronavirus challenge. J Immunol. 1994;152:3980–90. [PubMed] [Google Scholar]
- 14.VanCott JL, Brim TA, Simkins RA, Saif LJ. Isotype-specific antibody-secreting cells to transmissible gastroenteritis virus and porcine respiratory coronavirus in gut- and bronchus-associated lymphoid tissues of suckling pigs. J Immunol. 1993;150:3990–4000. [PubMed] [Google Scholar]
- 15.Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16:1–4. doi: 10.1016/s1074-7613(01)00261-8. [DOI] [PubMed] [Google Scholar]
- 16.Hieshima K, Ohtani H, Shibano M, et al. CCL28 has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J Immunol. 2003;170:1452–61. doi: 10.4049/jimmunol.170.3.1452. [DOI] [PubMed] [Google Scholar]
- 17.Fagarasan S, Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat Rev Immunol. 2003;3:63–72. doi: 10.1038/nri982. [DOI] [PubMed] [Google Scholar]
- 18.Kunkel EJ, Kim CH, Lazarus NH, Vierra MA, Soler D, Bowman EP, Butcher EC. CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. J Clin Invest. 2003;111:1001–10. doi: 10.1172/JCI17244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haverson K, Bailey M, Stokes CR. T-cell populations in the pig intestinal lamina propria: memory cells with unusual phenotypic characteristics. Immunology. 1999;96:66–73. doi: 10.1046/j.1365-2567.1999.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salmon H. Surface markers of porcine lymphocytes and distribution in various lymphoid organs. Int Arch Allergy Appl Immunol. 1979;60:262–74. doi: 10.1159/000232351. [DOI] [PubMed] [Google Scholar]
- 21.Salmon H. The intestinal and mammary immune system in pigs. Vet Immunol Immunopathol. 1987;17:367–88. doi: 10.1016/0165-2427(87)90155-3. [DOI] [PubMed] [Google Scholar]
- 22.Asanuma H, Inaba Y, Aizawa C, Kurata T, Tamura S. Characterization of mouse nasal lymphocytes isolated by enzymatic extraction with collagenase. J Immunol Meth. 1995;187:41–51. doi: 10.1016/0022-1759(95)00165-7. [DOI] [PubMed] [Google Scholar]
- 23.Asanuma H, Thompson AH, Iwasaki T, Sato Y, Inaba Y, Aizawa C, Kurata T, Tamura S. Isolation and characterization of mouse nasal-associated lymphoid tissue. J Immunol Meth. 1997;202:123–31. doi: 10.1016/s0022-1759(96)00243-8. [DOI] [PubMed] [Google Scholar]
- 24.Igarashi Y, Kaliner MA, Hausfeld JN, Irani AA, Schwartz LB, White MV. Quantification of resident inflammatory cells in the human nasal mucosa. J Allergy Clin Immunol. 1993;91:1082–93. doi: 10.1016/0091-6749(93)90223-3. [DOI] [PubMed] [Google Scholar]
- 25.Pawankar R, Okuda M. A comparative study of the characteristics of intraepithelial and lamina propria lymphocytes of the human nasal mucosa. Allergy. 1993;48:99–105. doi: 10.1111/j.1398-9995.1993.tb00693.x. [DOI] [PubMed] [Google Scholar]
- 26.Jahnsen FL, Farstad IN, Aanesen JP, Brandtzaeg P. Phenotypic distribution of T cells in human nasal mucosa differs from that in the gut. Am J Respir Cell Mol Biol. 1998;18:392–401. doi: 10.1165/ajrcmb.18.3.2995. [DOI] [PubMed] [Google Scholar]
- 27.Bradley PA, Bourne FJ, Brown PJ. The respiratory tract immune system in the pig. I. Distribution of immunoglobulin-containing cells in the respiratory tract mucosa. Vet Pathol. 1976;13:81–9. doi: 10.1177/030098587601300201. [DOI] [PubMed] [Google Scholar]
- 28.Mair TS, Stokes CR, Bourne FJ. Immunohistochemical study of the local humoral immune system of the equine respiratory mucosa. Res Vet Sci. 1988;45:160–5. [PubMed] [Google Scholar]
- 29.Resendiz-Albor AA, Esquivel R, Lopez-Revilla R, Verdin L, Moreno-Fierros L. Striking phenotypic and functional differences in lamina propria lymphocytes from the large and small intestine of mice. Life Sci. 2005;76:2783–803. doi: 10.1016/j.lfs.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 30.Jahnsen FL, Lund-Johansen F, Dunne JF, Farkas L, Haye R, Brandtzaeg P. Experimentally induced recruitment of plasmacytoid (CD123high) dendritic cells in human nasal allergy. J Immunol. 2000;165:4062–8. doi: 10.4049/jimmunol.165.7.4062. [DOI] [PubMed] [Google Scholar]
- 31.Lee BJ, Naclerio RM, Bochner BS, Taylor RM, Lim MC, Baroody FM. Nasal challenge with allergen upregulates the local expression of vascular endothelial adhesion molecules. J Allergy Clin Immunol. 1994;94:1006–16. doi: 10.1016/0091-6749(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 32.Brandtzaeg P, Johansen FE, Baekkevold ES, Carlsen HS, Farstad IN. The traffic of mucosal lymphocytes to extraintestinal sites. J Pediatr Gastroenterol Nutr. 2004;39:725–6. doi: 10.1097/00005176-200406003-00004. [DOI] [PubMed] [Google Scholar]
- 33.Briskin M, Winsor-Hines D, Shyjan A, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- 34.Picker LJ, Butcher EC. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–91. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- 35.Rott LS, Briskin MJ, Butcher EC. Expression of alpha4beta7 and E-selectin ligand by circulating memory B cells: implications for targeted trafficking to mucosal and systemic sites. J Leukoc Biol. 2000;68:807–14. [PubMed] [Google Scholar]
- 36.Xu B, Wagner N, Pham LN, Magno V, Shan Z, Butcher EC, Michie SA. Lymphocyte homing to bronchus-associated lymphoid tissue (BALT) is mediated by 1-selectin/PNAd, alpha4beta1 integrin/VCAM-1, and LFA-1 adhesion pathways. J Exp Med. 2003;197:1255–67. doi: 10.1084/jem.20010685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farstad IN, Halstensen TS, Lien B, Kilshaw PJ, Lazarovits AI, Brandtzaeg P. Distribution of beta 7 integrins in human intestinal mucosa and organized gut-associated lymphoid tissue. Immunology. 1996;89:227–37. doi: 10.1046/j.1365-2567.1996.d01-727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benson M, Carlsson B, Carlsson LM, Mostad P, Svensson PA, Cardell LO. DNA microarray analysis of transforming growth factor-beta and related transcripts in nasal biopsies from patients with allergic rhinitis. Cytokine. 2002;18:20–5. doi: 10.1006/cyto.2002.1012. [DOI] [PubMed] [Google Scholar]
- 39.Coste A, Lefaucheur JP, Wang QP, Lesprit E, Poron F, Peynegre R, Escudier E. Expression of the transforming growth factor beta isoforms in inflammatory cells of nasal polyps. Arch Otolaryngol Head Neck Surg. 1998;124:1361–6. doi: 10.1001/archotol.124.12.1361. [DOI] [PubMed] [Google Scholar]
- 40.Lee CH, Rhee CS, Min YG. Cytokine gene expression in nasal polyps. Ann Otol Rhinol Laryngol. 1998;107:665–70. doi: 10.1177/000348949810700807. [DOI] [PubMed] [Google Scholar]
- 41.Watelet JB, Gevaert P, Bachert C, Holtappels G, van Cauwenberge P. Secretion of TGF-betal, TGF-beta2, EGF and PDGF into nasal fluid after sinus surgery. Eur Arch Otorhinolaryngol. 2002;259:234–8. doi: 10.1007/s00405-002-0448-z. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe C, Miura S, Hokari R, et al. Spatial heterogeneity of TNF-alpha-induced T cell migration to colonic mucosa is mediated by MAdCAM-1 and VCAM-1. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1379–87. doi: 10.1152/ajpgi.00026.2002. [DOI] [PubMed] [Google Scholar]
- 43.Kunkel EJ, Campbell JJ, Haraldsen G, et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761–8. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meurens F, Whale J, Brownlie R, Dybvig T, Thompson DR, Gerdts V. Expression of mucosal chemokines TECK/CCL25 and MEC/CCL28 during fetal development of the ovine mucosal immune system. Immunology. 2007;120:544–55. doi: 10.1111/j.1365-2567.2006.02532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lazarus NH, Kunkel EJ, Johnston B, Wilson E, Youngman KR, Butcher EC. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J Immunol. 2003;170:3799–805. doi: 10.4049/jimmunol.170.7.3799. [DOI] [PubMed] [Google Scholar]
- 46.Meurens F, Berri M, Whale J, et al. Expression of TECK/CCL25 and MEC/CCL28 chemokines and their respective receptors CCR9 and CCR10 in porcine mucosal tissues. Vet Immunol Immunopathol. 2006;113:313–27. doi: 10.1016/j.vetimm.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 47.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 48.Berri M, Meurens F, Lefevre F, Chevaleyre C, Zanello G, Gerdts V, Salmon H. Molecular cloning and functional characterization of porcine CCL28. Possible involvement in homing of IgA antibody secreting cells into the mammary gland. Mol Immunol. doi: 10.1016/j.molimm.2007.04.026. [Epub Ahead of Print] [DOI] [PubMed] [Google Scholar]