Abstract
Adrenergic signalling of the immune system is one of the important modulator pathways of the inflammatory immune response realized via G protein-mediated pathways. The resulted signal depends on the type of the receptor-coupled G-protein (GPCR) that, according to the classical paradigm in the case of β-adrenergic receptor (β-AR), is Gs-type. Recently, alternate and/or multiple G protein coupling specificity of GPCRs have been demonstrated including a switch from Gs to Gi binding. The possibility of a Gs/Gi switch and its role in the immune response of macrophages has not been investigated yet. In this study, we demonstrate that β-adrenergic stimulation itself is able to induce a transient mitogen-activated protein kinase phosphorylation in murine peritoneal macrophages in a pertussis toxin-sensitive manner, suggesting that the Gs/Gi switch also occurs in the immune system. Although this process is very rapid, it can influence different signalling pathways and can reprogramme effector functions suggesting that sympathetic modulation of the defence mechanism of the innate immune system has an additional, Gs/Gi switch-dependent component.
Keywords: macrophages, TNF-α, G-proteins, β-adrenoceptor, MAP kinases
Introduction
β-adrenergic receptors (β-ARs) that represent a primary target for sympathetically derived catecholamines are expressed on a wide variety of tissues and cell-types, including the surface of various immune cells.1,2 Adrenergic signalling of the immune system originates from the sympathetic nervous system that innervates primary and secondary immune organs, providing them with its neurotransmitters.3,4 The inflammatory response induced by the interaction of macrophages with various pathogens is under a tonic sympathetic control that involves β-ARs expressed on macrophages.2,5–8
G protein coupled receptors (GPCRs) are seven transmembrane-spanning (7-TM) receptors that act via heterotrimeric guanine nucleotide regulatory proteins (G proteins). G proteins contain different subunits with different signalling properties (e.g. Gαs, Gαi, Gαo, Gαq, etc. and βγ).9 The GPCR β-AR was formerly considered to be associated exclusively with Gαs that increases adenylate cyclase activity. The resulted increase in intracellular cAMP level activates protein kinase A (PKA) that induces several cellular responses including the inactivation of the c-Raf-1/ERK pathway via inhibitory phosphorylation of c-Raf protein.10–12 It has recently become apparent that β-AR is also capable of transducing other signalling processes than those associated with the formerly recognized cAMP related pathways. Studies have shown that some aspects of signalling via β-AR and its subtypes are inhibited by pertussis toxin (PTX), indicating that they might be mediated by Gi/Go proteins. PKA mediated phosphorylation of the β-AR decreases its affinity for the Gαs subunit whereas increases Gαi binding switching the predominant coupling of β-AR from stimulatory guanine nucleotide regulatory protein Gs to inhibitory guanine nucleotide regulatory protein Gi.13,14 Gi signalling decreases the adenylate cyclase activity and increases the receptor-stimulated mitogen-activated-protein-kinase (MAPK) activation. Although the alternate and/or multiple G protein coupling specificity were demonstrated in several cell types (in cultured HEK293 cells,13 cardiac myocytes,15 Chinese hamster ovary cells16), the possibility of a Gs/Gi switch of certain 7-TM receptors and its possible role in the immune response of macrophages has not yet been investigated.
Macrophages play a central role in the inflammatory reaction by determining, initiating, and maintaining the specific immune response. The primary defence against pathogens is achieved by several routes, mainly by producing and releasing cytokines, enzymes, reactive free radicals, cytotoxic peptides, and by phagocytosis of the pathogen followed by antigen presentation to T lymphocytes.17,18 These effector functions can be induced by several stimuli, e.g. in response to activation of certain receptors by pathogens or other structures. Although in certain pathogen induced processes the CD14 and Toll-like receptor families are the first targets19 a number of other non-immune receptors, like β-AR, contribute to the postreceptor signalling and modulate the extension of the final immune response.2,20 The sympathetic control of the innate immune response is believed to be immunosuppressive, because the β-AR coupled Gs pathway via cAMP/PKA is known to suppress the inflammatory response.8,21,22
We recently published,23 that in peritoneal macrophages and in human myelomonocyte leukaemia cells (PLB-985) differentiated towards the macrophage lineage, the immunomodulatory effect of β-AR activation either suppressed or stimulated effector functions (tumour necrosis factor-α (TNF-α), interleukin (IL)-12 and NO production), depending on the nature of the applied inflammatory stimuli. We demonstrated, that isoproterenol pretreatment resulted in a reduced TNF-α expression in lipopolysaccharide (LPS)-treated cells, in good correlation with its known immunosuppressive effect. In contrast, the same isoproterenol pretreatment increased TNF-α expression in the case of phorbol 12-myristate 13-acetate (PMA) stimulation. In good correlation with the changes in TNF-α production, isoproterenol pretreatment significantly decreased the activation of extracellular signal-regulated kinase (ERK)1/2 and p38 MAPKs in LPS-stimulated cells, while it slightly increased the activation of these kinases in PMA-treated cells. All these effects could be completely inhibited by a pretreatment with the β-AR antagonist propranolol supporting the role of a β-receptor in these processes.23
Previous studies have proved that LPS-induced activation of inflammatory cells depends in part upon the activation of heterotrimeric Gi proteins,24,25 It is also known, that the MAPKs, which are the main effectors of Gi signalling regulate cytokine and NO production of macrophages.26,27 The possibility, that isoproterenol pretreatment induces Gs/Gi switch and a rapid change in MAPK activities may influence the inflammatory immune response may have considerable therapeutic importance. Because the possibility of a Gs/Gi switch has not yet been investigated in immunological competent cells, our aim was at first to test whether this change in the G-protein coupling specificity also occurs in peritoneal macrophages and then to investigate how this change may influence the immunomodulatory effect of isoproterenol on LPS or PMA-induced TNF-α production.
Materials and methods
Antibodies, chemicals and reagents
Primary rabbit anti-ERK1 immunoglobulin G (IgG; SC-94) and goat anti-p38 IgG (SC-728-G) antibodies and antiphospho-ERK monoclonal IgG (SC-7383) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and rabbit antiphospho-p38 IgG was purchased from Cellular Signalling (Beverly, MA). Horseradish peroxidase conjugates of anti-rabbit, anti-goat or anti-mouse IgG were obtained from Jackson ImmunoSearch (West Grove, PA) and were used as secondary antibodies. The PKC inhibitors bis-indol-maleinimide (Bim) and Gö6976 were purchased from Calbiochem (La Jolla, CA). Thioglycollate, PTX, KT-5720, and isoproterenol were purchased from Sigma (Saint Louis, MO). Dulbecco's modified Eagle's minimal essential medium (DMEM), RPMI, fetal bovine serum (FBS), penicillin, and streptomycin were obtained from Gibco BRL (Grovemont Circle, Gaithersburg, MD).
Preparation of peritoneal macrophages
BALB/c mice were purchased from Charles River Laboratories (Budapest, Hungary). Mice were kept in individual cages in the Animal Unit for at least 7 days before use. Animals received food and water ad libitum and lighting was maintained on a 12-hr cycle. All procedures were approved by the Institutional Animal Care and Use Committee of the Institute of Experimental Medicine. In each experiment 8–10 mice were pretreated intraperitoneally with 2 ml of 3% thioglycollate 4 days before the experiment. On the day of the experiment mice were anaesthetized with ether and macrophages were obtained by intraperitoneal lavage with 10 ml of DMEM supplemented with 10% FBS and 0.1% dipotassium ethylenediaminetetra-acetic acid under sterile conditions. Cells were pooled, washed with DMEM containing 10% FBS, plated into six-well plates for Western blot (WB) assays then left to adhere for 2 hr in the plate. The non-adherent cells were removed by rinsing the plates three times with 4.5% glucose-supplemented phosphate-buffered saline (PBS) solution. Macrophages were allowed to rest for 48 hr in freshly prepared complete media before treating them with the drugs.
Determination of TNF-α production
Living cells (2–3 × 105/well) were plated into 96-well plates in DMEM, without FBS. Cells were pretreated with 100 ng/ml PTX (dissolved in the tissue culture medium) overnight. Stimulation of the β-adrenergic receptor with 100 μm isoproterenol was performed for 30 min. Macrophage activation was achieved with 10 μg/ml LPS, supernatants were collected after 24 hr. The level of TNF-α of the supernatants was measured with an enzyme-linked immunosorbent assay (ELISA) kit from R & D Systems (Minneapolis, MN) in at least three parallel wells. Absorbance was read in both cases by Bio-Rad Microplate Reader Model 450 (BioRad Laboratories, Hercules, CA).
Preparation of cell extracts and analysis by Western blotting
Cells (2–6 × 106) were plated into 6-well plates in DMEM without FBS for WB analysis. Cells were settled for 2 hr then treated with various concentrations of isoproterenol for the time indicated in the Results. For determination of PTX sensitivity, cells were pretreated overnight with 100 ng/ml PTX, and then stimulated with β-adrenergic receptor agonist isoproterenol. Cells were lysed as described previously.28 Fifty μg of lysed cell protein was separated by sodium dodecyl sulphate (SDS)–polyacrylamide gel electrophoresis gel and transferred to polyvinylidene difluoride membrane (Bio-Rad) using the Mini-Protean III system (Bio-Rad). Membranes stained first with mouse monoclonal antiphospho-ERK antibodies were incubated in Tris buffered saline (TBS)/0·1% Tween-20 containing 0·5% H2O2 at room temperature for 30 min, then washed three times with TBS/0·1% Tween-20 and developed by rabbit polyclonal anti-ERK1 antibodies. In some cases membranes stained first with antiphospho-p38 rabbit polyclonal antibodies were treated with H2O2 as it is described above and then developed by the mouse monoclonal antiphospho-ERK1/2. These double-stained membranes were developed later by rabbit polyclonal anti-ERK1 or by goat polyclonal anti-p38 IgG after cleaning the membranes from the previously bound antibodies by heating them on 50 °C for 30 min in 62·5 mm Tris-HCl buffer (pH = 6·7) supplemented with 100 mmβ-mercaptoethanol and 2% SDS. The ECL system was used for chemiluminescence detection (Amersham, Amersham, UK). For quantitative analysis of Western blots, image analysis of X-ray films with the Bio-Rad Chem doc TM system was used with the Quantity One 4.4 program, following digitalisation with a Hewlett Packard 5100C scanner.
Statistical analysis
Statistical analysis of the data from three independent experiments was performed by one-way anova followed by Dunnett's test or by the Newman–Keuls test where appropriate P < 0·05 was considered as statistically significant (*P < 0·05, **P < 0·01, and *** indicates significance at level P < 0·001).
Results
β-AR mediated activation of ERK and p38 MAP kinases in murine peritoneal macrophages by isoproterenol
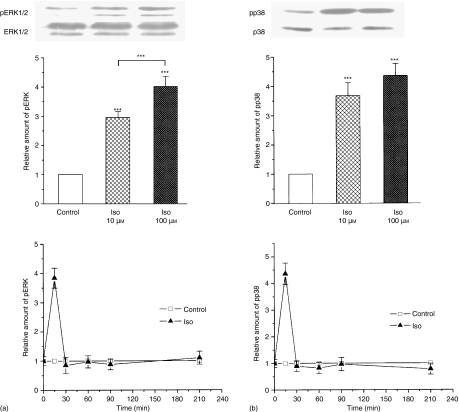
First we tested whether isoproterenol induces MAPKs activation in murine peritoneal macrophages. Isolated macrophages were incubated with various concentrations of isoproterenol for different times and phosphorylation of ERK and p38 proteins were followed in the cell lysates by Western blot analysis using phospho-specific antibodies (see Materials and methods). As demonstrated in Fig. 1 (upper and middle panels), the amount of both p-ERK and p-p38 were increased 10 min after isoproterenol administration. Quantitative evaluation (see middle parts of Fig. 1) showed that the effect of isoproterenol was significant on the activation of both MAPKs, even at the 10 μm concentration. For the quantitative analyses, optical density values obtained from Western blots were normalized to the total expression level of these kinases determined by nonphospho-specific antibodies and were further normalized to the untreated controls. The upper panels of Fig. 1(a and b) show the Western blots of one typical experiment, while middle panels summarize the results of three independent experiments. When macrophages were incubated for 5, 10, 30 60, 90 and 210 min with 100 μm isoproterenol, the isoproterenol-induced ERK (Fig. 1a lowest panel) or p38 (Fig. 1b lowest panel) phosphorylation was transient, reached a peak between 5 and 10 min, and after 30 min returned to the level of the untreated control and remained unchanged during the following 210 min.
Figure 1.
Isoproterenol-induced ERK (a) and p38 (b) phosphorylation in murine peritoneal macrophages. In each experiment, 5 × 106 peritoneal macrophages were treated and 50 µg of whole cell protein extracts were analysed by Western blot using specific antibodies recognizing phospho-ERK1/2, phospho-p38, or total ERK1/2 and p38 proteins, as described in Materials and methods. Experiments were repeated three times from separate pooled macrophage preparations and each experiment was analysed by Western blot two times. Macrophages were treated with 10 µm or 100 µm isoproterenol for 10 min (upper and middle parts) or for 5, 10, 30, 60, 90 and 210 min with 100 µm isoproterenol (lower parts). Upper parts: One typical immunoblot out of n = 6. Middle panels show the quantification of these experiments and lower panels show the quantification of ERK and p38 phosphorylation in time. In the middle and lower panels, data are given as the optical densities of the bands obtained by Western blot and determined by BioScan v1.0. Protein loading was standardized to the total ERK1/2 protein levels of the cellular extracts and values were normalized to the control, nontreated cells. Columns represent the mean values and error bars represent the ±SE where n = 6. The asterisks represent the significances as they are given in the Materials and methods section. ***Indicates significance at level P < 0·001.
PTX sensitivity of the β-AR mediated activation of ERK1/2 and p38
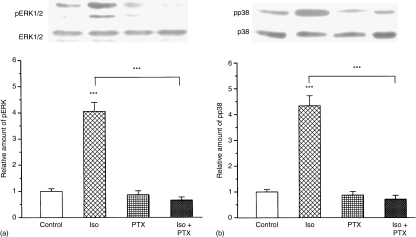
To test the involvement of the Gi pathway in the isoproterenol-induced ERK and p38 activation, we used the Gi inhibitor PTX. Preincubation of cells with 100 ng/ml PTX overnight and a following 10 min-long treatment with 100 μm isoproterenol, significantly inhibited ERK (Fig. 2a) or p38 (Fig. 2b) phosphorylation compared to samples treated with isoproterenol alone. For quantitative evaluation, data were standardized to the total ERK or p38 expression levels and were further normalized to the control levels. The upper panels of Fig. 2(a and b) show the Western blots of one typical experiment, while lower panels show quantitative analyses summarizing the results of three independent experiments evaluated similar to Fig. 1.
Figure 2.
PTX sensitivity of isoproterenol-induced ERK and p38 phosphorylation in peritoneal macrophages. Peritoneal macrophages (5 × 106) were pretreated with 100 ng/ml PTX overnight and that was followed by the administration of 100 µm isoproterenol for 10 min. Western blots were performed as described in the legend to Fig. 1. Immunoblot (upper panels) and quantification (lower panels) of ERK (a) and p38 (b) phosphorylation in macrophages are presented. Immunoblots show the results of one typical experiment from the three independent experiments, each with duplicate determination. Evaluation and standardization were carried out as described in the legend to Fig. 1. Columns represent the mean values and error bars represent the ±SE from the mean (n = 6). ***Indicates significance at level P < 0·001.
In summary, the results of the above experiments show a rapid, transient and PTX sensitive phosphorylation of MAPKs supporting the existing of a Gs/Gi switch in isoproterenol treated peritoneal macrophages.
Is the suppressive effect of isoproterenol on LPS-induced inflammatory TNF-α production and ERK phosphorylation PTX-sensitive in peritoneal macrophages?
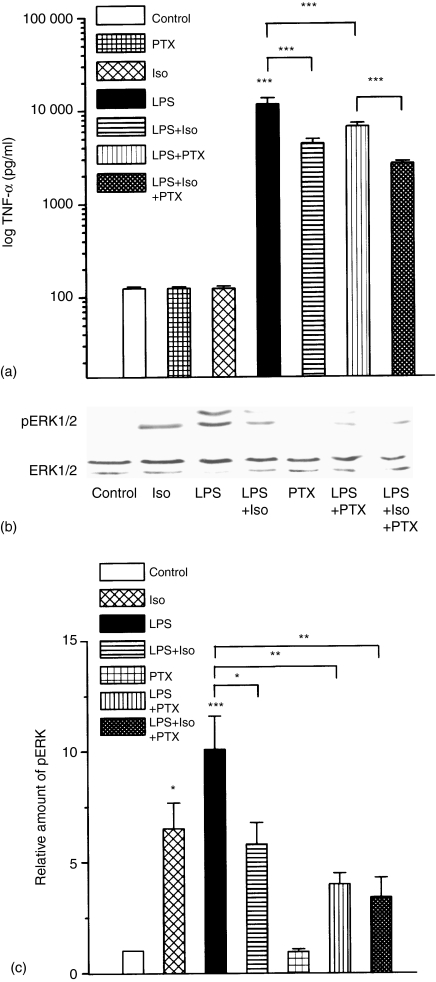
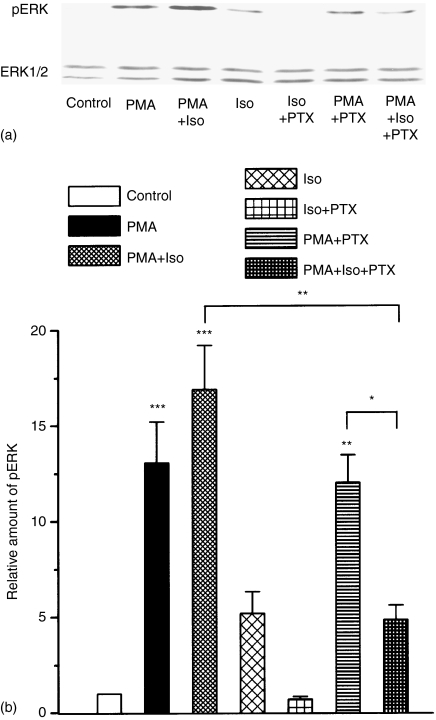
It is known that Gi-mediated signalling is involved in the effector pathways of LPS24,25 but the PTX sensitivity of LPS-induced TNF-α production is rather controversial.24,29 In the following experiments we tested the PTX sensitivity of the LPS-induced ERK1/2 activation and TNF-α production and we studied the involvement of Gi activation in the immunosuppressive effect of isoproterenol in LPS-activated peritoneal macrophages. Cells were preincubated with 100 ng/ml PTX overnight, then with 100 μm isoproterenol for 10 min and thereafter treated with 10 µg/ml LPS for 24 hr. The TNF-α levels were determined by ELISA (see Materials and methods). The results of TNF-α determinations are summarized on Fig. 3(a). As it is shown, neither PTX nor isoproterenol treatment alone changed the TNF-α production compared to the nontreated control cells. PTX pretreatment resulted in a significant but not complete decrease in LPS induced TNF-α production. In good correlation with its known immunosuppressive effect, the isoproterenol pretreatment resulted in a significant reduction in LPS induced TNF-α production and this decreased TNF-α level was maintained even in the case of PTX treatment prior to isoproterenol. Similar experiments were carried out to study the effect of PTX and/or isoproterenol pretreatment on the LPS-induced ERK1/2 phosphorylation by Western blotting. One typical immunoblot and the quantification of three independent experiments, each in duplicates are presented in Fig. 3(b) and Fig. 3(c), respectively.
Figure 3.
Effect of PTX and isoproterenol pretreatments on LPS induced TNF-α production and ERK phosphorylation in peritoneal macrophages. Peritoneal macrophages (2–3 × 105) were pretreated with or without 100 ng/ml PTX overnight and then with or without 100 µm isoproterenol for 10 min, followed by the stimulation with 10 µg/ml LPS(a) Supernatants were collected after 24 hr for determination of TNF-α levels. The quantitative results were obtained from five independent experiments each in triplicate. Error bars represent ±SE from the mean. **Indicates significance at level P < 0·01 and *** at level P < 0·001. (b and c) Peritoneal macrophages (5 × 106) were pretreated with or without 100 ng/ml PTX overnight and that was followed by the administration of 100 µm isoproterenol for 10 min, followed by the stimulation with 10 µg/ml LPS for 5 min 50 µg whole cell protein extracts were analysed by Western blot, immuno-stained with phospho-specific ERK1/2 antibodies and reprobed with antibodies raised against non-phosphorylated ERK1/2 to reveal total ERK1/2 protein levels. Immunoblot (b) and quantification (c) of three independent experiments, each with duplicate determination. Quantification and standardization of the data were carried out as described in the legend to Fig. 1. Error bars represent ±SE from the mean (n = 6). The asterisks represent the significances as they are given in the Materials and methods section.
In good correlation with the TNF-α levels presented in (a), overnight pretreatment with PTX considerably decreased the amount of LPS-activated p-ERK1/2 level in peritoneal macrophages. All these data support the involvement of the Gi pathway in LPS-induced processes. When the cells were pretreated with 100 μm isoproterenol for 10 min and thereafter treated with 10 µg/ml LPS for 5 min, the amount of phosphorylated ERK1/2 kinases were significantly suppressed. Similar to the TNF-α production, suppressed ERK1/2 phosphorylation could be observed when the cells were pretreated with PTX overnight prior to isoproterenol and LPS administration.
Thus, the PTX pretreatment did not attenuate further the isoproterenol suppressed TNF-α production and ERK phosphorylation in the LPS induced PTX-sensitive activation of peritoneal macrophages.
The stimulatory effect of isoproterenol on PMA-induced TNF-α production and ERK phosphorylation is PTX-sensitive in peritoneal macrophages
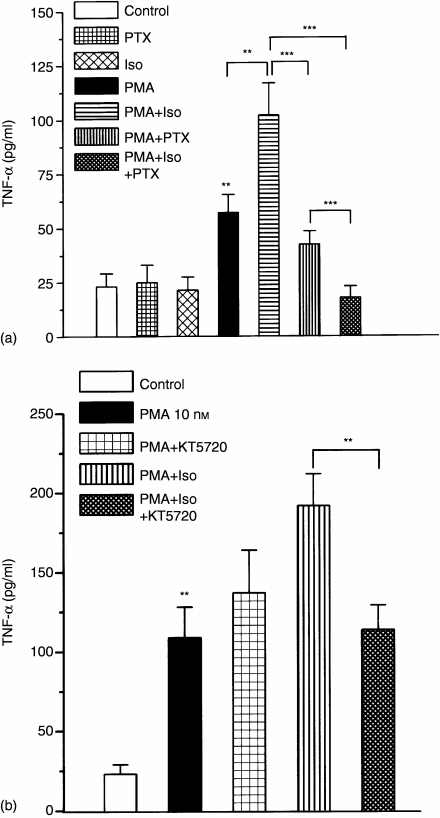
In the following experiments the cells were preincubated with (or without) 100 ng/ml PTX overnight, then with 100 μm isoproterenol for 10 min and thereafter treated with 100 nm PMA for 24 hr. The results of the TNF-α determinations are summarised on Fig. 4(a). In contrast with LPS, PMA-induced TNF-α production was significantly increased by isoproterenol (Fig. 4a) and a preceding PTX pretreatment significantly reduced that TNF-α level. The PMA-induced stimulation of TNF-α production itself was not sensitive to PTX but in combination with isoproterenol, PTX reversed the immunostimulatory effect of isoproterenol in PMA-stimulated macrophages. While isoproterenol activated cAMP/PKA is thought to be involved in Gs/Gi switch,14 we studied the effect of PKA inhibition on the isoproterenol-induced immunostimulation of PMA activated macrophages. When isoproterenol and PMA were added to cells previously treated with the PKA inhibitor KT-5720 (for 30 min), the increment of TNF-α production decreased almost to the level measured in the presence of PMA alone (Fig. 4b).
Figure 4.
Modulation of PMA-induced TNF-α production by isoproterenol. Effect of PTX or KT-5620 pretreatments. Peritoneal macrophages (2–3 × 105) were pretreated with or without 100 ng/ml PTX overnight and then with or without 100 µm isoproterenol for 10 min, followed by the stimulation of 100 nm PMA for 24 hr (a) Macrophages without PTX treatment were preincubated with 200 nm KT-5720 for 30 min prior to isoproterenol and PMA addition (b) Supernatants were collected after 24 hr for the determination of TNF-α. The quantitative results were obtained from three independent experiments, each tested in triplicate. Error bars represent ±SE from the mean (n = 9). The asterisks represent the significances as they are given in the Materials and methods section.
We also studied the effect of PTX on the isoproterenol treated, PMA-stimulated ERK activity by Western blotting. In our previous work23 we showed that in PMA stimulated peritoneal macrophages there is an early and transient activation of ERK, with a peak at 10 min after the treatment. In this work, macrophages were preincubated with 100 ng/ml PTX overnight, and then cells were treated with 100 μm isoproterenol for 10 min and thereafter 100 nm PMA was added for 5 min. The representative blot presented on Fig. 5(a) and the quantification of the three independent experiments, each in duplicates (Fig. 5b), show that the PMA-stimulated ERK phosphorylation did not decrease but slightly increased due to isoproterenol treatment, and this increase was inhibited in PTX preincubated macrophages. Data also reinforced the PTX sensitivity of isoproterenol induced ERK phosphorylation.
Figure 5.
Modulation of PMA-induced ERK phosphorylation by isoproterenol in macrophages. The effect of PTX. Peritoneal macrophages (5 × 106) were pretreated with or without 100 ng/ml PTX overnight that was followed by the administration of 100 µm isoproterenol for 10 min and stimulated with 100 nm PMA for 5 min 50 µg whole cell protein extracts were analysed by Western blot, immuno-stained with phospho-specific ERK1/2 antibodies and reprobed with antibodies raised against non-phosphorylated ERK1/2 to reveal total ERK1/2 protein levels. Immunoblot (a) and quantification (b) of three independent experiments, each with duplicate determination. Quantification and standardization of the data were carried out as described in the legend to Fig. 1. Error bars represent ±SE from the mean (n = 6). The asterisks represent the significances as they are given in the Materials and methods section.
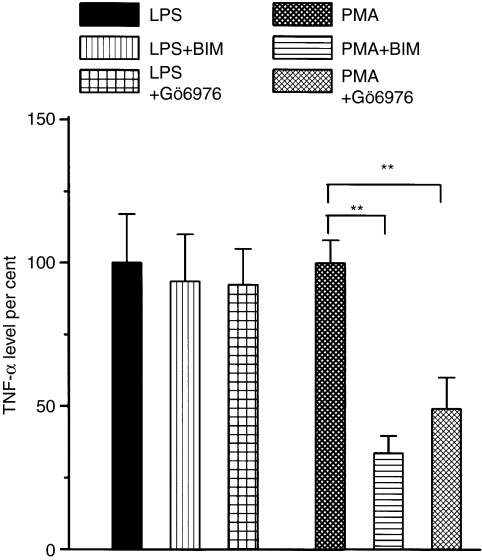
As it is also known that among the LPS-induced events activation of PKC was also described.30 Therefore we performed experiments in order to clarify the extent of the involvement of PKC activation in the LPS stimulated cells. We compare the TNF-α production measured in the presence or in the absence of two different PKC inhibitors in LPS or in PMA stimulated cells. These experiments demonstrated that both highly specific PKC inhibitors: Gö6850 (Bim) (an inhibitor for conventional and PKC zeta isoforms) and Gö6976 (an inhibitor of conventional Ca sensitive PKC isoforms), both in 50 nm concentration, inhibited the PMA-induced TNF-α production significantly but had only a minor effect (6–8% inhibition) on the LPS-induced response (Fig. 6). Our results also showed that neither of these PKC inhibitors influenced the basal TNF-α production of the untreated peritoneal macrophages.
Figure 6.
Effect of PKC inhibitors on the mitogen-stimulated TNF-α production in murine peritoneal macrophages. Cells were prepared and plated as described in the Methods section. 50 nm Bim or 50 nm Gö6976 were added to the cells 30 min before LPS (10 µg/ml) or PMA (100 nm) induction. Cells were incubated for 24 hr and TNF-α levels where determined from the supernatants by the ELISA technique. Columns represent the TNF-α level expressed as the percentage of the total stimulation observed in the presence of LPS or PMA alone. Results were obtained from three independent experiments, each tested in triplicate. Error bars represent ±SE from the mean (n = 9) and ** indicates significance at level P < 0·01 and *** at level P < 0·001.
These results suggest the involvement of a PKA dependent PTX sensitive Gi signalling in the immunostimulatory effect of isoproterenol in PMA-activated macrophages.
Discussion
β-AR and its subtypes (β1- and β2-ARs) represent the classically defined Gs coupled seven-membrane-spanning receptors. It has been suggested that both β1- and β2-AR are able to switch their G protein-coupling specificity from Gs to Gi because of phosphorylation of the receptor by PKA.13,14,31,32 Gs/Gi switch was reported for other GPCRs, such as receptors for vasoactive intestinal peptide, vasopressin, prostacyclin, parathyroid hormone, histamine, and also serotonin.33–36 This new Gs/Gi switch-related aspect of β-AR signalling is the Gi-protein mediated PTX-sensitive activation of MAPKs.13,14,31,32,37,38 Thus, a single β-AR is able to induce signals to both PKA and the MAPK pathways, which can cross-talk with each other regulating the signalling specificity of the receptor.39,40 PKA is known to have an inhibitory effect on the c-Raf-1/ERK pathway10–12 but it is also able to activate the ERK pathway in certain cell types via modification of B-Raf pathway.41–43 Accordingly, the β-AR-induced ERK activation can be either PTX insensitive or sensitive. This latter pathway sequentially involves Gi, Gβγ, MAPKs, phosphoinositide 3-kinase (PI3K) and Akt activation as it is described in cardiac myocytes.39,44,45 Relation between the PTX-sensitive or -insensitive signalling through β-AR and the MAPK pathways was described in various cell types,16,31,46 but until now, no data have been published for cells of the innate immunity. In this work we presented evidence that in the case of peritoneal murine macrophages, members of the MAPK family, i.e. ERK and p38 are simultaneously transiently activated by the β-AR agonist isoproterenol in a PTX-sensitive manner demonstrating that the Gs/Gi switch also exists in immunologically competent cells. Although simultaneous activation of ERKs and p38 is mostly connected to Gq signalling,47 such a synchronic activation of MAPKs has also been demonstrated related to a Gi protein coupled signalling pathway.48
The sympathetic modulation of the immune response via β-AR expressed on macrophages is one of the important regulatory mechanisms both in the physiological and in the pathological state. The down-regulation of LPS-induced TNF-α production by isoproterenol pretreatment was observed by different authors.7,8,49–51 This immunosuppressive effect is mainly explained by the isoproterenol induced cAMP/PKA activation via Gs protein and the consequent phosphorylation of Raf-1, which has an inhibitory effect on the MEK/ERK pathway.42,52
Recently we have demonstrated that depending on the inflammatory stimulus (e.g. PMA activation instead of the bacterial LPS), the sympathetic stimuli (isoproterenol pretreatment) might increase or decrease the production of the inflammatory mediators (TNF-α, IL-12, and NO) in correlation with the changes of MAPK-activities in peritoneal macrophages and in differentiated myelomonocyte leukaemia cells (PLB-983).23 The fact that isoproterenol pretreatment differently influenced MAPK-activities and TNF-α production in LPS or PMA treated cells raises the question whether an isoproterenol induced Gs/Gi switch may play a role in this stimulus-dependent immunomodulatory effect of isoproterenol.
PTX pretreatment of peritoneal macrophages previous to β-AR stimulation by isoproterenol inhibits all signalling events mediated by Gi proteins. The fact that PTX reduces the stimulatory effect of isoproterenol pretreatment on PMA induced TNF-α production and ERK phosphorylation demonstrated in this paper, implies that the isoproterenol induced Gs/Gi switch and MAPK activation are critical components of the increased inflammatory response. This observation is further supported by the results obtained with the PKA inhibitor KT-5720. As we demonstrated here, KT-5720 reduced the stimulatory effect of isoproterenol on PMA induced TNF-α production, similarly to PTX.
The highly potent inflammatory mediator LPS binds to Toll-like receptors (CD14-TLR4) on macrophages, which causes a Gi dependent activation of MAPKs53 (besides other signalling pathways). The MAP kinases play an important role in TNF-α expression via the c-Fos/AP-1 and NF-kappaB pathways.19,54,55 In good correlation with this, we demonstrated here, that LPS-induced ERK activation and TNF-α production are PTX sensitive per se in peritoneal macrophages, supporting the involvement of Gi in these processes. Similar results were obtained after LPS activation of different macrophage and promonocytic cell lines.24,25 As the LPS-induced TNF-α production and ERK phosphorylation already have a PTX-sensitive Gi component, the contribution of an isoproterenol induced Gs/Gi switch can not be distinguished. Still, the possibility that the isoproterenol induced Gs/Gi switch might reprogramme cellular effector functions of the cells via a rapid and transient ERK activation which may influence upstream signals desensitising Gi for a second stimulus, cannot be ruled out.
Activation of PKC signalling seems to be common in LPS- and PMA-activated peritoneal macrophages. It is known that LPS-induced ERK activation occurs, in part, through the activation of the atypical PKC isoform (PKC zeta).30 By measuring the LPS- or PMA-stimulated TNF-α production in the presence of different PKC inhibitors, we demonstrate in this paper that the LPS-induced activation of PKC is only a minor (7%) mediator of the signalling through the CD14-TLR complex. Thus, in contrast with PMA-stimulated macrophages, the isoproterenol acts on other parts of LPS-induced signal-transduction processes than the PKC.
The PTX dependent immunostimulatory effect of isoproterenol on PMA-activated peritoneal macrophages presented in this paper, may serve as a model for other stimuli that can promote inflammatory cytokine production via different activation of MAPKs as nerve growth factor56 or mycobacterial infection.57 The regulatory role of sphingosine kinase/phosphoinositide-specific phospholipase C/conventional protein kinase C pathway in the proinflammatory response of mycobacteria infected macrophages has recently been identified.57
In summary, here we demonstrate for the first time, that isoproterenol induces a Gs/Gi switch in peritoneal macrophages resulting in a PTX sensitive, rapid and transient activation of both ERK and p38 MAP kinases. Thus macrophages might be added to the growing list of cell types that exhibit changes in their function because of the switch of the G-protein subtype. We showed that this switch can contribute to the inflammatory responses of certain type of stimuli and intensifies inflammatory mediator production suggesting that sympathetic modulation of the innate immune system may have an additional, Gs/Gi switch-dependent component.
Acknowledgments
We are grateful to Ms. J. Benkõ, Ms. G. Bagó and Mr. Z. Ondrejó for their excellent technical assistance. This work was supported by OTKA grant nos. T046896 and T046965 as well as by ETT grant nos. 043/2006 and 298/2006.
Abbreviations:
- β-AR
β-adrenergic receptor
- 7-TM receptors
7-transmembrane receptors
- GPCR
receptor-coupled G-protein
- Gs
stimulatory G protein
- Gi
inhibitory G protein
- ip.
peritoneal, intraperitoneal
- cAMP
cyclic adenosine 3′,5′-monophosphate
- MAPK
mitogen-activated-protein-kinase
- ERK
extracellular signal-regulated kinase
- PTX
pertussis toxin
- LPS
bacterial lipopolysaccharide
- Iso
isoproterenol
- TNF
tumour necrosis factor
- PMA
phorbol myristyl acetate
- ELISA
enzyme-linked immunosorbent assay.
References
- 1.Besedovsky H, Sorkin E, Keller M, Muller J. Changes in blood hormone levels during the immune response. Proc Soc Exp Biol Med Soc Exp Biol Med (New York, N Y) 1975;150:466. doi: 10.3181/00379727-150-39057. [DOI] [PubMed] [Google Scholar]
- 2.Spengler RN, Allen RM, Remick DG, Strieter RM, Kunkel SL. Stimulation of alpha-adrenergic receptor augments the production of macrophage-derived tumor necrosis factor. J Immunol. 1990;145:1430. [PubMed] [Google Scholar]
- 3.Felten SY, Olschowka J. Noradrenergic sympathetic innervation of the spleen. II. Tyrosine hydroxylase (TH) -positive nerve terminals form synaptic like contacts on lymphocytes in the splenic white pulp. J Neurosci Res. 1987;18:37. doi: 10.1002/jnr.490180108. [DOI] [PubMed] [Google Scholar]
- 4.Felten DL, Felten SY, Bellinger DL, Carlson SL, Ackerman KD, Madden KS, Olschowki JA, Livnat S. Noradrenergic sympathetic neural interactions with the immune system: structure and function. Immunol Rev. 1987;100:225. doi: 10.1111/j.1600-065x.1987.tb00534.x. [DOI] [PubMed] [Google Scholar]
- 5.Vizi ES. Receptor-mediated local fine-tuning by noradrenergic innervation of neuroendocrine and immune systems. Ann N Y Acad Sci. 1998;851:388. doi: 10.1111/j.1749-6632.1998.tb09012.x. [DOI] [PubMed] [Google Scholar]
- 6.Szelenyi J, Kiss JP, Puskas E, Szelenyi M, Vizi ES. Contribution of differently localized alpha 2- and beta-adrenoceptors in the modulation of TNF-alpha and IL-10 production in endotoxemic mice. Ann N Y Acad Sci. 2000;917:145. doi: 10.1111/j.1749-6632.2000.tb05378.x. [DOI] [PubMed] [Google Scholar]
- 7.Szelenyi J, Kiss JP, Puskas E, Selmeczy Z, Szelenyi M, Vizi ES. Opposite role of alpha2- and beta-adrenoceptors in the modulation of interleukin-10 production in endotoxaemic mice. Neuroreport. 2000;11:3565. doi: 10.1097/00001756-200011090-00032. [DOI] [PubMed] [Google Scholar]
- 8.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve – an integrative interface between two super systems: the brain and the immune system. Pharmacol Rev. 2000;52:595. [PubMed] [Google Scholar]
- 9.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nature Rev. 2002;3:639. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 10.Cook SJ, McCormick F. Inhibition by cAMP of Ras-dependent activation of Raf. Science. 1993;262:1069. doi: 10.1126/science.7694367. [DOI] [PubMed] [Google Scholar]
- 11.Crespo P, Cachero TG, Xu N, Gutkind JS. Dual effect of beta-adrenergic receptors on mitogen-activated protein kinase. Evidence for a beta gamma-dependent activation and a G alpha s-cAMP-mediated inhibition. J Biol Chem. 1995;270:25259. doi: 10.1074/jbc.270.42.25259. [DOI] [PubMed] [Google Scholar]
- 12.Wu J, Dent P, Jelinek T, Wolfman A, Weber MJ, Sturgill TW. Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3′,5′-monophosphate. Science. 1993;262:1065. doi: 10.1126/science.7694366. [DOI] [PubMed] [Google Scholar]
- 13.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 14.Lefkowitz RJ, Pierce KL, Luttrell LM. Dancing with different partners: protein kinase a phosphorylation of seven membrane-spanning receptors regulates their G protein-coupling specificity. Mol Pharmacol. 2002;62:971. doi: 10.1124/mol.62.5.971. [DOI] [PubMed] [Google Scholar]
- 15.Zou Y, Komuro I, Yamazaki T, Kudoh S, Uozumi H, Kadowaki T, Yazaki Y. Both Gs and Gi proteins are critically involved in isoproterenol-induced cardiomyocyte hypertrophy. J Biol Chem. 1999;274:9760. doi: 10.1074/jbc.274.14.9760. [DOI] [PubMed] [Google Scholar]
- 16.Zamah AM, Delahunty M, Luttrell LM, Lefkowitz RJ. Protein kinase A-mediated phosphorylation of the beta 2-adrenergic receptor regulates its coupling to Gs and Gi. Demonstration in a reconstituted system. J Biol Chem. 2002;277:31249. doi: 10.1074/jbc.M202753200. [DOI] [PubMed] [Google Scholar]
- 17.Beutler B. TNF, immunity and inflammatory disease: lessons of the past decade. J Invest Med. 1995;43:227. [PubMed] [Google Scholar]
- 18.Bendtzen K. Cytokines and natural regulators of cytokines. Immunol Lett. 1994;43:111. doi: 10.1016/0165-2478(94)00153-7. [DOI] [PubMed] [Google Scholar]
- 19.Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 20.Abrass CK, O'Connor SW, Scarpace PJ, Abrass IB. Characterization of the beta-adrenergic receptor of the rat peritoneal macrophage. J Immunol. 1985;135:1338. [PubMed] [Google Scholar]
- 21.Chong YH, Shin YJ, Suh YH. Cyclic AMP inhibition of tumor necrosis factor alpha production induced by amyloidogenic C-terminal peptide of Alzheimer's amyloid precursor protein in macrophages: involvement of multiple intracellular pathways and cyclic AMP response element binding protein. Mol Pharmacol. 2003;63:690. doi: 10.1124/mol.63.3.690. [DOI] [PubMed] [Google Scholar]
- 22.van der Poll T, Jansen J, Endert E, Sauerwein HP, van Deventer SJ. Noradrenaline inhibits lipopolysaccharide-induced tumor necrosis factor and interleukin 6 production in human whole blood. Infect Immun. 1994;62:2046. doi: 10.1128/iai.62.5.2046-2050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szelenyi J, Selmeczy Z, Brozik A, Medgyesi D, Magocsi M. Dual beta-adrenergic modulation in the immune system. Stimulus-dependent effect of isoproterenol on MAPK activation and inflammatory mediator production in macrophages. Neurochem Int. 2006;49:94–103. doi: 10.1016/j.neuint.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Fan H, Williams DL, Breuel KF, Zingarelli B, Teti G, Tempel GE, Halushka PV, Cook JA. Gi proteins regulate lipopolysaccharide and Staphylococcus aureus induced cytokine production but not (1→3)-beta-D-glucan induced cytokine suppression. Front Biosci. 2006;11:2264. doi: 10.2741/1969. [DOI] [PubMed] [Google Scholar]
- 25.Fan H, Teti G, Ashton S, Guyton K, Tempel GE, Halushka PV, Cook JA. Involvement of G (i) proteins and Src tyrosine kinase in TNFalpha production induced by lipopolysaccharide, group B Streptococci and Staphylococcus aureus. Cytokine. 2003;22:126. doi: 10.1016/s1043-4666(03)00122-4. [DOI] [PubMed] [Google Scholar]
- 26.Nemeth ZH, Leibovich SJ, Deitch EA, Sperlagh B, Virag L, Vizi ES, Szabo C, Hasko G. Adenosine stimulates CREB activation in macrophages via a p38 MAPK-mediated mechanism. Biochem Biophys Res Commun. 2003;312:883. doi: 10.1016/j.bbrc.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Chio CC, Chang YH, Hsu YW, Chi KH, Lin WW. PKA-dependent activation of PKC, p38 MAPK and IKK in macrophage: implication in the induction of inducible nitric oxide synthase and interleukin-6 by dibutyryl cAMP. Cell Signal. 2004;16:565. doi: 10.1016/j.cellsig.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Kolonics A, Apati A, Janossy J, Brozik A, Gati R, Schaefer A, Magocsi M. Activation of Raf/ERK1/2 MAP kinase pathway is involved in GM-CSF-induced proliferation and survival but not in erythropoietin-induced differentiation of TF-1 cells. Cell Signal. 2001;13:743. doi: 10.1016/s0898-6568(01)00201-7. [DOI] [PubMed] [Google Scholar]
- 29.Altavilla D, Squadrito F, Canale P, et al. Endotoxin tolerance impairs a pertussis-toxin-sensitive G-protein regulating tumour necrosis factor release by macrophages from tumour-bearing rats. Pharmacol Res. 1996;33:203. doi: 10.1006/phrs.1996.0028. [DOI] [PubMed] [Google Scholar]
- 30.Monick MM, Carter AB, Flaherty DM, Peterson MW, Hunninghake GW. Protein kinase C zeta plays a central role in activation of the p42/44 mitogen-activated protein kinase by endotoxin in alveolar macrophages. J Immunol. 2000;165:4632. doi: 10.4049/jimmunol.165.8.4632. [DOI] [PubMed] [Google Scholar]
- 31.Martin NP, Whalen EJ, Zamah MA, Pierce KL, Lefkowitz RJ. PKA-mediated phosphorylation of the beta1-adrenergic receptor promotes Gs/Gi switching. Cell Signal. 2004;16:1397. doi: 10.1016/j.cellsig.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Luttrell LM, Daaka Y, Lefkowitz RJ. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol. 1999;11:177. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- 33.Kilts JD, Gerhardt MA, Richardson MD, et al. Beta (2)-adrenergic and several other G protein-coupled receptors in human atrial membranes activate both G (s) and G (i) Circ Res. 2000;87:705. doi: 10.1161/01.res.87.8.705. [DOI] [PubMed] [Google Scholar]
- 34.Ren XR, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz RJ. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci USA. 2005;102:1448. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gesty-Palmer D, Chen M, Reiter E, et al. Distinct beta-Arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 Activation. J Biol Chem. 2006;281:10856. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- 36.Clark JA, Black AR, Leontieva OV, et al. Involvement of the ERK signaling cascade in protein kinase C-mediated cell cycle arrest in intestinal epithelial cells. J Biol Chem. 2004;279:9233. doi: 10.1074/jbc.M312268200. [DOI] [PubMed] [Google Scholar]
- 37.Brodde OE. Beta-adrenoceptors in cardiac disease. Pharmacol Ther. 1993;60:405. doi: 10.1016/0163-7258(93)90030-h. [DOI] [PubMed] [Google Scholar]
- 38.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 39.Xiao RP, Ji X, Lakatta EG. Functional coupling of the beta 2-adrenoceptor to a pertussis toxin-sensitive G protein in cardiac myocytes. Mol Pharmacol. 1995;47:322. [PubMed] [Google Scholar]
- 40.Abramson SN, Martin MW, Hughes AR, Harden TK, Neve KA, Barrett DA, Molinoff PB. Interaction of beta-adrenergic receptors with the inhibitory guanine nucleotide-binding protein of adenylate cyclase in membranes prepared from cyc-S49 lymphoma cells. Biochem Pharmacol. 1988;37:4289. doi: 10.1016/0006-2952(88)90609-0. [DOI] [PubMed] [Google Scholar]
- 41.Calipel A, Mouriaux F, Glotin AL, Malecaze F, Faussat AM, Mascarelli F. Extracellular signal-regulated kinase-dependent proliferation is mediated through the protein kinase A/B-Raf pathway in human uveal melanoma cells. J Biol Chem. 2006;281:9238. doi: 10.1074/jbc.M600228200. [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi T, Nagao S, Wallace DP, Belibi FA, Cowley BD, Pelling JC, Grantham JJ. Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int. 2003;63:1983. doi: 10.1046/j.1523-1755.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 43.Dumaz N, Marais R. Integrating signals between cAMP and the RAS/RAF/MEK/ERK signalling pathways. Based on the anniversary prize of the Gesellschaft fur Biochemie und Molekularbiologie Lecture delivered on 5 July 2003 at the Special FEBS Meeting in Brussels. FEBS J. 2005;272:3491. doi: 10.1111/j.1742-4658.2005.04763.x. [DOI] [PubMed] [Google Scholar]
- 44.Xiao RP, Zhu W, Zheng M, Chakir K, Bond R, Lakatta EG, Cheng H. Subtype-specific beta-adrenoceptor signaling pathways in the heart and their potential clinical implications. Trends Pharmacol Sci. 2004;25:358. doi: 10.1016/j.tips.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Xiao RP. Beta-adrenergic signaling in the heart. dual coupling of the beta2-adrenergic receptor to G (s) and G (i) proteins. Science. 2001 doi: 10.1126/stke.2001.104.re15. STKE [electronic resource] (104) RE15. [DOI] [PubMed] [Google Scholar]
- 46.Heubach JF, Ravens U, Kaumann AJ. Epinephrine activates both Gs and Gi pathways, but norepinephrine activates only the Gs pathway through human beta2-adrenoceptors overexpressed in mouse heart. Mol Pharmacol. 2004;65:1313. doi: 10.1124/mol.65.5.1313. [DOI] [PubMed] [Google Scholar]
- 47.Feoktistov I, Goldstein AE, Biaggioni I. Role of p38 mitogen-activated protein kinase and extracellular signal-regulated protein kinase in adenosine A2B receptor-mediated interleukin-8 production in human mast cells. Mol Pharmacol. 1999;55:726. [PubMed] [Google Scholar]
- 48.Howlett AC. Handbook of Experimental Pharmacology. Heidelberg: Springer Berlin; 2005. Cannabinoid receptor signaling; p. 53. [DOI] [PubMed] [Google Scholar]
- 49.Izeboud CA, Mocking JA, Monshouwer M, van Miert AS, Witkamp RF. Participation of beta-adrenergic receptors on macrophages in modulation of LPS-induced cytokine release. J Recept Signal Transduct Res. 1999;19:191. doi: 10.3109/10799899909036645. [DOI] [PubMed] [Google Scholar]
- 50.Izeboud CA, Monshouwer M, van Miert AS, Witkamp RF. The beta-adrenoceptor agonist clenbuterol is a potent inhibitor of the LPS-induced production of TNF-alpha and IL-6 in vitro and in vivo. Inflamm Res. 1999;48:497. doi: 10.1007/s000110050493. [DOI] [PubMed] [Google Scholar]
- 51.Hasko G, Szabo C, Nemeth ZH, Salzman AL, Vizi ES. Stimulation of beta-adrenoceptors inhibits endotoxin-induced IL-12 production in normal and IL-10 deficient mice. J Neuroimmunol. 1998;88:57. doi: 10.1016/s0165-5728(98)00073-3. [DOI] [PubMed] [Google Scholar]
- 52.Weissinger EM, Oettrich K, Evans C, et al. Activation of protein kinase A (PKA) by 8-Cl-cAMP as a novel approach for antileukaemic therapy. Br J Cancer. 2004;91:186. doi: 10.1038/sj.bjc.6601909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fan H, Peck OM, Tempel GE, Halushka PV, Cook JA. Toll-like receptor 4 coupled GI protein signaling pathways regulate extracellular signal-regulated kinase phosphorylation and AP-1 activation independent of NFkappaB activation. Shock. 2004;22:57. doi: 10.1097/01.shk.0000129759.58490.d6. [DOI] [PubMed] [Google Scholar]
- 54.Feng WG, Wang YB, Zhang JS, Wang XY, Li CL, Chang ZL. cAMP elevators inhibit LPS-induced IL-12 p40 expression by interfering with phosphorylation of p38 MAPK in murine peritoneal macrophages. Cell Res. 2002;12:331. doi: 10.1038/sj.cr.7290135. [DOI] [PubMed] [Google Scholar]
- 55.Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy – from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005;1754:253. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 56.Barouch R, Kazimirsky G, Appel E, Brodie C. Nerve growth factor regulates TNF-alpha production in mouse macrophages via MAP kinase activation. J Leukoc Biol. 2001;69:1019. [PubMed] [Google Scholar]
- 57.Yadav M, Clark L, Schorey JS. Macrophage's proinflammatory response to a mycobacterial infection is dependent on sphingosine kinase-mediated activation of phosphatidylinositol phospholipase C, protein kinase C, ERK1/2, and phosphatidylinositol 3-kinase. J Immunol. 2006;176:5494. doi: 10.4049/jimmunol.176.9.5494. [DOI] [PubMed] [Google Scholar]