Abstract
Although chemotherapy remains among the best treatment options for most cancers, adjuvant therapies such as dendritic cell (DC)-based immunotherapy have been added to treatment protocols to destroy residual tumour cells. Combination treatment with low-dose temozolomide (TMZ) chemotherapy followed by vaccination with TAT-survivin-pulsed DCs enhanced T-cell responses specific for survivin and improved survival rate, as compared with DC alone or TMZ alone. Moreover, antigen-specific immunity appears to be mediated by CD8+ T cells, as determined by in vitro T-cell subset depletion. These studies demonstrated that a combination of low-dose TMZ chemotherapy and TAT-based DC immunotherapy may be a novel strategy for safe and effective treatment of malignant gliomas.
Keywords: dendritic cells, glioma, human immunodeficiency virus Tat, surviving, temozolomide
Introduction
Malignant gliomas are the most common primary brain tumour of the central nervous system in adults.1 The prognosis for patients who are diagnosed with a high-grade glioma is very poor regardless of the conventional treatments, including surgical removal, radiotherapy and chemotherapy.2
Temozolomide (TMZ) is a new orally administered, second-generation imidazotetrazine prodrug with an essentially 100% oral bioavailability.3,4 It has been reported that TMZ is active against malignant brain tumours, particularly chemoresistant malignant gliomas.5 Numerous studies have been focusing on the development of strategies to optimize the clinical efficacy of TMZ by different dosing schedules and combination with other antineoplastic agents. Recently, it has been demonstrated that integrating tumour vaccine with standard cytotoxic chemotherapy could have a profound pharmacodynamic influence on the vaccine-induced antitumour response.6 Therefore, further investigation of additional therapeutic strategies that combine TMZ chemotherapy and dendritic cell (DC)-based immunotherapy against malignant glioma may further enhance the survival.
Survivin, a member of the inhibitor of apoptosis proteins family, has a capacity to suppress apoptotic cell death and regulate cell division and it is present during normal fetal development but undetectable in terminally differentiated adult tissues.7–9 Significantly, survivin is aberrantly expressed in most of human cancers of epithelial and haematopoietic origin, including glioma.10,11 Recently, a number of reports revealed that survivin-specific cytotoxic T lymphocyte (CTL) responses can be induced in vitro with peptide-pulsed or RNA-transduced DCs12,13 and the inhibition of survivin expression in tumour cells leads to apoptosis,14 suggesting that this molecule could be an ideal target for tumour immunotherapy. In most trials, tumour-antigen-derived peptides are pulsed on to DCs in vitro and cells are administered to patients. A major limitation of this approach is that the human leucocyte antigen (HLA) type of the patient and the antigen-specific peptide that binds to the patient's HLA class I antigen must be known. As an alternative, many investigators have begun to explore the utility of bacterial recombinant proteins that bear a protein transduction domain (TAT-PTD) as DC-based tumour vaccines. It has been demonstrated that human immunodeficiency virus (HIV) TAT-PTD-containing whole protein antigen-transduced DCs stimulated antigen-specific CD8+ and CD4+ T cells when vaccinated for antigen-expressing tumour models.15,16
In the present study, we evaluated the in vivo effects of combined TMZ chemotherapy and immunotherapy with TAT-survivin-pulsed DCs and the role of CD4+ and CD8+ T cells in antitumour immunity induced by TAT-survivin recombinant protein.
Materials and methods
Animals and cell lines
Six- to eight-week-old female C57BL/6 (H-2b) mice were purchased from SLC (Shizuoka, Japan). The murine glioma cell line GL26 (H-2b) was kindly provided by Dr John S. Yu (Cedars Sinai Medical Center, Los Angeles, CA). The GL26 cells were cultured in Dulbecco's modified Eagle's minimum essential medium (Gibco BRL Co, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (Gibco BRL), 2 mm l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin.
Purification of recombinant HIV TAT PTD-containing survivin protein
The TAT survivin expression vectors, based on pET-15b (Novagen, Darmstadt, Germany), were constructed by a reverse trancription polymerase chain reaction (RT-PCR) using sense primer 5′-CGAACTCGAGATGGGTGCCCCGACGTTG-3′, and antisense primer 5′-GCAAGGATCCTCAATCCATGGCAGCCAG-3′. Subsequently, TAT survivin was amplified using sense primer 5′-GCAGCATATGTATGGAAGGAAGAAGCGGAGACAGCGACGAAGACTCGAGATGGGTGCCCCGACGTTG-3′ encoding YGRKKRRQRRR from HIV TAT47−57, and antisense primer. After sequencing, each vector was introduced into the host Escherichia coli BL21(DE3) to produce recombinant proteins. The purification was performed using Ni-nitrilotriacetic acid (NTA) resin (Qiagen, Valencia, CA), according to the manufacturer's instructions. The lysates and elutes from both the expression and control culture were analysed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE), followed by Coomassie Blue staining. Naked or TAT-PTD fused green fluorescence protein (Naked GFP and TAT GFP, respectively) were also purified as control (data not shown). The endotoxin level of the recombinant proteins was determined using the endotoxin detection kit (Sigma, St Louis, MO). The proteins were verified by Western blot analysis and were stored at −80° until use.
Western blotting and flow cytometry analysis
GL26 cells and DCs were used for Western blotting assay. The assay was performed as previously described with modification via chemiluminescence utilizing an Amersham ECL Advance system (Amersham) with semidry transfer. Detection was performed with an anti-surivin (clone D-8) antibody (Santa Cruz Biotechnologies, Santa Cruz, CA). For fluorescence-activated cell sorter (FACS) analysis, DCs were pulsed with TAT-PTD–survivin (2 μm) for 18 hr and analysed by FACSCalibur flow cytometry (Becton Dickinson, Franklin Lakes, NJ). Unpulsed DCs or naked survivin-pulsed DCs were used as negative control. Incubation of DCs with TAT-PTD–protein resulted in transduction of 90% of cells (Cho et al. manuscript in preparation and unpublished data), which was in agreement with previous findings showing that a TAT–β-gal fusion protein was capable of efficiently delivering peptide or proteins into different types of cells.17,18
Generation of bone-marrow-derived DCs
The DCs were prepared from bone marrow as described previously, with minor modifications.19 In brief, bone marrow cells were harvested from tibias and femurs of normal C57BL/6 mice. The cells were washed twice in serum-free RPMI-1640 (Gibco BRL) medium and cultured in six-well culture plates at 5 × 106 cells/well in complete RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, recombinant murine granulocyte–macrophage colony-stimulating factor (GM-CSF; 20 ng/ml; R & D Systems, Minneapolis, MN) and recombinant murine interleukin-4 (IL-4; 20 ng/ml; R & D Systems). On day 2, non-adherent granulocytes were gently removed and fresh medium with GM-CSF and IL-4 was added. On day 7, non-adherent and loosely adherent cells obtained from these cultures were considered to be immature bone-marrow-derived DCs.
In vivo antitumour effect of combined treatment
The GL26 intracranial (i.c.) model was used for experiments as described previously.20 Mice were treated intraperitoneally (i.p.) with TMZ (2·5 mg/kg/day) from 2 to 6 days and subcutaneously (s.c.) with DCs [1 × 106 cells/mouse in 200 μl phosphate-buffered saline (PBS)] or TAT-survivin-pulsed DCs (1 × 106 cells/mouse in 200 μl PBS; 2 μm for 18 hr) on days 13, 20 and 27 after i.c. GL26 cell inoculation. Six groups were studied: [control PBS, DCs alone, TMZ alone, TAT–survivin-pulsed D (TAT Surv./DC), combined TMZ and unpulsed DC injection (TMZ + DCs), and combined TMZ and TAT-survivin-pulsed DC injection (TMZ + TAT Surv./DCs)]. Seven mice, for each treatment group, were used for the experiments. Representative mice from each treatment group were killed at selected time-points to obtain tissue (spleen) for immunological analysis or tumour-specific splenocyte proliferation. To save the experimental animals according to The Guide for the Care and Use of Laboratory Animals (NRC1996), this study was carried out at the same time as another similar study21 because they used the same control groups, including Control, DCs, TMZ and TMZ + DCs.
Cytotoxicity assay
On day 45 after GL26 cell inoculation, splenocytes were harvested from the different treatment groups for preparation of a single-cell suspension. These splenocytes, restimulated in vitro with 4% paraformaldehyde-prefixed GL26 cells in the presence of recombinant murine IL-2 (20 U/ml) for 5 days, were used as effector cells. The GL26, survivin RNA-electroporated DCs and DC target cells were labelled with 51Cr (100 μCi/1 × 106 cells) for 1 hr, washed four times, and then added to each well in triplicate of 96-well, V-bottomed microtitre plates with various numbers of the effector cells. After incubation for 4 hr at 37°, 100 μl supernatant from each well was collected, and the radioactivity was counted with a gamma counter. The percentage specific lysis was calculated as described previously.19
Enzyme-linked immunospot (ELISPOT) assay
An ELISPOT assay, using a kit purchased from AID (Strassberg, Germany), was performed according to the manufacturer's instructions. In brief, the restimulated splenocytes were seeded into a 96-well multitestplate (MTP) coated with the anti-mouse interferon-γ (IFN-γ) antibody at a concentration of 1 × 105 cells/well in a cell culture medium. The plates were incubated for 24 hr at 37°. The cells were removed, and the plates were washed three times with a washing buffer (provided in the kit) and three times with a PBS-Tween buffer (provided in the kit). Then, 100 μl biotinylated anti-mouse IFN-γ monoclonal antibody (mAb; Detection antibody; provided in the kit) was added to the wells. The plates were incubated for 2·5 hr at room temperature, washed with a PBS-Tween buffer and 100 μl streptavidin–horseradish peroxidase was then added to each well and the wells were incubated for 2 hr at room temperature. The washing step was repeated, chromogenic substrate (provided in the kit) and H2O2 were then immediately added to each well. After developing the spots, the reaction was quenched with distilled water, and the plates were inverted and allowed to dry overnight in the dark. The number of spots corresponding to the IFN-γ-secreting cells was determined using an automatic AID-ELISPOT-Reader (Strassberg, Germany).
In vitro depletion of CD4+ and CD8+ T cells
Splenocytes were reacted with magnetic beads conjugated to monoclonal antibodies to CD4 or CD8 [magnetic antibody cell sorter (MACS), Miltenyi Biotec GmbH, Bergisch Gladbach, Germany] for 1 hr at 4°. Following incubation, the cells were washed with PBS and processed through a MACS magnetic separation column. Cell viability after depletion was determined by trypan blue dye exclusion; depletion was >98% specific T-cell subpopulation by FACS analysis.
Statistical analysis
The results are expressed as a mean ± SEM. Statistical analysis was performed using a Student's t-test, with the exception of the survival data, which were analysed using the Kaplan and Meier test. Survival data were compared using a log-rank test. A value of P < 0·05 was considered significant.
Results
Prolongation of mouse survival by combination treatment
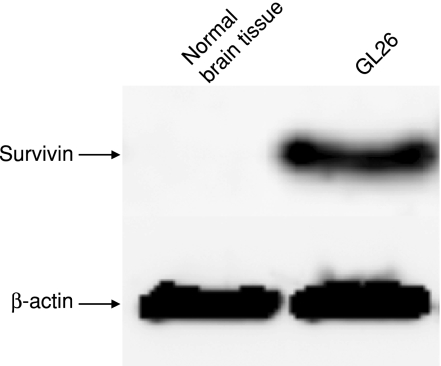
We previously evaluated a variety of doses to optimize an effective low-dose of TMZ.21 Survival was increased in all mice treated with a variety of doses (2·5 mg/kg/day, 5 mg/kg/day, and 10 mg/kg/day) of TMZ compared with the control mice. Toxicity, as measured by weight loss and neurological behaviour, was not observed in any of the TMZ-treated mice (data not shown). Therefore, we used TMZ at a dose of 2·5 mg/kg/day in the following experiments. Endogenous survivin expression was assessed in murine GL26 glioma cells by Western blot (Fig. 1). No survivin expression was detected in normal brain tissue.
Figure 1.
Survivin expression in GL26. Detection of survivin expression by Western blot analysis in GL26. Normal brain tissue was used as negative control.
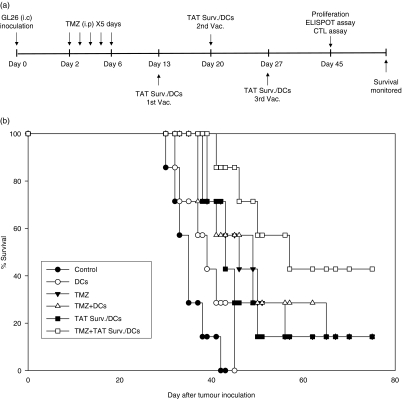
We evaluated the therapeutic efficacy of combination treatment in an established i.c. GL26 glioma model. The treatment schedule is shown schematically in Fig. 2(a). As shown in Fig. 2(b), treatment with TMZ alone, TAT Surv./DCs, and TMZ + DCs demonstrated a slightly longer survival compared with the PBS-treated control or DCs alone. Furthermore, combination treatment with TMZ + TAT Surv./DCs significantly prolonged survival compared with TMZ alone, TAT Surv./DCs, or TMZ + DCs.
Figure 2.
Prolongation of survival by a combination of GL26 glioma model with TMZ and TAT-survivin-pulsed DCs. (a) Experimental schedule of the survival. Mice were treated i.p. with TMZ (2·5 mg/kg/day) from 2 to 6 days and s.c. with DCs (1 × 106 cells/mouse in 200 μl PBS) or TAT-survivin-pulsed DCs [TAT Surv./DCs] (1 × 106 cells/mouse in 200 μl PBS) on days 13, 20 and 27 after i.c. GL26 cell inoculation. (b) Kaplan–Meier survival curve of the mice that received intracranial inoculation of GL26 cells. The significant differences (long-rank test) included: control versus TMZ, P = 0·0009; control versus TMZ + DCs, P = 0·0067; control versus TMZ + TAT Surv./DCs, P = 0·0004; TMZ versus TMZ + DCs, P = 0·7680; TMZ + DCs versus TMZ + TAT Surv./DCs, P = 0·0477; TAT Surv./DCs versus TMZ + TAT Surv./DCs, P = 0·0237. Seven mice were used in each experimental group.
Tumour-specific splenocyte proliferation and ELISPOT assay
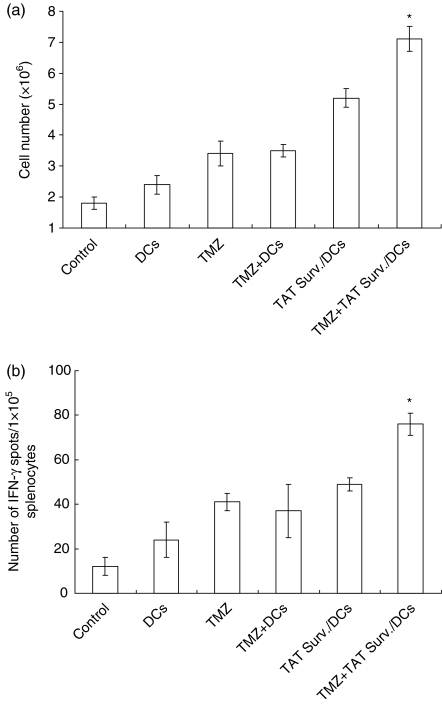
Splenocytes from the mice treated with PBS-treated control, DCs alone, TMZ alone, TAT Surv./DCs, TMZ + DCs, or TMZ + TAT Surv./DCs were examined for the proliferative response of their tumour-specific splenocytes. Splenocytes were harvested from different treatment groups on day 45 after GL26 cell inoculation and then were restimulated in vitro for 5 days with 4% paraformaldehyde prefixed 2 × 105 syngeneic GL26 cells. As shown in Fig. 3(a), the tumour-specific splenocyte proliferative responses were observed when the splenocytes were treated with TMZ + TAT Surv./DCs, and to a lesser extent, TAT Surv./DCs. By contrast, little splenocyte proliferative response was observed when the splenocytes were treated with DCs alone, TMZ alone, and TMZ + DCs. Therefore, the treatment of TMZ + TAT Surv./DCs showed a significantly enhanced tumour-specific T-cell response. In addition, because CTLs are known to produce the T helper type 1 (Th1) cytokine IFN-γ in an antigen-specific manner, we also assayed the presence of tumour-specific CTLs using the IFN-γ ELISPOT assay. As shown in Fig. 3(b), splenocytes from the mice treated with TMZ + TAT Surv./DCs showed significantly higher numbers of IFN-γ-secreting T cells than splenocytes from the mice treated with other preparations in the control group. This result suggests that treatment of mice with TMZ + TAT Surv./DCs enhanced the cell-mediated immune response to syngeneic tumour cells.
Figure 3.
Proliferation and IFN-γ ELISPOT assay of splenocytes in response to tumour antigen. (a) On day 45 after inoculation of GL26 (1 × 104) cells, splenocytes from each group were harvested in vitro and restimulated with 4% paraformaldehyde and prefixed with GL26 cells for 5 days as effector cells. The total number of cells obtained from each well was determined by counting using a haemocytometer. Data are representative of three independent experiments, three mice each, performed in triplicate. Results are given as means ± SE. (b) IFN-γ-secreting splenocytes from the treated mice, as described above, were measured using the ELISPOT assay after restimulation in vitro with 4% paraformaldehyde prefixed with GL26 cells for 5 days. Results represent the mean number of IFN-γ spots per 105 splenocytes from individually tested mice. Statistically significant at *P < 0·01 using a Student's t-test compared with all other groups. Results are given as means ± SE, and are representative of two independent experiments.
Enhanced tumour-specific immune responses by combination treatment
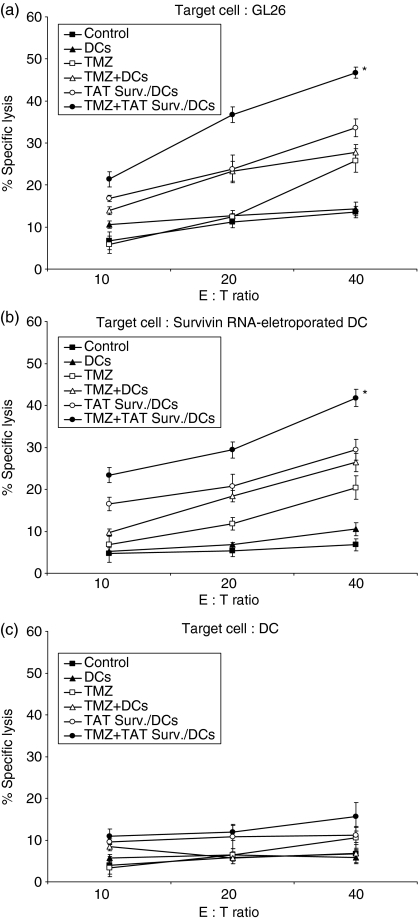
To examine the antitumour immune mechanism associated with combination treatment of TMZ + TAT Surv./DCs, splenocytes were harvested from different treatment groups on day 45 after GL26 cell inoculation, and a single-cell suspension was prepared. As shown in Fig. 4(a), the specific cytotoxicity of the effector cells, from the mice treated with TMZ alone, TAT Surv./DCs and TMZ + DCs, induced killing responses of CTLs against syngeneic GL26 target cells [25·36 ± 1·80%, 34·81 ± 1·15%, and 33·61 ± 2·14%, respectively; effector : target (E : T) ratio of 40 : 1]. In particular, the specific cytotoxicity of the effector cells from the mice treated with TMZ + TAT Surv./DCs significantly enhanced CTL activity against the GL26 target cells (46·7 ± 1·32%; E : T ratio of 40 : 1), but not against control DC target cells (Fig. 4c). Similar results were also achieved in the survivin RNA-transfected DC target cells (Fig. 4b). These results suggest that the combination treatment of TMZ chemotherapy and immunotherapy with TAT-PTD-based DCs enhances the tumour-specific immune response against GL26 glioma cells.
Figure 4.
Enhanced CTL activity by combination treatment with TMZ and TAT-survivin-pulsed DCs in the GL26 glioma model. (a–c) On day 45 after inoculation of GL26 (1 × 104) cells, splenocytes from each group were harvested in vitro and restimulated with 4% paraformaldehyde and prefixed with GL26 cells for 5 days as effector cells. These effector cells were then assessed for their cytolytic activity to target cells (GL26, Survivn RNA-electroporated DCs, and DCs) labelled with 51Cr. The 51Cr release assay was measured after 4 hr of incubation at a variety of E : T ratios. Statistically significant at *P < 0·01 using a Student's t-test compared with all other groups. Results are given as means ± SE, and are representative of two independent experiments.
CD8+ T cells mediated antigen-specific cellular responses by combination treatment
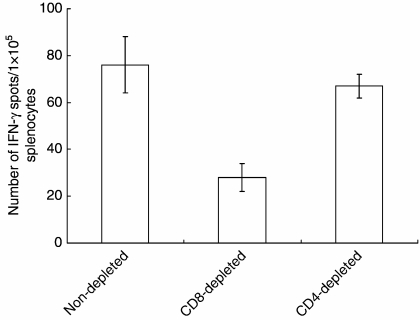
We sought to evaluate whether CD4+ or CD8+ T cells are responsible for the enhanced cell-mediated immune responses induced by TMZ + TAT Surv./DCs. After treatment, we depleted CD4+ or CD8+ T cells in vitro from splenocytes collected from treated mice and then tested the effectors of specific cell population on IFN-γ-secreting T cells using ELISPOT assay. As shown in Fig. 5, when CD8+ T cells were depleted, IFN-γ secretion was decreased to a background level, whereas CD4+ T-cell depletion resulted in the same enhancement of IFN-γ secretion as whole splenocytes from TMZ + TAT Surv./DCs. This indicated that CD8+ T cells are responsible for IFN-γ secretion in a major histocompatibility complex (MHC) class I-dependent manner.
Figure 5.
IFN-γ secretion level by in vitro CD4+ or CD8+ T-cell subset depletion in mice on combined treatment. One week after the last injection, mice were killed and spleen cells were pooled. CD4+ or CD8+ T cells were depleted in vitro from splenocytes and then stimulated in vitro with survivin RNA-electroporated DCs for 18 hr. The number of IFN-γ-secreting T cells specific for survivin was determined by ELISPOT assay on splenocytes from individual mice. Results are given as means ± SE, and are representative of two independent experiments.
Discussion
Chemotherapy and immunotherapy are expected to provide important novel strategies for improved future treatment of malignant gliomas.4,22 Chemotherapy alone frequently fails because of the development of drug resistance or serious toxicity;23 immunotherapy has been unable to maintain effective antitumour immune responses in the presence of a bulky tumour burden because of the effects of tumour-derived immunosuppressive factors such as transforming growth factor-β24 and IL-10.25 Many studies have reported that the combination of chemotherapy and immunotherapy may be more effective than single modality treatment alone.26,27 Here, we investigated the antitumour immunity of combined treatment with low-dose TMZ chemotherapy and TAT-based immunotherapy in a GL26 glioma animal model.
Recently, DCs transduced with HIV TAT protein transduction domain (TAT-PTD)-containing antigen induced CTL in vivo and vaccinated against antigen-expressing tumours.28,29 This 11-amino acid TAT (residues 47–57; YGRKKRRQRR R) PTD was pioneered by Dowdy et al.30 Earlier studies showed that TAT-PTD possessed significantly enhanced protein transduction potential into cells both in vitro and in vivo.31,32 These studies showed that a TAT-PTD-mediated protein can be processed and presented by MHC class I molecules after delivery into DCs (antigen-presenting cells) and can generate an antigen-specific CTL.
With regard to the in vitro activity of TMZ on the glioma cells, Roos et al.33 reported that TMZ inhibited cell proliferation, induced G2/M arrest and apoptosis in U87 human glioma cells. In the present study, TMZ inhibited the proliferation of GL26 cells in a dose-dependent manner at a variety of concentrations (data not shown). Regarding the doses of TMZ chemotherapy, Tentori et al.34 reported that TMZ (100 mg/kg/day i.p. for 3 days) showed antitumour activity against malignant melanoma, gliomas or lymphomas growing in murine brain tumour models. In our GL26 mouse model TMZ at a low-dose (2·5 mg/kg/day) demonstrated survival prolongation without toxicity, and TMZ at higher doses (50 mg/kg and 100 mg/kg) was associated with high mortality (data not shown).
In this study, we demonstrated that combination treatment with low-dose TMZ and TAT–survivin-pulsed DCs significantly prolonged survival and enhanced the tumour-specific immune responses in a GL26 tumour model (Figs 2b, 3b and 4a). This is compatible with our previous observation that combination treatment with low-dose TMZ chemotherapy followed by vaccination with survivin RNA-transfected DCs is required for induction of therapeutic vaccine efficacies in glioma.21 It can be speculated that chemotherapeutic drugs may induce apoptosis of established tumours, leading to cross-priming of tumour-associated antigens.35 In our previous report, DC vaccination induced a cross-priming effect in mice treated with TMZ.21 Furthermore, TMZ treatment could increase expression of survivin and other tumour antigens and antigen presentation on tumour cells. The additional studies will be required to address this important issue.
Some investigators have suggested another possible explanation. Machiels et al.36 and Ghiringhelli et al.37 reported that chemotherapy with cyclophosphamide increased the antitumour immune responses, following DC-based immunotherapy, by suppressing regulatory T cells and increasing the Th1-type immune responses. These regulatory T cells are crucial for maintaining T-cell tolerance to self-antigen in the tumour microenvironment including brain tumours.38 It is also speculated that TMZ chemotherapy may functionally reduce the population of regulatory T cells and induce homeostatic proliferation. Additional studies are necessary to determine whether TMZ reduces the function of regulatory T cells.38
We additionally evaluated a possible role of CD4+ versus CD8+ T cells for induced CTL from TMZ + TAT Surv./DCs-treated mice. This indicated that CD8+ T cells are responsible for enhanced cell-mediated immunity through combined treatment (Fig. 5).
We also experimented with in vitro depletion of CD8+ T cells and CD4+ T cells using TAT–survivin-pulsed DCs as stimulator cells instead of survivin RNA/DC in the ELISPOT assay system. Similar results were achieved in the TAT-survivin-pulsed DCs (data not shown). However, direct assay, such as in vivo depletion studies, should be performed to determine the population responsible for the antitumour effects of the TMZ ± TAT-survivin/DCs in present study.
Although the mechanism for internalization and processing of HIV TAT-PTD-containing protein antigen by DCs has not yet been defined, these data suggested that HIV TAT PTD-containing bacterial recombinant protein might efficiently transduce DCs, prolong the process by proteasomes for MHC class I-restricted presentation to CTL by DCs, and induce antitumour immunity.28,29 Although we have not tested the ability of CTL response and antitumour immunity compared with the naked survivin-pulsed DCs, the antitumour immunity was greater in the TAT-survivin-pulsed DCs than in the naked survivin-pulsed DCs against the murine brain tumour model (Cho et al., manuscript in preparation and unpublished data).
For our in vivo experiments, we started to administer the TMZ at an early stage of tumour development, from day 2 after the i.c. GL26 cell inoculation, at which time the tumour burden is thought to be minimal; this may explain the significant in vivo antitumour effects of the combined treatment in our experiments. These findings support the concept of improved therapeutic effects of chemotherapy and immunotherapy on a smaller tumour burden or early-stage tumours compared to a larger tumour burden or advanced late-stage tumours.
In conclusion, the combination of low-dose TMZ chemotherapy and TAT-survivin-pulsed DC immunotherapy may be a novel strategy for safe and effective treatment of malignant gliomas. Development of the most effective combination protocols, including treatment doses, treatment numbers, treatment timing, administration routes, and treatment schedules of TMZ chemotherapy with DC-based immunotherapy, remain to be determined.
Acknowledgments
This study was supported in part by a grant from the Korea Research Foundation Grant (KRF-2006–005-J00602).
References
- 1.Nazzaro JM, Neuwelt EA. The role of surgery in the management of supratentorial intermediate and high-grade astrocytomas in adults. J Neurosurg. 2006;73:331–44. doi: 10.3171/jns.1990.73.3.0331. [DOI] [PubMed] [Google Scholar]
- 2.Surawicz TS, Davis F, Freels S, Laws ER, Jr, Menck HR. Brain tumor survival: results from the National Cancer Data Base. J Neurooncol. 1998;40:15–60. doi: 10.1023/a:1006091608586. [DOI] [PubMed] [Google Scholar]
- 3.Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin Cancer Res. 2000;6:2585–97. [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin Cancer Res. 2000;6:2585–97. [PubMed] [Google Scholar]
- 6.Emens LA, Jaffee EM. Leveraging the activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res. 2005;65:8059–64. doi: 10.1158/0008-5472.CAN-05-1797. [DOI] [PubMed] [Google Scholar]
- 7.Gianani R, Jarboe E, Orlicky D, et al. Expression of survivin in normal, hyperplastic, and neoplastic colonic mucosa. Hum Pathol. 2001;32:119–25. doi: 10.1053/hupa.2001.21897. [DOI] [PubMed] [Google Scholar]
- 8.Velculescu VE, Madden SL, Zhang L, et al. Analysis of human transcriptosomes. Nat Genet. 1999;23:387–8. doi: 10.1038/70487. [DOI] [PubMed] [Google Scholar]
- 9.Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 10.Chakravarti A, Noll E, Black PM, et al. Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J Clin Oncol. 2002;20:1063–8. doi: 10.1200/JCO.2002.20.4.1063. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda S, Pelus LM. Survivin, a cancer target with an emerging role in normal adult tissues. Mol Cancer Ther. 2006;5:1087–98. doi: 10.1158/1535-7163.MCT-05-0375. [DOI] [PubMed] [Google Scholar]
- 12.Andersen MH, Pedersen LO, Capeller B, Brocker EB, Becker JC, thor Straten P. Spontaneous cytotoxic T-cell responses against survivin-derived MHC class I-restricted T-cell epitopes in situ as well as ex vivo in cancer patients. Cancer Res. 2001;61:5964–8. [PubMed] [Google Scholar]
- 13.Zeis M, Siegel S, Wagner A, et al. Generation of cytotoxic responses in mice and human individuals against hematological malignancies using survivin-RNA-transfected dendritic cells. J Immunol. 2003;170:5391–7. doi: 10.4049/jimmunol.170.11.5391. [DOI] [PubMed] [Google Scholar]
- 14.Blanc-Brude OP, Mesri M, Wall NR, Plescia J, Dohi T, Altieri DC. Therapeutic targeting of the survivin pathway in cancer. Initiation of mitochondrial apoptosis and suppression of tumor-associated angiogenesis. Clin Cancer Res. 2003;9:2683–92. [PubMed] [Google Scholar]
- 15.Shibagaki N, Udey MC. Dendritic cells transduced with protein antigen induce cytotoxic lymphocytes and elicit antitumor immunity. J Immunol. 2002;168:2393–401. doi: 10.4049/jimmunol.168.5.2393. [DOI] [PubMed] [Google Scholar]
- 16.Wang HY, Fu T, Wang G, et al. Induction of CD4+ T cell-dependent antitumor immunity by TAT-mediated tumor antigen delivery into dendritic cells. J Clin Invest. 2002;109:1463–70. doi: 10.1172/JCI15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fawell S, Seery J, Daikh Y, Moore C, Chen LL, Pepinsky B, Barsoum J. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci USA. 1994;91:664–8. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–72. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 19.Kim CH, Hong MJ, Park SD, et al. Enhancement of anti-tumor immunity specific to murine glioma by vaccination with tumor cell lysate-pulsed dendritic cells engineered to produce interleukin-12. Cancer Immunol Immunother. 2006;55:1309–19. doi: 10.1007/s00262-006-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong YK, Chung DS, Joe YA, et al. Efficient inhibition of in vivo human malignant glioma growth and angiogenesis by interferon-beta treatment at early stage of tumor development. Clin Cancer Res. 2000;6:3354–60. [PubMed] [Google Scholar]
- 21.Park SD, Kim CH, Kim CK, Park JA, Sohn HJ, Hong YK, Kim TG. Cross-priming by temozolomide enhances anti-tumor immunity of dendritic cells vaccination in murine brain tumor model. Vaccine. 2007;25:3485–91. doi: 10.1016/j.vaccine.2006.12.060. [DOI] [PubMed] [Google Scholar]
- 22.Yamanaka R, Yajima N, Abe T, et al. Dendritic cell-based glioma immunotherapy. Int J Oncol. 2003;23:5–15. [PubMed] [Google Scholar]
- 23.Shah MA, Schwartz GK. Cell cycle-mediated drug resistance: an emerging concept in cancer therapy. Clin Cancer Res. 2001;7:2168–81. [PubMed] [Google Scholar]
- 24.Constam DB, Philipp J, Malipiero UV, ten Dijke P, Schachner M, Fontana A. Differential expression of transforming growth factor-beta 1, -beta 2, and -beta 3 by glioblastoma cells, astrocytes, and microglia. J Immunol. 1992;148:1404–10. [PubMed] [Google Scholar]
- 25.Chen Q, Daniel V, Maher DW, Hersey P. Production of IL-10 by melanoma cells: examination of its role in immunosuppression mediated by melanoma. Int J Cancer. 1994;56:755–60. doi: 10.1002/ijc.2910560524. [DOI] [PubMed] [Google Scholar]
- 26.Lake RA, Robinson BW. Immunotherapy and chemotherapy – a practical partnership. Nat Rev Cancer. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 27.Bourquin C, Schreiber S, Beck S, Hartmann G, Endres S. Immunotherapy with dendritic cells and CpG oligonucleotides can be combined with chemotherapy without loss of efficacy in a mouse model of colon cancer. Int J Cancer. 2006;118:2790–5. doi: 10.1002/ijc.21681. [DOI] [PubMed] [Google Scholar]
- 28.Kim DT, Mitchell DJ, Brockstedt DG, et al. Introduction of soluble proteins into the MHC class I pathway by conjugation to an HIV tat peptide. J Immunol. 1997;159:1666–8. [PubMed] [Google Scholar]
- 29.Mitsui H, Inozume T, Kitamura R, Shibagaki N, Shimada S. Polyarginine-mediated protein delivery to dendritic cells presents antigen more efficiently onto MHC class I and class II and elicits superior antitumor immunity. J Invest Dermatol. 2006;126:1804–12. doi: 10.1038/sj.jid.5700335. [DOI] [PubMed] [Google Scholar]
- 30.Schwarze SR, Hruska KA, Dowdy SF. Protein transduction: unrestricted delivery into all cells? Trends Cell Biol. 2000;10:290–5. doi: 10.1016/s0962-8924(00)01771-2. [DOI] [PubMed] [Google Scholar]
- 31.Morris MC, Depollier J, Mery J, Heitz F, Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotechnol. 2001;19:1173–6. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
- 32.Ho A, Schwarze SR, Mermelstein SJ, Waksman G, Dowdy SF. Synthetic protein transduction domains: enhanced transduction potential in vitro and in vivo. Cancer Res. 2001;61:474–7. [PubMed] [Google Scholar]
- 33.Roos WP, Batista LF, Naumann SC, et al. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O(6)-methylguanine. Oncogene. 2007;26:186–97. doi: 10.1038/sj.onc.1209785. [DOI] [PubMed] [Google Scholar]
- 34.Tentori L, Leonetti C, Scarsella M, et al. Systemic administration of GPI 15427, a novel poly (ADP-ribose) polymerase-1 inhibitor, increases the anti-tumor activity of temozolomide against intracranial melanoma, glioma, lymphoma. Clin Cancer Res. 2003;9:5370–9. [PubMed] [Google Scholar]
- 35.Nowak AK, Lake RA, Marzo AL, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170:4905–13. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 36.Machiels JP, Reilly RT, Emens LA, et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the anti-tumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–97. [PubMed] [Google Scholar]
- 37.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide, which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–44. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 38.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]