Abstract
Osteopontin (OPN) is important for the function of fibroblasts, macrophages and lymphocytes during inflammation and wound healing. In recent studies of experimental colitis we demonstrated exacerbated tissue destruction in OPN-null mice, associated with reduced tumour necrosis factor-α expression and increased myeloperoxidase activity. The objective of this investigation therefore was to determine the importance of OPN expression in neutrophil function. Although, in contrast to macrophages, neutrophils expressed low levels of OPN with little or no association with the CD44 receptor, intraperitoneal recruitment of neutrophils in OPN-null mice was impaired in response to sodium periodate. The importance of exogenous OPN for neutrophil recruitment was demonstrated by a robust increase in peritoneal infiltration of PMNs in response to injections of native or recombinant OPN. In vitro, OPN–/– neutrophils exhibited reduced chemokinesis and chemotaxis towards N-formyl methionyl leucyl phenylalanine (fMLP), reflecting a reduction in migration speed and polarization. Exogenous OPN, which was chemotactic for the neutrophils, rescued the defects in polarization and migration speed of the OPN–/– neutrophils. In contrast, the defensive and cytocidal activities of OPN–/– neutrophils, measured by assays for phagocytosis, generation of reactive oxygen species, cytokine production and matrix metalloproteinase-9, were not impaired. These studies demonstrate that, while exogenous OPN may be important for the recruitment and migration of neutrophils, expression of OPN by neutrophils is not required for their destructive capabilities.
Keywords: CD44, migration, neutrophils, osteopontin, phagocytosis
Introduction
Osteopontin (OPN) is a multifunctional cytokine that is up-regulated in a broad range of inflammatory diseases involving the brain, liver, gastrointestinal tract, lung, bone, cardiac tissue, joints and kidney.1–4 In recent studies we observed an exacerbation of intestinal tissue destruction in acute colitis induced in OPN-null mice.5 These findings contrast with the attenuation of experimental inflammatory disease observed in non-luminal tissues in OPN-null mice,6–11 and emphasize the importance of OPN in mucosal protection.4 Notably, OPN expression during inflammation is not limited to specific cell lineages but involves many different cells, including epithelial, mesenchymal, as well as immune, cells.3 The increased expression of OPN is reflected in elevated concentrations of OPN in tissue fluids and plasma and has been associated with increased cell mobilization, survival and activity. Many of the effects of OPN on macrophages and fibroblasts, which have a central role in regulating inflammatory and fibrotic responses, involve interactions with integrins and the CD44 receptor and are mediated by the cytoskeleton.12
Polymorphonuclear leucocytes (neutrophils, PMNs) and macrophages are rapidly activated to migrate to sites of infection in response to inflammatory signals. Migration of these cells is dependent upon cytoskeletal rearrangements that allow the cells to adopt a polarized morphology and directional movement in response to chemotactic stimuli. Neutrophils are primarily responsible for the elimination of endogenous and exogenous noxious stimuli using a combination of phagocytosis, respiratory burst and release of proteases and cytotoxic mediators as the first line of host defence. Respiratory burst agonists include lipopolysaccharides (LPS), N-formyl methionyl leucyl phenylalanine (fMLP), and phorbol 12-myristate 13-acetate (PMA), which also stimulate the release of proinflammatory cytokines and phagocytosis. Under inflammatory conditions, rolling neutrophils attach to endothelial cells by reverse binding to transmembrane cell surface glycoprotein receptors. Although activated neutrophils migrating through the endothelial lining can cause tissue damage in facilitating access of immune cells to diseased sites, their presence is normally transient, allowing tissue remodelling to repair the damaged tissues. However, the persistent presence of hyperactivated neutrophils can cause irreparable tissue damage that impacts on organ function, as observed in colitis and other inflammatory diseases.
Previous studies have shown that OPN has a pivotal role in the development and maintenance of immune responses through its regulation of macrophage, lymphocyte and fibroblast activities. When challenged, all of these cells express high levels of OPN and exhibit impaired migration and cytokine production in the absence of OPN expression.4,7,11,13–16 OPN regulates cytokine expression by macrophages through interactions with cell surface integrins and CD44 receptors15 while extracellular and intracellular interactions between OPN and CD44 have been implicated in the formation of cell processes and macrophage migration.17,18 However, whereas CD44 is also required for the polarization and directed migration of neutrophils, which migrate more rapidly than macrophages, the expression and localization of CD44 is largely independent of OPN,4 suggesting that OPN may function differently in neutrophils.
We have observed an exacerbation of intestinal tissue destruction in OPN-null mice in response to colitis, which correlated with a suppressed tumour necrosis factor (TNF-α) response and increased myeloperoxidase activity,5 indicative of increased neutrophil activity. Notably, a similar pattern of destruction caused by the persistence of neutrophils has been observed in experimental colitis induced in TNF-α null mice.20 Thus, the objective of this investigation was to study the relationship between OPN expression and neutrophil function. Whereas recruitment of neutrophils was found to be impaired in OPN-null mice, the absence of OPN expression showed only modest effects on the polarization and directed migration of neutrophils and did not influence their destructive potential.
Materials and methods
Animals and cell preparations
Generation of the OPN-null mice has been described previously.21 The original 129sv F2 mice were subsequently back-crossed (10 times) into a C57BL/6 J background. Adult, 8-week-old OPN-null mice and their matched C57BL/6 J wild-type (WT) controls were used for these experiments. Animal experiments were conducted according to guidelines established by the Animal Care Committee of the University of Toronto. Femurs and tibias were dissected and after flushing out the bone marrow cells the neutrophils were isolated on an 82%/65%/55% Percoll gradient (Sigma, Oakville, ON, Canada). Following Wright–Giemsa staining,22 the cell preparations were shown to consistently contain >90% neutrophils.
Analysis of OPN expression by reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from WT and OPN–/– neutrophils and from RAW 264.7 macrophages using the Absolutely RNA miniprep kit (Stratagene, Sedar Creek, TX) and the purity was assessed spectrophotometrically. First-strand cDNA reactions were performed on 1 μg RNA in 20 μl 1 × PCR buffer II, containing 5 mm MgCl2, 1 mm each of the dNTPs, 1 U/μl RNAse inhibitor, 2·5 U/μl murine leukaemia virus (MuLV) reverse transcriptase 2·5 μm random hexamers (Applied Biosystems, Foster City, CA) at 42° for 60 min. The cDNA (2·5 μl) was then amplified by PCR using Eppendorf MasterMix (Eppendorf North America Inc., New York, NY) in a 25-μl reaction volume in a GeneAmp PCR system 2400 (Perkin Elmer; Life Sciences, Boston, MA) for 35 cycles after an initial 30-second denaturation at 94°, annealing for 30 seconds at 55° and extending for 30 seconds at 72°. The sequences of the primers used for the amplification of murine OPN were: 5′-AGCCAAGCTATCACCTCGG-3′ and 5′-GGTTTGCAGTCTTCTGCGGC-3′, which generated an amplicon of 408 base pairs (bp). The PCR products were resolved by electrophoresis on a 2% Nuseive gel and visualized by ethidium bromide staining. Quantitative real-time PCR was perfomed using Taqman PCR master mix and the ABI 7900 HT Fast Real-Time PCR system (Applied Biosystems, Foster City, CA), and primer sets from ABI. The amplifications were performed as follows: 2 min at 50°, 10 min at 95°, then 95° for 15 seconds and 60° for 60 seconds. The results were normalized to an internal control transcript encoding glyceraldehyde 3-phosphate dehydrogenase (GAPDH). No PCR products were generated from RNA before reverse transcription, indicating the absence of genomic DNA.
Immunofluorescence staining and confocal microscopy
Neutrophils were plated in eight-well chamber slides (Laboratory-TekTM; BD Falcon, Bedford, MA) supplemented with α-minimum essential medium (α-MEM) containing 5% fetal bovine serum and antibiotics (100 μg/ml penicillin G, 50 μg/ml gentamicin sulphate and 0·3 μg/ml fungizone). Neutrophils were allowed to attach, washed gently before fixing with paraformaldehyde [2% in phosphate-buffered saline (PBS), pH 7·4 for 1 hr], and then stained with a rabbit anti-porcine OPN antibody18 and a biotin-conjugated rat anti-mouse CD44 (Clone KM201, Cedarlane, Laboratories, Hornby, ONT, Canada), followed by Texas red fluor-tagged goat anti-rabbit F(ab′)2 fragment and fluorescein isothiocyanate (FITC) –streptavidin, respectively. Other cells were stained with tetramethylrhodamine isothiocyanate (TRITC) -phalloidin (Sigma Co., St Louis, MO). The immunostained cells were examined by laser confocal microscopy as described previously.18
Migration assays
Transwell chamber assays
For each group of mice, a neutrophil suspension (1 × 106/ml PBS) was plated onto fetal bovine serum-coated 3·0-μm pore polycarbonate membrane inserts in two wells of a 24-well Transwell plate (Corning Incorporated Life Sciences, Acton, MA), with 10−6 m fMLP (or PBS control) in the lower compartment as chemoattractant. Migration assays were also conducted to examine the effects of native OPN purified from RAW 264.7 cells on the migration when chemoattractants were included in the upper as well as the lower compartments. Neutrophils were allowed to migrate for 30 min at 37° through the membrane and attach to round, glass coverslips (12 mm diameter) placed at the bottom of the well. The number of attached cells on each coverslip was counted in 10 fields for each of the three replicate wells.
Zigmond chamber assays
Bone marrow neutrophils were suspended in PBS and 1% gelatin for Zigmond chamber chemotaxis analyses using 10−6 m fMLP as the chemoattractant; analyses were performed as described previously.22,23 To quantify the proportion of cells that were polarized, the total cells and the number of cells that were polarized in any direction or towards the chemotactic gradient (< 45° from the direction to the gradient) were counted in four areas of images using time-lapse video microscopy (Nikon Eclipse E400) equipped with differential interference contrast optics and a ×40 objective. Images were captured at 60-second intervals and analysed with Retrac software (http://mc11.mcri.ac.uk/) to characterize neutrophil chemotaxis. The data were expressed as means ± SD for each time-point.
In vivo migration
Peritoneal cell recruitment was induced in three groups of three 8-week-old mice per group with a single injection of PBS containing either: 20 μg native rodent macrophage OPN purified to homogeneity from RAW 264.7 cell-conditioned medium, 20 μg full-length recombinant rat OPN 5 mm sodium periodate (Sigma, Oakville, ON, Canada) or PBS alone (control). After 3·5 hr the animals were killed and 5 ml PBS was used as a lavage of the peritoneal cavity to collect the peritoneal cell infiltrate. The total cell number was counted and the relative percentage of neutrophils or mononuclear cells was determined by Wright–Giemsa staining.
Phagocytosis assays
General and specific phagocytosis by neutrophils stimulated with LPS (10 μg/ml in PBS) for 1 hr at 37° was assessed. For phagocytosis of polystyrene beads, Nile red (535/575) 1·0-μm carboxylate modified microspheres (Cat. No. T-8819; Molecular Probes, Eugene, OR) were opsonized with fetal calf serum, mixed with neutrophils at a ratio of 10 : 1 and incubated for 25 min at 37° in PBS. For measurement of phagocytosis through Fcγ or complement C5 receptors, sheep erythrocytes (sRBCs; ICN/Cappel, Aurora, OH) were washed with PBS and then opsonized with rabbit immunoglobulin G (IgG: ICN/Cappel; 1 : 2500) for 1 hr at 37°. Alexa 488-labelled monoclonal goat anti-rabbit secondary antibodies (Molecular Probes; 1 : 500) were added to label the sRBCs. For the complement pathway the sRBCs were opsonized with rabbit IgG (ICN/Cappel; 1 : 2500), and incubated with complement C5-deficient human serum (Sigma) for 30 min at 37°. Opsonized sRBC were labelled with Texas Red–sulphonyl chloride dye (Molecular Probes; 1 : 5) for 20 min at 4° and rinsed well. The labelled sRBCs were mixed with neutrophils at a ratio of 5 : 1 and incubated for 25 min at 37°; neutrophils were washed to remove non-phagocytosed RBCs before analyses. Phagocytosis was determined by flow cytometric analysis (Guava Personal Cell Analysis System; Guava Technologies Inc., Hayward, CA). The mean fluorescence index of >104 cells for triplicate samples was evaluated and the results were further confirmed by fluorescence microscopy (Nikon Eclipse CF160). The number of neutrophils with ingested erythrocytes/beads was counted for 100 neutrophils from each of the samples evaluated by flow cytometry.
Measurement of neutrophil NADPH oxidase activity
Neutrophil H2O2 production was assessed using the conversion of non-fluorescent dihydrorhodamine-123 reagent (DHR; Molecular Probes, Inc.) into rhodamine-123. Cells were incubated at 37° for 15 min with dihydrorhodamine-123 in the presence of fMLP or LPS; then, the mean fluorescence intensity of rhodamine 123 was detected by flow cytometry on a single-cell basis using a 490-nm filter; it was also confirmed by microscopy, as described above.
Cytokine analysis and gelatin enzymography
Suspended neutrophils (3 × 106 cells/ml PBS) were incubated with 0·1 μm PMA at 37° for 15 min and centrifuged for 5 min at 16 000 g. Samples of the supernatant (500 μl) were mixed with an equal volume of assay buffer and incubated for 1 hr at 21° with a murine cytokine array blot (TranSignal Mouse Cytokine Antibody Array 1·0; Panomics Inc., Fremont, CA). They were developed using chemiluminescence according to the manufacturer's instructions. Samples of supernatant (20 μl) and an equivalent sample of the cell pellets made soluble with lysis buffer were also electrophoresed on a non-reducing sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) gel (10% acrylamide and 0·1% gelatin) to analyse for gelatinase activity. The gels were washed twice for 10 min in 2·5% Triton X-100 to remove SDS and incubated in assay buffer (50 mm Tris–HCl, 0·2 m NaCl, 5 mm CaCl2, 0·5 μl/ml Brij35) for 24 hr at 21° before staining with 0·5% Coomassie brilliant blue solution.
Statistical analyses
The results were expressed as means ± SD unless stated otherwise. For all experiments, at least three replicates were included and experiments were repeated at least three times. For multiple comparisons, analysis of variance (anova) was performed.
Results
Expression of OPN in neutrophils
The expression of OPN mRNA in neutrophils was measured by semi-quantitative RT-PCR and real-time quantitative (q) RT-PCR. Much lower levels of OPN cDNA were amplified from the neutrophils compared to macrophages (Fig. 1a) and when analysed by qRT-PCR OPN expression was around 75 times lower than in RAW 264.7 cells and about 25 times less than in peritoneal macrophages (not shown). The distribution of OPN and its relationship to CD44 in neutrophils was also markedly different from that observed in activated macrophages and in fibroblasts18,24 (Fig. 1b). OPN expression (green) in neutrophils was observed in a punctate pattern throughout the cytoplasm while CD44 expression (red) localized to one end (uropod) of polarized neutrophils. Thus, as observed previously,23 there appears to be little specific association between the OPN and CD44 apparent in neutrophils, and the lack of OPN expression did not influence the expression of CD44. Moreover, staining for F-actin showed no difference in stress fibre formation or in the size or shape of polarized neutrophils (Fig. 1c).
Figure 1.
Expression of OPN in neutrophils. (a) OPN mRNA levels in neutrophils (PMNs) were measured by RT-PCR and compared with OPN expression by RAW 264.7 macrophages, using β-actin as a control. A single 408-bp amplicon was amplified with much lower amounts in the neutrophils (+ RT). Only a trace of product was observed in the absence of reverse transcriptase (– RT). Quantitative PCR revealed that OPN mRNA in the neutrophils was 75-fold less than in the RAW 264.7 cells. (b) Confocal immunofluorescence analysis of polarized neutrophils stained for cell-surface surface (non-permeablized cells) CD44 (red) and cellular (permeabilized cells) OPN (green). No significant colocalization of the punctate OPN with the CD44, which is concentrated in the uropod, was evident. (c) Immunofluorescence staining of neutrophils with TRITC-phalloidin to examine F-actin distribution showed no differences between the OPN–/– null and WT cells regardless of their polarization state.
Analysis of neutrophil chemotaxis and chemokinesis
To determine whether OPN functions in the migration of neutrophils, studies of chemokinesis and chemotaxis were performed in modified Boyden chambers (Transwell chambers®) and in Zigmond chambers. Addition of the chemoattractant fMLP (10−6 mol/l) to the lower side or both sides of the Transwell chambers increased both chemotactic and chemokinetic migration of freshly isolated WT neutrophils, respectively (Fig. 2a). Whereas the basal migration (PBS control) was not significantly different from WT cells, both chemotaxis and chemokinesis were markedly reduced in the OPN–/– neutrophils. The impaired chemotaxis of the OPN–/– cells could be rescued dose-dependently by the addition of exogenous OPN to 0·25 μg/ml. However, at higher concentrations (1·0 μg/ml), the effect of OPN was reduced. Chemotaxis was also suppressed by the addition of OPN to the WT neutrophils, indicating that OPN was exerting a chemotactic effect that competed with the response of the cells to the fMLP gradient. That OPN is chemotactic for neutrophils was demonstrated by a dose-dependent increase in migration for both the WT and OPN–/– neutrophils when OPN was added to the lower chamber of the Transwell system (Fig. 2b).
Figure 2.
Migration of neutrophils in transwell chambers. Neutrophils were analysed for migration in response to 10−6 mol/l fMLP stimulation and OPN using BSA as a control. The number of neutrophils migrating through the 3·0-μm pores after 30 min in Transwell chambers was determined and the means ± SD were calculated for triplicate samples. (a) Migration toward fMLP, which was consistently decreased in OPN–/– neutrophils, could be partially rescued by low concentrations of exogenous macrophage OPN added to both sides (BS) of the membrane, but decreased migration in both WT cells and in OPN–/– cells at higher concentrations. (b) A dose-dependent increase in migration towards OPN added to the lower chamber (LC) was observed for both WT and OPN–/– cells, the OPN–/– neutrophils responding more strongly. The results from one of three replicate experiments are shown.
In vitro neutrophil polarization and migration
Migration analyses in Zigmond chambers allowed cell polarization and migration speed to be analysed, together with measurements of chemotaxis by individual cells.25 The proportion of WT and OPN–/– neutrophils that polarized in any direction (random) and in the direction of the chemoattractant was examined before and during the first 15 min of fMLP stimulation (Fig. 3a). While the proportion of WT and OPN–/– neutrophils that migrated randomly and directionally after 15 min was similar for both phenotypes there was a much lower proportion of directionally migrating cells compared to randomly migrating cells (Fig. 3a). The addition of macrophage OPN (1 μg/ml and 2·5 μg/ml) to OPN–/– cells increased both random and directed migration reproducibly but with low significance (P < 0·1). When the cells were analysed for speed of polarization, there was little difference for random polarization, whereas directed polarization to fMLP was always less in the OPN–/– cells (Fig. 3b). Although these differences were not significant at the P < 0·05 level at any time-point, they were a consistent finding in replicate experiments. In the presence of exogenous OPN the speed of polarization of OPN–/– cells was increased, but this was significant only for random polarization (P < 0·05). In agreement with the Transwell assays, OPN–/– neutrophils had a slower migration speed, which was markedly increased in the presence of exogenous OPN (P < 0·001) (Fig. 3c).
Figure 3.
Polarization and migration of neutrophils in a Zigmond chamber. (a) The proportion of neutrophils that polarized randomly and in the direction of fMLP was determined after 15 min. Fewer cells were seen to display directed migration for both WT and OPN–/– neutrophils. Addition of exogenous macrophage OPN to OPN–/– cells increased both random and directed migration reproducibly, but not significantly (P > 0·1). (b) Analysis of neutrophils over the 15-min time interval consistently showed a lower directed polarization for the OPN–/– cells, but the differences were not statistically significant. Exogenous OPN increased both random and directed polarization to similar levels observed in WT neutrophils but the increases were only significant (*P < 0·05) for the random polarization. (c) The average migration speed of OPN–/– neutrophils was lower than the WT cells (*P < 0·05). While fMLP stimulation increased the average speed of both WT and OPN–/– neutrophils, the effect was less in the OPN–/– neutrophils. Addition of exogenous OPN increased migration of OPN–/– cells approximately three-fold (***P < 0·001). Results are expressed as means ± SD.
Recruitment of neutrophils in vivo
Since OPN has been shown to be required for normal migration of macrophages, we first studied the recruitment of neutrophils into the peritoneal cavity using sodium periodate. The total number of cells recovered in the peritoneal lavage following periodate stimulation was more than two-fold lower in OPN-null mice and the percentage of neutrophils recovered was more than three-fold lower than in the WT controls (Fig. 4a), indicating that the absence of OPN has an impact on the recruitment of neutrophils. In contrast, the proportion of other immune cells, such as monocytes and lymphocytes, was not lowered. Notably, no differences in the total number of cells recovered, or in the fractions of different populations including neutrophils, were observed following longer stimulation (48 hr) with 1% Brewer's thioglycollate medium (not shown), which is used for macrophage peritoneal preparations.18 The importance of exogenous OPN for the in vivo recruitment of neutrophils was demonstrated by peritoneal injection of both native and recombinant OPN (Fig. 4b) inducing a peritoneal infiltrate comprising predominantly PMNs (> 65% and 90%, respectively) whereas lower numbers of PMN (< 3%) were present in the vehicle-injected controls.
Figure 4.
Recruitment of neutrophils in vivo. The total number of immune cells in peritoneal exudates following intraperitoneal injections of 5 mm periodate or 20 μg/ml OPN was determined in Wright–Giemsa-stained slides using a haemocytometer. (a) Recruitment of immune cells by periodate was more than three-fold lower (open bars) in OPN–/– null mice with a disproportionately lower recruitment of neutrophils (black bars). (b) In response to administration of full-length native or recombinant OPN an infiltrate containing predominantly neutrophils was obtained, whereas PBS vehicle control did not recruit any significant numbers of neutrophils. Results are expressed as mean ± SD.
Phagocytosis assays
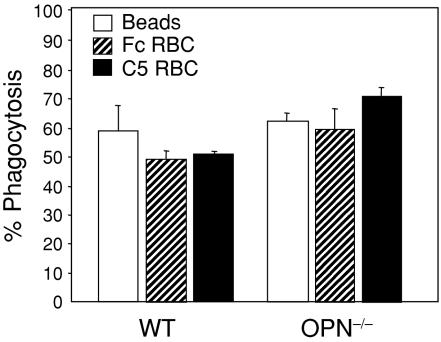
To determine whether the functional activities of neutrophils were affected by the absence of OPN expression, superoxide and matrix metalloproteinase production were determined together with their phagocytic activity. The general phagocytic activity of the neutrophils was analysed by incubating neutrophils with fluorescent beads and determining the percentage of neutrophils with phagocytosed beads. Selective phagocytosis through both Fcγ and complement receptors was analysed by incubating neutrophils with sRBCs coated with IgG and IgM ligands, respectively. No impairment was evident in the ability of neutrophils derived from OPN-null mice to phagocytose uncoated beads, or ligand-coated beads (Fig. 5). Instead, there was an indication that specific phagocytosis was increased in the OPN–/– cells, suggesting that OPN expression in neutrophils is not required for either general or specific phagocytosis.
Figure 5.
Phagocytosis assays. The ability of LPS-stimulated neutrophils to phagocytose polystyrene beads was assessed by incubating cells for 25 min at 37° with fluorescent beads and the number of beads internalized by neutrophils was determined by flow cytometry. No differences between cells derived from OPN–/– null mice or WT controls in the phagocytosis of beads were observed. Specific phagocytosis mediated through Fc or complement receptors (C5) was analysed using red blood cells labelled with Texas Red–sulphonyl chloride dye. No significant differences were observed between cells derived from OPN–/– mice or WT controls in the phagocytosis of beads. Samples from three separate experiments were analysed by fluorescence microscopy and flow cytometry and the results are expressed as the percentage of cells containing one or more beads (mean ± SD).
Superoxide production
Production of superoxide, which is an integral part of the bactericidal activity of neutrophils,26 was measured by NADPH oxidase activity, which generates H2O2. Neutrophils were stimulated with either LPS or fMLP and NADPH oxidase activity was measured as mean fluorescence intensity after 15 min. No significant differences in superoxide production were evident between the WT and OPN–/– neutrophils with and without stimulation by LPS or fMLP (Fig. 6a). The lack of differences was verified by normalizing the data to the unstimulated controls for each cell type (Fig. 6b).
Figure 6.
Neutrophil production of H2O2 was assessed from the mean fluorescence intensity (MFI) of rhodamine-123 and used as a measure of the oxidative burst. Samples were analysed by fluorescence microscopy and flow cytometry (mean ± SD). (a) No differences were observed between the WT and OPN–/– neutrophils stimulated with either fMLP or LPS. (b) The ratio of fluorescence between stimulated and unstimulated PMNs confirmed the lack of significant differences in oxidative burst ability.
Analysis of cytokines and matrix metalloproteinase 9 (MMP-9)
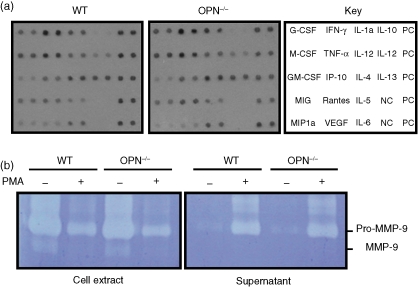
Cytokine production by neutrophils is an integral part of the innate immune response. The cytokines are stored in granules and released when neutrophils are activated. The cytokines released by PMA in WT and OPN–/– were assessed using a cytokine blot (Panomics). Although the amounts of different cytokines varied, with higher levels of interferon-γ (IFN-γ), TNF-α, interleukin-1α (IL-1α) and IL-4, there were no significant differences in the pattern of cytokines released by WT and OPN–/– neutrophils (Fig. 7a). Neutrophils characteristically produce the gelatinase, MMP-9, which functions in inflammation-associated tissue destruction27 that can be attenuated by the selective inhibition of MMP-9.28 MMP-9 was analysed in cell extracts and conditioned PBS with and without de-granulation with PMA, as shown in a representative enzymogram (Fig. 7b). The majority of enzyme was present inside the cells in the latent, 92 000 molecular weight pro-MMP form, with only a trace of activated MMP-9. More than half of the pro-MMP was released by PMA treatment of the neutrophils. However, no significant differences were observed in the activation or release of pro-MMP-9 by PMA treatment of WT and OPN–/– cells.
Figure 7.
Cytokine release and gelatin enzymography. Neutrophils were incubated with 0·1 µm PMA to stimulate neutrophil degranulation. (a) Cytokines released into PBS were analysed on a cytokine blot. The key to duplicate spots for cytokines and both positive (PC) and negative (NC) controls is shown in the right panel. No differences in the release of cytokines by WT and OPN–/– cells were evident in these assays. (b) Gelatinolytic enzymes in cell extracts and in conditioned PBS with and without stimulation with PMA were analysed by enzymography. Only pro-MMP-9 and activated MMP-9 were detected, together with an unidentified slower migrating protein with gelatinase activity. Most of the activity corresponded to the pro-MMP-9, which was effectively released into the PBS by the PMA treatment. No differences in MMP-9 expression between the WT and OPN–/– neutrophils were evident in replicate experiments.
Discussion
Ablation of OPN expression has been shown to reduce the magnitude of the immune response in several models of inflammatory diseases including arthritis,9 multiple sclerosis,10 kidney disease8 and lung fibrosis,6 in which granuloma formation and T-cell responses predominate in the established inflammatory lesion. Although it is evident that OPN produced by macrophages and T lymphocytes has an important role in cell-mediated immunity, its function in neutrophils in the early stages of the innate response has not been reported. To determine the basis of the exacerbated inflammatory destruction observed in experimental colitis of OPN-null mice, which is associated with an increase of myeloperoxidase activity,5 we investigated the effects of OPN on neutrophil function. These studies have revealed defective recruitment of neutrophils in OPN-null mice, which may relate to impaired chemotaxis or the requirement for exogenous OPN. However, the functional activities of the OPN–/– neutrophils, including cytocidal activities, phagocytosis, generation of reactive oxygen species (ROS; oxidative burst), cytokine and MMP-9 production, were not impaired. Thus, while OPN may be important for neutrophil recruitment, in experimental colitis the rich blood supply to the gut is likely to circumvent this deficit and provide enough neutrophils in the inflamed tissues to compensate for the impaired activity of macrophages and lymphocytes.5 Moreover, the elevated destruction of the inflamed gut tissues can be explained by the persistence of the neutrophils29 because of the reduced clearance by the defective macrophages in the OPN-null mice.4
Neutrophils are highly motile cells that function in the immediate innate immune response, phagocytosing foreign materials and protecting the host by production of cytotoxic agents such as ROS and secretion of proteolytic enzymes. In regulated innate responses neutrophils are active at the injured site for up to 24 hr, after which time they undergo apoptosis and are cleared by macrophages. In certain inflammatory pathologies, such as oxidative stress, neutrophils can persist or exhibit hyperactivity, resulting in excessive damage to the injured tissue.20,30–32 Recent studies have shown that OPN–/– macrophages exhibit cytoskeletal defects that affect their migration18 and exhibit impaired cytokine production, survival and phagocytic activity (authors unpublished). However, neutrophils express much lower amounts of OPN in comparison to macrophages (Fig. 1) and secretion of OPN by neutrophils could not be demonstrated in our studies. Moreover, unlike macrophages, neutrophils do not demonstrate an intracellular association of OPN with the CD44 receptor and the morphological changes reflecting F-actin rearrangements seen in OPN–/– macrophages18 were not evident in OPN–/– neutrophils. Thus, if neutrophils express an intracellular form of OPN12 that is thought to regulate the formation of cell processes and cell motility in fibroblasts24 and macrophages,18,33 and has been observed to regulate IFN-α production in plasmacytoid dendritic cells,34 the association with CD44 and effects on the cell cytoskeleton are not evident. As discussed previously,23 the difference in OPN association with CD44 may reflect differences in the basic mechanisms involved in the migration of neutrophils, which express lower levels of integrins.35 Thus, in neutrophils, which migrate more rapidly than macrophages, the expression and localization of CD44 is largely independent of OPN,4 (Fig. 1), suggesting that OPN may function differently in these cells.
That OPN–/– neutrophils exhibit impaired acute/short-term peritoneal recruitment probably relates to the observed defects in chemotactic and chemokinetic migration, which appear to be associated with the absence of exogenous OPN (Figs 2–4). Although the recovery of total inflammatory cells in the peritoneal lavage was significantly lower in OPN-null mice, there was a disproportionate decrease in neutrophils compared to monocytes and lymphocytes (Fig. 4a) in response to periodate stimulation. Since marrow preparations of neutrophils for the in vitro analyses did not show any difference in cell counts in general, or neutrophils in particular, when the WT and OPN–/– cells were compared (data not shown), the lowered fraction of neutrophils following acute stimulation does not appear to represent a defect in the production of neutrophils in the marrow. Notably, no decrease in the proportion of neutrophils was evident in the peritoneum 48–72 hr after administration of dithioglycollate, indicating an impairment in the initial recruitment of neutrophils, but not in the subsequent accumulation of cells. Consistent with the robust and selective recruitment of neutrophils into the peritoneum (Fig. 4b) and the demonstration that neutrophils are the main cell to be recruited by extracellular OPN in alcohol-induced hepatitis,19 our in vitro studies showed that exogenous OPN is chemotactic for neutrophils (Fig. 2b) and, while providing a partial rescue of polarization, markedly increased migration (Fig. 3). Thus, the impaired recruitment of the OPN–/– neutrophils in the OPN-null mice can be attributed to the lack of both chemotactic and migration effects on the neutrophils.
Although it is difficult to separate the effects of exogenous OPN from the OPN expressed by the neutrophils to identify the role of the neutrophil OPN, it is clear that the exogenous OPN has a more prominent effect on neutrophil recruitment. Moreover, the exogenous OPN, which is released by resident and immune cells at sites of injury, appears to exert its functions through the CD44 receptor. This is indicated by the ability of recombinant OPN, lacking post-translational modifications, to show the same efficacy as native OPN for peritoneal recruitment (Fig. 4b), and our previous demonstration that CD44 receptor expression is important for the directed migration and polarization of neutrophils.23 Notably, post-translational modifications have been shown to be important for OPN signalling through integrins but not for CD44-mediated activities.15
In contrast to the defects in motility, OPN–/– neutrophils did not show any impairment in their phagocytic, cytotoxic, matrix degradative activities or cytokine expression. Thus, unspecific phagocytosis of fluorescent beads, and receptor-associated phagocytosis through Fc or complement receptors, was equally efficient in the OPN–/– neutrophils, with some higher values for the OPN–/– neutrophils (Fig. 6). As phagocytosis is a cytoskeleton-dependent process, in which re-organization of their cytoskeleton in response to stimuli is required to extend membranal protuberances supported by F-actin, the absence of cytoskeletal defects in the OPN–/– cells (Fig. 1b) is in agreement with the lack of defects in phagocytosis. Similarly, ROS production and MMP-9 production are not impaired in the OPN–/– neutrophils (Figs 6 and 7). The ability to generate ROS is one of the important killing mechanisms of neutrophils and impaired ROS generation results in pathologies such as chronic granulomatous disease.26 MMP-9 is a prominent matrix-degrading enzyme typically expressed at high levels in activated neutrophils. The gelatinase activity expressed by neutrophils has a marked impact in destructive inflammatory diseases and is used as a marker for disease activity,27 while its selective inhibition can protect against inflammatory injury by neutrophils.28 The sustained production of ROS and MMP-9 by neutrophils is likely to be responsible for the massive destruction of the intestinal crypts when colitis is induced in OPN-null mice.5
In summary, these studies have demonstrated the importance of OPN expression in general and exogenous OPN in particular for the migration and recruitment of neutrophils. However, no significant affects of OPN were observed on the phagocytic, cytotoxic or matrix degradative activities of neutrophils. Thus, while the initial recruitment of neutrophils may be impaired, their destructive effects are not limited by OPN expression and are likely to be highly dependent on their removal from inflammatory sites.
Acknowledgments
This work was funded by grants MOP-36333 and MOP-457134 from the Canadian Institutes of Health Research to J.S. and R.Z. and by Sick Kids Foundation grant MOP-459476 to R.Z.
References
- 1.Giachelli CM, Steitz S. Osteopontin: a versatile regulator of inflammation and biomineralization. Matrix Biol. 2000;19:615–22. doi: 10.1016/s0945-053x(00)00108-6. [DOI] [PubMed] [Google Scholar]
- 2.O'Regan A, Berman JS. Osteopontin: a key cytokine in cell-mediated and granulomatous inflammation. Int J Exp Pathol. 2000;81:373–90. doi: 10.1046/j.1365-2613.2000.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denhardt D, Noda M, Aw OR, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–61. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sodek J, Batista Da Silva AP, Zohar R. Osteopontin and mucosal protection. J Dent Res. 2006;85:404–15. doi: 10.1177/154405910608500503. [DOI] [PubMed] [Google Scholar]
- 5.da Silva AP, Pollett A, Rittling SR, Denhardt DT, Sodek J, Zohar R. Exacerbated tissue destruction in DSS-induced acute colitis of OPN-null mice is associated with downregulation of TNF-alpha expression and non-programmed cell death. J Cell Physiol. 2006;208:629–39. doi: 10.1002/jcp.20701. [DOI] [PubMed] [Google Scholar]
- 6.O'Regan AW, Hayden JM, Body S, Liaw L, Mulligan N, Goetschkes M, Berman JS. Abnormal pulmonary granuloma formation in osteopontin-deficient mice. Am J Respir Crit Care Med. 2001;164:2243–7. doi: 10.1164/ajrccm.164.12.2104139. [DOI] [PubMed] [Google Scholar]
- 7.Miyazaki Y, Tashiro T, Higuchi Y, Setoguchi M, Yamamoto S, Nagai H, Nasu M, Vassalli P. Expression of osteopontin in a macrophage cell line and in transgenic mice with pulmonary fibrosis resulting from the lung expression of a tumor necrosis factor-alpha transgene. Ann N Y Acad Sci. 1995;760:334–41. doi: 10.1111/j.1749-6632.1995.tb44651.x. [DOI] [PubMed] [Google Scholar]
- 8.Noiri E, Dickman K, Miller F, et al. Reduced tolerance to acute renal ischemia in mice with a targeted disruption of the osteopontin gene. Kidney Int. 1999;56:74–82. doi: 10.1046/j.1523-1755.1999.00526.x. [DOI] [PubMed] [Google Scholar]
- 9.Yumoto K, Ishijima M, Rittling SR, et al. Osteopontin deficiency protects joints against destruction in anti-type II collagen antibody-induced arthritis in mice. Proc Natl Acad Sci USA. 2002;99:4556–61. doi: 10.1073/pnas.052523599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chabas D, Baranzini SE, Mitchell D, et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–5. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 11.Jansson M, Panoutsakopoulou V, Baker J, Klein L, Cantor H. Cutting edge: attenuated experimental autoimmune encephalomyelitis in eta-1/osteopontin-deficient mice. J Immunol. 2002;168:2096–9. doi: 10.4049/jimmunol.168.5.2096. [DOI] [PubMed] [Google Scholar]
- 12.Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Medical. 2000;11:279–303. doi: 10.1177/10454411000110030101. [DOI] [PubMed] [Google Scholar]
- 13.Diao H, Kon S, Iwabuchi K, et al. Osteopontin as a mediator of NKT cell function in T-cell-mediated liver diseases. Immunity. 2004;21:539–50. doi: 10.1016/j.immuni.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Sato T, Nakai T, Tamura N, et al. Osteopontin/Eta-1 upregulated in Crohn's disease regulates the Th1 immune response. Gut. 2005;54:1254–62. doi: 10.1136/gut.2004.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashkar S, Weber GF, Panoutsakopoulou V, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–4. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 16.Matsui Y, Rittling SR, Okamoto H, et al. Osteopontin deficiency attenuates atherosclerosis in female apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1029–34. doi: 10.1161/01.ATV.0000074878.29805.D0. Epub. [DOI] [PubMed] [Google Scholar]
- 17.Weber GF, Ashkar S. Molecular mechanisms of tumor dissemination in primary and metastatic brain cancers. Brain Res Bull. 2000;53:421–4. doi: 10.1016/s0361-9230(00)00379-8. [DOI] [PubMed] [Google Scholar]
- 18.Zhu B, Suzuki K, Goldberg HA, Rittling SR, Denhardt DT, McCulloch CA, Sodek J. Osteopontin modulates CD44-dependent chemotaxis of peritoneal macrophages through G-protein-coupled receptors: evidence of a role for an intracellular form of osteopontin. J Cell Physiol. 2004;198:155–67. doi: 10.1002/jcp.10394. [DOI] [PubMed] [Google Scholar]
- 19.Banerjee A, Apte U, Smith R, Ramaiah S. Higher neutrophil infiltration mediated by osteopontin is a likely contributing factor to the increased susceptibility of females to alcoholic liver disease. J Pathol. 2006;208:473–85. doi: 10.1002/path.1917. [DOI] [PubMed] [Google Scholar]
- 20.Naito Y, Takagi T, Handa O, et al. Enhanced intestinal inflammation induced by dextran sulfate sodium in tumor necrosis factor-alpha deficient mice. J Gastroenterol Hepatol. 2003;18:560–9. doi: 10.1046/j.1440-1746.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 21.Rittling SR, Matsumoto HN, McKee MD, et al. Mice lacking osteopontin show normal development and bone structure but display altered osteoclast formation in vitro. J Bone Miner Res. 1998;13:1101–11. doi: 10.1359/jbmr.1998.13.7.1101. [DOI] [PubMed] [Google Scholar]
- 22.Sun CX, Downey GP, Zhu F, Koh AL, Thang H, Glogauer M. Rac1 is the small GTPase responsible for regulating the neutrophil chemotaxis compass. Blood. 2004;104:3758–65. doi: 10.1182/blood-2004-03-0781. [DOI] [PubMed] [Google Scholar]
- 23.Alstergren P, Zhu B, Glogauer M, Mak T, Ellen R, Sodek J. Polarization and directed migration of murine neutrophils is dependent on cell-surface expression of CD44. J Cell Immunol. 2004;231:146–57. doi: 10.1016/j.cellimm.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Zohar R, Suzuki N, Suzuki K, Arora P, Glogauer M, McCulloch CA, Sodek J. Intracellular osteopontin is an integral component of the CD44-ERM complex involved in cell migration. J Cell Physiol. 2000;184:118–30. doi: 10.1002/(SICI)1097-4652(200007)184:1<118::AID-JCP13>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 25.Koh AL, Sun CX, Zhu F, Glogauer M. The role of Rac1 and Rac2 in bacterial killing. Cell Immunol. 2005;235:92–7. doi: 10.1016/j.cellimm.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Roos D, van Bruggen R, Meischl C. Oxidative killing of microbes by neutrophils. Microbes Infect. 2003;5:1307–15. doi: 10.1016/j.micinf.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Peake NJ, Foster HE, Khawaja K, Cawston TE, Rowan AD. Assessment of the clinical significance of gelatinase activity in patients with juvenile idiopathic arthritis using quantitative protein substrate zymography. Ann Rheum Dis. 2006;65:501–7. doi: 10.1136/ard.2005.039032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JH, Suk MH, Yoon DW, et al. Inhibition of matrix metalloproteinase-9 prevents neutrophilic inflammation in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291:L580–7. doi: 10.1152/ajplung.00270.2005. [DOI] [PubMed] [Google Scholar]
- 29.Haslett C, Savill JS, Whyte MK, Stern M, Dransfield I, Meagher LC. Granulocyte apoptosis and the control of inflammation. Philos Trans R Soc Lond B Biol Sci. 1994;345:327–33. doi: 10.1098/rstb.1994.0113. [DOI] [PubMed] [Google Scholar]
- 30.Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science. 2002;296:155–8. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- 31.Kruidenier L, Kuiper I, Lamers CB, Verspaget HW. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J Pathol. 2003;201:28–36. doi: 10.1002/path.1409. [DOI] [PubMed] [Google Scholar]
- 32.Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61:647–53. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki K, Zhu B, Rittling SR, Denhardt DT, Goldberg HA, McCulloch CA, Sodek J. Colocalization of intracellular osteopontin with CD44 is associated with migration, cell fusion, and resorption in osteoclasts. J Bone Miner Res. 2002;17:1486–97. doi: 10.1359/jbmr.2002.17.8.1486. [DOI] [PubMed] [Google Scholar]
- 34.Shinohara ML, Lu L, Bu J, Werneck MB, Kobayashi KS, Glimcher LH, Cantor H. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol. 2006;7:498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedl P, Brocker EB, Zanker KS. Integrins, cell matrix interactions and cell migration strategies: fundamental differences in leukocytes and tumor cells. Cell Adhes Commun. 1998;6:225–36. doi: 10.3109/15419069809004478. [DOI] [PubMed] [Google Scholar]