Abstract
Post-translational modifications of histone proteins are major mechanisms that modify chromatin structure and regulate gene expression in eukaryotes. Activation of histone acetyltransferases or inhibition of histone deacetylases (HDACs) is generally believed to allow chromatin to assume a more open state, permitting transcriptional activity. We report here the surprising observation that treatment of murine dendritic cells with the HDAC inhibitors trichostatin A (TSA) or suberoylanilide hydroxamic acid (SAHA) in non-apoptotic concentrations strongly inhibited induction of both interleukin-12 protein p40 (IL-12p40) mRNA and protein upon stimulation of Toll-like receptors (TLRs). Moreover, TLR-mediated up-regulation of costimulatory molecules was also inhibited. Up-regulation of tumour necrosis factor-α mRNA and protein in response to TLR agonists was only affected upon prolonged exposure to HDAC inhibitors and regulation of IL-1β was not affected. Similar effects were apparent in murine and human macrophages. Regarding the mode of action, HDAC inhibition increased the acetylation status at the IL-12p40 locus. Nevertheless, IL-12p40 chromatin remodelling, binding of Rel-A and IRF1 to the IL-12p40 promoter and transcriptional activation were abrogated. In contrast, HDAC inhibitors had no effects on upstream nuclear factor-κB and mitogen-activated protein kinase activation. Thus HDACs positively regulate the expression of a subset of cytokine genes by enabling transcription factor recruitment.
Keywords: chromatin modifications, dendritic cells, histone deacetylases, interleukin-12, Toll-like receptors
Introduction
Innate immune cells, such as macrophages and dendritic cells (DCs), employ pattern recognition receptors to initiate host immune responses against infectious agents. Toll-like receptors (TLRs) play a pivotal role in pattern recognition.1 TLRs contain an intracellular Toll/interleukin-1 receptor (TIR) domain, which initiates downstream signalling by recruiting one or more TIR-containing adapter proteins.1 In turn, three important groups of signalling pathways are activated: the nuclear factor-κB (NF-κB) pathway, the group of mitogen-activated protein kinases (MAP kinases) and members of the interferon regulatory factor family (IRF).2–4 More recently, we, and others, have identified inducible chromatin modifications as an important additional restriction point in TLR-regulated gene expression.5,6
It is generally accepted that acetylation of key amino acids on histone proteins decreases their affinity for DNA, thus resulting in a DNA structure that is permissive for the subsequent recruitment of transcriptional activators.7 Indeed, histone acetyltransferases, such as p300 and cyclic adenosine monophosphate response element-binding protein-binding protein (CBP), are important coactivators that positively regulate several cytokine genes, including interleukin-12 (IL-12).8–12 Conversely, histone deacetylases (HDACs) catalyse the deacetylation of histone proteins, and are classically associated with the inactivation of gene expression.13 The TLR ligands initiate rapid and dramatic changes in gene expression, thus implying the involvement of chromatin remodelling events. Indeed, lipopolysaccharide (LPS)-induced expression of IL-12p40 was accompanied by histone acetylation at the IL-12p40 locus, enabling transcription factor recruitment.6 However, the impact of histone modifications on TLR-regulated gene expression has not been extensively studied.
The small molecule HDAC inhibitors suberoylanilide hydroxamic acid (SAHA) and trichostatin A (TSA) repress histone deacetylation, thereby causing general hyperacetylation of histones.14 In keeping with the role of HDACs as negative regulators of gene expression, HDAC inhibitors induce the expression of several genes.15–18 The reprogramming of gene expression by HDAC inhibitors generally causes growth arrest, induction of differentiation and/or apoptosis. Molecules that target HDACs are therefore being explored for the treatment of certain forms of cancer.14
HDAC inhibitors also have anti-inflammatory effects in a range of disease models in the mouse, including systemic lupus erythematosus,19 septic shock,20 asthma21 and experimental autoimmune encephalomyelitis.22 In these models, HDAC inhibitors generally reduce the production of proinflammatory cytokines that drive disease. This contrasts with the accepted role of HDACs as repressors of gene expression. Both we23 and others24 recently identified sets of TLR-inducible genes that are targeted by HDAC inhibitors in macrophages and DCs. In this study, we use the IL-12p40 gene as a model to characterize the mechanisms by which SAHA and TSA inhibit TLR-inducible gene expression, and identify further cytokine genes that are targeted by HDAC inhibitors.
Materials and methods
Materials
The phosphothioate-modified CpG-oligonucleotide 1668 (TCC ATG ACG TTC CTG ATG CT) was custom synthesized by TIB Molbiol (Berlin, Germany). The LPS from Salmonella minnesota was provided by U. Seydel (Borstel, Germany) or was purchased from Sigma (St Louis, MO). Both SAHA and Pam3Cys-Ser-(Lys)4 (Pam3CSK4) were obtained from ALEXIS (Lausen, Switzerland). The TSA was purchased from Calbiochem (Schwalbach, Germany) or Sigma. CAAT/enhancer binding protein β (C/EBPβ), NF-κB/Rel-A, IRF1, IRF2 and IRF7 antibodies were received from Santa Cruz Biotechnology (Heidelberg, Germany) and antibodies against inhibitor of nuclear factor κBα (IκBα)-, phosphorylated extracellular regulators of kinases (pERK)-, phosphorylated c-Jun NH2-terminal kinase (pJNK), phosphorylated protein 38 (pp38)-, signal transducers and activators of transcription 1 (STAT1)- and pY701-STAT1 were purchased from Cell Signaling Technology (Danvers, MA). Recombinant human macrophage colony-stimulating factor (M-CSF/CSF-1) was a gift from Chiron, Emeryville, CA. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent was obtained from Sigma.
Generation of primary DCs and macrophages
Bone marrow-derived dendritic cells (BMDCs) were generated from BALB/c and IFNAR–/– mice, the latter were obtained from Heike Weighardt (Munich, Germany). The DCs were prepared from female, 4- to 10-week-old mice as described by Inaba25 with minor modifications. Briefly, bone marrow cells were placed in 70 cm2 tissue-culture flasks in differentiation medium [RPMI-1640 medium supplemented with 10% fetal calf serum (FCS), 50 mm 2-mercaptoethanol, antibiotics penicillin G (100 IU/ml) and streptomycin sulphate (100 IU/ml) and 200 U/ml granulocyte–macrophage colony-stimulating factor (GM-CSF)]. After 24 hr, non-adherent cells were collected and washed; 107 of these cells were seeded into 175 cm2 tissue-culture flasks in differentiation medium. At day 5 fresh differentiation medium was added and at day 9 non-adherent, immature DCs (CD11c+ B220–) were harvested. Culture supernatant of a GM-CSF-transfected cell line was used equally as a source of GM-CSF. Bone marrow-derived macrophages (BMDM) were generated by culture of murine bone marrow cells, from 6- to 8-week-old male C57BL/6 or BALB/c mice, with complete RPMI medium containing recombinant human CSF-1 (10 000 U/ml; 100 ng/ml) on bacteriological plastic plates for 7 days. CD14+ monocytes, positively selected from healthy donors using magnetic antibody cell sorting (MACS) technology (Miltenyi Biotec Inc., Auburn, CA), were cultured in Iscove's modified Dulbecco's medium supplemented with 10% FCS, 20 U/ml penicillin (Invitrogen, Carlsbad, CA), 20 μg/ml streptomycin (Invitrogen) and 2 mm l-glutamine (Invitrogen) for 7 days in the presence of CSF-1 (10 000 U/ml; 100 ng/ml) to generate human monocyte-derived macrophages.
Western blotting
After stimulation, cells were lysed for 30 min on ice in 250 μl lysis buffer [50 mm Tris–HCl, pH 7·4; 1% Igepal; 0·25% sodium deoxycholate; 150 mm NaCl; 1 mm ethylenediaminetetraacetic acid (EDTA); 1 mm phenylmethylsulfonylfluoride (PMSF); 1 μg/ml each aprotinin, leupeptin, and pepstatin; 1 mm Na3VO4; and 1 mm NaF]. Lysates were cleared by centrifugation at 4° for 10 min at 11 000 g. Equal amounts of the lysates were fractionated by 12% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and electrotransferred to polyvinylidene difluoride membranes. The membranes were blocked with tris buffered saline (TBS) (100 mm Tris pH 7·8, 150 mm NaCl)/5% non-fat dry milk/0·05% Tween-20, and were blotted with the indicated antibodies. Detection was by enhanced chemiluminescence (ECL; Amersham, Freiburg, Germany). Antibodies to MAP kinases were used according to the manufacturer's protocol.
Determination of cytokine secretion
Cell-free supernatant was harvested and analysed for cytokines by commercially available enzyme-linked immunosorbent assay (ELISA) kits (OptEIA; Becton Dickinson, Heidelberg, Germany).
Quantitative reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated using Qiagen RNeasy kits (Qiagen, Valencia, CA) or HighPure RNA kits (Roche, Mannheim, Germany), which included DNase I digestion. A total of 1 μg RNA was reverse-transcribed into cDNA (MBI Fermentas, St Leon-Rot, Germany) using an oligo-dT primer. The cDNA was diluted 1 : 4 and 2·5 μl was used as template in 25 μl TaqMan-PCR mix, according to the manufacturer's protocol (reagents from AbGene, Hamburg, Germany; platform: ABI Prism 7700, Applied Biosystems, Darmstadt, Germany). Quantifications were made using fluorogenic probes (FAM/TAMRA; Eurogentec, Seraing, Belgium). The specificity of RT-PCR was controlled by no-template and no-reverse transcriptase (no-RT) controls. Quantitative PCR results were expressed relative to the housekeeping gene β-actin  or
or  . Alternatively, mRNA levels were quantified using the Platinum SybrGreen System (Invitrogen) and an ABI Prism 7500 sequence detection system. For experiments using the SybrGreen System, mRNA levels were expressed relative to hypoxanthine guanine phosphoribosyl transferase (hprt) mRNA. All primer sequences are available on request.
. Alternatively, mRNA levels were quantified using the Platinum SybrGreen System (Invitrogen) and an ABI Prism 7500 sequence detection system. For experiments using the SybrGreen System, mRNA levels were expressed relative to hypoxanthine guanine phosphoribosyl transferase (hprt) mRNA. All primer sequences are available on request.
Pre-mRNA RT-PCR
Total RNA was isolated and reverse transcribed as described above. The RT-PCR assays were performed using primer pairs amplifying an amplicon spanning the intron2/exon3 intron3 part of the IL-12p40 gene. Quantifications were made using fluorogenic probes using a sequence in exon 3. The isolated cDNA was carefully checked for contamination with genomic DNA by analysing a control without reverse transcriptase (no-RT) and also by performing a PCR to amplify non-transcribed regions within the IL-12p40 promoter26.
Chromatin immunoprecipitation assay (ChIP)
ChIP was carried out as described by the group of Natoli.27,28 Briefly, cells were fixed by adding formaldehyde to a final concentration of 1% (HCHO 37% in 10% methanol, Calbiochem). After 7 min, cross-linking was stopped by adding glycine to a final concentration of 125 mm. The cells were transferred to a 1·5-ml tube, centrifuged and washed three times with ice-cold phosphate-buffered saline (PBS). Nuclei were isolated by incubating cells in L1 buffer (50 mm Tris–HCl pH 8·0, 2 mm EDTA, 0·1% Nonidet P-40, 10% glycerol supplemented with protease inhibitors) for 5 min on ice and by centrifuging for 5 min at 1000 g and 4°. Nuclei were resuspended in L2 buffer (50 mm Tris–HCl pH 8·0, 5 mm EDTA, 1% SDS). For chromatin fragmentation samples were treated with ultrasonic power four times for 12 seconds (DNA fragments between 0·5 and 1·2 kilobase pairs). Samples were centrifuged for 5 min, at 16 000 gat 4°, supernatants transferred to new tubes and diluted 10 times with d-buffer (50 mm Tris–HCl pH 8·0, 5 mm EDTA, 200 mm NaCl, 0·5% nonidet P-40). Extracts were precleared for 3 hr with 80 μl protein A-agarose. Immunoprecipitations were carried out overnight at 4° using 2 μg antibody. Immuncomplexes were collected with 20 μl salmon-sperm-saturated protein A-agarose for 30 min. Cells were washed four times (5 min) with high-salt buffer (20 mm Tris–HCl pH 8·0, 2 mm EDTA, 0·1% SDS, 1% nonidet P-40 and 0·5 mm NaCl) and three times with no salt buffer (1 ×TE). Immune complexes were extracted in 120 μl 1 × TE containing 2% SDS and protein/DNA cross-links were reverted by heating at 65°C. DNA was purified using a QIAquick kit (Qiagen, Hilden, Germany). One-twentieth of the immunoprecipitated DNA was used in quantitative PCR. For normalization, DNA was isolated from the precleared primary solution and one-twentieth of the DNA was used in PCR. Results were normalized by subtracting the obtained input DNA Ct value from that of the specific target gene (= ΔCt). The ΔCt value inversely correlated with transcription factor association at the region being amplified.
Flow cytometry
For intracellular cytokine staining, cells were stimulated in the presence of 10 μg/ml brefeldin A (Sigma). Cells were fixed in 4% paraformaldehyde (PFA)/PBS for 10 min. Subsequently, cells were permeabilized in PBS containing 0·5% saponin and 2% FCS for 1 hr and then stained with fluorescein isothiocyanate (FITC)-labelled anti-IL12p40 (BD, Heidelberg, Germany). For surface stainings cells were washed in PBS/2% FCS and stained directly with conjugated monoclonal antibodies (anti-I-Ad/Ed: clone 2G9, anti-CD86: clone GL1, anti-CD40: clone HM40-3, all antibodies from BD). Cells were analysed on a fluorescence-activated cell sorter (FACS) Canto (BD).
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared as described by Andrews and Faller.27,29 Protein concentration was determined by bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL). The EMSAs were performed using the LightShift® Chemiluminescent EMSA Kit (Pierce) according to the manufacturer's protocol with minor modifications; instead of the provided binding buffer, a 5 × binding buffer containing 100 mm HEPES pH 8·0, 250 mm KCl, 2·5 mm dithiothreitol, EDTA 250 μm, 5 mm MgCl2, 25% glycerin was used. The sequence of the biotinylated NF-κB probe was 5′-AGC TTC AGA GGG GAC TTT CCG AGA GGT CGA-3′.
Chromatin accessibility measured by real-time PCR assays
To quantify nucleosome remodelling, chromatin accessibility measured by real-time PCR (ChART) assay was performed as described for the IL-2 promoter.30 Cells (2 × 106) were stimulated and nuclei were prepared and tested for restriction enzyme accessibility as described by Weinmann et al.6 Digestion reactions were carried out with 25 U MseI for 10 min at 37°C. Subsequently, DNA was purified using the DNeasy Tissue kit (Qiagen, Hilden, Germany). DNA from restriction enzyme accessibility assays was used in three different quantitative PCR approaches using TaqMan technology. One primer set spanned the restriction sites for MseI. A second primer set amplified outside this region and a third primer set was for β-actin. The two latter primer sets were used for normalization of DNA amounts, whereas the first primer set reported accessibility of the locus. A decrease in the amount of amplicon generated by the first set indicated increased accessibility and thus nucleosome remodelling. Data were calculated and plotted as percentage of non-stimulated cells.
Statistical analysis
All experiments were repeated at least three times. If not stated otherwise mean +SD is shown. Significant differences were evaluated by the unpaired Student t-test with two-tailed distributions. P-values below 0·05 were considered to be significant and are indicated by one asterisk.
Results
HDAC inhibition diminishes TLR-induced IL-12p40 secretion in DCs
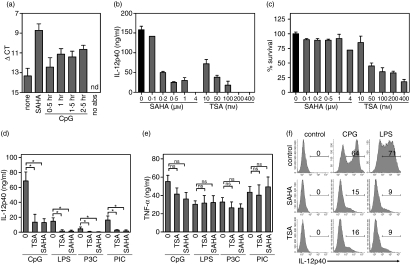
We have previously shown that chromatin remodelling is required for TLR9-mediated IL-12p40 mRNA induction. Given the importance of histone acetylation in gene regulation, we assessed the role of HDACs in regulating IL-12p40 gene expression. Using ChIP assays with an anti-acetyl histone H4 antibody, we observed an increasing but weak acetylation of the IL-12p40 locus after stimulation with CpG-DNA (Fig. 1a). Treatment with the HDAC inhibitor SAHA resulted in strong histone H4 acetylation, which exceeded that of CpG-DNA treatment.
Figure 1.
The HDAC inhibitors SAHA and TSA inhibit TLR-induced IL-12p40. (a) BMDCs were incubated with the histone deacetylase inhibitor SAHA (4 μm) for 1 hr or stimulated with CpG-DNA (1 μm) for the indicated time. ChIP assays were carried out from nuclear extracts with an anti-acetyl histone H4 antibody and acetylation of the IL-12p40 promoter was measured by quantitative PCR (one of three experiments). (b) BMDCs were preincubated for 1 hr with SAHA or TSA and stimulated with CpG-DNA (1 μm) for 14 hr. Levels of IL-12p40 in culture supernatants were estimated by ELISA. (c) Additionally, cell viability was measured at 16 hr by MTT assay. (d, e) BMDCs were preincubated with SAHA (1 μm) or TSA (10 nm) for 1 hr and stimulated with CpG-DNA (1 μm), LPS (100 ng/ml), Pam3CSK4 (5 μg/ml) or poly(I : C) (50 μg/ml) for 8 hr. IL-12p40 (d) and TNF-α (e) were measured in the supernatant (mean + SEM, n = 3; ns: not significant). (f) BMDCs were preincubated with SAHA (1 μm) or TSA (10 nm) for 1 hr and stimulated with CpG-DNA (1 μm) or LPS (100 ng/ml). After 6 hr of incubation, cells were stained and analysed for intracytoplasmatic IL-12p40 by flow cytometry.
Despite this enhancement of histone acetylation at the IL-12p40 promoter, the HDAC inhibitors SAHA and TSA actually greatly reduced IL-12p40 secretion in response to CpG-DNA (Fig. 1b). HDAC inhibitors also affect cellular viability by causing growth inhibition and/or apoptosis of proliferating cells.14,31 Although treatment of DC with higher doses of either SAHA or TSA for 16 hr decreased cell viability as assessed by MTT assay (Fig. 1c), low doses of either HDAC inhibitor (1 μm SAHA or 10 nm TSA) efficiently suppressed IL-12p40 secretion (Fig. 1b), without affecting cell viability (Fig. 1c). Further experiments were performed with these non-cytotoxic concentrations of SAHA and TSA, unless otherwise stated.
TLR agonists differ in their capacity to induce IL-12p40, whereas tumour necrosis factor-α (TNF-α) is a commonly induced pro-inflammatory gene in DCs. Pretreatment of DCs with SAHA and TSA for 1 hr not only inhibited CpG-DNA- (TLR9) induced IL-12p40, but also had a strong inhibitory effect on LPS- (TLR4), poly (I:C)- (TLR3) and Pam3CSK4- (TLR2/1) induced IL12p40 secretion (Fig. 1d). In contrast, SAHA or TSA did not significantly affect TNF-α production by TLR agonists (Fig. 1e). Intracellular cytokine staining confirmed that production of IL-12p40 protein in response to CpG-DNA or LPS was strongly diminished in the presence of SAHA or TSA (Fig. 1f), demonstrating that HDAC inhibitors did not merely block the release of IL-12p40 from DC.
HDAC inhibition interferes with TLR-induced IL-12p40 transcription
Next we examined at which level HDAC inhibitors interfere with TLR signalling. Both TSA (Fig. 2a) and SAHA (data not shown) inhibited LPS- or CpG-DNA-induced IL-12p40 mRNA levels by 5- to 10-fold in DC (Fig. 2a). Neither inhibitor affected basal IL-12p40 mRNA levels. When BMM are primed with CSF-1, LPS also elicits high levels of IL-12p40 from these cells.32 As with DCs, TSA dramatically inhibited IL-12p40 mRNA expression in BMM (Fig. 2b). To confirm that the effect of HDAC inhibitors on the IL-12p40 mRNA expression was the result of a reduced transcription rate, rather than decreased mRNA stability, we measured the IL-12p40 pre-mRNA concentration (unspliced, or partially spliced RNAs) (Fig. 2c). In DCs, HDAC inhibitors decreased LPS- and CpG-DNA-induced IL-12p40 pre-mRNA expression efficiently. No contaminating genomic DNA was detected (data not shown).
Figure 2.
HDAC inhibitors diminish TLR-induced IL-12p40 transcription. (a) BMDCs were pretreated for 1 hr with TSA, then stimulated with LPS (100 ng/ml) or CpG-DNA (1 μm). After 3 hr mRNA was isolated and expression of IL-12p40 was quantified by quantitative RT-PCR. The relative expression of IL-12p40 mRNA in comparison to the mRNA encoding the housekeeping gene β-actin is shown (mean + SEM, n = 4). (b) BMM were treated with medium, LPS (10 ng/ml), TSA (500 nm) or LPS + TSA. After 3 hr, mRNA was isolated and expression of IL-12p40 was estimated, relative to hprt, by quantitative RT-PCR. (c) Total RNA was isolated from BMDCs preincubated with TSA and stimulated with CpG or LPS. RT-PCR assays were performed using primer pairs that amplify pre-mRNA from intron sequences. The isolated cDNA was checked for contamination with genomic DNA applying a control cDNA preparation without reverse transcriptase (no RT) and also with primers against non-transcribed regions within the IL-12p40 promoter. Data are triplicates of quantitative RT-PCR results from one of three independent experiments (mean + SD).
HDAC inhibition abolishes TLR-induced IL-12p40 chromatin remodelling and transcription factor recruitment
A prerequisite for efficient IL-12p40 transcription is chromatin remodelling, namely nucleosomal sliding, followed by Rel-A binding within the promoter.5,6 To gain further insight into the mechanism(s) underlying the suppressive effect of HDAC inhibitors on TLR-dependent activation of IL-12p40 expression, we assessed Rel-A recruitment to the IL-12p40 promoter using ChIP assays. Stimulation of dendritic cells with CpG-DNA enhanced the association of Rel-A to the IL-12p40 promoter (close to the TATA-Box), an effect that was suppressed by either SAHA (Fig. 3a) or TSA (data not shown). C/EBP has been reported to synergize with Rel-A in IL-12p40 induction.33 Interestingly, recruitment of C/EBP to the IL-12p40 promoter was not inhibited by treatment with SAHA (Fig. 3c). Apart from NF-κB, members of the IRF-family have been reported to regulate IL-12p40.34–36 We therefore examined the effect of HDAC inhibitors on the CpG-DNA-induced recruitment of IRF1, IRF2 and IRF7 to the IL12-p40 promoter region by ChIP assays. We could not detect binding of these transcription factors to a well-defined region close to the TATA-box of the IL-12p40 promoter (data not shown). However, an additional DNAse hypersensitive site 11 kb upstream of the TATA box has been described in the IL-12p40 promoter.37 CpG-DNA induced the recruitment of IRF1 (Fig. 3b), but not IRF2, IRF7 or Rel-A (data not shown), to this regulatory region in the IL-12p40 gene, as assessed by ChIP assays. Further, treatment with HDAC inhibitors blocked the CpG-DNA-induced recruitment of IRF1 to this site. As transcription factor access was decreased upon SAHA or TSA treatment, we next examined nucleosome remodelling within the IL-12p40 promoter. HDAC inhibitors significantly reduced CpG-DNA induced chromatin remodelling as assessed by CHART assays (Fig. 3d), consistent with the inhibitory effect on recruitment of Rel-A and IRF-1 to regulatory regions within the IL-12p40 promoter.
Figure 3.
TLR-induced nucleosome remodelling and transcription factor recruitment is targeted by HDAC inhibitors. BMDCs were preincubated with SAHA (1 μm) for 1 hr and stimulated with CpG-DNA (1 μm) for 2 hr. ChIP assays were carried out from nuclear extracts with anti-RelA (a), anti-IRF1 (b), C/EBPβ (c), anti-IRF2 and anti-IRF7 (data not shown) antibodies and recruitment to the IL-12p40 promoter was measured by quantitative PCR using primers against (a, c) the known NF-κB binding site,52 and (b) a hypersensitive site in the IL-12p40 promoter 11 kb upstream of the TATA box.37 (d) BMDCs were preincubated with SAHA (1 μm) or TSA (10 nm) for 1 hr and stimulated with CpG-DNA. Nuclear extracts were tested for restriction enzyme accessibility by ChART assay. Chromatin remodelling is plotted as a percentage of non-stimulated cells. Data are triplicates of PCR results from two independent experiments.
The effect of HDAC inhibitors on TLR-induced cytokine expression is independent from MAP kinase and NF-κB activation. The demonstration that TSA and SAHA suppressed the induction of IL-12p40 in response to multiple TLR agonists (Fig. 1d) suggested that HDAC inhibitors may target a common activation pathway. We therefore investigated the influence of TSA on early TLR signalling events. Stimulation of DCs with LPS resulted in a time-dependent phosphorylation of JNK, ERK and p38, and TSA did not affect these responses (Fig. 4a). This experiment was performed with a higher concentration of 50 nm TSA to avoid missing weak effects. Similar results were obtained for SAHA and for stimulation with CpG-DNA (data not shown).
Figure 4.
HDAC inhibitors do not affect upstream MAP kinase and NF-κB signalling. BMDCs were preincubated with TSA (50 nm) for 1 hr and stimulated with LPS (10 ng/ml) for the time indicated. Cell lysates were prepared and immunoblotting was performed using (a) phosphospecific antibodies against JNK, ERK and p38 as well as antibody against IκBα. Equivalent protein loading was checked using an antibody against β-actin. (b) Stimulated cells from above were also analysed by EMSA. Nuclear extracts were prepared and analysed for DNA-binding activities of NF-κB using an oligonucleotide with a canonical NF-κB-binding motif. Results are representative of three independent experiments.
Next we examined the influence of HDAC inhibitors on NF-κB activation. Stimulation of dendritic cells with LPS resulted in a time-dependent increase of nuclear NF-κB Rel-A/c-Rel DNA-binding activity as determined by EMSA. Again, TSA had no obvious effects on NF-κB translocation (Fig. 4b). Similarly, LPS-induced IκBα degradation was unaffected by TSA (Fig. 4a). Thus, classical signalling events that are upstream of IL-12p40 gene expression were not affected by HDAC inhibition.
HDAC inhibitors differentially affect regulation of further TLR-induced target genes
Since HDAC inhibitors impaired both the recruitment of IRF1 to the IL-12p40 promoter and IL-12p40 mRNA expression, we postulated that they would also inhibit the expression of other TLR-inducible IRF1 target genes. In DC, induction of interferon-β (IFN-β) by CpG-DNA is dependent on IRF138 and Fig. 5(a) shows that TSA inhibited CpG-DNA-induced IFN-β mRNA expression in these cells. Corroborating this finding, STAT-1 tyrosine phosphorylation, which is indicative of autocrine IFN-β signalling, was also abolished (data not shown). In contrast, TLR-mediated up-regulation of IL-1β and TNF-α mRNA expression was not affected by TSA in DC, again indicating that the effect was selective. Induction of IL-6 mRNA was only modestly affected.
Figure 5.
SAHA and TSA differentially inhibit TLR-induced mRNA expression of various proinflammatory cytokines and affect regulation of costimulatory molecules. (a) BMDCs were preincubated with TSA (10 nm) for 1 hr and stimulated with CpG-DNA (1 μm) for 3 hr. Messenger RNA expression of IFN-β, TNF-α, IL-6 and IL-1β was quantified by quantitative RT-PCR. The relative expression of target genes in comparison to β-actin is shown. (b) BMM were treated with medium, LPS (10 ng/ml), TSA (500 nm) or LPS + TSA for 3 hr. Levels of IFN-β, TNF-α, IL-6 and IL-1β mRNA were estimated by quantitative RT-PCR. The relative expression of target genes in comparison to hprt is shown. (c) HMDM were treated with medium, LPS (10 ng/ml) or LPS + TSA (500 nm) for 4 hr. Levels of IFN-β, IL-6 and IL-1β mRNA, relative to hprt mRNA, were estimated by quantitative RT-PCR. (d) BMDCs were stimulated with LPS in the presence of SAHA or TSA overnight. Expression of CD86, MHC-class II and CD40 was determined on CD11c+ cells by flow cytometry. Unstimulated (filled grey); LPS (black lines).
In macrophages, CpG DNA is a weak stimulus for IFN-β production.38 However, LPS, which can signal in a MyD88-independent fashion, elicits robust IFN-β production via activation of IRF3 in this cell type. We wondered whether HDAC inhibitors might also target the action of IRF family members other than IRF1, and thus assessed the effects of TSA on LPS-induced IFN-β mRNA expression in BMM. Indeed, TSA significantly inhibited this response (Fig. 5b). Consequently, type I IFN-dependent genes (nitric oxide synthetase and guanylate-binding protein 2) were also targeted by TSA in BMM (data not shown). LPS-induced expression of IL-6 mRNA was also dramatically impaired, and this effect was much more potent than was observed in DCs using CpG-DNA as the stimulus (Fig. 5a,b). However, as with DCs, the inhibitory effects on TLR-inducible gene expression in BMMs were selective; TSA did not affect TLR-mediated induction of IL-1β or TNF-α. We also assessed the effect of TSA on LPS-regulated gene expression in human monocyte-derived macrophages. Figure 5(c) shows that the effects of HDAC inhibition were conserved between mouse and human macrophages; TSA targeted LPS-inducible expression of IL-6 and IFN-β, but not IL-1β.
HDAC inhibitors negatively affect TLR-induced DC maturation
Type I IFN signalling has also been implicated in the up-regulation of costimulatory molecules on DCs upon TLR stimulation.39 Consistent with this, LPS-mediated up-regulation of CD86 was blocked by SAHA or TSA (Fig. 5d). There was also an inhibitory effect on cell surface MHC-class II expression. Regarding CD40, SAHA and TSA by themselves actually up-regulated expression, but also inhibited the additional increase of CD40 expression by LPS.
Additional effects of long-term HDAC inhibitor pretreatment in DC
Although our data indicated that TLR-inducible expression of TNF-α was not affected by HDAC inhibition, others have reported that this gene was sensitive to SAHA.20 We therefore investigated whether prolonged pretreatment with HDAC inhibitors would affect TLR-inducible TNF-α. Figure 6(a) shows that while pretreatment of DC with TSA for 1 hr impaired TLR-inducible IL-12p40, but not TNF-α expression, longer pretreatment times (3 hr or 5 hr, Fig. 6a and data not shown) inhibited both IL-12p40 and TNF-α expression. Similarly, IL-6 was more efficiently reduced with prolonged TSA-pretreatment (data not shown). In contrast, induction of IL-1β again was not impaired by TSA irrespective of the pretreatment time. Consistent with the mRNA expression data, prolonged pretreatment of DC with TSA or SAHA for 3 hr instead of 1 hr inhibited the secretion of TNF-α protein (Fig. 6b).
Figure 6.
Longer pretreatment with HDAC inhibitors reveals additional effects on TLR-regulated gene expression. (a) BMDCs were preincubated with HDAC inhibitors SAHA or TSA for 1 or 3 hr and stimulated with CpG-DNA or LPS for an additional 3 hr. IL-12p40, TNF-α and IL-1β mRNA levels were quantified by quantitative RT-PCR. (b) BMDCs were pretreated as above with TSA or SAHA and further stimulated with CpG-DNA or LPS for 14 hr. TNF-α in culture supernatant was measured by ELISA (mean + SEM, n = 3).
Discussion
Few studies have assessed the contribution of HDACs to TLR-regulated gene expression. Some studies have identified TLR-inducible genes in which HDACs act in a classical fashion to down-regulate or inactivate gene expression; such genes are typically up-regulated by HDAC inhibitors. For example, HDACs act to shut down a set of LPS-inducible genes including Cox-2 in macrophages.23 Similarly, the IL-12p40 promoter is also known to be negatively regulated by HDAC1.10 HDAC1 belongs to the class I HDAC family, and emerging literature implicate class I HDACs as negative regulators of TLR-inducible genes.40–42
Despite the literature implicating HDACs as negative regulators of TLR-inducible genes, we show here that HDACs are also required for efficient TLR-mediated induction of the IL-12p40 gene in DCs and macrophages (Figs 1 and 2a,b). This finding is supported by similar observations in simian virus 40-transformed lung epithelial cells.43 Other studies have recently identified additional genes that also require HDAC activity for efficient induction in response to LPS.20,23,24 We further extend these findings to show that the effects of HDAC inhibitors were apparent with different TLRs; we observed a reduction in IL-12p40 expression after stimulation with CpG-DNA (TLR9), LPS (TLR4), poly (I:C) (TLR3) and Pam3CSK4 (TLR2/1).
Although the studies above have documented the requirement for HDACs in LPS-inducible gene expression, they have not characterized the mechanisms responsible. We used the IL-12p40 gene as a model to address this question. Firstly, we showed that the targeting of TLR-induced IL-12p40 protein production by HDAC inhibitors (Fig. 1d) did not occur at the level of cytokine secretion (Fig. 1f). Next we showed that HDAC inhibitors most likely act by directly targeting IL-12p40 transcription or a process upstream from transcription (Fig. 2). In additional experiments we excluded the possibility of HDAC inhibitors affecting TLR-mediated activation of the MAP-kinases JNK, ERK and p38 or NF-κB (Fig. 4). These findings strongly support the assumption that HDAC inhibitors must affect either one or more of the following processes: transcription factor binding, nucleosomal remodelling, formation of the transcription initiation complex or transcription itself.
Certainly, histone acetylation is known to be involved in activation of the IL-12p40 promoter in response to TLR stimulation44,45 (Fig. 1a). As might be predicted, treatment with HDAC inhibitors resulted in a continuous increase of histone H4 acetylation over the time period of the experiments. Histone H4 acetylation has been shown to correlate with activation of LPS-inducible gene expression and contrasts with our observation that HDAC inhibitors actually impaired TLR-inducible IL-12p40 expression. We conclude that, while HDACs can negatively regulate IL-12p40 expression via histone deacetylation, HDAC activity is also absolutely required for the induction of IL-12p40 expression via alternative mechanisms.
Our data suggest that HDAC inhibitors most probably act by targeting transcription factor recruitment. CpG-DNA-induced nucleosome remodelling was impaired by HDAC inhibitors (Fig. 3d). Using ChIP-assays we showed that HDAC inhibitors abrogated TLR-induced Rel-A and IRF-1 recruitment to the IL-12p40 promoter. Interestingly, C/EBPβ recruitment was not altered by SAHA treatment. In different cellular systems, HDAC inhibitors have been reported by other groups to reduce the recruitment of transcription factors to various promoters. Yamaguchi and co-workers showed that phorbol 12-myristate 13-acetate (PMA) -induced expression of cyclo-oxygenase-2 (Cox-2), cyclin D1 and collagenase-1, all of which are c-jun-dependent genes, was strongly inhibited by TSA and SAHA.46 Corroborating our findings, no effect of TSA on PMA-induced signalling pathways was observed in this system. Sakamoto and co-workers reported that TSA inhibited selected IFN-β-stimulated immediate early genes42 by reducing the recruitment of RNA-polymerase II to the ISG54 promoter.
One mechanism by which HDACs may positively regulate transcription factor recruitment to promoters is via direct post-translational modification. Recent studies have revealed that, similar to histone proteins, transcription factors such as p53, GATA, Smad7, Stat 3 and NF-κB are subject to reversible acetylation.47 Histone acetyltransferases, such as p300/CBP, not only target histone proteins but also modify various non-histone proteins. Such post-translational modifications are involved in the regulation of the respective transcriptional activity. Certain HDACs, as exemplified for HDAC3, are involved in deacetylation of non-histone proteins.48,49 Thus, it is likely that HDAC inhibitors like SAHA and TSA also alter the acetylation status of various transcription factors.
On the basis of HDAC inhibitor sensitivity, we identified three distinct classes of TLR-induced cytokines. One group of TLR-induced cytokines/chemokines was strongly inhibited by HDAC inhibitors. As well as IL-12p40, this group included IFN-β, IP-10, MCP5, inducible nitric oxide synthetase, guanylate-binding protein 2 and IL-12p35 (Fig. 5 and data not shown). This effect on IFN-β, as well as IFN-β-dependent gene expression, is consistent with a recent report that demonstrated that HDAC activity was required for IFN-β expression in response to virus challenge.50 A second group of genes including TNF-α needed a prolonged pretreatment with HDAC inhibitors to be significantly inhibited. Interleukin-6 behaved slightly differently, needing prolonged HDAC inhibitor treatment in some cells while being directly sensitive in other cells. The need for prolonged HDAC inhibitor treatment suggests that indirect mechanisms may be responsible and indeed, HDAC inhibitors have been reported to indirectly target LPS-induced expression of the chemokines Ccl2 and Ccl7.23 Finally, we observed that IL-1β mRNA expression was not affected by HDAC inhibitors under any experimental conditions. In murine bone marrow-derived macrophages, no stimulus-induced chromatin alterations occurred in the IL-1 promoter, in contrast to findings reported for the IFN-β and IL-12p40 promoters. Furthermore, it has been shown that the IL-1β promoter shows no, or only minor, stimulus-induced alterations in histone acetylation.42,51 These data support our findings that show that the induction of IL-1β expression was not targeted by HDAC inhibitors.
To date, dual functions of HDACs have mainly been reported for IFN signaling.40–42 In summary, we have extended this literature to show that HDACs are required for nucleosome remodelling and for the recruitment of IRF and Rel family transcription factors to a subset of TLR-target genes. Given that HDAC inhibitors show promise as anti-inflammatory agents, the identification of specific HDACs that positively regulate pro-inflammatory gene expression may guide the design of more selective HDAC inhibitors for therapeutic applications in the inflammation field.
Acknowledgments
This project has been supported by: Grant from the Deutsche Forschungsgemeinschaft to A.D. (DFG Da 592/2), grant from the NHMRC, Australia to M.S. and research grant for the Scripps NeuroAIDS Preclinical Studies Center (SNAPS) from the National Institute of Mental Health (NIMH), grant number: 2P30MH062261-07 to T.R. We appreciate the excellent technical support by Adelina Dillmann, Stefanie Penati and Rene Karagyilan.
Abbreviations:
- AP-1
activator protein-1
- BMM
bone-marrow-derived macrophages
- BMDC
bone-marrow-derived dendritic cells
- ChIP
chromatin immunoprecipitation
- COX
cyclo-oxygenase
- CBP
CREB-binding protein
- CREB
cyclic adenosine monophosphate [cAMP]-response element binding protein
- EMSA
electrophoretic mobility shift
- HDAC
histone deacetylase
- hprt
hypoxanthine guanine phosphoribosyl transferase
- IFN
interferon
- IRF
interferon regulatory factor
- LPS
lipopolysaccharide
- poly (I:C)
poly inosyl : cytosine
- PMA
phorbol 12-myristate 13-acetate
- Pam3CSK4
Pam3Cys-Ser-(Lys)4
- SAHA
suberoylanilide hydroxamic acid
- TLR
Toll-like receptors
- TSA
trichostatin A.
References
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–58. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- 3.O'Neill LA. How Toll-like receptors signal: what we know and what we don't know. Curr Opin Immunol. 2006;18:3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 5.Albrecht I, Tapmeier T, Zimmermann S, Frey M, Heeg K, Dalpke A. Toll-like receptors differentially induce nucleosome remodelling at the IL-12p40 promoter. EMBO Report. 2004;5:172–7. doi: 10.1038/sj.embor.7400078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinmann AS, Plevy SE, Smale ST. Rapid and selective remodeling of a positioned nucleosome during the induction of IL-12 p40 transcription. Immunity. 1999;11:665–75. doi: 10.1016/s1074-7613(00)80141-7. [DOI] [PubMed] [Google Scholar]
- 7.Chan HM, La Thangue NB. p300/CBP proteins. HATs for transcriptional bridges and scaffolds. J Cell Sci. 2001;114:2363–73. doi: 10.1242/jcs.114.13.2363. [DOI] [PubMed] [Google Scholar]
- 8.Avots A, Buttmann M, Chuvpilo S, et al. CBP/p300 integrates Raf/Rac-signaling pathways in the transcriptional induction of NF-ATc during T cell activation. Immunity. 1999;10:515–24. doi: 10.1016/s1074-7613(00)80051-5. [DOI] [PubMed] [Google Scholar]
- 9.Bannert N, Avots A, Baier M, Serfling E, Kurth R. GA-binding protein factors, in concert with the coactivator CREB binding protein/p300, control the induction of the interleukin 16 promoter in T lymphocytes. Proc Natl Acad Sci USA. 1999;96:1541–6. doi: 10.1073/pnas.96.4.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu J, Sun H, Wang X, Liu C, Xu X, Li F, Huang B. Interleukin-12 p40 promoter activity is regulated by the reversible acetylation mediated by HDAC1 and p300. Cytokine. 2005;31:46–51. doi: 10.1016/j.cyto.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Sisk TJ, Gourley T, Roys S, Chang CH. MHC class II transactivator inhibits IL-4 gene transcription by competing with NF-AT to bind the coactivator CREB binding protein (CBP)/p300. J Immunol. 2000;165:2511–17. doi: 10.4049/jimmunol.165.5.2511. [DOI] [PubMed] [Google Scholar]
- 12.Vanden Berghe W, De Bosscher K, Boone E, Plaisance S, Haegeman G. The nuclear factor-kappaB engages CBP/p300 and histone acetyltransferase activity for transcriptional activation of the interleukin-6 gene promoter. J Biol Chem. 1999;274:32091–8. doi: 10.1074/jbc.274.45.32091. [DOI] [PubMed] [Google Scholar]
- 13.Sengupta N, Seto E. Regulation of histone deacetylase activities. J Cell Biochem. 2004;93:57–67. doi: 10.1002/jcb.20179. [DOI] [PubMed] [Google Scholar]
- 14.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 15.Hoshikawa Y, Kwon HJ, Yoshida M, Horinouchi S, Beppu T. Trichostatin A induces morphological changes and gelsolin expression by inhibiting histone deacetylase in human carcinoma cell lines. Exp Cell Res. 1994;214:189–97. doi: 10.1006/excr.1994.1248. [DOI] [PubMed] [Google Scholar]
- 16.Miyashita T, Yamamoto H, Nishimune Y, Nozaki M, Morita T, Matsushiro A. Activation of the mouse cytokeratin A (endo A) gene in teratocarcinoma F9 cells by the histone deacetylase inhibitor Trichostatin A. FEBS Lett. 1994;353:225–9. doi: 10.1016/0014-5793(94)01034-x. [DOI] [PubMed] [Google Scholar]
- 17.Futamura M, Monden Y, Okabe T, Fujita-Yoshigaki J, Yokoyama S, Nishimura S. Trichostatin A inhibits both ras-induced neurite outgrowth of PC12 cells and morphological transformation of NIH3T3 cells. Oncogene. 1995;10:1119–23. [PubMed] [Google Scholar]
- 18.Kim YB, Ki SW, Yoshida M, Horinouchi S. Mechanism of cell cycle arrest caused by histone deacetylase inhibitors in human carcinoma cells. J Antibiot (Tokyo) 2000;53:1191–200. doi: 10.7164/antibiotics.53.1191. [DOI] [PubMed] [Google Scholar]
- 19.Reilly CM, Mishra N, Miller JM, et al. Modulation of renal disease in MRL/lpr mice by suberoylanilide hydroxamic acid. J Immunol. 2004;173:4171–8. doi: 10.4049/jimmunol.173.6.4171. [DOI] [PubMed] [Google Scholar]
- 20.Leoni F, Zaliani A, Bertolini G, et al. The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc Natl Acad Sci USA. 2002;99:2995–3000. doi: 10.1073/pnas.052702999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi JH, Oh SW, Kang MS, Kwon HJ, Oh GT, Kim DY. Trichostatin A attenuates airway inflammation in mouse asthma model. Clin Exp Allergy. 2005;35:89–96. doi: 10.1111/j.1365-2222.2004.02006.x. [DOI] [PubMed] [Google Scholar]
- 22.Camelo S, Iglesias AH, Hwang D, et al. Transcriptional therapy with the histone deacetylase inhibitor trichostatin A ameliorates experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;164:10–21. doi: 10.1016/j.jneuroim.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Aung HT, Schroder K, Himes SR, et al. LPS regulates proinflammatory gene expression in macrophages by altering histone deacetylase expression. FASEB J. 2006;20:1315–27. doi: 10.1096/fj.05-5360com. [DOI] [PubMed] [Google Scholar]
- 24.Brogdon J, Xu Y, Szabo S, An S, Buxton F, Cohen D, Huang Q. Histone deacetylase activities are required for innate immune cell control of Th1 but not Th2 effector cell function. Blood. 2007;109:1123–30. doi: 10.1182/blood-2006-04-019711. [DOI] [PubMed] [Google Scholar]
- 25.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray PJ. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proc Natl Acad Sci USA. 2005;102:8686–91. doi: 10.1073/pnas.0500419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saccani S, Pantano S, Natoli G. p38-dependent marking of inflammatory genes for increased NF-kappa B recruitment. Nat Immunol. 2002;3:69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- 28.Bosisio D, Marazzi I, Agresti A, Shimizu N, Bianchi ME, Natoli G. A hyper-dynamic equilibrium between promoter-bound and nucleoplasmic dimers controls NF-kappaB-dependent gene activity. EMBO J. 2006;25:798–810. doi: 10.1038/sj.emboj.7600977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrews NC, Faller DV. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucl Acids Res. 1991;19:2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao S, Procko E, Shannon MF. Chromatin remodeling, measured by a novel real-time polymerase chain reaction assay, across the proximal promoter region of the IL-2 gene. J Immunol. 2001;167:4494–503. doi: 10.4049/jimmunol.167.8.4494. [DOI] [PubMed] [Google Scholar]
- 31.Dai Y, Rahmani M, Dent P, Grant S. Blockade of histone deacetylase inhibitor-induced RelA/p65 acetylation and NF-κB activation potentiates apoptosis in leukemia cells through a process mediated by oxidative damage, XIAP downregulation, and c-Jun N-terminal kinase 1 activation. Mol Cell Biol. 2005;25:5429–44. doi: 10.1128/MCB.25.13.5429-5444.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sweet MJ, Campbell CC, Sester DP, et al. Colony-stimulating factor-1 suppresses responses to CpG DNA and expression of Toll-like receptor 9 but enhances responses to lipopolysaccharide in murine macrophages. J Immunol. 2002;168:392–9. doi: 10.4049/jimmunol.168.1.392. [DOI] [PubMed] [Google Scholar]
- 33.Plevy SE, Gemberling JH, Hsu S, Dorner AJ, Smale ST. Multiple control elements mediate activation of the murine and human interleukin 12 p40 promoters. Evidence of functional synergy between C/EBP and Rel proteins. Mol Cell Biol. 1997;17:4572–88. doi: 10.1128/mcb.17.8.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Negishi H, Fujita Y, Yanai H, et al. Evidence for licensing of IFN-gamma-induced IFN regulatory factor 1 transcription factor by MyD88 in Toll-like receptor-dependent gene induction program. Proc Natl Acad Sci USA. 2006;103:15136–41. doi: 10.1073/pnas.0607181103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takaoka A, Yanai H, Kondo S, et al. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434:243–9. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- 36.Salkowski CA, Kopydlowski K, Blanco J, Cody MJ, McNally R, Vogel SN. IL-12 is dysregulated in macrophages from IRF-1 and IRF-2 knockout mice. J Immunol. 1999;163:1529–36. [PubMed] [Google Scholar]
- 37.Zhou L, Nazarian AA, Smale ST. Interleukin-10 inhibits interleukin-12 p40 gene transcription by targeting a late event in the activation pathway. Mol Cell Biol. 2004;24:2385–96. doi: 10.1128/MCB.24.6.2385-2396.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitz F, Heit A, Guggemoos S, et al. Interferon-regulatory-factor 1 controls Toll-like receptor 9-mediated IFN-beta production in myeloid dendritic cells. Eur J Immunol. 2007;37:315–27. doi: 10.1002/eji.200636767. [DOI] [PubMed] [Google Scholar]
- 39.Hoebe K, Janssen EM, Kim SO, Alexopoulou L, Flavell RA, Han J, Beutler B. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nat Immunol. 2003;4:1223–9. doi: 10.1038/ni1010. [DOI] [PubMed] [Google Scholar]
- 40.Klampfer L, Huang J, Swaby LA, Augenlicht L. Requirement of histone deacetylase activity for signaling by STAT1. J Biol Chem. 2004;279:30358–68. doi: 10.1074/jbc.M401359200. [DOI] [PubMed] [Google Scholar]
- 41.Nusinzon I, Horvath CM. Positive and negative regulation of the innate antiviral response and beta interferon gene expression by deacetylation. Mol Cell Biol. 2006;26:3106–13. doi: 10.1128/MCB.26.8.3106-3113.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakamoto S, Potla R, Larner AC. Histone deacetylase activity is required to recruit RNA polymerase II to the promoters of selected interferon-stimulated early response genes. J Biol Chem. 2004;279:40362–7. doi: 10.1074/jbc.M406400200. [DOI] [PubMed] [Google Scholar]
- 43.Iwata K, Tomita K, Sano H, Fujii Y, Yamasaki A, Shimizu E. Trichostatin A, a histone deacetylase inhibitor, down-regulates interleukin-12 transcription in SV-40-transformed lung epithelial cells. Cell Immunol. 2002;218:26–33. doi: 10.1016/s0008-8749(02)00523-3. [DOI] [PubMed] [Google Scholar]
- 44.Sun H, Lu J, Wei L, Wang X, Xu X, Dong M, Huang B. Histone acetyltransferase activity of p300 enhances the activation of IL-12 p40 promoter. Mol Immunol. 2004;41:1241–6. doi: 10.1016/j.molimm.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Weinmann AS, Mitchell DM, Sanjabi S, Bradley MN, Hoffmann A, Liou HC, Smale ST. Nucleosome remodeling at the IL-12 p40 promoter is a TLR-dependent, Rel-independent event. Nat Immunol. 2001;2:51–7. doi: 10.1038/83168. [DOI] [PubMed] [Google Scholar]
- 46.Yamaguchi K, Lantowski A, Dannenberg AJ, Subbaramaiah K. Histone deacetylase inhibitors suppress the induction of c-Jun and its target genes including COX-2. J Biol Chem. 2005;280:32569–77. doi: 10.1074/jbc.M503201200. [DOI] [PubMed] [Google Scholar]
- 47.Chen LF, Greene WC. Regulation of distinct biological activities of the NF-kappaB transcription factor complex by acetylation. J Mol Med. 2003;81:549–57. doi: 10.1007/s00109-003-0469-0. [DOI] [PubMed] [Google Scholar]
- 48.Yuan R, Guan R, Shen W, Zheng J. Photocatalytic degradation of methylene blue by a combination of TiO2 and activated carbon fibers. J Colloid Interface Sci. 2005;282:87–91. doi: 10.1016/j.jcis.2004.08.143. [DOI] [PubMed] [Google Scholar]
- 49.Chen L, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293:1653–7. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- 50.Nusinzon I, Horvath CM. Interferon-stimulated transcription and innate antiviral immunity require deacetylase activity and histone deacetylase. Proc Natl Acad Sci USA. 2003;100:14742–7. doi: 10.1073/pnas.2433987100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang MD, Zhang Y, McDevit D, Marecki S, Nikolajczyk BS. The interleukin-1beta gene is transcribed from a poised promoter architecture in monocytes. J Biol Chem. 2006;281:9227–37. doi: 10.1074/jbc.M510700200. [DOI] [PubMed] [Google Scholar]
- 52.Murphy TL, Cleveland MG, Kulesza P, Magram J, Murphy KM. Regulation of interleukin 12 p40 expression through an NF-kappa B half-site. Mol Cell Biol. 1995;15:5258–67. doi: 10.1128/mcb.15.10.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]