Abstract
Interleukin-31 (IL-31) is a novel T-helper-lymphocyte-derived cytokine that plays an important role in allergic skin inflammation and atopic dermatitis. It has recently been implicated in bronchial inflammation. We investigated the functions and mechanisms of IL-31-induced activation of human bronchial epithelial cells. The gene and protein expressions of candidate cytokines/chemokines from IL-31-stimulated human bronchial epithelial BEAS-2B cells were first quantified by quantitative real-time polymerase chain reaction and enzyme-linked immunosorbent assay, respectively. The activity of different mitogen-activated protein kinases (MAPKs) in IL-31-stimulated BEAS-2B cells was assessed by Western blot. The IL-31 could significantly elevate the gene and protein expressions of epidermal growth factor (EGF), vascular endothelial growth factor (VEGF) and monocyte chemoattractant protein-1 (MCP-1/CCL2) of BEAS-2B cells in both time-dependently and dose-dependently. Combination of IL-31 with either IL-4 or IL-13 further enhanced VEGF and CCL2 production while IL-31 could synergistically augment the release of EGF, VEGF, CCL2, IL-6 and IL-8 in cocultures of BEAS-2B cells and eosinophils. In addition, IL-31 could activate p38 MAPK, extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) of BEAS-2B cells. Selective inhibitors of p38 MAPK (SB203580), ERK (PD98059), and JNK (SP600125) could differentially inhibit the production of EGF, VEGF and CCL2, thereby suggesting a role for MAPKs in IL-31 functions. In conclusion, the activation of MAPKs can be crucial for IL-31-mediated activation of bronchial epithelial cells, thereby providing an immunological role for IL-31 in bronchial inflammation, at least partly, via epithelial EGF, VEGF and CCL2 production.
Keywords: allergy, chemokines/monokines, cytokines/interleukins, epithelial cells, signalling/signal transduction
Introduction
Allergic asthma is a chronic allergic inflammatory disease of the airway characterized by bronchial infiltration of eosinophils, elevated plasma concentration of allergen-specific immunoglobulin E and T helper type 2 (Th2) cytokines, reversible bronchial obstruction, mucus hypersecretion and bronchial hyperreactivity (BHR).1 Activated bronchial epithelial cells are potent sources of a wide variety of proinflammatory cytokines, growth factors and chemokines, such as tumour necrosis factor-α, vascular endothelial growth factor (VEGF) and monocyte chemoattractant protein 1 (MCP-1/CCL2).1–6 Secretion of inflammatory mediators, together with the recruitment and interaction of different immune effector cells such as eosinophils with the bronchial epithelium, will eventually contribute to bronchial inflammation, tissue damage and remodelling of pulmonary structure in asthma.1,6–9
Interkeukin-31 (IL-31) is a novel Th2 effector cytokine that has recently been cloned and shown to induce skin inflammation, pruritus and severe dermatitis in over-expressing transgenic mice.10 Further studies have demonstrated that IL-31 is associated with atopic-dermatitis-induced skin inflammation and pruritus in humans.11,12 The activity of human IL-31 is mediated through a heterodimeric receptor composed of IL-31 receptor A (IL-31RA) and oncostatin M receptor (OSMR),13 which has recently been demonstrated to activate the signal transducer and activator of transcription factor 3 (STAT-3), extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and Akt signalling pathways in human alveolar epithelial cells transfected with IL-31RA.14 The IL-31RA has homology to gp130-like type 1 cytokine receptors and it is specific for IL-31 receptor signalling pathways.10 Human epidermal keratinocytes and bronchial epithelial cells expressing both IL-31RA and OSMR are potential targets of IL-31.10,14 The expression of IL-31RA and OSMR on human epidermal keratinocytes and the number of IL-31-expressing, skin-infiltrating Th2 and CD45RO+ (memory) cutaneous lymphocyte antigen (CLA)+ T cells in atopic dermatitis patients was higher than in normal subjects.11 Interleukin-31 heterodimeric receptors are also present in many tissues including testis, brain, bone marrow, thymus, skin and intestine.10,12,15–17 Target cells expressing IL-31RA for IL-31 in these tissues include activated monocytes, macrophages, myeloid progenitor cells, keratinocytes, eosinophils and epithelial cells.10–12,17,18
Recent evidence has suggested that IL-31 might be involved in promoting allergic inflammation and an airway epithelial response that may characterize allergic asthma.14 Although the intracellular signalling pathways of IL-31RA in human alveolar epithelial cells have recently been characterized, the function of IL-31 in bronchial inflammation or allergic asthma is still not clear.14 In an attempt to further understand the role of IL-31 in modulating bronchial inflammation, we investigated the effects of IL-31 on the production of cytokines and chemokines, as well as the activity of mitogen-activated protein kinases (MAPKs) in human bronchial epithelial cells.
Materials and methods
Reagents
Recombinant human IL-31, IL-4 and IL-13 were obtained from PeproTec Inc. (PeproTec House, London, UK). Goat polyclonal antibody to IL-31RA was purchased from R & D Systems Inc. (Minneapolis, MN). Mouse monoclonal antibodies to phospho-p38 MAPK, phospho-ERK1,2, and phospho-JNK1,2 were purchased from BD Biosciences Pharmingen (San Jose, CA), while rabbit polyclonal antibodies to total p38 MAPK, ERK1,2 and JNK1,2 were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). The p38 MAPK inhibitor SB203580 (SB), ERK inhibitor PD98059 (PD), JNK inhibitor SP600125 (SP) and protease inhibitor phenylmethylsulphonyl fluoride (PMSF) were purchased from Calbiochem Corp. (San Diego, CA). The SB was dissolved in distilled water, while PD and SP were dissolved in dimethyl sulphoxide (DMSO). In all studies, the concentration of DMSO was equal to or lower than 0·1% (v/v).
Purification of human blood eosinophils from buffy coat and eosinophil culture
Fresh human buffy coats obtained from the Hong Kong Red Cross Blood Transfusion Service were diluted 1 : 2 with phosphate-buffered saline (PBS) and centrifuged using an isotonic Percoll solution (density 1·082 g/ml; GE Healthcare Bio-Sciences Corp, Piscataway, NJ) at 4° for 30 min at 1000 g. The eosinophil-rich granulocyte fraction was collected and washed twice with PBS containing 2% fetal calf serum (Gibco Invitrogen Corp, Grand Island, NY). The cells were then incubated with anti-CD16 magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) at 4° for 45 min and CD16-positive neutrophils were depleted by passage through an LS+ column (Miltenyi Biotec) within a magnetic field. With this preparation, the drop-through fraction contained eosinophils with a purity of at least 98%, as assessed by the Hemacolor rapid blood smear stain (E. Merck Diagnostica, Darmstadt, Germany). The isolated eosinophils were cultured in RPMI-1640 medium (Invitrogen) supplemented with 10% defined fetal bovine serum (FBS; Invitrogen).
Human bronchial epithelial cell culture
BEAS-2B cells are an adenovirus 12–simian virus 40 hybrid (Ad12SV40) transformed human bronchial epithelial cell line, which was isolated from normal human bronchial epithelium obtained at autopsy of non-cancerous individuals. It was purchased from the American Type Culture Collection (ATCC; Manassas, VA). The cell line was maintained in Dulbecco's modified Eagle's minimal essential medium/F12 medium (Invitrogen Corp., Carlsbad, CA) supplemented with 10% FBS in 5% CO2/95% humidified air at 37°. Before each experiment, 6× 105 and 1·5 × 105 BEAS-2B cells were seeded and cultured in 6- and 24-well plates, respectively, overnight, to a confluent monolayer. For coculturing with eosinophils, the medium was replaced with RPMI-1640 medium containing 10% FBS with eosinophils.19
Quantitative analysis of epidermal growth factor, VEGF and CCL2
Epidermal growth factor (EGF), VEGF and CCL2 concentrations in culture supernatants of BEAS-2B cells were quantified by enzyme-linked immunosorbent assay (ELISA) with a detection limit of 0·7 pg/ml for EGF, and 5·0 pg/ml for VEGF and CCL2, respectively (R & D Systems).
Quantitative real time polymerase chain reaction (qRT-PCR)
Total RNA was extracted using Tri-Reagent (Molecular Research Center Inc, Cincinnati, OH). Extracted RNA was reverse transcribed into first-strand cDNA from 4 μg total RNA using random hexanucleotide primer and MultiScribe reverse transcriptase (Applied Biosystems, Foster City, CA). Quantitative RT-PCR amplification of EGF, VEGF and CCL2 was performed using SYBR Green PCR Core reagents kit (Applied Biosystems). The PCR amplification was performed in a 96-well optical tray, each well containing 25-μl reaction mixture that comprised 200 nm of each primer, 0·025 U/μl Ampli-Taq Gold Polymerase, 200 μm each of dATP, dCTP and dGTP, 400 μm dUTP and 5·5 mm MgCl2. The PCR primers were as follows: CCL2 sense 5′-AATGCCCCAGTCACCTGCTGTTAT-3′ and antisense 5′-GCAATTTCCCCAAGTCTCTGTATC-3′, yielding a 427-base-pair (bp) product20; VEGF sense 5′-GCCTCGCCTTGCTGCTCTACC-3′ and antisense 5′-CACACTCCAGGCCCTCGTCATTG-3′, yielding a 252-bp product20; EGF sense 5′-CAGGGAAGATGACCACCACT-3′ and antisense 5′-CAGTTCCCACCACTTCAGGT-3′, yielding a 187-bp; β-actin sense 5′-AGCGGGAAATCGTGCGTG-3′ and antisense 5′-CAGGGTACATGGTGGTGCC-3′, yielding a 300-bp product.20 The thermal cycling conditions were 10 min at 95°, followed by 40 cycles of 95° for 15 seconds and 56° (for CCL2 and VEGF) or 60° (for EGF) for 1 min. The emission intensity from SYBR green was detected with an iCycler detection system (Bio-Rad Laboratories, Hercules, CA). Threshold cycles were averaged from triplicate reactions and the expression was determined relative to the mean value derived from untreated BEAS-2B cells. The gene expression of samples was normalized with the internal reference gene (β-actin, ACTB).
Western blot
After the preceding treatment, BEAS-2B cells (1 × 107) were washed with PBS and lysed in 0·3 ml lysis buffer [20 mm Tris–HCl (pH 7·5), 150 mm NaCl, 1 mm sodium ethylenediaminetetraacetic acid, 1 mm ethyleneglycoltetraacetic acid, 1% Triton, 2·5 mm sodium pyrophosphate, 1 mm beta-glycerophosphate, 1 mm sodium vanadate, 1 μg/ml leupeptin] (Cell Signaling Technology Inc, Beverly, MA) with 1 mm PMSF. Cell debris was removed by centrifugation at 14 000 g for 10 min, and the supernatants were boiled in Laemmli sample buffer (Bio-Rad Laboratories) for 5 min. An equal amount of proteins was subjected to sodium dodecyl sulphate−10% polyacrylamide gel electrophoresis before blotting onto a PVDF membrane (GE Healthcare Bio-Sciences Corp., Piscataway, NJ). The membrane was blocked with 5% skimmed milk in Tris-buffered saline with 0·05% Tween-20, pH 7·6 for 1 hr at room temperature, probed with primary rabbit anti-human β-actin (Santa Cruz Biotechnology), mouse anti-human total or phospho-p38 MAPK, total or phospho-ERK1,2, total or phospho-JNK (BD Biosciences Pharmingen) or goat anti-human IL-31RA antibodies (R & D Systems) at 4° overnight. The membrane was washed and incubated with secondary goat anti-mouse, goat anti-rabbit (GE Healthcare Bio-Sciences) or donkey anti-goat antibodies (R & D Systems) coupled to horseradish peroxidase for 1 hr at room temperature. Antibody–antigen complexes were then detected using ECL Plus™ chemiluminescent detection system according to the manufacturer's instructions (GE Healthcare Bio-Sciences).
Statistical analysis
All results were expressed as the mean ± SEM of the indicated number of experiments. Differences between groups were assessed by Kruskal–Wallis test or Student's t-test as appropriate. A probability of P < 0·05 was considered significantly different. All analyses were performed using the Statistical Package for the SocialSciences (SPSS) statistical software for Windows, version 10·1.4 (SPSS Inc, Chicago, IL).
Results
Cytokine and chemokine production in BEAS-2B cells upon IL-31 stimulation
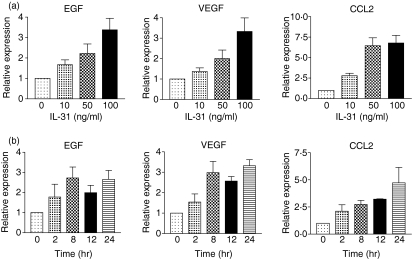
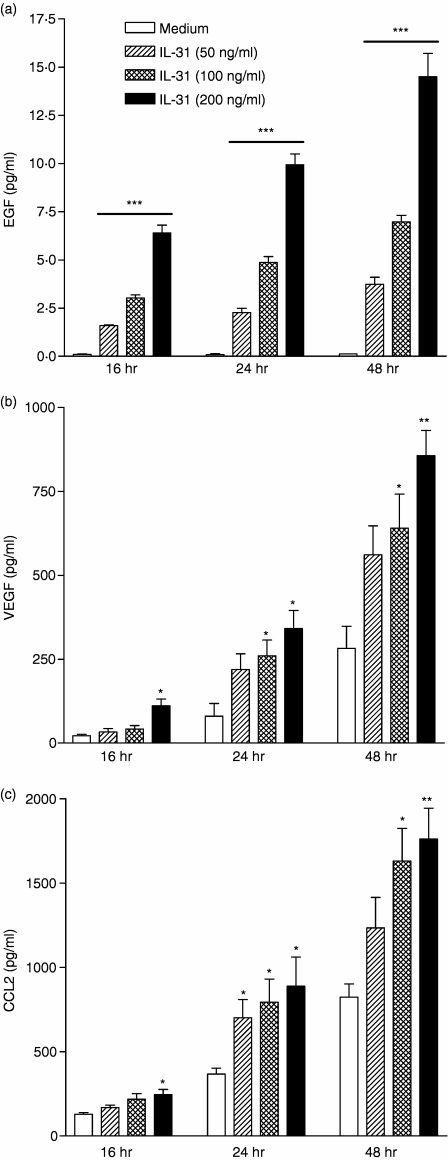
Using an antibody-based cytokine membrane array (RayBiotech Inc., Norcross, GA) to screen for the cytokine protein expression profile of IL-31-activated BEAS-2B cells, results indicated that the chemokine CCL2, and the growth factors VEGF and EGF were induced from BEAS-2B cells upon 24-hr IL-31 stimulation (data not shown). As shown in Fig. 1(a), candidate genes detected by cytokine array were validated by qRT-PCR. The gene expression EGF, VEGF and CCL2 was up-regulated dose-dependently upon IL-31 stimulation from 10 to 100 ng/ml after 8 hr. IL-31 could also significantly increase the gene expression of the above cytokines in a time-dependent manner from 2 to 24 hr (Fig. 1b). Regarding the protein expression, IL-31 could significantly induce the release of EGF, VEGF and CCL2 in both dose- and time-dependently from BEAS-2B cells (all P < 0·05) (Fig. 2).
Figure 1.
Gene expressions of EGF, VEGF and CCL2 in BEAS-2B cells after IL-31 treatment. Total RNA was extracted from BEAS-2B cells (5 × 105/well) treated with or without IL-31 at (a) 10–100 ng/ml for 8 hr and (b) 100 ng/ml for 2–24 hr. The mRNA was then reverse transcribed and analysed by qRT-PCR. Results are expressed as the arithmetic mean plus SEM from three independent experiments. Relative expression levels of the genes were expressed with β-actin housekeeping gene as internal reference.
Figure 2.
IL-31 induced release of EGF, VEGF and CCL2 from BEAS-2B cells. BEAS-2B cells (1·5 × 105/well) were cultured with or without IL-31 (10–200 ng/ml) for 16, 24 and 48 hr in a 24-well plate. (a) EGF (b) VEGF and (c) CCL2 released into the culture supernatant were determined by ELISA. Results are expressed as the arithmetic mean plus SEM from three independent experiments. Differences between groups of different doses of IL-31 and incubation time were assessed by Student's t-test and Kruskal–Wallis test, respectively. *P < 0·05, **P < 0·005 and ***P < 0·0005 when compared with the medium control.
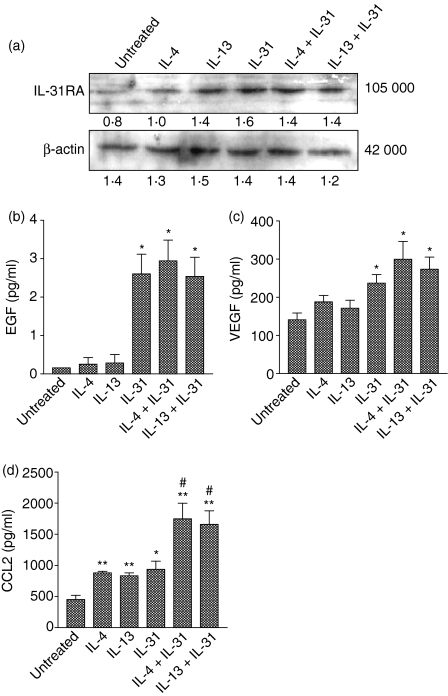
Effects of IL-4 and IL-13 on the protein expression of IL-31RA and release of EGF, VEGF and CCL2 from IL-31-treated BEAS-2B cells
As shown in Fig. 3(a), IL-31RA was expressed in resting and activated BEAS-2B cells. IL-4, IL-13, IL-31 alone and the combination of IL-31 with either IL-4 or IL-13 could augment the protein expression of IL-31RA in BEAS-2B cells compared with an untreated cell control. These results confirmed the expression of IL-31RA in BEAS-2B cells and suggested that IL-31 could mediate its effect through IL-31RA signalling pathways in BEAS-2B cells. As shown in Fig. 3(b,c), IL-4 and IL-13 alone did not have a significant effect on the release of both EGF and VEGF, but IL-31 alone could induce and augment the protein release of EGF and VEGF, respectively. The combinations of IL-31 with IL-4 or IL-13 had no significant effect on the release of EGF and VEGF compared with cells treated with IL-31 alone. However, stimulation of the BEAS-2B cells with IL-4, IL-13 and IL-31 significantly up-regulated the release of CCL2 (Fig. 3d). The combined treatment of IL-31 with either IL-4 or IL-13 was found to significantly augment CCL2 expression when compared with IL-31-treated cells (P < 0·05). In this experiment, the concentration of IL-4 and IL-13 was chosen at 20 ng/ml according to our previous study.21 This was the lowest concentration exerting the highest activity on CCL2 induction in BEAS-2B cells.21
Figure 3.
Effect of IL-4 and IL-13 on the expression of IL-31RA protein and the release of EGF, VEGF and CCL2 in BEAS-2B cells. BEAS-2B cells (1·5 × 105/well) were treated with individual and combined cytokines of IL-4 (20 ng/ml), IL-13 (20 ng/ml) and IL-31 (50 ng/ml) for 24 hr. (a) Total protein was extracted from the cells and samples with equal protein amounts were analysed using Western blot. Triplicate experiments were performed with essentially identical results and a representative blot is shown. β-actin was used as protein loading control. The release of (b) EGF, (c) VEGF and (d) CCL2 in the culture supernatant was determined by ELISA. Results are expressed as the arithmetic mean plus SEM from three independent experiments. Differences between groups were assessed by Student's t-test. *P < 0·05 and **P < 0·005 when compared with the untreated group; #P < 0·05 when compared with IL-31-treated group. Figure under each band shown in (a) represents corresponding relative density determined by densitometry.
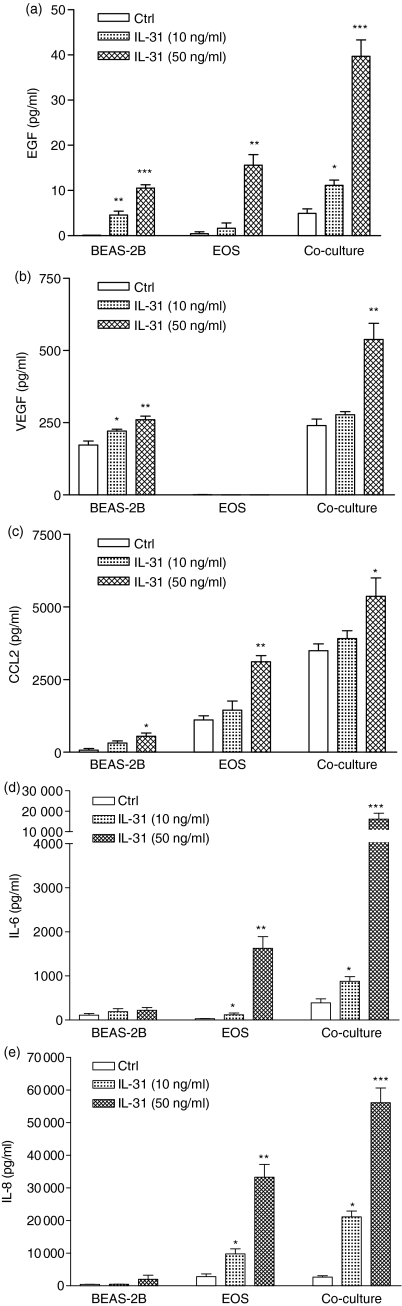
Release of EGF, VEGF, CCL2, IL-6 and IL-8 upon the interaction of BEAS-2B cells and eosinophils stimulated by IL-31
Figure 4 shows that untreated BEAS-2B cells and eosinophils produce a relatively low concentration of EGF, VEGF, CCL2, IL-6 and IL-8 after 18 hr incubation. IL-31 (50 ng/ml) could significantly activate the release of EGF, VEGF, CCL2 but not IL-6 and IL-8 from BEAS-2B cells after 18 hr. Moreover, IL-31 could significantly activate eosinophils to release EGF, CCL2, IL-6 and IL-8 in a dose-dependent manner (all P < 0·05). In a coculture of eosinophils and bronchial epithelial cells, the induction of EGF, VEGF, CCL2, IL-6 and IL-8 was synergistically up-regulated upon activation by IL-31 (50 ng/ml).
Figure 4.
Induction of the release of (a) EGF, (b) VEGF, (c) CCL2, (d) IL-6 and (e) IL-8 upon the interaction of eosinophils and BEAS-2B cells with or without IL-31. Confluent BEAS-2B cells (1·5 × 105/well) were cultured with or without eosinophils (5 × 105/well) in the presence or absent of IL-31 in different concentrations for 24 hr in a 24-well plate. Cytokine release in culture supernatant was determined by ELISA. Results are expressed as the arithmetic mean plus SEM from three independent experiments. *P < 0·05, **P < 0·005, ***P < 0·0005 when compared with the BEAS-2B and eosinophils control. Ctrl, medium control; EOS, eosinophils.
Activation of MAPKs in BEAS-2B cells upon IL-31 stimulation
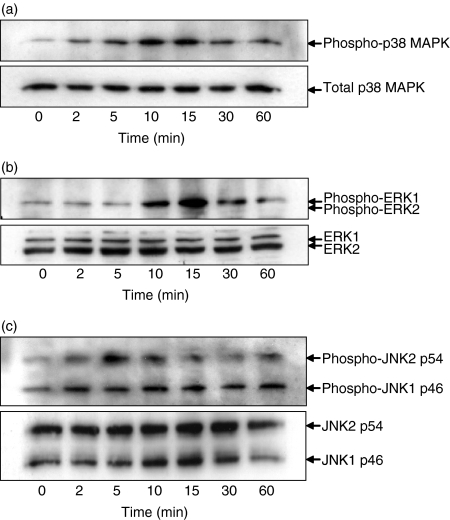
As shown in Fig. 5, IL-31 (100 ng/ml) could activate p38 MAPK, ERK1,2 and JNK1,2 signalling pathways in BEAS-2B cells. Western blot analysis showed that phosphorylation levels of p38 MAPK and JNK1,2 were elevated after 2 min, while phosphorylation levels of ERK1 were increased after 10 min. Peak phosphorylation levels of p38 MAPK and ERK1 occurred after 10–15 min, and decreased thereafter (Fig. 5a,b). Moreover, the phosphorylation level of JNK2 reached a maximal level at 5 min, whereas the phosphorylation level of JNK1 reached a maximum at 2 min that was sustained for 1 hr in BEAS-2B cells upon IL-31 stimulation (Fig. 5c).
Figure 5.
Activation of p38 MAPK, ERK1,2 and JNK1,2 in BEAS-2B cells. BEAS-2B cells (1 × 106/well) were cultured with or without IL-31 (100 ng/ml) for different incubation times as indicated. Total cellular proteins were extracted from the cells for the measurement of (a) phosphorylated and total p38 MAPK, (b) phosphorylated and total ERK1,2 and (c) phosphorylated and total JNK1,2 proteins by Western blot analysis. Experiments were performed in three independent replicates with essentially identical results, and representative results are shown. Total p38 MAPK, ERK1,2, and JNK1,2 were used as protein control to ensure equal protein loading.
Effects of kinase inhibitors on IL-31-induced protein expressions of EGF, VEGF and CCL2
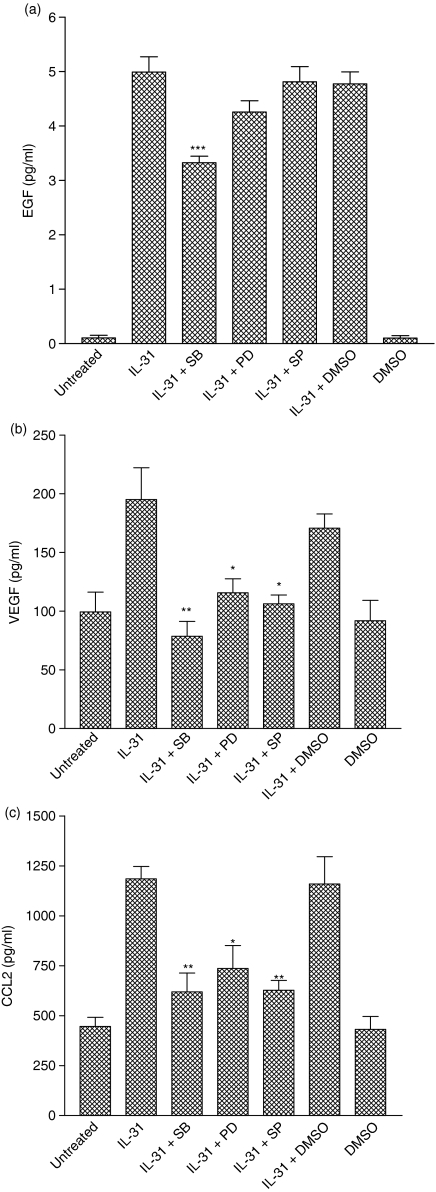
To further examine whether the activation of p38 MAPK, ERK1 and JNK1,2 signalling pathways were necessary for the IL-31 stimulation of EGF, VEGF and CCL2 release, BEAS-2B cells were stimulated with IL-31 in the presence or absence of different kinase inhibitors. In our recent study, the concentrations of the different inhibitors being used had been optimized so that the inhibitory effect of each inhibitor was maximal without significant cytotoxicity to the cells.21 As shown in Fig. 6(a–c), treatment of the cells with the solvent DMSO did not affect the cytokine release. Therefore, the effects of these inhibitors on BEAS-2B cells were neither the result of the cytotoxicity of the compounds nor of the solvent. Preincubation of cells with a p38 MAPK inhibitor, SB203580 (20 μm), could significantly diminish the IL-31-induced release of all three cytokines EGF, VEGF and CCL2. SB203580 could completely inhibit the IL-31-induced release of VEGF and CCL2, whereas it could partially suppress the release of EGF from BEAS-2B cells. On the other hand, preincubation of the cells with either a MEK inhibitor, PD98059 (20 μm), or a JNK inhibitor, SP600125 (2 μm) could significantly inhibit the IL-31-induced release of VEGF and CCL2 but not EGF (Fig. 6a–c). These results suggested that IL-31 can regulate the release of VEGF and CCL2 in BEAS-2B cells through the activation of MAPK signalling pathways.
Figure 6.
Effect of SB203580, PD98059 and SP600125 on IL-31-induced production of EGF, VEGF and CCL2. BEAS-2B cells (1·5 × 105/well) were pretreated with SB203580 (20 μm), PD98059 (20 μm) or SP600125 (2 μm) for 1 hr followed by incubation with or without IL-31 (100 ng/ml) for a further 24 hr. Release of cytokines (a) EGF, (b) VEGF and (c) CCL2 in the culture supernatant was determined by ELISA. Results are expressed as the arithmetic mean plus SEM from three independent experiments. DMSO (0·1%) was used as the solvent control. Differences between groups were assessed by Student's t-test. *P < 0·05, **P < 0·005 and ***P < 0·001 when compared with the IL-31 treated group. SB, SB203580; PD, PD98059; SP, SP600125.
Discussion
The present study suggested a functional role for IL-31, a member of the IL-6 cytokine family, in bronchial inflammation and remodelling. Human bronchial epithelial cells are one of the targets of IL-31, because of the constitutive expression of IL-31RA in various normal and immortalized human bronchial epithelial cells.14 Although the expression level of functional IL-31RA is low relative to that of OSMR, it could be detected in our experimental model of BEAS-2B cells by Western blot (Fig. 4a). Interleukin-31 was previously described as stimulating the gene expression of several chemokines, including growth-related oncogene (GRO)1/CXCL1, thymus and activation-regulated chemokine (TARC)/CCL17, monocyte-derived chemokine (MDC)/CCL22, I-309/CCL1 and macrophage inflammatory protein 1 (MIP-1/CCL3,4) in normal human epidermal keratinocytes.10 Of interest were CCL17 and CCL22, which had been reported as markers for and been associated with allergic asthma.22–24 However, IL-31 could not stimulate the production of these two chemokines in human bronchial epithelial cells as determined by cytokine array and ELISA (data not shown). Instead, IL-31 could stimulate bronchial epithelial cells to produce another CC chemokine CCL2, as well as the growth factors VEGF and EGF, which might favour airway hypersensitivity and remodelling in allergic asthma, respectively (Figs 1, 2). VEGF is a potent angiogenic factor mediating endothelial cell survival, proliferation, migration and tubule formation;25 while EGF is thought to have a stimulatory effect on the proliferation of epithelial cells and smooth muscle which is crucial in airway remodelling.26,27
CCL2 and VEGF were constitutively expressed in bronchial epithelial cells and they were up-regulated by IL-31 (Figs 1, 2b,c). On the other hand, EGF was not produced in BEAS-2B cells but its release was induced upon IL-31 stimulation (Fig. 2a). Evidence had suggested that CCL2, VEGF and EGF could function in the recruitment of inflammatory cells, and were key regulators of bronchial inflammation and remodelling in bronchial asthma.25,26 Increased expressions of these cytokines were reported in bronchial tissue and bronchoalveolar lavage fluid of asthmatic patients.25–27 It was known that CCL2 mediated the activation and recruitment of monocytes, mast cells, basophils and eosinophils as well as Th2 lymphocytes, whereas VEGF enhanced the recruitment of monocytes and neutrophils from the vascular compartment to inflammatory sites in the bronchoalveolar spaces.27,28
Gonzalo et al. has previously shown that antibodies against CCL2 profoundly reduce the number of inflammatory cells in bronchoalveolar lavage fluid and reduce BHR drastically in a mouse model of asthma.29 Furthermore, our recent findings have demonstrated that CCL2 could be up-regulated by the Th2 cytokines IL-4 and IL-13, which are the predominant cytokines in BHR.21 These findings suggested that CCL2 might mediate the development of BHR in allergic asthma. Since IL-31 could induce CCL2, VEGF and EGF from the activated bronchial epithelium, it might have functions in, at least in part, inducing bronchial inflammation and remodelling. It is known that the adhesion molecule intercellular adhesion molecule-1 (ICAM-1) plays an important role in leucocyte trafficking at inflammatory sites. One might speculate the effect of IL-31 on the expression of ICAM-1 in bronchial epithelial cells. However, we could not find any significant effect of IL-31 on ICAM-1 expression in BEAS-2B cells (data not shown). Actually, the effect of IL-31 on the release of CCL2 and VEGF from bronchial epithelial cells also appeared to be relatively weak, in comparison with tumour necrosis factor-α which stimulated CCL2 and VEGF 10 times more strongly than IL-31.2,30 We suggest that the relatively weak activities of IL-31 might operate alone or together with other mediators to fine-tune the cellular response under an inflammatory condition which might favour an allergic profile for an ongoing inflammatory process.
IL-4 and IL-13 are significantly expressed and have been shown to play critical roles in BHR and bronchial remodelling of allergic asthma.21,22,31,32 These Th2 cytokines could up-regulate the production of CCL2 in BEAS-2B cells as illustrated by our recent findings.21 Besides, a recent report had demonstrated that IL-31 expression was significantly increased and correlated with IL-4 and IL-13 in allergic contact dermatitis.33 Therefore, we speculated that IL-4 or IL-13 might have a synergistic effect on IL-31 activity, regarding the expression of CCL as well as VEGF and EGF. Although both IL-4 and IL-13 alone could moderately up-regulate the expression of IL-31RA in BEAS-2B cells (Fig. 3a), our current findings could not demonstrate any synergistic effect of IL-4 or IL-13 on IL-31-induced expression of IL-31RA, CCL2, VEGF, EGF (Fig. 3), or surface expression of ICAM-1 (data not shown). Neither IL-4 nor IL-13 caused a significant increase in EGF or VEGF secretion, while both stimulated the secretion of CCL2. Moreover, both IL-4 and IL-13 caused an additive stimulation of CCL2 secretion when added in combination with IL-31 (Fig. 3d). Although these two cytokines could activate both STAT-3 and MAPKs,21 results might suggest that they might not have interaction between their receptor signalling pathways and that of IL-31. Therefore, more investigations are needed to elucidate any potential inflammatory mediators and their receptor signalling pathways that could cross-talk with that of IL-31RA and subsequently mediate the biological functions of IL-31 synergistically.
We also observed that IL-31 could not only induce EGF, CCL2, IL-6 and IL-8 in human eosinophils but also exhibit a synergistic effect on the induction of EGF, VEGF, CCL2, IL-6 and IL-8 in cocultures of eosinophils and BEAS-2B cells (Fig. 4). In conjunction with the effect of IL-31 on BEAS-2B cells, IL-31 can play a crucial role in the allergic inflammation of the bronchi characterized by the infiltration of eosinophils.
Chattopadhyay et al. demonstrated that IL-31 might mediate its biological activities through the activation of the STAT-3 signalling pathway and MAPK signalling molecules, including ERK, JNK and Akt, in human bronchial epithelial cells.14 In addition, Dambacher et al. also demonstrated that IL-31 might mediate ERK-1,2, Akt, STAT-1 and STAT-3 activation in human intestine epithelial cells.17 In concurrence with these recent findings, we demonstrated the activation of ERK1, JNK1,2 and p38 MAPK signalling molecules in IL-31-stimulated human bronchial epithelial cells (Fig. 5). However, the downstream targets of these signalling pathways mediating the functions of IL-31 are still uncertain. As shown in Fig. 6, IL-31-induced release of CCL2 and VEGF might be mediated by the activation of p38 MAPK, ERK1 and JNK1,2 signalling molecules, which could be inhibited by three kinase inhibitors p38 MAPK inhibitor SB203580, MEK1,2 inhibitor PD98059 and JNK1,2 inhibitor SP600125, respectively. We also demonstrated the involvement of p38 MAPK, but not of ERK1 and JNK1,2 in IL-31-induced release of EGF in BEAS-2B cells. However, induction of EGF was not completely inhibited by SB203580, suggesting that one or more additional signalling pathways (e.g. Akt and STAT signalling pathways) might also be involved and remain to be determined.
In conclusion, this study demonstrated for the first time that IL-31 alone or in combination with Th2 cytokines (IL-4 and IL-13) and eosinophils modulates the function of bronchial epithelial cells through, at least in part, activation of MAPK signalling pathways and production of inflammatory mediators. These results suggested a new role for IL-31 in the development of allergic airway inflammation. It would be useful to determine whether IL-31 and IL-31RA are overexpressed in samples from human patients with allergic asthma. IL-31 may also be a new target for development of therapeutic agent for treatment in allergic asthma.
Acknowledgments
This study was supported by a Research Grant Committee Earmarked Research Grant, Hong Kong (CUHK4281/04 m), and Direct Grant for Research, The Chinese University of Hong Kong.
References
- 1.Chiappara G, Gagliardo R, Siena A, Bonsignore MR, Bousquet J, Bonsignore G, Vignola AM. Airway remodelling in the pathogenesis of asthma. Curr Opin Allergy Clin Immunol. 2001;1:85–93. doi: 10.1097/01.all.0000010990.97765.a1. [DOI] [PubMed] [Google Scholar]
- 2.Koyama S, Sato E, Tsukadaira A, Haniuda M, Numanami H, Kurai M, Nagai S, Izumi T. Vascular endothelial growth factor mRNA and protein expression in airway epithelial cell lines in vitro. Eur Respir J. 2002;20:1449–56. doi: 10.1183/09031936.02.00089802. [DOI] [PubMed] [Google Scholar]
- 3.Meyer-Hoffert U, Lezcano-Meza D, Bartels J, Montes-Vizuet AR, Schroder JM, Teran LM. Th2- and to a lesser extent Th1-type cytokines upregulate the production of both CXC (IL-8 and gro-alpha) and CC (RANTES, eotaxin, eotaxin-2, MCP-3 and MCP-4) chemokines in human airway epithelial cells. Int Arch Allergy Immunol. 2003;131:264–71. doi: 10.1159/000072138. [DOI] [PubMed] [Google Scholar]
- 4.Komiya A, Nagase H, Yamada H, et al. Concerted expression of eotaxin-1, eotaxin-2, and eotaxin-3 in human bronchial epithelial cells. Cell Immunol. 2003;225:91–100. doi: 10.1016/j.cellimm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Reibman J, Hsu Y, Chen LC, Bleck B, Gordon T. Airway epithelial cells release MIP-3alpha/CCL20 in response to cytokines and ambient particulate matter. Am J Respir Cell Mol Biol. 2003;28:648–54. doi: 10.1165/rcmb.2002-0095OC. [DOI] [PubMed] [Google Scholar]
- 6.Wong CK, Wang CB, Ip WK, Tian YP, Lam CWK. Role of p38 MAPK and NF-κB for chemokine release in coculture of human eosinophils and bronchial epithelial cells. Clin Exp Immunol. 2005;139:90–100. doi: 10.1111/j.1365-2249.2005.02678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lampinen M, Carlson M, Hakansson LD, Venge P. Cytokine-regulated accumulation of eosinophils in inflammatory disease. Allergy. 2004;59:793–805. doi: 10.1111/j.1398-9995.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- 8.Broide DH. Molecular and cellular mechanisms of allergic disease. J Allergy Clin Immunol. 2001;108:S65–71. doi: 10.1067/mai.2001.116436. [DOI] [PubMed] [Google Scholar]
- 9.Wong CK, Li ML, Wang CB, Ip WK, Tian YP, Lam CW. House dust mite allergen Der p I elevates the release of inflammatory cytokines and expression of adhesion molecules in co-culture of human eosinophils and bronchial epithelial cells. Int Immunol. 2006;18:1327–35. doi: 10.1093/intimm/dxl065. [DOI] [PubMed] [Google Scholar]
- 10.Dillon SR, Sprecher C, Hammond A, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–60. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 11.Bilsborough J, Leung DY, Maurer M, et al. IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2006;117:418–25. doi: 10.1016/j.jaci.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 12.Sonkoly E, Muller A, Lauerma AI, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117:411–7. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 13.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chattopadhyay S, Tracy E, Liang P, Robledo O, Rose-John S, Baumann H. Interleukin-31 and oncostatin-M mediate distinct signaling reactions and response patterns in lung epithelial cells. J Biol Chem. 2007;282:3014–26. doi: 10.1074/jbc.M609655200. [DOI] [PubMed] [Google Scholar]
- 15.Diveu C, Lak-Hal AH, Froger J, et al. Predominant expression of the long isoform of GP130-like (GPL) receptor is required for interleukin-31 signaling. Eur Cytokine Netw. 2004;15:291–302. [PubMed] [Google Scholar]
- 16.Dreuw A, Radtke S, Pflanz S, Lippok BE, Heinrich PC, Hermanns HM. Characterization of the signaling capacities of the novel gp130-like cytokine receptor. J Biol Chem. 2004;279:36112–020. doi: 10.1074/jbc.M401122200. [DOI] [PubMed] [Google Scholar]
- 17.Dambacher J, Beigel F, Seiderer J, et al. Interleukin-31 mediates MAP kinase and STAT1/3 activation in intestinal epithelial cells and its expression is up-regulated in inflammatory bowel disease. Gut. 2007 doi: 10.1136/gut.2006.118679. epub ahead of printing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broxmeyer HE, Li J, Hangoc G, et al. Regulation of myeloid progenitor cell proliferation/survival by IL-31 receptor and IL-31. Exp Hematol. 2007;35:78–86. doi: 10.1016/j.exphem.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang CB, Wong CK, Ip WK, Li ML, Tian YP, Lam CW. Induction of IL-6 in co-culture of bronchial epithelial cells and eosinophils is regulated by p38 MAPK and NF-kappaB. Allergy. 2005;60:1378–85. doi: 10.1111/j.1398-9995.2005.00884.x. [DOI] [PubMed] [Google Scholar]
- 20.Wong CK, Tsang CM, Ip WK, Lam CW. Molecular mechanisms for the release of chemokines from human leukemic mast cell line (HMC)-1 cells activated by SCF and TNF-alpha: roles of ERK, p38 MAPK, and NF-kappaB. Allergy. 2006;61:289–97. doi: 10.1111/j.1398-9995.2006.00972.x. [DOI] [PubMed] [Google Scholar]
- 21.Ip WK, Wong CK, Lam CW. Interleukin (IL) -4 and IL-13 up-regulate monocyte chemoattractant protein-1 expression in human bronchial epithelial cells: involvement of p38 mitogen-activated protein kinase, extracellular signal-regulated kinase 1/2 and Janus kinase-2 but not c-Jun NH2-terminal kinase 1/2 signalling pathways. Clin Exp Immunol. 2006;145:162–72. doi: 10.1111/j.1365-2249.2006.03085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroegel C, Julius P, Matthys H, Virchow JC, Jr, Luttmann W. Endobronchial secretion of interleukin-13 following local allergen challenge in atopic asthma: relationship to interleukin-4 and eosinophil counts. Eur Respir J. 1996;9:899–904. doi: 10.1183/09031936.96.09050899. [DOI] [PubMed] [Google Scholar]
- 23.Leung TF, Wong CK, Chan IH, Ip WK, Lam CW, Wong GW. Plasma concentration of thymus and activation-regulated chemokine is elevated in childhood asthma. J Allergy Clin Immunol. 2002;110:404–9. doi: 10.1067/mai.2002.126378. [DOI] [PubMed] [Google Scholar]
- 24.Leung TF, Wong CK, Lam CW, Li AM, Ip WK, Wong GW, Fok TF. Plasma TARC concentration may be a useful marker for asthmatic exacerbation in children. Eur Respir J. 2003;21:616–20. doi: 10.1183/09031936.03.00083303. [DOI] [PubMed] [Google Scholar]
- 25.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 26.Amishima M, Munakata M, Nasuhara Y, Sato A, Takahashi T, Homma Y, Kawakami Y. Expression of epidermal growth factor and epidermal growth factor receptor immunoreactivity in the asthmatic human airway. Am J Respir Crit Care Med. 1998;157:1907–12. doi: 10.1164/ajrccm.157.6.9609040. [DOI] [PubMed] [Google Scholar]
- 27.Romagnani S. Cytokines and chemoattractants in allergic inflammation. Mol Immunol. 2002;38:881–5. doi: 10.1016/s0161-5890(02)00013-5. [DOI] [PubMed] [Google Scholar]
- 28.Zittermann SI, Issekutz AC. Endothelial growth factors VEGF and bFGF differentially enhance monocyte and neutrophil recruitment to inflammation. J Leukoc Biol. 2006;80:247–57. doi: 10.1189/jlb.1205718. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalo JA, Lloyd CM, Wen D, et al. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J Exp Med. 1998;188:157–67. doi: 10.1084/jem.188.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Propst SM, Denson R, Rothstein E, Estell K, Schwiebert LM. Proinflammatory and Th2-derived cytokines modulate CD40-mediated expression of inflammatory mediators in airway epithelia: implications for the role of epithelial CD40 in airway inflammation. J Immunol. 2000;165:2214–21. doi: 10.4049/jimmunol.165.4.2214. [DOI] [PubMed] [Google Scholar]
- 31.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004;202:175–90. doi: 10.1111/j.0105-2896.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 32.Chatila TA. Interleukin-4 receptor signaling pathways in asthma pathogenesis. Trends Mol Med. 2004;10:493–9. doi: 10.1016/j.molmed.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Neis MM, Peters B, Dreuw A, et al. Enhanced expression levels of IL-31 correlate with IL-4 and IL-13 in atopic and allergic contact dermatitis. J Allergy Clin Immunol. 2006;118:930–7. doi: 10.1016/j.jaci.2006.07.015. [DOI] [PubMed] [Google Scholar]