Abstract
It is still controversial whether malaria protection is mediated by conventional immunity associated with T and B cells or by innate immunity associated with extrathymic T cells and autoantibody-producing B cells. Given this situation, it is important to examine the mechanism of malaria protection in β2-microglobulin-deficient (β2m(–/–)) mice. These mice lack major histocompatibility complex class I and CD1d antigens, which results in the absence of CD8+ T cells and natural killer T (NKT) cells. When C57BL/6 and β2m(–/–) mice were injected with parasitized (Plasmodium yoelii 17XNL) erythrocytes, both survived from the infection and showed a similar level of parasitaemia. The major expanding T cells were NK1.1– αβΤ-cell receptorint cells in both mice. The difference was a compensatory expansion of NK and γδT cells in β2m(–/–) mice, and an elimination experiment showed that these lymphocytes were critical for protection in these mice. These results suggest that malaria protection might be events of the innate immunity associated with multiple subsets with autoreactivity. CD8+ T and NKT cells may be partially related to this protection.
Keywords: rodent, natural killer cells, parasitic-protozoan, MHC, cell activation
Introduction
There have been many reports postulating that malaria protection is the result of immunological events associated with certain lymphocyte subsets and humoral antibodies. Among lymphocyte subsets, CD8+ cytotoxic T cells are known to be related to cytotoxicity against malaria parasites or malaria-infected erythrocytes.1–6 CD4+ helper T cells are also known to be responsible for the supportive function of the generation of CD8+ T cells7–10 and B cells.2,11–14 On the other hand, antibodies against some antigens on malaria parasites are also known to be responsible for malaria protection.15–19 Earlier studies on malaria protection have considered such protection to be mainly mediated by cytotoxic T cells and conventional B cells (which produce antibodies against malaria parasite antigens).
In recent studies in humans and mice, another concept of malaria protection has been proposed: Lymphocytes other than conventional T cells, namely, extrathymic T cells (i.e. interleukin (IL)-2Rβ+ T-cell receptor (TCR)int cells), have been indicated to be the major lymphocyte subsets associated with malaria protection in mice.20–24 Such counterparts in humans might be CD56+ T cells.25 When these T cells were expanding during malarial infection, thymic atrophy was induced, resulting in the arrest of mainstream of T-cell differentiation in the thymus (for conventional T cells).22
In parallel with the expansion of extrathymic T cells, malarial infection was accompanied by autoantibody production in both humans and mice.26–30 In other words, it is suggested that autoantibody-producing B-1 cells are important lymphocytes for malaria protection. It is conceivable that extrathymic T cells with autoreactivity and autoantibody-producing B-1 cells might play crucial roles in attacking abnormal self-cells infected with intracellular pathogens such as malaria parasites.
However, it cannot be denied that lymphocytes other than extrathymic T cells and B-1 cells, namely, CD8+ cytotoxic T cells (major histocompatibility complex (MHC) class I-restricted) and natural killer T (NKT) cells (CD1d-restricted),31–34 are also responsible for the malaria protection. In this regard, we used β2-microglobulin-deficient (β2m(–/–)) mice, which lack both CD8+T cells and NKT cells, to examine this possibility.
Materials and methods
Mice and parasites
C57BL/6 (B6) and β2m(–/–) mice (B6 background) at the age of 8–14 weeks were used. β2m(–/–) mice, obtained by backcrossing the original β2m(–/–) mice eight times with B6 mice, were provided by Chiba University School of Medicine (Chiba, Japan).35 These mice were maintained at the animal facility of Niigata University (Niigata, Japan) under specific pathogen-free conditions. Plasmodium yoelii 17XNL (non-lethal strain), a generous gift of Dr S. Waki (Gunma Prefectural College of Health Science, Maebashi, Japan), was used.20 Parasites were maintained by routine in vivo passage in mice. Mice were infected by an intraperitoneal (i.p.) injection of 104 parasitized erythrocytes per mouse. Parasitaemia in the blood was observed by Giemsa staining every 2 or 3 days and the mice were killed at the indicated days after infection. Lymphocytes were obtained from the liver, spleen and thymus in control and infected mice.
Cell preparation
Hepatic mononuclear cells (MNC) were isolated by a previously described method.36 Briefly, the liver was removed, pressed through 200-gauge stainless steel mesh, and suspended in Eagle's minimal essential medium (Nissui Pharmaceutical, Tokyo, Japan) supplemented with 5 mm HEPES and 2% heat-inactivated newborn calf serum. After being washed once with medium, the cells were fractionated by centrifugation in 15 ml of 35% Percoll solution (Amersham Pharmacia Biotech, Piscataway, NJ) for 15 min at 424 g. The pellet was resuspended in erytherocyte lysing solution (155 mm NH4Cl, 10 mm KHCO3, 1 mm ethylenediaminetetraacetic acid-Na, and 17 mm Tris (pH 7·3)). Splenocytes and thymocytes were obtained by forcing the spleen and thymus through stainless steel mesh. Splenocytes were used after erythrocyte lysing.
Immunofluorescence test
The surface phenotypes of lymphocytes were identified by two- or three-colour immunofluorescence tests.37 Fluoroscein isothiocyanate (FITC)-, phycoerythrin (PE)-, or biotin-conjugated reagents of monoclonal antibodies (mAb) were used, and the latter being biotin-conjugated reagents were developed with TRI-colour conjugated streptavidin (Caltag Laboratories, Burlingame, CA). The mAb used here were anti-CD3 (145-2C11), anti-IL-2Rβ (TM-β1), anti-NK1.1 (PK136), anti-αβTCR (H57-597), anti-γδTCR (GL3), anti-B220 (RA3-6B2), anti-CD5 (53-7.3) and anti-CD69 (H1.2F3) mAbs (BD Biosciences, Mountain View, CA). Cells were analysed by FACScan (BD Biosciences). To prevent non-specific binding of mAbs, CD16/32 (2.4G2; BD PharMingen, San Jose, CA) was added before staining with labelled mAb. Dead cells were excluded by forward scatter, side scatter and propidium iodide gating.
Cell depletion experiments
Mice received an i.p. injection of 250 µg of anti-NK1.1 (PK136) mAb, or 500 µg of anti-γδTCR (UC7-13D5) mAb twice per week from 3 days before infection until recovery. Anti-NK1.1 (PK136) mAb and anti-γδTCR (UC7-13D5, a gift from Dr Matsuzaki, Center of Molecular Biosciences, University of the Ryukyus, Okinawa, Japan) mAb were prepared from ascites of mice injected with the corresponding hybridoma and precipitated with saturated ammonium sulphate.
Reverse transcription–polymerase chain reaction (RT–PCR) analysis
Total RNA was extracted from cells of B6 and β2m(–/–) mice. To detect mRNAs of TCRγ chain, RNA was reverse transcribed using the primers of these genes and such cDNA was further amplified by PCR methods. Briefly, total RNA was prepared from cells with RNeasy Mini Kit (QIAGEN GmbH, Germany). cDNA was synthesized using 5 µg RNA with illustraTM Ready-to-Go RT–PCR Beads (GE Healthcare UK Ltd, Buckinghamshire, UK) and Oligo (dT) 15 Primer (Promega, Madison, WI). PCR amplification of synthesized cDNA was then conducted. Forward primers for Vγ1, Vγ2, Vγ4, Vγ5 and Vγ6 were paired with a reverse primer for a constant region sequence that is shared by all TCRγ clusters. Their sequences are as follows: Vγ1, 5′-ACA CAG CTA TAC ATT GGT AC-3′; Vγ2, 5′-TGT CCT TGC AAC CCC TAC CC-3′; Vγ4, 5′-TGT CCT TGC AAC CCC TAC CC-3′; Vγ5, 5′-TGT GCA CTG GTA CCA ACT GA-3′; Vγ6, 5′-GGA ATT CAA AAG AAA ACA TTG TCT-3′; and Cγ (constant region) reverse, 5′-CTT ATG GAG ATT TGT TTC AGC-3′; G3PDH, forward 5′-GCG AGA CCC CAC TAA CAT CAA ATG-3′, reverse 5′-CAG TGG ATG CAG GGA TGA TGT TCT-3′. PCR products were visualized on 2% agarose gel stained with ethidium bromide under UV illumination.
Statistical analysis
Differences between the results obtained in control mice and mice infected with malaria were analysed by using the Student's t-test. A value of P < 0·05 was considered to be significant.
Results
Time-kinetics of parasitaemia and the number of lymphocytes after infection
B6 and β2m(–/–) mice were injected with 104 malaria-infected erythrocytes, and parasitaemia was examined during infection (Fig. 1a). In B6 mice, parasitaemia appeared on day 3 after infection, reached a peak on day 17–19 and then declined. The pattern of parasitaemia in β2m(–/–) mice was almost the same as that of B6 mice, although parasitaemia of β2m(–/–) mice was slightly prolonged up to day 35.
Figure 1.
Time-kinetic study after malarial infection. (a) Parasitaemia. (b) Number of lymphocytes yielded by the liver and spleen. B6 and β2m(–/–) mice were infected with 104 parasitized erythrocytes for the blood stage of malarial infection. At each point of time, four mice were used to produce the mean and one SD. *P < 0·05.
To know how β2m(–/–) mice lacking MHC class I antigens were able to survive against malarial infection, the number of lymphocytes yielded by the liver and spleen was compared between B6 and β2m(–/–) mice (Fig. 1b). It was confirmed that the number of lymphocytes increased in the liver and spleen in both mouse strains during malarial infection. However, the number of lymphocytes in the liver and spleen of β2m(–/–) mice increased more prominently on day 14 than in those of B6 mice (P < 0·05).
Identification of the lymphocyte subsets expanding in B6 and β2m(–/–) mice with malaria
We then investigated whether expanding lymphocyte subsets were different between B6 and β2m(–/–) mice during malarial infection. Lymphocytes were isolated from the liver and spleen on day 7, 14 and 21 after infection and two-colour staining for CD3 and IL-2Rβ was conducted (Fig. 2a). In this staining, NK cells were CD3– IL-2Rβ+, extrathymic T cells were CD3int IL-2Rβ+ and conventional T cells were CD3high IL-2Rβ–.
Figure 2.
Phenotypic characterization of lymphocytes in immunofluorescence tests. (a) Two-colour staining for CD3 and IL-2Rβ. (b) Two-colour staining for γδTCR and NK1.1. Lymphocytes were isolated from the liver and spleen of B6 and β2m(–/–) mice before and after malarial infection. Numbers in the figure represent the percentages of immunofluorescence-positive cells. Representative results from three experiments were depicted.
As shown previously21–25 as well as in this study, the major expanding lymphocyte subsets were CD3int IL-2Rβ+ cells in the liver (day 7–21) and in the spleen (day 21) of B6 mice. However, the pattern of expansion was slightly different in β2m(–/–) mice, namely, on day 7, NK cells expanded in the liver of these mice (indicated by an arrow). The expansion of CD3int IL-2Rβ+ cells was commonly seen in both mouse strains.
Two-colour staining for NK1.1 and αβTCR (or γδTCR) was conducted to identify whether expanding CD3int cells were αβTCR+ or γδTCR+ (Fig. 2b). It was confirmed that NK cells (NK1.1+ αβTCR–) were abundant on day 7 in the β2m(–/–) mice (indicated by an arrow). In the case of B6 mice, expanding lymphocytes were mainly αβT cells. However, in β2m(–/–) mice, some γδT cells as well as αβT cells were found to be expanding (indicated by an arrowhead). This figure also shows that there were NK1.1+αβT cells (i.e. NKT cells) in B6 mice but that such cells were few in β2m(–/–) mice.
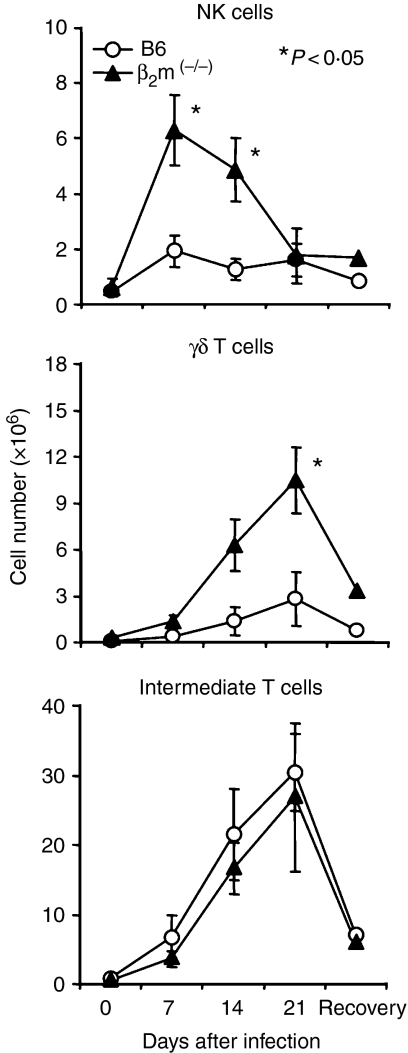
Time-kinetics in the absolute number of NK cells, γδT cells and CD3int cells (i.e. intermediate T cells) during malaria infection were enumerated in the liver by repeated experiments (n = 4) (Fig. 3). The number of NK cells in the liver of B6 mice remained unchanged even after malarial infection. On the other hand, the number of NK cells increased in β2m(–/–) mice on days 7 and 14 (P < 0·05). A similar pattern was seen for γδT cells, namely, the number of γδT cells was highly elevated in β2m(–/–) mice during infection. The number of CD3int IL-2Rβ+ cells was almost the same in B6 and β2m(–/–) mice.
Figure 3.
Variation in the number of NK cells, γδT cells and intermediate T cells in the liver during malarial infection. B6 and β2m(–/–) were used. The mean and one SD were produced from four mice. *P < 0·05.
Requirement of NK cells and γδT cells for malaria protection in β2m(–/–) mice
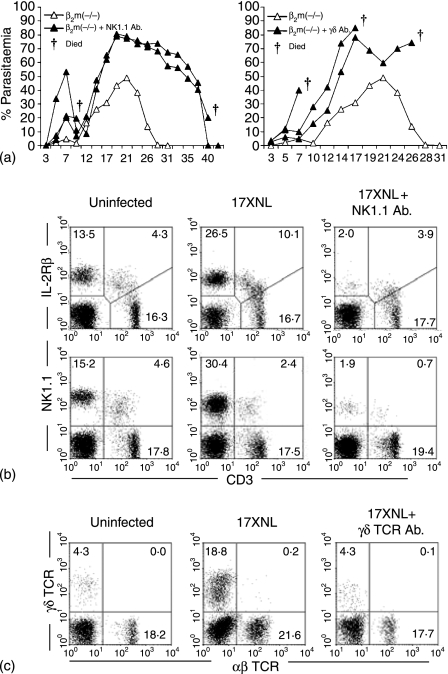
Because NK cells and γδT cells became abundant in β2m(–/–) mice during malaria infection, the necessity of these lymphocyte subsets for malaria protection was examined. Elimination of NK and γδT cells was performed using in vivo injections of anti-NK1.1 antibody and anti-γδTCR antibody, respectively (Fig. 4a). When anti-NK1.1 antibody was injected, the level of parasitaemia in the blood increased prominently in β2m(–/–) mice. Finally, some β2m(–/–) mice survived from malaria infection, while others died.
Figure 4.
Experiments in which NK cells and γδT cells were eliminated in mice with malaria. (a) Parasitaemia. (b) Elimination of NK cells. (c) Elimination of γδT cells. NK cells were eliminated by anti-NK1.1 mAb, whereas γδT cells were eliminated by anti-γδTCR mAb. Representative results of three mice are depicted in each experiment. Some mice in which NK cells or γδT cells were eliminated died during malarial infection. Experiments (a) and (b) were two-colour immunofluorescence tests to detect NK cells and γδT cells.
In the case of the injections with anti-γδTCR antibody, parasitaemia increased and all mice failed to recover and died. All tested β2m(–/–) mice showed the same tendency, irrespective of the level of parasitaemia.
To confirm the elimination of NK cells or γδT cells, two-colour staining for NK1.1 (or IL-2Rβ) and CD3 (Fig. 4b) and that for γδTCR and αβTCR (Fig. 4c) were conducted. Even after malarial infection, the levels of NK and γδT cells were low due to the effective elimination by antibodies.
Characteristics of expanding γδT cells in mice with malaria
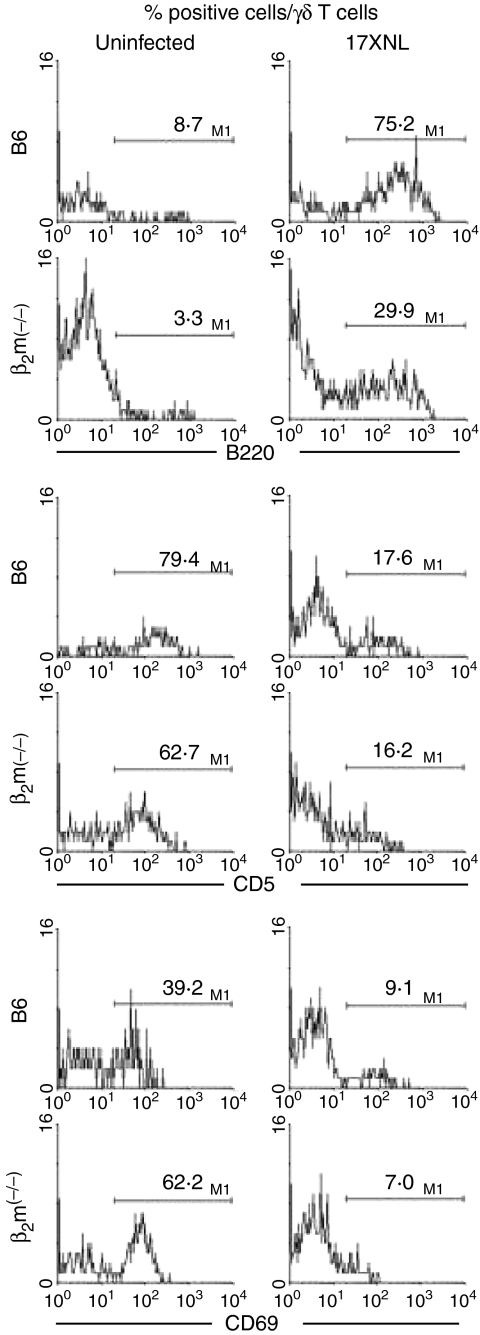
To characterize the phenotype of γδT cells, three-colour staining for γδTCR, αβTCR and B220 (or CD5 or CD69) was conducted (Fig. 5). Lymphocytes were isolated from the liver of normal mice or malaria-infected mice (day 21 after infection). By gated analysis, the expression of B220, CD5 or CD69 in γδT cells was estimated.
Figure 5.
Further characterization of the phenotype of γδT cells in mice with or without malaria. Three-colour staining of hepatic lymphocytes for IL-2Rβ, γδTCR and B220 (or CD5 or CD69) were conducted. Malaria-infected mice were used on day 21 after the infection. By gated analysis, the expressions of B220, CD5 and CD69 on γδT cells were estimated.
Primarily, γδT cells did not express B220 in either B6 or β2m(–/–) mice. However, the expression of B220 antigens was newly generated in γδT cells at a high level in B6 mice and at a low level in β2m(–/–) mice (Fig. 5, top). In the case of CD5 antigens, the majority of γδT cells expressed CD5 antigens before malaria infection, but many γδT cells lost CD5 antigens after the infection. This tendency was the same in B6 and β2m(–/–) mice. A decreased level of CD69 antigens was also seen in γδT cells after malarial infection in both of B6 and β2m(–/–) mice. The number of analysed cells were a very few for the analysis. However, the repeated experiments showed that the data were reproducible.
Usage of γδTCR by γδT cells after malarial infection
Usage of γδTCR by γδT cells was examined by RT-PCR method (Fig. 6). Usage of γδTCR became less polyclonal after malarial infection in B6 mice, because the signs of Vγ1, Vγ2 and Vγ4 were prominent but Vγ5 and Vγ6 became less prominent in comparison with those of control mice. In the case of β2m(–/–) mice, the sign of Vγ usage was only Vγ6. After malarial infection, the signs of Vγ usage were Vγ1, Vγ2, Vγ4 and Vγ6. There was a similarity of Vγ usage between B6 and β2m(–/–) mice after malarial infection. Usage of Vγ7 was not detected in both mice with and without malarial infection (data not shown).
Figure 6.
Usage of γδTCR by γδT cells identified by RT-PCR method. The signs of Vγ1, Vγ2, Vγ4, Vγ5 and Vγ6 were determined by RT–PCR method before and after malarial infection in B6 and β2m(–/–) mice. The sign of glyceraldehyde-3-phosphate dehydrogenase (G3PDH) was a positive control.
Discussion
In the present study, we used β2m(–/–) mice to examine how these mice recovered from or died of malarial infection. In contrast to control B6 mice, β2m(–/–) mice lack CD8+ cytotoxic T cells and NKT cells because of the absence of both MHC class I antigens and CD1d antigens (MHC class I-like molecule with β2-microglobulin component).35 The present results showed that β2m(–/–) mice showed a level of parasitaemia similar to that of B6 mice during malarial infection and were able to recover from malaria, as did B6 mice. In other words, mice were found to recover from malaria in the absence of CD8+ T cells and NKT cells.
During malarial infection, however, β2m(–/–) mice showed a different pattern of lymphocyte activation in the liver and spleen. As previously reported,20–24 hepatomegaly and lymphocytosis in the liver always accompanied malarial infection. This pattern was confirmed in B6 mice in this study. In the case of β2m(–/–) mice, hepatomegaly and splenomegaly were more prominent than in B6 mice. Indeed, the number of lymphocytes yielded by the liver and spleen was extremely high in β2m(–/–) mice. A phenotypic study demonstrated that NK cells in β2m(–/–) mice greatly expanded in the early phase of malarial infection, while γδT cells greatly expanded in the late phase. A major expansion of IL-2Rβ+ TCRint cells was common in both B6 and β2m(–/–) mice. These results suggest that there is a compensatory phenomenon in the immunity of β2m(–/–) mice. Under conditions in the absence of CD8+ T cells and NKT cells, NK cells and γδT cells play a compensatory function for malarial protection.
In order to directly examine the functional importance of NK cells and γδT cells which newly appeared in β2m(–/–) mice for malarial protection, experiments in which NK cells and γδT cell were eliminated were conducted by using anti-NK1.1 and anti-γδTCR mAb. Increased, prolonged levels parasitaemia were induced in β2m(–/–) mice treated with anti-NK1.1 mAbs. However, these mice tended to recover from malaria. In sharp contrast, the treatment of anti-γδTCR mAb rendered β2m(–/–) mice sensitive to malarial death. It is concluded that the compensatory function of γδT cells was more potent than that of NK cells in β2m(–/–) mice with malaria.
The characteristics of γδT cells that appeared in the liver of β2m(–/–) mice infected with malaria were further examined in immunofluorescence tests. Conventional γδ T cells in the liver of both B6 and β2m(–/–) mice were mainly B220– CD5+ CD69±. However, after malarial infection a considerable proportion of γδT cells became B220+ CD5– CD69–. Unconventional T cells38 or activated γδT cells24 are known to express B220 antigen. On the other hand, there was a report that splenic γδT cells are CD5+ but intestinal γδT cells are CD5–.39 Therefore, the phenotype of γδT cells after malarial infection somewhat resemble activated or intestinal γδT cells. The loss of CD69 (an early activation maker) antigen40 on hepatic γδT cells after malarial infection was interesting. Both γδT cells seen in the liver of B6 and β2m(–/–) mice were double-negative CD4– 8– (data not shown). In other words, hepatic γδT cells were CD4– 8– whereas intestinal γδT cells were CD8+. This detailed comparison was done in our previous study.41 We also examined the usage of γδTCR by γδT cells in B6 mice and β2m(–/–) mice. There was a polyclonal expansion of γδT cells using Vγ1, Vγ2, Vγ4 and Vγ6 in both B6 and β2m(–/–) mice. In early reports, the skewed usage of Vγ1Vδ6 or Vγ4Vδ4 (Vγ2Vδ4 Garman's classification) by γδT cells was seen after malarial infection.42–44 Although Vγ1+ and Vγ2+γδT cells seemed to expand in B6 and β2m(–/–) mice after malarial infection even in this study, the restriction to Vγ1 and Vγ2 was not seen in our experimental protocol.
In our previous studies,21–23 we reported that the major function for the blood stage of malaria protection is performed by NK1.1– αβTCRint cells. This lymphocyte population belongs to T cells seen in athymic nude mice, namely, extrathymic T cells. In parallel with the expansion of extrathymic T cells, the autoantibodies produced by B-1 cells were always present during malarial infection.21–23 We speculate that autoreactive NK1.1– αβTCRint cells and B-1 cell autoantibodies may attack erythrocytes invaded by intracellular pathogens and that macrophages finally process such denatured, infected erythrocytes. Therefore, hepatomegaly and splenomegaly appear in this disease.
In addition to this major protection, some conventional T cells (CD8+ T cells or CD4+ T cells) and B-2 cells (which produce direct antibodies against the malaria parasite) may play additional roles, especially in the hepatic stage of malaria protection. There is a report that CD8+ T cells were partially associated with the protection against the malaria infection, using β2(–/–) mice and anti-CD8 mAb.45 However, NK cells,46–48 NKT cells31–34 and γδT cells49–52 were also known to play compensatory functions of malaria protection. We have to emphasize the evidence that malaria protection occurred in the absence of MHC class I antigens and CD1d antigens in the present study. However, this raises the possibility that β2m(–/–) mice showed a decreased period of memory as secondary responses against malaria protection (our preliminary study). Further experiments on the importance of MHC class II antigens in malaria protection will also be conducted in the near future.
Acknowledgments
We wish to thank Mrs Yuko Kaneko for manuscript preparation. This work was supported by a grant-in-aid for scientific research from the Ministry of Education, Science, and Culture, Japan.
Abbreviations:
- β2m
β2-microglobulin
- CD3int (TCRint)
intermediate levels of CD3 (TCR) for antigen
- IL-2Rβ
IL-2 receptor β-chain
- MNC
mononuclear cell.
References
- 1.Guebre-Xabier M, Schwenk R, Krzych U. Memory phenotype CD8+ T cells persist in livers of mice protected against malaria by immunization with attenuated Plasmodium berghei sporozoites. Eur J Immunol. 1999;29:3978–86. doi: 10.1002/(SICI)1521-4141(199912)29:12<3978::AID-IMMU3978>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Wang R, Epstein J, Baraceros FM, et al. Induction of CD4+ T cell-dependent CD8+ type 1 responses in humans by a malaria DNA vaccine. Proc Natl Acad Sci USA. 2001;98:10817–22. doi: 10.1073/pnas.181123498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrot A, Zavala F. Effector and memory CD8+ T cells as seen in immunity to malaria. Immunol Rev. 2004;201:291–303. doi: 10.1111/j.0105-2896.2004.00175.x. [DOI] [PubMed] [Google Scholar]
- 4.Morrot A, Zavala F. Regulation of the CD8+ T cell responses against Plasmodium liver stages in mice. Int J Parasitol. 2004;34:1529–34. doi: 10.1016/j.ijpara.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Plebanski M, Hannan CM, Behboudi S, Flanagan KL, Apostolopoulos V, Sinden RE, Hill AV. Direct processing and presentation of antigen from malaria sporozoites by professional antigen-presenting cells in the induction of CD8 T-cell responses. Immunol Cell Biol. 2005;83:307–12. doi: 10.1111/j.1440-1711.2005.01325.x. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues MM, Cordey AS, Arreaza G, Corradin G, Romero P, Maryanski JL, Nussenzweig RS, Zavala F. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int Immunol. 1991;3:579–85. doi: 10.1093/intimm/3.6.579. [DOI] [PubMed] [Google Scholar]
- 7.Podoba JE, Stevenson MM. CD4+ and CD8+ T lymphocytes both contribute to acquired immunity to blood-stage Plasmodium chabaudi AS. Infect Immun. 1991;59:51–8. doi: 10.1128/iai.59.1.51-58.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quin SJ, Seixas EM, Cross CA, Berg M, Lindo V, Stockinger B, Langhorne J. Low CD4+ T cell responses to the C-terminal region of the malaria merozoite surface protein-1 may be attributed to processing within distinct MHC class II pathways. Eur J Immunol. 2001;31:72–81. doi: 10.1002/1521-4141(200101)31:1<72::aid-immu72>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho LH, Sano G, Hafalla JC, Morrot A, Curotto de Lafaille MA, Zavala F. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat Med. 2002;8:166–70. doi: 10.1038/nm0202-166. [DOI] [PubMed] [Google Scholar]
- 10.Weiss WR, Sedegah M, Berzofsky JA, Hoffman SL. The role of CD4+ T cells in immunity to malaria sporozoites. J Immunol. 1993;151:2690–8. [PubMed] [Google Scholar]
- 11.Fossati L, Merino J, Izui S. CD4+ T cells play a major role for IgM and IgG anti-DNA production in mice infected with Plasmodium yoelii. Clin Exp Immunol. 1990;79:291–6. doi: 10.1111/j.1365-2249.1990.tb05193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meding SJ, Langhorne J. CD4+ T cells and B cells are necessary for the transfer of protective immunity to Plasmodium chabaudi chabaudi. Eur J Immunol. 1991;21:1433–8. doi: 10.1002/eji.1830210616. [DOI] [PubMed] [Google Scholar]
- 13.Stephens R, Albano FR, Quin S, et al. Malaria-specific transgenic CD4+ T cells protect immunodeficient mice from lethal infection and demonstrate requirement for a protective threshold of antibody production for parasite clearance. Blood. 2005;106:1676–84. doi: 10.1182/blood-2004-10-4047. [DOI] [PubMed] [Google Scholar]
- 14.Bastian M, Lozano JM, Patarroyo ME, Pluschke G, Daubenberger CA. Characterization of a reduced peptide bond analogue of a promiscuous CD4 T cell epitope derived from the Plasmodium falciparum malaria vaccine candidate merozoite surface protein 1. Mol Immunol. 2004;41:775–84. doi: 10.1016/j.molimm.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Xu H, Hodder AN, Yan H, Crewther PE, Anders RF, Good MF. CD4+ T cells acting independently of antibody contribute to protective immunity to Plasmodium chabaudi infection after apical membrane antigen 1 immunization. J Immunol. 2000;165:389–96. doi: 10.4049/jimmunol.165.1.389. [DOI] [PubMed] [Google Scholar]
- 16.Reece WH, Pinder M, Gothard PK, et al. A CD4+ T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat Med. 2004;10:406–10. doi: 10.1038/nm1009. [DOI] [PubMed] [Google Scholar]
- 17.Cirrier J, Beck HP, Currie B, Good MF. Antigens released at schizont burst stimulate Plasmodium falciparum-specific CD4+ T cells from non-exposed donors: potential for cross-reactive memory T cells to cause disease. Int Immunol. 1995;7:821–33. doi: 10.1093/intimm/7.5.821. [DOI] [PubMed] [Google Scholar]
- 18.Calvo-Calle JM, Oliveira GA, Nardin EH. Human CD4+ T cells induced by synthetic peptide malaria vaccine are comparable to cells elicited by attenuated Plasmodium falciparum sporozoites. J Immunol. 2005;175:7575–85. doi: 10.4049/jimmunol.175.11.7575. [DOI] [PubMed] [Google Scholar]
- 19.Urban BC, Ing R, Stevenson MM. Early interactions between blood-stage plasmodium parasites and the immune system. Curr Top Microbiol Immunol. 2005;297:25–70. doi: 10.1007/3-540-29967-x_2. [DOI] [PubMed] [Google Scholar]
- 20.Weerasinghe A, Sekikawa H, Watanabe H, et al. Association of intermediate T cell receptor cells, mainly their NK1.1– subset, with protection from malaria. Cell Immunol. 2001;207:28–35. doi: 10.1006/cimm.2000.1737. [DOI] [PubMed] [Google Scholar]
- 21.Mannoor MK, Weerasinghe A, Halder RC, Morshed SRM, Ariyasinghe A, Watanabe H, Sekikawa H, Abo T. Resistance to malarial infection is achieved by the cooperation of NK1.1+ and NK1.1– subsets of intermediate TCR cells which are constituents of innate immunity. Cell Immunol. 2001;211:96–104. doi: 10.1006/cimm.2001.1833. [DOI] [PubMed] [Google Scholar]
- 22.Mannoor MK, Halder RC, Morshed SRM, et al. Essential role of extrathymic T cells in protection against malaria. J Immunol. 2002;169:301–6. doi: 10.4049/jimmunol.169.1.301. [DOI] [PubMed] [Google Scholar]
- 23.Halder RC, Abe T, Mannoor MK, et al. Onset of hepatic erythropoiesis after malarial infection in mice. Parasitol Int. 2003;52:259–68. doi: 10.1016/s1383-5769(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 24.Bakir HY, Tomiyama-Miyaji C, Watanabe H, Nagura T, Kawamura T, Sekikawa H, Abo T. Reasons why DBA/2 mice are resistant to malarial infection. expansion of CD3int B220+γδT cells with double-negative CD4– 8– phenotype in the liver. Immunology. 2006;117:127–35. doi: 10.1111/j.1365-2567.2005.02273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe H, Weerasinghe A, Miyaji C, et al. Expansion of unconventional T cells with natural killer markers in malaria patients. Parasitol Int. 2003;52:61–70. doi: 10.1016/s1383-5769(02)00085-5. [DOI] [PubMed] [Google Scholar]
- 26.Wenisch C, Wenisch H, Bankl HC, Exner M, Graninger W, Looareesuwan S, Rumpold H. Detection of anti-neutrophil cytoplasmic antibodies after acute Plasmodium falciparum malaria. Clin Diagn Laboratory Immunol. 1996;3:132–4. doi: 10.1128/cdli.3.1.132-134.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lloyd CM, Collins I, Belcher AJ, Manuelpillai N, Wozencraft AO, Staines NA. Characterization and pathological significance of monoclonal DNA-binding antibodies from mice with experimental malaria infection. Infect Immun. 1994;62:1982–8. doi: 10.1128/iai.62.5.1982-1988.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ribeiro CD, Alfred C, Monjour L, Gentilini M. Normal frequency of anti-thyroglobulin antibodies in hyperendemic areas of malaria: relevance to the understanding of autoantibody formation in malaria. Trop Geogr Med. 1984;36:323–8. [PubMed] [Google Scholar]
- 29.Kataaha PK, Facer CA, Mortazavi-Milani SM, Stierle H, Holborow EJ. Stimulation of autoantibody production in normal blood lymphocytes by malaria culture supernatants. Parasite Immunol. 1984;6:481–92. doi: 10.1111/j.1365-3024.1984.tb00818.x. [DOI] [PubMed] [Google Scholar]
- 30.Ribeiro CT, de Roquefeuil S, Druilhe P, Monjour L, Homberg JC, Gentilini M. Abnormal anti-single stranded (ss) DNA activity in sera from Plasmodium falciparum infected individuals. Trans R Soc Trop Med Hyg. 1984;78:742–6. doi: 10.1016/0035-9203(84)90005-1. [DOI] [PubMed] [Google Scholar]
- 31.Pied S, Roland J, Louise A, Voegtle D, Soulard V, Mazier D, Cazenave PA. Liver CD4– CD8– NK1.1+ TCR alpha beta intermediate cells increase during experimental malaria infection and are able to exhibit inhibitory activity against the parasite liver stage in vitro. J Immunol. 2000;164:1463–9. doi: 10.4049/jimmunol.164.3.1463. [DOI] [PubMed] [Google Scholar]
- 32.Romero JF, Eberl G, MacDonald HR, Corradin G. CD1d-restricted NKT cells are dispensable for specific antibody responses and protective immunity against liver stage malaria infection in mice. Parasite Immunol. 2001;23:267–9. doi: 10.1046/j.1365-3024.2001.00381.x. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Aseguinolaza G, Van Kaer L, Bergmann CC, et al. Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J Exp Med. 2002;195:617–24. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korten S, Anderson RJ, Hannan CM, Sheu EG, Sinden R, Gadola S, Taniguchi M, Hill AV. Invariant Valpha14 chain NKT cells promote Plasmodium berghei circumsporozoite protein-specific gamma interferon- and tumor necrosis factor alpha-producing CD8+ T cells in the liver after poxvirus vaccination of mice. Infect Immun. 2005;73:849–58. doi: 10.1128/IAI.73.2.849-858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adachi Y, Koseki H, Zijlstra M, Taniguchi M. Positive selection of invariant Vα14+ T cells by non-major histocompatibility complex-encoded class I-like molecules expressed on bone marrow-derived cells. Proc Natl Acad Sci U S A. 1995;92:1200–4. doi: 10.1073/pnas.92.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato K, Hasegawa K, Yamagiwa S, et al. Abundance of CD4+ T cells and NK cells but their deficiency in anti-hepatoma cytotoxicity in the liver of LEC rats. Biomed Res. 1997;18:211–20. [Google Scholar]
- 37.Watanabe T, Kawamura T, Kawamura H, Haga M, Shirai K, Watanabe H, Eguchi S, Abo T. Intermediate TCR cells in mouse lung. Their effector function to induce pneumonitis in mice with autoimmune-like graft-versus-host disease. J Immunol. 1997;158:5805–14. [PubMed] [Google Scholar]
- 38.Seki S, Abo T, Ohteki T, Sugiura K, Kumagai K. Unusual αβ-T cells expanded in autoimmune lpr mice are probably a counterpart of normal T cells in the liver. J Immunol. 1991;147:1214–21. [PubMed] [Google Scholar]
- 39.Mizoguci A, Mizoguci E, de Jong YP, Takedatsu H, Preffer FI, Terhorst C, Bhan AK. Role of the CD5 molecule on TCR γδT cell-mediated immune functions: development of germinal centers and chronic intestinal inflammation. Int Immunol. 2002;15:97–108. doi: 10.1093/intimm/dxg006. [DOI] [PubMed] [Google Scholar]
- 40.Gao YL, Rajan AJ, Raine CS, Brosnan CF. γδT cells express activation makers in the central nervous system of mice with chronic-relapsing experimental autoimmune encephalomyelitis. J Autoimmun. 2001;17:261–71. doi: 10.1006/jaut.2001.0547. [DOI] [PubMed] [Google Scholar]
- 41.Abo T, Kawamura T, Watanabe H. Physiological responses of extrathymic T cells in the liver. Immunol Rev. 2000;174:135–49. doi: 10.1034/j.1600-0528.2002.017415.x. [DOI] [PubMed] [Google Scholar]
- 42.Seixas EMG, Langhorne J. γδT cells contribute to control of chronic parasitemia in Plasmodium chabaudi infections in mice. J Immunol. 1999;162:2837–41. [PubMed] [Google Scholar]
- 43.Hassan R, Franco SAL, Stefanoff CG, Romano SO, Diamond HR, Franco LGP, Seuanez HN, Zalcberg IR. Hepatosplenic γδ T-cell lymphoma following seven malaria infections. Pathol Int. 2006;56:668–73. doi: 10.1111/j.1440-1827.2006.02027.x. [DOI] [PubMed] [Google Scholar]
- 44.van der Heyde HC, Batchelder JM, Sandor M, Weidanz WP. Splenic γδT cells regulated by CD4+T cells are required to control chronic Plasmodium chabaudi malaria in the B-cell-deficient mouse. Infect Immun. 2006;74:2717–25. doi: 10.1128/IAI.74.5.2717-2725.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Heyde HC, Manning DD, Roopenian DC, Weidanz WP. Resolution of blood-stage malarial infections in CD8+ cell-deficient β2-m0/0 mice. J Immunol. 1993;151:3187–91. [PubMed] [Google Scholar]
- 46.Solomon JB, Forbes MG, Solomon GR. A possible role for natural killer cells in providing protection against Plasmodium berghei in early stages of infection. Immunol Lett. 1985;9:349–52. doi: 10.1016/0165-2478(85)90061-6. [DOI] [PubMed] [Google Scholar]
- 47.Korbel DS, Finney OC, Riley EM. Natural killer cells and innate immunity to protozoan pathogens. Int J Parasitol. 2004;34:1517–28. doi: 10.1016/j.ijpara.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Korbel DS, Newman KC, Almeida CR, Davis DM, Riley EM. Heterogeneous human NK cell responses to Plasmodium falciparum-infected erythrocytes. J Immunol. 2005;175:7466–73. doi: 10.4049/jimmunol.175.11.7466. [DOI] [PubMed] [Google Scholar]
- 49.van der Heyde HC, Elloso MM, Chang WL, Kaplan M, Manning DD, Weidanz WP. Gamma delta T cells function in cell-mediated immunity to acute blood-stage Plasmodium chabaudi adami malaria. J Immunol. 1995;154:3985–90. [PubMed] [Google Scholar]
- 50.Jones SM, Goodier MR, Langhorne J. The response of gamma delta T cells to Plasmodium falciparum is dependent on activated CD4+ T cells and the recognition of MHC class I molecules. Immunology. 1996;89:405–12. doi: 10.1046/j.1365-2567.1996.d01-762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yonez DM, Batchelder J, van der Heyde HC, Manning DD, Weidanz WP. Gamma delta T-cell function in pathogenesis of cerebral malaria in mice infected with Plasmodium berghei ANKA. Infect Immun. 1999;67:446–8. doi: 10.1128/iai.67.1.446-448.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seixas E, Fonseca L, Langhorne J. The influence of gammadelta T cells on the CD4+ T cells and antibody response during a primary Plasmodium chabaudi chabaudi infection in mice. Parasite Immunol. 2002;24:131–40. doi: 10.1046/j.1365-3024.2002.00446.x. [DOI] [PubMed] [Google Scholar]